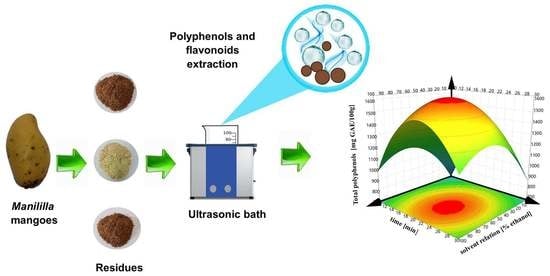
Effect of Ultrasound-Assisted Extraction Parameters on Total Polyphenols and Its Antioxidant Activity from Mango Residues (Mangifera indica L. var. Manililla)
Abstract
:1. Introduction
2. Materials and Methods
2.1. Plant Material and Obtaining Residues Flours
2.2. Extraction of Bioactives Compounds via Maceration
2.3. Extraction Assisted by Ultrasound Bath
2.4. Quantification of Total Polyphenols
2.5. Quantification of Total Flavonoids
2.6. Analysis of Mangiferin
2.7. Antioxidant Capacity Assay in Mango Residue Extracts
2.7.1. ABTS• + Scavenging Ability
2.7.2. DPPH Radical Scavenging Assay
2.8. Design of Experiments
3. Results
3.1. Residue Type
3.2. Full Factorial with Central Points Design Analysis
3.3. Effect of the Solvent Relation
3.4. Effect of Percentage Amplitude
3.5. Effect of Sonication Time
4. Mangiferin Quantification
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Lauricella, M.; Emanuele, S.; Calvaruso, G.; Giuliano, M.; D’Anneo, A. Multifaceted Health Benefits of Mangifera indica L. (Mango): The Inestimable Value of Orchards Recently Planted in Sicilian Rural Areas. Nutrients 2017, 9, 525. [Google Scholar] [CrossRef]
- Gálvez-López, C.; Adriano-Anaya, D.; Villarreal-Treviño, M.L.; Mayek-Pérez, C.; Salvador-Figueroa, N. Diversidad isoenzimática de mangos criollos de Chiapas, México. Rev. Chapingo Ser. Hortic. 2007, 13, 71–76. [Google Scholar]
- Monribot-Villanueva, J.L.; Elizalde-Contreras, J.M.; Aluja, M.; Segura-Cabrera, A.; Birke, A.; Guerrero-Analco, J.A. Endorsing and extending the repertory of nutraceutical and antioxidant sources in mangoes during postharvest shelf life. Food Chem. 2019, 285, 119–129. [Google Scholar] [CrossRef] [PubMed]
- Asif, A.; Farooq, U.; Akram, K.; Hayat, Z.; Shafi, A.; Sarfraz, F. Therapeutic potentials of bioactive compounds from mango fruit wastes. Trends Food Sci. Technol. 2016, 53, 102–112. [Google Scholar] [CrossRef]
- Ross, I.A. Chemical Constituents, Traditional and Modern Medicinal Uses. In Medicinal Plants of the World; Humana Press: Totowa, NJ, USA, 2003; Volume 1, pp. 315–328. [Google Scholar]
- Parmar, H.S.; Kar, A. Protective role of Mangifera indica, Cucumis melo and Citrullus vulgaris peel extracts in chemically induced hypothyroidism. Chem. Biol. Interact. 2009, 177, 254–258. [Google Scholar] [CrossRef]
- Morales, P.; Ferreira, I.C.F.R.; Carvalho, A.M.; Sánchez-Mata, M.C.; Cámara, M.; Fernández-Ruiz, V. Mediterranean non-cultivated vegetables as dietary sources of compounds with antioxidant and biological activity. LWT-Food Sci. Technol. 2014, 55, 389–396. [Google Scholar] [CrossRef] [Green Version]
