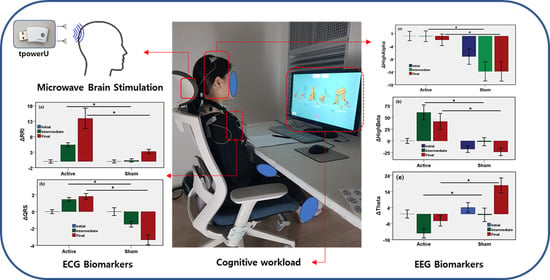
Quantifying Physiological Biomarkers of a Microwave Brain Stimulation Device
Abstract
:1. Introduction
2. Materials and Methods
2.1. Microwave Brain Stimulation Device
2.2. Study Design
2.3. Randomization
2.4. Participants of the Experiment
2.5. Data Acquisition
2.5.1. ECG Data Acquisition
2.5.2. EEG Data Acquisition
2.6. Pre-Processing
2.7. Feature Extraction
2.7.1. ECG Features
2.7.2. EEG Features
2.8. Statistical Analysis
3. Results
3.1. Effects of MBS on Neurological Outcome
3.2. Effects of MBS on Autonomic Nervous System
3.3. Effects of MBS on ECG Fiducial Features
4. Discussion
5. Conclusions
6. Patents
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Lozano, A.M.; Lipsman, N.; Bergman, H.; Brown, P.; Chabardes, S.; Chang, J.W.; Matthews, K.; McIntyre, C.C.; Schlaepfer, T.E.; Schulder, M.; et al. Deep brain stimulation: Current challenges and future directions. Nat. Rev. Neurol. 2019, 15, 148–160. [Google Scholar] [CrossRef]
- Roth, B.; Basser, P. A model of the stimulation of a nerve fiber by electromagnetic induction. IEEE Trans. Biomed. Eng. 1990, 37, 588–597. [Google Scholar] [CrossRef] [PubMed]
- Tehovnik, E.J.; Tolias, A.S.; Sultan, F.; Slocum, W.M.; Logothetis, N.K. Direct and Indirect Activation of Cortical Neurons by Electrical Microstimulation. J. Neurophysiol. 2006, 96, 512–521. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Polanía, R.; Nitsche, M.A.; Ruff, C.C. Studying and modifying brain function with non-invasive brain stimulation. Nat. Neurosci. 2018, 21, 174–187. [Google Scholar] [CrossRef] [PubMed]
- Llinás, R.R. The intrinsic electrophysiological properties of mammalian neurons: Insights into central nervous system function. Science 1988, 242, 1654–1664. [Google Scholar] [CrossRef] [PubMed]
- Ros, T.; Bernard, J.; Baars, R.; Lanius, A.; Vuilleumier, P. Tuning Pathological Brain Oscillations with Neu-rofeedback: A Systems Neuroscience Framework. Front. Human Neurosci. 2014, 8, 1008. [Google Scholar] [CrossRef] [Green Version]
- Assenza, G.; Capone, F.; di Biase, L.; Ferreri, F.; Florio, L.; Guerra, A.; Marano, M.; Paolucci, M.; Ranieri, F.; Di Lazzaro, V.; et al. Oscillatory Activities in Neurological Disorders of Elderly: Biomarkers to Target for Neuromodulation. Front. Aging Neurosci. 2017, 9, 189. [Google Scholar] [CrossRef]
- Brunoni, A.R.; Nitsche, M.A.; Bolognini, N.; Bikson, M.; Wagner, T.; Merabet, L.; Edwards, D.J.; Valero-Cabre, A.; Rotenberg, A.; Pascual-Leone, A.; et al. Clinical research with transcranial direct current stimulation (tDCS): Challenges and future directions. Brain Stimul. 2012, 5, 175–195. [Google Scholar] [CrossRef] [Green Version]
- Hallett, M. Transcranial Magnetic Stimulation: A Primer. Neuron 2007, 55, 187–199. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Paulus, W. Transcranial electrical stimulation (tES—tDCS; tRNS, tACS) methods. Neuropsychol. Rehabil. 2011, 21, 602–617. [Google Scholar] [CrossRef]
- Fioravanti, A.; Nerucci, F.; Collodel, G.; Markoll, R.; Marcolongo, R. Biochemical and morphological study of human articular chondrocytes cultivated in the presence of pulsed signal therapy. Ann. Rheum. Dis. 2002, 61, 1032–1033. [Google Scholar] [CrossRef] [Green Version]
