Biodegradable Hydrogels: Evaluation of Degradation as a Function of Synthesis Parameters and Environmental Conditions
Abstract
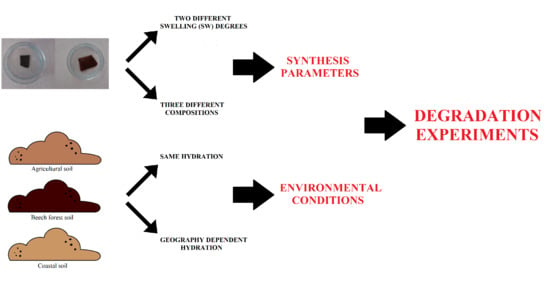
:1. Introduction
- physical crosslinking: weak bonds formation (hydrogen or electrostatic interactions) [7].
- hydrogels based on synthetic polymers;
- hydrogels based on natural polymers;
- hybrid hydrogels (based on both synthetic and natural polymers).
- cellulose derivatives (carboxymethyl cellulose sodium salt (NaCMC));
- clay mineral (sodium bentonite);
- humic acids sodium salt (AHum).
2. Materials and Methods
2.1. Chemicals
2.2. Hydrogel Synthesis Protocol
Hydrogels Preparation: General Procedure
2.3. Degradation Analysis Systems
- Two 50 mL Falcon tubes perforated on the bottom: the upper one with a larger hole to facilitate the introduction of deionized water, and the lower tube with many narrower holes to avoid any leakage of soil when present and to allow the water percolation;
- 2 Falcon caps from which the upper part was removed to allow the positioning of the discs of synthetic fabric;
- 2 discs of synthetic fabric with fine mesh (900 holes per cm2) placed in the upper part of each Falcon and held in place by the caps of the tubes;
- 1 square of absorbent paper crumpled and placed on top of the synthetic fabric in the upper tube, used to avoid the passage of soil through the synthetic fabric;
- 1 square of absorbent paper crumpled and placed at the base of the lower Falcon to prevent the leakage of soil when present;
- rubber bands.
- Systems with soil sample: the upper Falcon tube was filled with 10 g of soil. In the lower tube a piece of absorbent paper was shaped like a bowl and 4 g of soil was put in it to ensure contact between the soil and the overlaying synthetic fabric. This arrangement was adopted to allow the drainage of the deionized water through the lower Falcon tube and to mimic the placement of the hydrogel in the soil;
- Control systems: the upper Falcon tube was filled with on sheet of absorbent paper and two were placed in the lower Falcon.
2.4. Relationship Assessment between Curing Process and Swelling Degree (SW24)
2.5. Degradation Analysis Experiments
3. Results and Discussion
3.1. Relationship between Curing Process and Swelling Degree (SW24)
3.2. Degradation Analysis Experiments
- No significant differences in the resistance to degradation appeared among any of the cases under examination at these SW24 values;
- Comparing the different soil treatments, the coastal soil treatment was found to be the least effective on hydrogels degradation in all the studied conditions; the agricultural soil treatment, conversely, was the most degrading one, as well as the only one that displayed statistically significant differences from the others in most of the conditions tested in terms of AHum percent concentration and gel curing time.
- In the agricultural soil (organic C/N ratio 6.7): the low C/N ratio value, caused by the overexploitation of the soil, led to a mineralization of the nutrients (e.g., nitrogen). The low amount of organic matter present in this soil was supposedly the factor that led to the fast degradation of the studied hydrogels, which are made primarily of organic compounds.
- In the beech forest soil (organic C/N ratio 12.7), the higher C/N ratio value is due to the cyclic yearly addition of organic matter (e.g., leaves) and its slow decomposition, which leads to an increase in the acidity of the soil. Both these factors can affect hydrogels degradation, in part by a competition between the organic matter already present in the soil and the hydrogels and in part by a general slowing of the degradation activities caused by the acidity of the soil. Nevertheless, this soil is, by constitution, pre-enriched in microorganisms able to degrade organic matter, which partly occurs and contributes to the second-place score of this soil.
- The coastal soil (C/N ratio 8.8), like the agricultural soil, was subjected to cropping, but contains a higher organic matter amount. Although the soil in its bulk does not feature a marked salinity (electrical conductivity (EC 5:1) 0.375 mS/cm), its top-layer bacteria are prone to osmotic stress due to its location and the consequently frequent marine aerosols. This can slow the microorganisms’ metabolism, making them incapable of degrading the organic matter and the hydrogels as fast as in the other soil samples, leading to the least degradative effect among all cases examined.
- For both the agricultural and the beech forest soils, the degradation effect on the hydrogel was significantly higher than that observed in their respective controls at each of the AHum percentage concentrations and swelling values.
- The highest difference was displayed by the agricultural soil. For both agricultural and beech forest soils, the longer the curing time (corresponding to a lower swelling), the higher the degradation value.
- The treatment with the coastal soil showed no significant difference in comparison to its control in each of the combinations tested.
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| NaCMC | Carboxymethyl cellulose sodium salt |
| AHum | Humic acids |
| SW | Swelling degree |
| SW24 | Swelling degree after 24 h of immersion in deionized water |
References
- Worldometer. Available online: https://www.worldometers.info/world-population/ (accessed on 28 June 2021).