- Ribeiro, S.M.R.; Schieber, A. Bioactive Compounds in Mango (Mangifera indica L.). In Bioactive Foods in Promoting Health; Elsevier: New York, NY, USA, 2010; pp. 507–523. [Google Scholar]
- Fernández-Ponce, M.T.; Parjikolaei, B.R.; Lari, H.N.; Casas, L.; Mantell, C.; Martínez de la Ossa, E.J. Pilot-plant scale extraction of phenolic compounds from mango leaves using different green techniques: Kinetic and scale up study. Chem. Eng. J. 2016, 299, 420–430. [Google Scholar] [CrossRef]
- Chemat, F.; Zill, e.-H.; Khan, M.K. Applications of ultrasound in food technology: Processing, preservation and extraction. Ultrason. Sonochem. 2011, 18, 813–835. [Google Scholar] [CrossRef] [PubMed]
- Al-Dhabi, N.A.; Ponmurugan, K.; Jeganathan, P.M. Development and validation of ultrasound-assisted solid-liquid extraction of phenolic compounds from waste spent coffee grounds. Ultrason. Sonochem. 2017, 34, 206–213. [Google Scholar] [CrossRef] [PubMed]
- Hossain, M.B.; Brunton, N.P.; Patras, A.; Tiwari, B.; O’Donnell, C.P.; Martin-Diana, A.B. Optimization of ultrasound assisted extraction of antioxidant compounds from marjoram (Origanum majorana L.) using response surface methodology. Ultrason. Sonochem. 2012, 19, 582–590. [Google Scholar] [CrossRef] [Green Version]
- Martínez-Ramos, T.; Benedito-Fort, J.; Watson, N.J.; Ruiz-López, I.I.; Che-Galicia, G.; Corona-Jiménez, E. Effect of solvent composition and its interaction with ultrasonic energy on the ultrasound-assisted extraction of phenolic compounds from Mango peels (Mangifera indica L.). Food Bioprod. Process. 2020, 122, 41–54. [Google Scholar] [CrossRef]
- Kulkarni, V.M.; Rathod, V.K. Mapping of an ultrasonic bath for ultrasound assisted extraction of mangiferin from Mangifera indica leaves. Ultrason. Sonochem. 2014, 21, 606–611. [Google Scholar] [CrossRef] [PubMed]
- Baqueiro-Peña, I.; Guerrero-Beltrán, J. Physicochemical and antioxidant characterization of Justicia spicigera. Food Chem. 2017, 218, 305–312. [Google Scholar] [CrossRef] [PubMed]
- Safdar, M.N.; Kausar, T.; Nadeem, M. Comparison of Ultrasound and Maceration Techniques for the Extraction of Polyphenols from the Mango Peel. J. Food Process. Preserv. 2017, 41, 1–13. [Google Scholar] [CrossRef]
- Prior, R.L.; Wu, X.; Schaich, K. Standardized methods for the determination of antioxidant capacity and phenolics in foods and dietary supplements. J. Agric. Food Chem. 2005, 53, 4290–4302. [Google Scholar] [CrossRef]
- Zhishen, J.; Mengcheng, T.; Jianming, W. The determination of flavonoid contents in mulberry and their scavenging effects on superoxide radicals. Food Chem. 1999, 64, 555–559. [Google Scholar] [CrossRef]