- Hinrikus, H.; Parts, M.; Lass, J.; Tuulik, V. Changes in Human Eeg Caused by Low Level Modulated Microwave Stimulation. Bioelectromagnetics 2004, 25, 431–440. [Google Scholar] [CrossRef]
- Grossman, N.; Bono, D.; Dedic, N.; Kodandaramaiah, S.B.; Rudenko, A.; Suk, H.J.; Cassara, A.M.; Neufeld, E.; Kuster, N.; Boyden, E.S.; et al. Noninvasive Deep Brain Stim-ulation Via Temporally Interfering Electric Fields. Cell 2017, 169, 1029–1041. [Google Scholar] [CrossRef] [Green Version]
- Seo, T.; Oh, S.; Jung, D.; Huh, Y.; Cho, J.; Kwon, Y. Noninvasive Brain Stim-ulation Using a Modulated Microwave Signal. J. Electromagn. Eng. Sci. 2018, 18, 70–72. [Google Scholar] [CrossRef] [Green Version]
- Bachmann, M.; Lass, J.; Ioannides, A.A.; Hinrikus, H. Brain Stimulation by Modulated Microwave Radiation: A Fea-sibility Study. In Proceedings of the EMF-Med 1st World Conference on Biomedical Applications of Electromagnetic Fields (EMF-Med), Split, Croatia, 10–13 September 2018. [Google Scholar]
- Nepa, P.; Buffi, A. Near-Field-Focused Microwave Antennas: Near-field shaping and implementation. IEEE Antennas Propag. Mag. 2017, 59, 42–53. [Google Scholar] [CrossRef]
- Suhhova, A.; Bachmann, M.; Karai, D.; Lass, J.; Hinrikus, H. Effect of microwave radiation on human EEG at two different levels of exposure. Bioelectromagnetics 2012, 34, 264–274. [Google Scholar] [CrossRef] [PubMed]
- Beason, R.C.; Semm, P. Responses of neurons to an amplitude modulated microwave stimulus. Neurosci. Lett. 2002, 333, 175–178. [Google Scholar] [CrossRef]
- Cantello, R.; Tarletti, R.; Civardi, C. Transcranial Magnetic Stimulation and Parkinson’s Disease. Brain Res. Rev. 2002, 38, 309–327. [Google Scholar] [CrossRef]
- Abbruzzese, G.; Marchese, R.; Buccolieri, A.; Gasparetto, B.; Trompetto, C. Abnormalities of Sensorimotor Integration in Focal Dystonia: A Transcranial Magnetic Stimulation Study. Brain 2001, 124, 537–545. [Google Scholar] [CrossRef] [Green Version]
- Gironell, A.; Kulisevsky, J.; Lorenzo, J.; Barbanoj, M.; Pascual-Sedano, B.; Otermin, P. Tran-scranial Magnetic Stimulation of the Cerebellum in Essential Tremor: A Controlled Study. Arch. Neurol. 2002, 59, 413–417. [Google Scholar] [CrossRef] [Green Version]
- Tergau, F.; Naumann, U.; Paulus, W.; Steinhoff, B.J. Low-Frequency Repetitive Transcranial Mag-netic Stimulation Improves Intractable Epilepsy. Lancet 1999, 353, 2209. [Google Scholar] [CrossRef]
- O’Reardon, J.P.; Solvason, H.B.; Janicak, P.G.; Sampson, S.; Isenberg, K.E.; Nahas, Z.; McDonald, W.M.; Avery, D.; Fitzgerald, P.B.; Loo, C.; et al. Efficacy and Safety of Transcranial Magnetic Stimulation in the Acute Treatment of Major Depression: A Multisite Randomized Controlled Trial. Biol. Psychiatry 2007, 62, 1208–1216. [Google Scholar] [CrossRef]
- Cotelli, M.; Manenti, R.; Cappa, S.F.; Zanetti, O.; Miniussi, C. Transcranial magnetic stimulation improves naming in Alzheimer disease patients at different stages of cognitive decline. Eur. J. Neurol. 2008, 15, 1286–1292. [Google Scholar] [CrossRef]
- Hussain, I.; Park, S.J. HealthSOS: Real-Time Health Monitoring System for Stroke Prognostics. IEEE Access 2020, 8, 213574–213586. [Google Scholar] [CrossRef]
