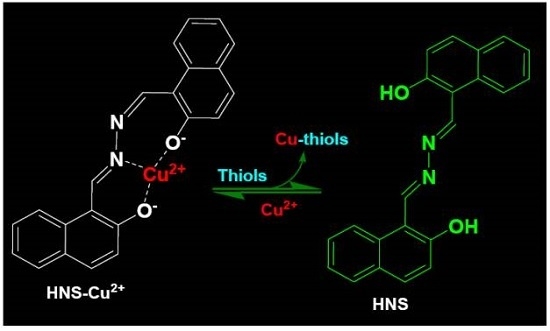
Synthesis and Application of an Aldazine-Based Fluorescence Chemosensor for the Sequential Detection of Cu2+ and Biological Thiols in Aqueous Solution and Living Cells
Abstract
:1. Introduction

2. Experimental Section
2.1. Chemicals
2.2. Apparatus
2.3. General Procedures of Spectra Detection
2.4. Association Constant Calculation
2.5. Synthesis and Characterization the Fluorescent Chemosensor HNA
2.6. Fluorescence Imaging of Biothiols in A549 Cells
3. Results and Discussion
3.1. Spectroscopic Studies of HNA in the Presence of Cu2+


3.2. Spectra Recognition of Thiols by HNA-Cu2+ Ensemble






3.3. Living-Cell Fluorescence Imaging Studies


4. Conclusions
Supplementary Files
Supplementary File 1Acknowledgments
Author Contributions
Conflicts of Interest
References
- Liu, J.; Xie, Y.-Q.; Lin, Q.; Shi, B.-B.; Zhang, P.; Zhang, Y.-M.; Wei, T.-B. Bioactive paper platform for colorimetric phenols detection. Sens. Actuators B Chem. 2013, 186, 657–662. [Google Scholar] [CrossRef]
- Gunnlaugsson, T.; Glynn, M.; Tocc, G.M.; Kruger, P.E.; Pfeffer, F.M. Anion recognition and sensing in organic and aqueous media using luminescent and colorimetric sensors. Coord. Chem. Rev. 2006, 250, 3094–3117. [Google Scholar] [CrossRef]
- Du, J.; Hu, M.; Fan, J.; Peng, X. Fluorescent chemodosimeters using “mild” chemical events for the detection of small anions and cations in biological and environmental media. Chem. Soc. Rev. 2012, 41, 4511–4535. [Google Scholar] [CrossRef] [PubMed]
- Chen, K.; Shu, Q.; Schmittel, M. Design strategies for lab-on-a-molecule probes and orthogonal sensin. Chem. Soc. Rev. 2015, 44, 136–160. [Google Scholar] [CrossRef] [PubMed]
- Kaur, N.; Kumar, S. Diastereo- and enantioselective aldol reaction of granatanone (pseudopelletierine). Tetrahedron 2011, 67, 9233–9439. [Google Scholar] [CrossRef]
- Gupta, V.K.; Singha, A.K.; Ganjalic, M.R.; Norouzic, P.; Faridbodc, F.; Mergu, N. Comparative study of colorimetric sensors based on newly synthesized Schiff bases. Sens. Actuators B Chem. 2013, 182, 642–651. [Google Scholar] [CrossRef]
- Nolan, E.M.; Lippard, S.J. Tools and Tactics for the Optical Detection of Mercuric Ion. Chem. Rev. 2008, 108, 3443–3480. [Google Scholar] [CrossRef] [PubMed]
- Gupta, V.K.; Prasad, R.; Kumar, A. Preparation of ethambutol–copper (II) complex and fabrication of PVC based membrane potentiometric sensor for copper. Talanta 2003, 60, 149–160. [Google Scholar] [CrossRef]
- Carter, K.P.; Young, A.M.; Palmer, A.E. Fluorescent Sensors for Measuring Metal Ions in Living Systems. Chem. Rev. 2014, 114, 4564–4601. [Google Scholar] [CrossRef] [PubMed]
- Hariharan, P.S.; Anthony, S.P. Substitutional group dependent colori/fluorimetric sensing of Mn2+, Fe3+ and Zn2+ ions by simple Schiff base chemosensor. Spectrochim. Acta A 2015, 136, 1658–1665. [Google Scholar] [CrossRef] [PubMed]
- Grynkiewicz, G.; Poenie, M.; Tsien, R.Y. A new generation of Ca2+ indicators with greatly improved fluorescence properties. J. Biol. Chem. 1985, 260, 3440–3450. [Google Scholar] [PubMed]
- He, G.J.; Guo, D.; He, C.; Zhang, X.L.; Zhao, X.W.; Duan, C.Y. A Color-Tunable Europium Complex Emitting Three Primary Colors and White Light. Angew. Chem. Int. Ed. 2009, 48, 6132–6135. [Google Scholar] [CrossRef] [PubMed]