- Durazzo, A. Study aproach of antioxidant properties in foods: Update and considerations. Foods 2017, 6, 17. [Google Scholar] [CrossRef] [Green Version]
- Apak, R. Current Issues in Antioxidant Measurement. J. Agric. Food Chem. 2019, 67, 9187–9202. [Google Scholar] [CrossRef]
- Butkhup, L.; Samappito, W.; Samappito, S. Phenolic composition and antioxidant activity of white mulberry (Morus alba L.) fruits. Int. J. Food Sci. Technol. 2013, 48, 934–940. [Google Scholar] [CrossRef]
- Miller, N.J.; Rice-Evans, C.; Davies, M.J.; Gopinathan, V.; Milner, A. A Novel Method for Measuring Antioxidant Capacity and its Application to Monitoring the Antioxidant Status in Premature Neonates. Clin. Sci. 1993, 84, 407–412. [Google Scholar] [CrossRef] [Green Version]
- Fu, L.; Xu, B.T.; Gan, R.Y.; Zhang, Y.; Xu, X.R.; Xia, E.Q. Total Phenolic Contents and Antioxidant Capacities of Herbal and Tea Infusions. Int. J. Mol. Sci. 2011, 12, 2112–2124. [Google Scholar] [CrossRef] [Green Version]
- Brand-Williams, W.; Cuvelier, M.E.; Berset, C. Use of a Free Radical Method to Evaluate Antioxidant Activity. LWT-Food Sci. Technol. 1995, 28, 25–30. [Google Scholar] [CrossRef]
- Morales, M.; Zapata, K.; Sagaste, C.A.; Angulo, A.A.; Rojano, B. Optimization of the ultrasound-assisted extraction of polyphenol, mangiferin, and its antioxidant expression in mango peel (Mangifera indica) using response surface methodology. Acta Sci. Pol. Technol. Aliment. 2020, 19, 5–14. [Google Scholar]
- Jirasuteeruk, C.; Theerakulkait, C. Ultrasound-assisted extraction of phenolic compounds from mango (Mangifera indica cv. Chok Anan) peel and its inhibitory effect on enzymatic browning of potato puree. Food Technol. Biotechnol. 2019, 57, 350–357. [Google Scholar] [CrossRef]
- Torres-León, C.; Rojas, R.; Contreras-Esquivel, J.C.; Serna-Cock, L.; Belmares-Cerda, R.E.; Aguilar, C.N. Mango seed: Functional and nutritional properties. Trends Food Sci. Technol. 2016, 55, 109–117. [Google Scholar] [CrossRef]
- Tobón-Arroyave, N.C. Extracción Asistida por Ultrasonido de Compuestos Fenólicos de la Pulpa de Café (Coffea arabica L.) Variedad Castillo. Bachelors thesis, Facultad de ingenierías, Corporación Universitaria Lasallista, Caldas, Antioquia, Colobia, 2015. [Google Scholar]
- Qian, J.Y.; Liu, D.; Huang, A.G. The efficiency of flavonoids in polar extracts of Lycium chinense Mill fruits as free radical scavenger. Food Chem. 2004, 87, 283–288. [Google Scholar] [CrossRef]