- Park, S.J.; Hong, S.; Kim, D.; Seo, Y.; Hussain, I.; Hur, J.H.; Jin, W. Development of a Real-Time Stroke Detection System for Elderly Drivers Using Quad-Chamber Air Cushion and IoT Devices; SAE International: Warrendale, PA, USA, 2018. [Google Scholar] [CrossRef]
- Park, S.J.; Hussain, I.; Hong, S.; Kim, D.; Park, H.; Benjamin, H.C.M. Real-time Gait Monitoring System for Consumer Stroke Prediction Service. In Proceedings of the 2020 IEEE International Conference on Consumer Electronics (ICCE), Las Vegas, NV, USA, 4–6 January 2020; pp. 1–4. [Google Scholar] [CrossRef]
- McCall, I.C.; Lau, C.; Minielly, N.; Illes, J. Owning Ethical Innovation: Claims about Commercial Wearable Brain Technologies. Neuron 2019, 102, 728–731. [Google Scholar] [CrossRef]
- Fehér, K.D.; Wunderlin, M.; Maier, J.G.; Hertenstein, E.; Schneider, C.L.; Mikutta, C.; Züst, M.A.; Klöppel, S.; Nissen, C. Shaping the slow waves of sleep: A systematic and integrative review of sleep slow wave modulation in humans using non-invasive brain stimulation. Sleep Med. Rev. 2021, 58, 101438. [Google Scholar] [CrossRef]
- Mertens, A.; Raedt, R.; Gadeyne, S.; Carrette, E.; Boon, P.; Vonck, K. Recent advances in devices for vagus nerve stimulation. Expert Rev. Med. Devices 2018, 15, 527–539. [Google Scholar] [CrossRef] [PubMed]
- Park, S.J.; Hong, S.; Kim, D.; Hussain, I.; Seo, Y.; Kim, M.K. Physiological Evaluation of a Non-invasive Wearable Vagus Nerve Stimulation (VNS) Device. In Advances in Human Factors in Wearable Technologies and Game Design. AHFE 2019; Proceedings of the International Conference on Applied Human Factors and Ergonomics, Washington, DC, USA, 24–28 July 2019; Ahram, T., Ed.; Springer: Cham, Switzerland, 2019; pp. 57–62. [Google Scholar] [CrossRef]
- Thut, G.; Miniussi, C. New insights into rhythmic brain activity from TMS–EEG studies. Trends Cogn. Sci. 2009, 13, 182–189. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bailey, W.H.; Bodemann, R.; Bushberg, J.; Chou, C.; Cleveland, R.; Faraone, A.; Foster, K.R.; Gettman, K.E.; Graf, K.; Har-rington, T.; et al. Synopsis of Ieee Std C95.1™-2019 “IEEE Standard for Safety Levels with Respect to Human Exposure to Electric, Magnetic, and Electromagnetic Fields, 0 Hz to 300 Ghz”. IEEE Access 2019, 7, 171346–1713456. [Google Scholar] [CrossRef]
- Camm, A.J.; Marek, M.; Bigger, J.T.; Breithardt, G.; Cerutti, S.; Cohen, R.J.; Coumel, P.; Fallen, E.L.; Kennedy, H.L.; Kleiger, R.E. Heart Rate Variability. Standards of Measurement, Physiological Interpre-tation, and Clinical Use. Eur. Heart J. 1996, 17, 354–381. [Google Scholar]
- Billman, G.E. The LF/HF ratio does not accurately measure cardiac sympatho-vagal balance. Front. Physiol. 2013, 4, 26. [Google Scholar] [CrossRef] [Green Version]
- Pan, J.; Tompkins, W.J. A Real-Time Qrs Detection Algorithm. IEEE Trans. Biomed. Eng. 1985, BME-32, 230–236. [Google Scholar] [CrossRef] [PubMed]
- Klimesch, W. EEG alpha and theta oscillations reflect cognitive and memory performance: A review and analysis. Brain Res. Rev. 1999, 29, 169–195. [Google Scholar] [CrossRef]