- Huang, J.; Xu, Y.; Qian, X. A red-shift colorimetric and fluorescent sensor for Cu2+ in aqueous solution: Unsymmetrical 4,5-diaminonaphthalimide with N-H deprotonation induced by metal ions. Org. Biomol. Chem. 2009, 7, 1299–1303. [Google Scholar] [CrossRef] [PubMed]
- Madhu, S.; Ravikanth, M. Boron-Dipyrromethene Based Reversible and Reusable Selective Chemosensor for Fluoride Detection. Inorg. Chem. 2014, 53, 1646–1653. [Google Scholar] [CrossRef] [PubMed]
- Lin, K.-K.; Wu, S.-C.; Hsu, K.-M.; Hung, C.-H.; Liaw, W.-F.; Wang, Y.-M. A N-(2-Aminophenyl)-5-(dimethylamino)-1-naphthalenesulfonic Amide (Ds-DAB) Based Fluorescent Chemosensor for Peroxynitrite. Org. Lett. 2013, 15, 4242–4245. [Google Scholar] [CrossRef] [PubMed]
- Hu, B.; Hu, L.-L.; Chen, M.-L.; Wang, J.-H. A FRET ratiometric fluorescence sensing system for mercury detection and intracellular colorimetric imaging in live Hela cells. Biosens. Bioelectron. 2013, 49, 499–505. [Google Scholar] [CrossRef] [PubMed]
- Yin, C.; Huo, F.; Zhang, J.; Martínez-Míñez, R.; Yang, Y.; Lv, H.; Li, S. Thiol-addition reactions and their applications in thiol recognition. Chem. Soc. Rev. 2013, 42, 6032–6059. [Google Scholar] [CrossRef] [PubMed]
- Hyman, L.M.; Franz, K.J. Probing oxidative stress: Small molecule fluorescent sensors of metal ions, reactive oxygen species, and thiols. Coord. Chem. Rev. 2012, 256, 2333–2356. [Google Scholar] [CrossRef] [PubMed]
- Chen, X.; Zhou, Y.; Peng, X.; Yoon, J. Fluorescent and colorimetric probes for detection of thiols. Chem. Soc. Rev. 2010, 39, 2120–2135. [Google Scholar] [CrossRef] [PubMed]
- Lee, H.Y.; Choi, Y.P.; Kim, S.; Yoon, T.; Guo, Z.; Lee, S.; Swamy, K.M.K.; Kim, G.; Lee, J.Y.; Shin, I.; Yoon, J. Selective homocysteine turn-on fluorescent probes and their bioimaging applications. Chem. Commun. 2014, 50, 6967–6969. [Google Scholar] [CrossRef] [PubMed]
- Wood, Z.A.; Schroder, E.; Robin Harris, J.; Poole, L.B. Structure, mechanism and regulation of peroxiredoxins. Trends Biochem. Sci. 2003, 28, 32–40. [Google Scholar] [CrossRef]
- Pullela, P.K.; Chiku, T.; Carvan III, M.J.; Sem, D.S. Fluorescence-based detection of thiols in vitro and in vivo using dithiol probes. Anal. Biochem. 2006, 352, 265–273. [Google Scholar] [CrossRef] [PubMed]
- Rahman, I.; MacNee, W. Regulation of redox glutathione levels and gene transcription in lung inflammation: The rapeutic approaches. Free Radic. Biol. Med. 2000, 28, 1405–1420. [Google Scholar] [CrossRef]
- Voet, D.; Voet, J.G. Biochemistry, 2nd ed.; John Wiley & Sons: New York, NY, USA, 1995. [Google Scholar]
- Xue, S.; Ding, S.; Zhai, Q.; Zhang, H.; Feng, G. A readily available colorimetric and near-infrared fluorescent turn-on probe for rapid and selective detection of cysteine in living cells. Biosens. Bioelectron. 2015, 68, 316–321. [Google Scholar] [CrossRef] [PubMed]
- Zhang, H.; Zhang, C.; Liu, R.; Yi, L.; Sun, H. A highly selective and sensitive fluorescent thiol probe through dual-reactive and dual-quenching groups. Chem. Commun. 2015, 51, 2029–2032. [Google Scholar] [CrossRef] [PubMed]
- Stamler, J.S.; Slivka, A. Biological chemistry of thiols in the vasculature and in vascular-related disease. Nutr. Rev. 1996, 54, 1–30. [Google Scholar] [CrossRef] [PubMed]