- Velderrain-Rodríguez, G.; Torres-Moreno, H.; Villegas-Ochoa, M.; Ayala-Zavala, J.; Robles-Zepeda, R.; Wall-Medrano, A. Gallic Acid Content and an Antioxidant Mechanism Are Responsible for the Antiproliferative Activity of ‘Ataulfo’ Mango Peel on LS180 Cells. Molecules 2018, 23, 695. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gorinstein, S.; Martín-Belloso, O.; Park, Y.S.; Haruenkit, R.; Lojek, A.; Íž, M. Comparison of some biochemical characteristics of different citrus fruits. Food Chem. 2001, 74, 309–315. [Google Scholar] [CrossRef]
- Chung, K.T.; Wei, C.I.; Johnson, M.G. Are tannins a double-edged sword in biology and health? Trends Food Sci. Technol. 2002, 9, 168–175. [Google Scholar] [CrossRef]
- Pitchaon, M. Antioxidant capacity of extracts and fractions from mango (Mangifera indica Linn.) seed kernels. Int. Food Res. J. 2011, 18, 523–528. [Google Scholar]
- Ajila, C.M.; Naidu, K.A.; Bhat, S.G.; Rao, U.J.S.P. Bioactive compounds and antioxidant potential of mango peel extract. Food Chem. 2007, 105, 982–988. [Google Scholar] [CrossRef]
- Lundstedt, T.; Seifert, E.; Abramo, L.; Thelin, B.; Nyström, Å.; Pettersen, J. Experimental design and optimization. Chemom. Intell. Lab. Syst. 1998, 42, 3–40. [Google Scholar] [CrossRef]
- Verma, R.; Pavithra, P.; Sreevidya, N. Antibacterial and antioxidant activity of methanol extract of Evolvulus nummularius. Indian J. Pharmacol. 2009, 41, 233–236. [Google Scholar] [CrossRef] [Green Version]
- Prakash Maran, J.; Manikandan, S.; Vigna Nivetha, C.; Dinesh, R. Ultrasound assisted extraction of bioactive compounds from Nephelium lappaceum L. fruit peel using central composite face centered response surface design. Arab. J. Chem. 2017, 10, 1145–1157. [Google Scholar] [CrossRef] [Green Version]
- Lim, K.J.A.; Cabajar, A.A.; Lobarbio, C.F.Y.; Taboada, E.B.; Lacks, D.J. Extraction of bioactive compounds from mango (Mangifera indica L. var. Carabao) seed kernel with ethanol–water binary solvent systems. J. Food Sci. Technol. 2019, 56, 2536–2544. [Google Scholar] [CrossRef]
- Pérez-Nájera, V.C.; Lugo-Cervantes, E.C.; Gutiérrez-Lomel, M.; Del-Toro-Sánchez, C.L. Extracción de compuestos fenólicos de la cáscara de lima (citrus limetta risso) y determinación de su actividad antioxidante. Biotecnia 2013, 15, 18–22. [Google Scholar] [CrossRef] [Green Version]
- Taiz, L.; Zeiger, E. Fisiología vegetal. Universitat Jaume I. 2006, 3, 1338. [Google Scholar]
- Berardini, N.; Knödler, M.; Schieber, A.; Carle, R. Utilization of mango peels as a source of pectin and polyphenolics. Innov. Food Sci. Emerg. Technol. 2005, 6, 442–452. [Google Scholar] [CrossRef]
- Yu, L.; Haley, S.; Perret, J.; Harris, M.; Wilson, J.; Qian, M. Free radical scavenging properties of wheat extracts. J. Agric. Food Chem. 2002, 50, 1619–1624. [Google Scholar] [CrossRef] [PubMed]