- Matthews, G.; Reinerman-Jones, L.; Abich, J.; Kustubayeva, A. Metrics for individual differences in EEG response to cognitive workload: Optimizing performance prediction. Personal. Individ. Differ. 2017, 118, 22–28. [Google Scholar] [CrossRef]
- Seo, S.-H.; Lee, J.-T. Stress and Eeg. In Convergence and Hybrid Information Technologies; Crisan, M., Ed.; Intech: Rijeka, Croatia, 2010. [Google Scholar]
- Borghini, G.; Astolfi, L.; Vecchiato, G.; Mattia, D.; Babiloni, F. Measuring Neurophysio-logical Signals in Aircraft Pilots and Car Drivers for the Assessment of Mental Workload, Fatigue and Drowsiness. Neurosci. Biobehav. Rev. 2014, 44, 58–75. [Google Scholar] [CrossRef]
- Gola, M.; Magnuski, M.; Szumska, I.; Wróbel, A. EEG beta band activity is related to attention and attentional deficits in the visual performance of elderly subjects. Int. J. Psychophysiol. 2013, 89, 334–341. [Google Scholar] [CrossRef]
- Malliani, A.; Lombardi, F.; Pagani, M. Power spectrum analysis of heart rate variability: A tool to explore neural regulatory mechanisms. Br. Heart J. 1994, 71, 1–2. [Google Scholar] [CrossRef] [Green Version]






| EEG Feature | Phase | Mean Value | Change from Baseline (%) | ||||
|---|---|---|---|---|---|---|---|
| Active | Sham | Active | Sham | Difference | p-Value | ||
| High Alpha (Relative Power) | Baseline | 0.10 | 0.11 | - | - | - | - |
| Intermediate | 0.10 | 0.11 | −0.08 | −13.82 | 13.74 | 0.0001 * | |
| Final | 0.10 | 0.11 | −1.51 | −13.82 | 12.31 | 0.0001 * | |
| Low Beta (Relative Power) | Baseline | 0.12 | 0.15 | - | - | - | - |
| Intermediate | 0.13 | 0.15 | 12.05 | −10.83 | 22.88 | 0.0001 * | |
| Final | 0.12 | 0.14 | 4.55 | −17.79 | 22.33 | 0.052 | |
| Low Alpha (Relative Power) | Baseline | 0.20 | 0.18 | - | - | - | - |
| Intermediate | 0.18 | 0.16 | −8.56 | −8.64 | 0.08 | 0.96 | |
| Final | 0.19 | 0.18 | −4.53 | 0.72 | −5.25 | 0.0001* | |
| Theta (Relative Power) | Baseline | 0.46 | 0.38 | - | - | - | - |
| Intermediate | 0.41 | 0.37 | −12.01 | −0.50 | −11.50 | 0.0001 * | |
| Final | 0.44 | 0.42 | −4.29 | 17.84 | −22.13 | 0.0001 * | |
| High Beta (Relative Power) | Baseline | 0.12 | 0.18 | - | - | - | - |
| Intermediate | 0.18 | 0.21 | 60.62 | −1.68 | 62.31 | 0.0001 * | |
| Final | 0.15 | 0.17 | 41.28 | −23.49 | 64.76 | 0.0001 * | |
| HRV Feature | Phase | Mean Value | Change from Baseline (%) | ||||
|---|---|---|---|---|---|---|---|
| Active | Sham | Active | Sham | Difference | p-Value | ||
| LF/(LF+HF) | Baseline | 0.59 | 0.63 | - | - | - | - |
| Intermediate | 0.60 | 0.60 | 3.62 | −12.85 | 16.47 | 0.32 | |
| Final | 0.59 | 0.58 | −2.56 | −14.44 | 11.87 | 0.51 | |
| HF/(LF+HF) | Baseline | 0.41 | 0.37 | - | - | - | - |
| Intermediate | 0.40 | 0.40 | 2.44 | −0.21 | 2.65 | 0.92 | |
| Final | 0.41 | 0.42 | −3.68 | 8.87 | −12.55 | 0.61 | |
| LF/HF | Baseline | 2.17 | 2.66 | - | - | - | - |
| Intermediate | 1.70 | 1.97 | −14.59 | −60.44 | 45.85 | 0.33 | |
| Final | 1.86 | 1.80 | −36.40 | −55.92 | 19.52 | 0.69 | |
| ECG Fiducial Feature | Phase | Mean Value | Change from Baseline (%) | ||||
|---|---|---|---|---|---|---|---|
| Active | Sham | Active | Sham | Difference | p-Value | ||
| RR Interval, s | Baseline | 0.79 | 0.86 | - | - | - | - |
| Intermediate | 0.83 | 0.87 | 5.37 | 0.32 | 5.05 | 0.0001 * | |