- Shi, J.; Wang, Y.; Tang, X.; Liu, W.; Jiang, H.; Dou, W.; Liu, W. A colorimetric and fluorescent probe for thiols based on 1, 8-naphthalimide and its application for bioimaging. Dyes Pigm. 2014, 100, 255–260. [Google Scholar] [CrossRef]
- Barve, A.; Lowry, M.; Escobedo, J.O.; Huynh, K.T.; Hakuna, L.; Strongin, R.M. Differences in heterocycle basicity distinguish homocysteine from cysteine using aldehyde-bearing fluorophores. Chem. Commun. 2014, 50, 8219–8222. [Google Scholar] [CrossRef] [PubMed]
- Wu, G.Y.; Fang, Y.Z.; Yang, S.; Lupton, J.R.; Turner, N.D. Glutathione metabolism and its implications for health. J. Nutr. 2004, 134, 489–492. [Google Scholar] [PubMed]
- Shahrokhian, S. Lead Phthalocyanine as a Selective Carrier for Preparation of a Cysteine-Selective Electrode. Anal. Chem. 2001, 73, 5972–5978. [Google Scholar] [CrossRef] [PubMed]
- Hao, W.; McBride, A.; McBride, S.; Gao, J.P.; Wang, Z.Y. Colorimetric and near-infrared fluorescence turn-on molecular probe for direct and highly selective detection of cysteine in human plasma. J. Mater. Chem. 2011, 21, 1040–1048. [Google Scholar] [CrossRef]
- Su, D.; Teoh, C.L.; Sahu, S.; Das, R.K.; Chang, Y.-T. Live cells imaging using a turn-on FRET-based BODIPY probe for biothiols. Biomaterials 2014, 35, 6078–6085. [Google Scholar] [CrossRef] [PubMed]
- Liu, B.; Wang, J.; Zhang, G.; Bai, R.; Pang, Y. Flavone-Based ESIPT Ratiometric Chemodosimeter for Detection of Cysteine in Living Cells. ACS Appl. Mater. Interfaces 2014, 6, 4402–440. [Google Scholar] [CrossRef] [PubMed]
- Martínez-Sierra, J.G.; Sanz, F.M.; Espílez, P.H.; Santamaria-Fernandez, R.; Gayón, J.M.M.; Alonso, J.I.G. Evaluation of different analytical strategies for the quantification of sulfur-containing biomolecules by HPLC-ICP-MS: Application to the characterisation of 34S-labelled yeast. J. Anal. Atom. Spectrom. 2010, 25, 989–997. [Google Scholar] [CrossRef]
- Brodbelt, J.S. Photodissociation mass spectrometry: New tools for characterization of biological molecules. Chem. Soc. Rev. 2014, 43, 2757–2783. [Google Scholar] [CrossRef] [PubMed]
- Xiao, C.; Chen, J.; Liu, B.; Chu, X.; Wu, L.; Yao, S. Sensitive and selective electrochemical sensing of L-cysteine based on a caterpillar-like manganese dioxide–carbon nanocomposite. Phys. Chem. Chem. Phys. 2011, 13, 1568–1574. [Google Scholar] [CrossRef] [PubMed]
- Zhang, R.; Yu, X.; Yin, Y.; Ye, Z.; Wang, G.; Yuan, J. Development of a heterobimetallic Ru(II)–Cu(II) complex for highly selective and sensitive luminescence sensing of sulfide anions. Anal. Chim. Acta 2011, 691, 83–88. [Google Scholar] [CrossRef] [PubMed]
- Wang, H.; Zhou, G.; Mao, C.; Chen, X. A fluorescent sensor bearing nitroolefin moiety for the detection of thiols and its biological imaging. Dyes Pigm. 2013, 96, 232–263. [Google Scholar] [CrossRef]
- Hong, V.; Kislukhin, A.A.; Finn, M.G. Thiol-Selective Fluorogenic Probes for Labeling and Release. J. Am. Chem. Soc. 2009, 131, 9986–9994. [Google Scholar] [CrossRef] [PubMed]
- Hu, M.; Fan, J.; Li, H.; Song, K.; Wang, S.; Cheng, G.; Peng, X. Fluorescent chemodosimeter for Cys/Hcy with a large absorption shift and imaging in living cells. Org. Biomol. Chem. 2011, 9, 980–983. [Google Scholar] [CrossRef] [PubMed]