- Do, Q.D.; Angkawijaya, A.E.; Tran-Nguyen, P.L.; Huynh, L.H.; Soetaredjo, F.E.; Ismadji, S. Effect of extraction solvent on total phenol content, total flavonoid content, and antioxidant activity of Limnophila aromatica. J. Food Drug Anal. 2014, 22, 296–302. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Spigno, G.; Tramelli, L.; De Faveri, D.M. Effects of extraction time, temperature and solvent on concentration and antioxidant activity of grape marc phenolics. J. Food Eng. 2007, 81, 200–208. [Google Scholar] [CrossRef]
- Nepote, V.; Grosso, N.R.; Guzmán, C.A.; Guzmán, G. Optimization of extraction of phenolic antioxidants from peanut skins. J. Sci. Food Agric. 2005, 85, 33–38. [Google Scholar] [CrossRef]
- Rivas-Pérez, B.N.; Colina, J.C. Compuestos fenólicos y actividad antioxidante en extractos de cuatro especies de orégano. Rev. Técnica de la Fac. de Ing. Univ. del Zulia 2017, 40, 134–142. [Google Scholar]
- Bochi, V.C.; Barcia, M.T.; Rodrigues, D.; Speroni, C.S.; Giusti, M.M.; Godoy, H.T. Polyphenol extraction optimisation from Ceylon gooseberry (Dovyalis hebecarpa) pulp. Food Chem. 2014, 164, 347–354. [Google Scholar] [CrossRef] [Green Version]
- Trabelsi, N.; Megdiche, W.; Ksouri, R.; Falleh, H.; Oueslati, S.; Soumaya, B. Solvent effects on phenolic contents and biological activities of the halophyte Limoniastrum monopetalum leaves. LWT-Food Sci. Technol. 2010, 43, 632–639. [Google Scholar] [CrossRef]
- Bychkov, A.L.; Ryabchikova, E.I.; Korolev, K.G.; Lomovsky, O.I. Ultrastructural changes of cell walls under intense mechanical treatment of selective plant raw material. Biomass Bioenerg. 2012, 47, 260–267. [Google Scholar] [CrossRef]
- Medina-Torres, N.; Ayora-Talavera, T.; Espinosa-Andrews, H.; Sánchez-Contreras, A.; Pacheco, N. Ultrasound Assisted Extraction for the Recovery of Phenolic Compounds from Vegetable Sources. Agronomy 2017, 7, 47. [Google Scholar] [CrossRef]
- Şahin, S.; Şamli, R. Optimization of olive leaf extract obtained by ultrasound-assisted extraction with response surface methodology. Ultrason. Sonochem. 2013, 20, 595–602. [Google Scholar] [CrossRef]
- Tao, Y.; Wu, D.; Zhang, Q.A.; Sun, D.W. Ultrasound-assisted extraction of phenolics from wine lees: Modeling, optimization and stability of extracts during storage. Ultrason. Sonochem. 2014, 21, 706–715. [Google Scholar] [CrossRef]
- Arranz-Martínez, S. Compuestos Polifenólicos (Extraíbles y no Extraíbles) en Alimentos de la Dieta Española: Metodología para su Determinación e Identificación. Doctoral thesis, Facultad de Farmacia, Universidad Complutense de Madrid, Madrid, España, 2010. [Google Scholar]
- Zou, T.B.; Xia, E.Q.; He, T.P.; Huang, M.Y.; Jia, Q.; Li, H.W. Ultrasound-assisted extraction of mangiferin from mango (Mangifera indica L.) leaves using response surface methodology. Molecules 2014, 19, 1411–1421. [Google Scholar] [CrossRef]