| Final | 0.90 | 0.89 | 13.91 | 3.21 | 10.70 | 0.0001 | |
| QRS Interval, s | Baseline | 0.11 | 0.11 | - | - | - | - |
| Intermediate | 0.11 | 0.11 | 1.44 | −1.50 | 2.94 | 0.0001 * | |
| Final | 0.11 | 0.11 | 1.81 | −3.35 | 5.16 | 0.0001 * | |
| QT Interval, s | Baseline | 0.45 | 0.43 | - | - | - | - |
| Intermediate | 0.47 | 0.42 | 4.44 | −2.33 | 6.77 | 0.0001 * | |
| Final | 0.45 | 0.44 | −0.67 | 1.16 | −1.83 | 0.08 | |
| QTc Interval, s | Baseline | 0.51 | 0.46 | - | - | - | - |
| Intermediate | 0.52 | 0.45 | 1.73 | −2.27 | 4.01 | 0.0001 * | |
| Final | 0.49 | 0.46 | −2.06 | 0.25 | −2.30 | 0.0001 * | |
| ST Interval, s | Baseline | 0.36 | 0.34 | - | - | - | - |
| Intermediate | 0.38 | 0.33 | 5.43 | −3.31 | 8.74 | 0.0001 * | |
| Final | 0.36 | 0.35 | 2.02 | 1.74 | 0.28 | 0.732 | |
| Study | Stimulation Characteristics | Study Sample | Physiology Domain | Outcomes |
|---|---|---|---|---|
| Suhhova et al. [17] | 450 MHz microwave stimulation with 25 Hz modulation frequency at two exposure rates | 15 healthy volunteers | EEG frequency spectral domain | Change in the power of alpha, beta1, and beta2 with exposure level. |
| Bachmann et al. [15] | 450 MHz microwave stimulation with 40 Hz harmonics | 14 healthy subjects | EEG frequency spectral domain | Increase in power of 10 Hz, 20 Hz, and 30Hz compared to the sham (without) stimulation. |
| Seo et al. [14] | 6.5 GHz microwave stimulation with a skin-contact stimulator. | One mouse | Action potential and Nerve firing rate (FR) | FR excited during 1 Hz stimulation and inhibited during 50 Hz stimulation. |
| Beason et al. [18] | 900 MHz microwave stimulation using cell phone at 217 Hz modulation. | 34 adult birds (zebra finches) | Action potential and Nerve firing rate (FR) | FR increased for 76%of the responding cells. |
| Hinrikus et al. [12] | 400 MHz microwave stimulation with 7 Hz on-off modulation. | 20 healthy volunteers | EEG frequency spectral domain | No significant changes were found in EEG bands power. |
| Proposed work | 2.4 GHz and 5.2 GHz microwave stimulation with 35 Hz modulation. | 10 healthy volunteers (Double-blind sham-controlled trial) | EEG frequency spectral domain, ECG time domain, and HRV time and frequency-domain analysis | Significant association of EEG spectral band power and ECG fiducial features are found with active microwave simulation relative to sham group. No association found for HRV features. |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hussain, I.; Young, S.; Kim, C.H.; Benjamin, H.C.M.; Park, S.J. Quantifying Physiological Biomarkers of a Microwave Brain Stimulation Device. Sensors 2021, 21, 1896. https://0-doi-org.brum.beds.ac.uk/10.3390/s21051896
Hussain I, Young S, Kim CH, Benjamin HCM, Park SJ. Quantifying Physiological Biomarkers of a Microwave Brain Stimulation Device. Sensors. 2021; 21(5):1896. https://0-doi-org.brum.beds.ac.uk/10.3390/s21051896
Chicago/Turabian StyleHussain, Iqram, Seo Young, Chang Ho Kim, Ho Chee Meng Benjamin, and Se Jin Park. 2021. "Quantifying Physiological Biomarkers of a Microwave Brain Stimulation Device" Sensors 21, no. 5: 1896. https://0-doi-org.brum.beds.ac.uk/10.3390/s21051896