- Guo, F.; Tian, M.; Miao, F.; Zhang, W.; Song, G.; Liu, Y.; Yu, X.; Sun, J.Z.; Wong, W.-Y. Lighting up cysteine and homocysteine in sequence based on the kinetic difference of the cyclization/addition reaction. Org. Biomol. Chem. 2013, 11, 7721–7728. [Google Scholar] [CrossRef] [PubMed]
- Tang, B.; Xing, Y.; Li, P.; Zhang, N.; Yu, F.; Yang, G. A Rhodamine-Based Fluorescent Probe Containing a Se-N Bond for Detecting Thiols and Its Application in Living Cells. J. Am. Chem. Soc. 2007, 129, 11666–11667. [Google Scholar] [CrossRef] [PubMed]
- Li, M.; Wu, X.; Wang, Y.; Li, Y.; Zhu, W.; James, T.D. A near-infrared colorimetric fluorescent chemodosimeter for the detection of glutathione in living cells. Chem. Commun. 2014, 50, 1751–1753. [Google Scholar] [CrossRef] [PubMed]
- Das, P.; Mandal, A.K.; Reddy, U.G.; Baidy, M.; Ghosh, S.K.; Das, A. Designing a thiol specific fluorescent probe for possible use as a reagent for intracellular detection and estimation in blood serum: Kinetic analysis to probe the role of intramolecular hydrogen bonding. Org. Biomol. Chem. 2013, 11, 6604–6614. [Google Scholar] [CrossRef] [PubMed]
- Zheng, L.-Q.; Li, Y.; Yu, X.-D.; Xu, J.-J.; Chen, H.-Y. A sensitive and selective detection method for thiol compounds using novel fluorescence probe. Anal. Chim. Acta 2014, 850, 71–77. [Google Scholar] [CrossRef] [PubMed]
- Huo, F.-J.; Yang, Y.-T.; Su, J.; Sun, Y.-Q.; Yin, C.-X.; Yan, X.-X. Indicator approach to develop a chemosensor for the colorimetric sensing of thiol-containing water and its application for the thiol detection in plasma. Analyst 2011, 136, 1892–1897. [Google Scholar] [CrossRef] [PubMed]
- Zhang, D.Q. Highly selective colorimetric detection of cysteine and homocysteine in water through a direct displacement approach. Inorg. Chem. Commun. 2009, 12, 1255–1258. [Google Scholar] [CrossRef]
- Wang, Y.; Sun, H.; Hou, L.; Shang, Z.; Dong, Z.; Jin, W. 1,4-Dihydroxyanthraquinone–Cu2+ ensemble probe for selective detection of sulfide anion in aqueous solution. Anal. Methods 2013, 5, 5493–5500. [Google Scholar] [CrossRef]
- Lee, Y.H.; Park, N.; Park, Y.B.; Hwang, Y.J.; Kang, C.; Kim, J.S. Organelle-selective fluorescent Cu2+ ion probes: Revealing the endoplasmic reticulum as a reservoir for Cu-overloading. Chem. Comm. 2014, 50, 3197–3200. [Google Scholar] [CrossRef] [PubMed]
- You, Q.-H.; Lee, A.W.-M.; Chan, W.-H.; Zhu, X.-M.; Leung, K.C.-F. A coumarin-based fluorescent probe for recognition of Cu2+ and fast detection of histidine in hard-to-transfect cells by a sensing ensemble approach. Chem. Commun. 2014, 50, 6207–6210. [Google Scholar] [CrossRef] [PubMed]
- Meng, Q.; Jia, H.; Succar, P.; Zhao, L.; Zhang, R.; Duan, C.; Zhang, Z. A highly selective and sensitive ON–OFF–ON fluorescence chemosensor for cysteine detection in endoplasmic reticulum. Biosens. Bioelectron. 2015, 74, 461–468. [Google Scholar] [CrossRef] [PubMed]
- He, G.; Zhang, X.; He, C.; Zhao, X.; Duan, C. Ratiometric fluorescence chemosensors for copper(II) and mercury(II) based on FRET systems. Tetrahedron 2010, 66, 9762–9768. [Google Scholar] [CrossRef]
- Mañes, J.; Campillos, P.; Font, G. Extraction-spectrophotometric determination of hydrazine with 2-hydroxy-1-naphthaldehyde. Aanlyst 1987, 112, 1183–1184. [Google Scholar] [CrossRef]