- Barreira, J.C.M.; Ferreira, I.C.F.R.; Oliveira, M.B.P.O.; Pereira, J.A. Effects of different phenols extraction conditions on antioxidant activity of almond (prunus dulcis) fruits. J. Food Biochem. 2009, 3, 763–776. [Google Scholar] [CrossRef]
- Rostagno, M.A.; Palma, M.; Barroso, C.G. Ultrasound-assisted extraction of soy isoflavones. Anal. Chim. Act. 2007, 597, 265–272. [Google Scholar] [CrossRef]
- Ying, Z.; Han, X.; Li, J. Ultrasound-assisted extraction of polysaccharides from mulberry leaves. Food Chem. 2011, 127, 1273–1279. [Google Scholar] [CrossRef] [PubMed]
- Vilkhu, K.; Mawson, R.; Simons, L.; Bates, D. Applications and opportunities for ultrasound assisted extraction in the food industry. A review. Innov. Food Sci. Emerg. Technol. 2008, 9, 161–169. [Google Scholar] [CrossRef]
- Toma, M.; Vinatoru, M.; Paniwnyk, L.; Mason, T.J. Investigation of the effects of ultrasound on vegetal tissues during solvent extraction. Ultrason. Sonochem. 2001, 8, 137–142. [Google Scholar] [CrossRef]





| n° | Solvent Relation | Amplitude | Sonication Time | Total | Total | ABTS | DPPH | |
|---|---|---|---|---|---|---|---|---|
| Ethanol | Polyphenols | Flavonoids | ||||||
| (%) | (%) | (min) | mg GAE/100 g | mg QE/100 g | Inhibition (%) | Inhibition (%) | ||
| Peel | C | 100 | - | - | 1261 ± 0.03 | 573 ± 0.01 | 66.7 ± 0.00 | 55.37 ± 0.07 |
| 1 | 0 (−1) | 30 (−1) | 10 (−1) | 320.1 ±7 0.00 | 254.5 ± 2.12 | 52.7 ± 5.80 | 13.6 ± 0.54 | |
| 2 | 100 (1) | 30 (−1) | 10 (−1) | 521.1 ±99.70 | 623.2 ± 6.71 | 82.2 ± 8.35 | 42.3 ± 6.57 | |
| 3 | 0 (−1) | 90 (1) | 10 (−1) | 203.1 ± 10.60 | 223.5 ± 3.53 | 54.1 ± 5.05 | 14.4 ± 1.49 | |
| 4 | 100 (1) | 90 (1) | 10 (−1) | 561.6 ± 0.00 | 626.4 ± 110.1 | 70.1 ± 0.20 | 44.1 ± 2.19 | |
| 5 | 0 (−1) | 30 (−1) | 30 (1) | 390.6 ± 76.36 | 387.2 ± 44.19 | 43.3 ± 19.21 | 20.6 ± 0.91 | |
| 6 | 100 (1) | 30 (−1) | 30 (1) | 843.6 ± 16.97 | 604.7 ± 26.51 | 73.0 ± 2.02 | 59.1 ± 4.45 | |
| 7 | 0 (−1) | 90 (1) | 30 (1) | 366.6 ± 42.42 | 438.5 ± 106.0 | 37.2 ± 2.34 | 23.3 ± 2.88 | |
| 8 | 100 (1) | 90 (1) | 30 (1) | 752.1 ± 2.12 | 704.5 ± 5.65 | 56.3 ± 2.30 | 49.9 ± 8.47 | |
| 9 | 50 (0) | 60 (0) | 20 (0) | 1485.6 ± 50.91 | 916 ± 98.99 | 60.0 ± 6.97 | 78.4 ± 0.91 | |
| 10 | 50 (0) | 60 (0) | 20 (0) | 1814.1 ± 258.19 | 1178.5 ± 67.17 | 82.1 ± 4.47 | 83.2 ± 0.54 | |
| 11 | 50 (0) | 60 (0) | 20 (0) | 1515.6 ± 76.36 | 1236 ± 381.83 | 51.8 ± 7.69 | 63.4 ± 23.55 | |
| Endocarp | C | 100 | - | - | 183 ± 0.00 | 53 ± 0.01 | 2.19 ± 0.00 | 0.75 ± 0.01 |
| 1 | 0 (−1) | 30 (−1) | 10 (−1) | 125.3 ± 15.90 | 616.1 ± 19.64 | 10.5 ± 0.33 | 0.58 ± 0.25 | |
| 2 | 100 (1) | 30 (−1) | 10 (−1) | 143.2 ± 0.75 | 348.0 ± 25.53 | 9.7 ± 0.33 | 0.19 ± 0.09 | |
| 3 | 0 (−1) | 90 (1) | 10 (−1) | 178.2 ± 1.26 | 298.0 ± 9.82 | 10.8 ± 0.00 | 0.16 ± 0.07 | |
| 4 | 100 (1) | 90 (1) | 10 (−1) | 151.4 ± 2.27 | 402.2 ± 11.78 | 9.1 ± 0.00 | 0.99 ± 1.34 | |
| 5 | 0 (−1) | 30 (−1) | 30 (1) | 213.2 ± 4.79 | 299.4 ± 3.92 | 9.5 ± 0.31 | 0.79 ± 0.87 | |
| 6 | 100 (1) | 30 (−1) | 30 (1) | 200.2 ± 0.50 | 381.3 ± 9.82 | 10.1 ± 0.75 | 1.06 ± 0.03 | |
| 7 | 0 (−1) | 90 (1) | 30 (1) | 273.2 ± 0.75 | 356.3 ± 25.53 | 11.3 ± 0.33 | 0.99 ± 0.34 | |
| 8 | 100 (1) | 90 (1) | 30 (1) | 122.3 ± 0.00 | 463.3 ± 0.00 | 8.5 ± 0.20 | 1.27 ± 1.13 | |
| 9 | 50 (0) | 60 (0) | 20 (0) | 469.8 ± 2.52 | 386.9 ± 5.89 | 14.4 ± 0.15 | 1.46 ± 0.34 | |
| 10 | 50 (0) | 60 (0) | 20 (0) | 469.5 ± 7.57 | 580 ± 3.92 | 13.3 ± 0.44 | 0.33 ± 0.28 | |
| 11 | 50 (0) | 60 (0) | 20 (0) | 385.3 ± 49.24 | 400.8 ± 1.96 | 12.3 ± 0.02 | 0.68 ± 0.40 | |
| Kernel | C | 100 | - | - | 670.8 ± 0.03 | 428 ± 0.00 | 53.94 ± 0.01 | 33.74 ± 0.01 |
| 1 | 0 (−1) | 30 (−1) | 10 (−1) | 345.2 ± 22.72 | 781.3 ± 9.82 | 49.5 ± 2.85 | 16.2 ± 0.00 | |
| 2 | 100 (1) | 30 (−1) | 10 (−1) | 252 ± 30.30 | 548.0 ± 9.82 | 63.7 ± 0.00 | 20.5 ± 0.50 | |
| 3 | 0 (−1) | 90 (1) | 10 (−1) | 292.7 ± 12.12 | 455 ± 82.49 | 71.2 ± 1.16 | 26.9 ± 1.65 | |
| 4 | 100 (1) | 90 (1) | 10 (−1) | 362.3 ± 4.54 | 774.4 ± 0.00 | 62.6 ± 0.00 | 22.7 ± 1.27 | |
| 5 | 0 (−1) | 30 (−1) | 30 (1) | 359.1 ± 12.12 | 816.1 ± 15.71 | 69.8 ± 0.57 | 31.6 ± 1.65 | |
| 6 | 100 (1) | 30 (−1) | 30 (1) | 307.7 ± 39.39 | 830 ± 1.60 | 74.6 ± 0.00 | 30.0 ± 0.38 | |
| 7 | 0 (−1) | 90 (1) | 30 (1) | 372 ± 33.33 | 585.5 ± 0.00 | 83.7 ± 0.93 | 39.0 ± 0.00 | |
| 8 | 100 (1) | 90 (1) | 30 (1) | 315.2 ± 1.51 | 778.6 ± 17.67 | 60.9 ± 0.57 | 21.3 ± 0.63 | |
| 9 | 50 (0) | 60 (0) | 20 (0) | 672 ± 15.15 | 860.5 ± 1.13 | 63.8 ± 0.00 | 55.8 ± 1.65 | |
| 10 | 50 (0) | 60 (0) | 20 (0) | 618.4 ± 3.03 | 886.9 ± 1.96 | 61.9 ± 0.51 | 57.5 ± 0.38 | |
| 11 | 50 (0) | 60 (0) | 20 (0) | 418.0 ± 4.54 | 868.8 ± 27.49 | 65.3 ± 0.83 | 59.3 ± 0.00 |
| Residues | Model Variables | Total Polyphenols mg GAE/100 g | Total Flavonoids mg QE/100 g | ABTS Inhibition (%) | DPPH Inhibition (%) |
|---|---|---|---|---|---|
| PEEL | β | 367 | 224 | 54.8 | 10.1 |
| β1 | −0.60 | 4.31 | 0.370 | 0.202 | |
| β2 | −2.73 | −1.20 | 0.086 | 0.000 | |
| β3 | 1.2 | 4.6 | −0.281 | 0.310 | |
| β12 | 0.0045 | 0.0045 | −0.00250 | 0.00122 | |
| β13 | 0.182 | −0.079 | −0.0006 | 0.0081 | |
| β23 | 0.077 | 0.069 | −0.0062 | 0.0015 | |
| β123 | −0.00187 | 0.00012 | 0.000024 | −0.000106 | |
| CP | 1110.2 | 627.3 | 6.02 | 41.61 | |