- Jung, H.S.; Han, J.H.; Kim, Z.H.; Kang, C.; Kim, J.S. Coumarin-Cu(II) Ensemble-Based Cyanide Sensing Chemodosimeter. Org. Lett. 2011, 13, 5056–5059. [Google Scholar] [CrossRef] [PubMed]
- Choi, M.G.; Cha, S.; Lee, H.; Jeon, H.L.; Chang, S.-K. Sulfide-selective chemosignaling by a Cu2+ complex of dipicolylamine appended fluorescein. Chem. Comm. 2009, 47, 7390–7392. [Google Scholar] [CrossRef] [PubMed]
- Zhang, R.; Son, B.; Dai, Z.; Ye, Z.; Xiao, Y.; Liu, Y.; Yuan, J. Highly sensitive and selective phosphorescent chemosensors for hypochlorous acid based on ruthenium(II) complexes. Biosens. Bioelectron. 2013, 50, 1–7. [Google Scholar] [CrossRef] [PubMed]
- Koteeswari, R.; Ashokkumar, P.; Malar, E.J.P.; Ramakrishnan, V.T.; Ramamurthy, P. Highly selective, sensitive and quantitative detection of Hg2+ in aqueous medium under broad pH range. Chem. Comm. 2011, 27, 7695–7697. [Google Scholar] [CrossRef] [PubMed]
- Dean, J.A. Langès Handbook of Chemistry, 15th ed.; McGraw-Hill: New York, NY, USA, 1999. [Google Scholar]
- Jung, H.S.; Han, J.H.; Pradhan, T.; Kim, S.; Lee, S.W.; Sessler, J.L.; Kim, T.W.; Kang, C.; Kim, J.S. A cysteine-selective fluorescent probe for the cellular detection of cysteine. Biomaterials 2012, 33, 945–953. [Google Scholar] [CrossRef] [PubMed]
- Son, S.-H.; Kim, Y.; Heo, M.B.; Lim, Y.T.; Lee, T.S. A fluorescence turn-on probe for the detection of thiol-containing amino acids in aqueous solution and bioimaging in cells. Tetrahedron 2014, 70, 2034–2039. [Google Scholar] [CrossRef]
- Hwang, C.; Sinskey, A.J.; Lodish, H.F. Oxidized redox state of glutathione in the endoplasmic reticulum. Science 1992, 257, 1496–1502. [Google Scholar] [CrossRef] [PubMed]
- Zhang, L.; Duan, D.; Liu, Y.; Ge, C.; Cui, X.; Sun, J.; Fang, J. Highly Selective Off–On Fluorescent Probe for Imaging Thioredoxin Reductase in Living Cells. J. Am. Chem. Soc. 2014, 136, 226–233. [Google Scholar] [CrossRef] [PubMed]
- Meng, Q.; Jia, H.; Gao, X.; Wang, Y.; Zhang, R.; Wang, R.; Zhang, Z. Reversible and Selective Fluorescence Detection of Histidine Using a Naphthalimide-Based Chemosensing Ensemble. Chem. Asian J. 2015, 10, 2411–2418. [Google Scholar] [CrossRef] [PubMed]
© 2016 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons by Attribution (CC-BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Jia, H.; Yang, M.; Meng, Q.; He, G.; Wang, Y.; Hu, Z.; Zhang, R.; Zhang, Z. Synthesis and Application of an Aldazine-Based Fluorescence Chemosensor for the Sequential Detection of Cu2+ and Biological Thiols in Aqueous Solution and Living Cells. Sensors 2016, 16, 79. https://0-doi-org.brum.beds.ac.uk/10.3390/s16010079
Jia H, Yang M, Meng Q, He G, Wang Y, Hu Z, Zhang R, Zhang Z. Synthesis and Application of an Aldazine-Based Fluorescence Chemosensor for the Sequential Detection of Cu2+ and Biological Thiols in Aqueous Solution and Living Cells. Sensors. 2016; 16(1):79. https://0-doi-org.brum.beds.ac.uk/10.3390/s16010079
Chicago/Turabian StyleJia, Hongmin, Ming Yang, Qingtao Meng, Guangjie He, Yue Wang, Zhizhi Hu, Run Zhang, and Zhiqiang Zhang. 2016. "Synthesis and Application of an Aldazine-Based Fluorescence Chemosensor for the Sequential Detection of Cu2+ and Biological Thiols in Aqueous Solution and Living Cells" Sensors 16, no. 1: 79. https://0-doi-org.brum.beds.ac.uk/10.3390/s16010079