| S | 131.514 | 152.582 | 11.1711 | 9.34335 | |
| R2 | 0.964 | 0.8784 | 0.6807 | 0.9125 | |
| R2adj | 0.942 | 0.8036 | 0.4842 | 0.8587 | |
| R2pred | 0.939 | 0.7981 | 0.2818 | 0.8442 | |
| ENDOCARP | β | 56.8 | 1027 | 11.28 | 0.85 |
| β1 | 0.323 | −7.16 | −0.0170 | −0.0163 | |
| β2 | 0.821 | −8.43 | −0.0070 | −0.0122 | |
| β3 | 4.21 | −25.21 | −0.0873 | −0.0052 | |
| β12 | 0.0003 | 0.0910 | 0.000061 | 0.000303 | |
| β13 | 0.0079 | 0.2618 | 0.001332 | 0.000629 | |
| β23 | 0.0060 | 0.3125 | 0.001225 | 0.000516 | |
| β123 | −0.000777 | −0.00289 | −0.000021 | −0.00001 | |
| CP | 265.7 | 60.3 | 3.424 | 0.065 | |
| S | 30.6913 | 61.0593 | 0.655224 | 0.669933 | |
| R2 | 0.9657 | 0.776 | 0.9185 | 0.2865 | |
| R2adj | 0.9446 | 0.6382 | 0.8684 | 0 | |
| R2pred | 0.9486 | 0.6533 | 0.8434 | 0 | |
| KERNEL | β | 381 | 951.2 | 26.74 | 2.42 |
| β1 | −2.38 | −7.267 | 0.2893 | 0.0966 | |
| β2 | −1.42 | −6.238 | 0.4251 | 0.2053 | |
| β3 | −0.94 | −0.66 | 1.203 | 0.849 | |
| β12 | 0.0412 | 0.12326 | −0.003382 | −0.000795 | |
| β13 | 0.0629 | 0.2170 | −0.00343 | −0.00108 | |
| β23 | 0.0545 | 0.0799 | −0.00642 | −0.00270 | |
| β123 | −0.00140 | −0.003113 | −0.000041 | −0.000063 | |
| CP | 243.7 | 176 | −3.320 | 31.515 | |
| S | 76.6281 | 26.3773 | 1.34219 | 1.33077 | |
| R2 | 0.7876 | 0.9792 | 0.9848 | 0.9955 | |
| R2adj | 0.6569 | 0.9663 | 0.9754 | 0.9927 | |
| R2pred | 0.6627 | 0.9254 | 0.9597 | 0.9896 |
| Residue | Control (Maceration 24 h) (mg/g) | Ultrasound-Assisted Extraction (mg/g) |
|---|---|---|
| Peel | 0 | 150 ± 12.42 |
| Seed | 0 | 0.025 ± 0.0002 |
| Kernel | 0 | 0 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Borrás-Enríquez, A.J.; Reyes-Ventura, E.; Villanueva-Rodríguez, S.J.; Moreno-Vilet, L. Effect of Ultrasound-Assisted Extraction Parameters on Total Polyphenols and Its Antioxidant Activity from Mango Residues (Mangifera indica L. var. Manililla). Separations 2021, 8, 94. https://0-doi-org.brum.beds.ac.uk/10.3390/separations8070094
Borrás-Enríquez AJ, Reyes-Ventura E, Villanueva-Rodríguez SJ, Moreno-Vilet L. Effect of Ultrasound-Assisted Extraction Parameters on Total Polyphenols and Its Antioxidant Activity from Mango Residues (Mangifera indica L. var. Manililla). Separations. 2021; 8(7):94. https://0-doi-org.brum.beds.ac.uk/10.3390/separations8070094
Chicago/Turabian StyleBorrás-Enríquez, Anahí J., Elizabeth Reyes-Ventura, Socorro J. Villanueva-Rodríguez, and Lorena Moreno-Vilet. 2021. "Effect of Ultrasound-Assisted Extraction Parameters on Total Polyphenols and Its Antioxidant Activity from Mango Residues (Mangifera indica L. var. Manililla)" Separations 8, no. 7: 94. https://0-doi-org.brum.beds.ac.uk/10.3390/separations8070094