- Matson, P.A.; Parton, W.J.; Power, A.G.; Swift, M.J. Agricultural intensification and ecosystem properties. Science 1997, 277, 504–509. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sannino, A.; Demitri, C.; Madaghiele, M. Biodegradable cellulose-based hydrogels: Design and applications. Materials 2009, 2, 353–373. [Google Scholar] [CrossRef]
- Peppas, N.A.; Bures, P.; Leobandung, W.; Ichikawa, H. Hydrogels in pharmaceutical formulations. Eur. J. Pharm. Biopharm. 2000, 50, 27–46. [Google Scholar] [CrossRef]
- Prasanthi, N.L.; Roy, H.; Jyothi, N.; Vajrapriya, V.S. A Brief Review on Chitosan and Application in Biomedical Field. Am. J. PharmTech Res. 2016, 6, 41–51. [Google Scholar]
- Ullah, F.; Bisyrul, M.; Javed, F.; Akil, H. Classification, processing and application of hydrogels: A review. Mater. Sci. Eng. C 2015, 57, 414–433. [Google Scholar] [CrossRef] [PubMed]
- Chang, C.; Zhang, L. Cellulose-based hydrogels: Present status and application prospects. Carbohydr. Polym. 2011, 84, 40–53. [Google Scholar] [CrossRef]
- Maitra, J.; Shukla, V.K. Cross-linking in Hydrogels—A Review. Am. J. Polym. Sci. 2014, 4, 25–31. [Google Scholar]
- Aminabhavi, T.M.; Deshmukh, A.S. Polysaccharide-Based Hydrogels as Biomaterials; Springer International Publishing: Cham, Switzerland, 2016. [Google Scholar]
- Heinze, T.; el Seoud, O.A.; Koschella, A. Cellulose Derivatives: Synthesis, Structure, and Properties; Heinze, T., el Seoud, O.A., Koschella, A., Eds.; Springer International Publishing: Cham, Switzerland, 2018; pp. 39–172. [Google Scholar]
- Esposito, F.; del Nobile, M.A.; Mensitieri, G.; Nicolais, L. Water sorption in cellulose-based hydrogels. J. Appl. Polym. Sci. 1996, 60, 2403–2407. [Google Scholar] [CrossRef]
- Bentonite. Available online: https://bentonite.it/proprieta-bentonite.php (accessed on 14 January 2021).
- Belchinskaya, L.I.; Novikova, L.A.; Khokhlov, V.Y.; Tkhi, J.L.; Kartel, M.T. Surface Chemistry and Porosity of Natural and Activated Aluminosilicate from Montmorillonite and Clinoptilolite. Хімія, фізика та технoлoгія пoверхні 2015, 4, 358–365. [Google Scholar]
- Abdullahi, S.L.; Audu, A.A. Comparative Analysis on Chemical Composition of Bentonite Clays Obtained from Ashaka and Tango Deposits in Gombe State, Nigeria. ChemSearch J. 2017, 8, 35–40. [Google Scholar]
- Valde, B. Origin and Mineralogy of Clays: Clays and the Environment, 1st ed.; Springer: Berlin, Germany; Heidelberg, Germany, 1995. [Google Scholar]
- Mi, J.; Gregorich, E.G.; Xu, S.; Mclaughlin, N.B.; Ma, B.; Liu, J. Field Crops Research Effect of bentonite amendment on soil hydraulic parameters and millet crop performance in a semi-arid region. Field Crop Res. 2017, 212, 107–114. [Google Scholar] [CrossRef]
- Canellas, L.P.; Olivares, F.L.; Aguiar, N.O.; Jones, D.L.; Nebbioso, A.; Mazzei, P.; Piccolo, A. Scientia Horticulturae Humic and fulvic acids as biostimulants in horticulture. Sci. Hortic. 2015, 196, 15–27. [Google Scholar] [CrossRef]
- Pettit, R.E. Organic matter, humus, humate, humic acid, fulvic acid and humin: Their importance in soil fertility and plant health. CTI Res. 2004, 10, 1–7. [Google Scholar]
- Zhou, L.; Monreal, C.M.; Xu, S.; Mclaughlin, N.B.; Zhang, H. Geoderma Effect of bentonite-humic acid application on the improvement of soil structure and maize yield in a sandy soil of a semi-arid region. Geoderma 2019, 338, 269–280. [Google Scholar] [CrossRef]







| Soil Sample | Description |
|---|---|
| Agricultural soil | pH 8.1 |
| Organic carbon 0.6% w/w | |
| Total nitrogen 0.09% w/w | |
| Organic C/N: 6.7 | |
| Olsen P2O5: 0.18% w/w, | |
| Texture: sand: 35%; silt: 48%; clay: 17% | |
| Electrical conductivity: 0.145 mS/cm | |
| Beech forest soil | pH 6.6 |
| Organic carbon 6.73% w/w (humic carbon 4.22%) | |
| Total nitrogen: 0.53% w/w | |
| Organic C/N: 12.7 | |
| Olsen P2O5: 0.26% w/w | |
| Texture: sand: 55%; silt: 22%; clay: 23% | |
| Electrical conductivity 0.112 µS/cm | |
| Coastal soil | pH 7.8 |
| Organic carbon 1.52% w/w | |
| Total nitrogen 0.17% w/w | |
| Organic C/N: 8.8 | |
| Olsen P2O5: 0.16% w/w | |
| Texture: sand: 34%; silt: 23%; clay: 43% | |
| Electric conductivity: 0.375 mS/cm |
| Suspension | Curing Temperature | Curing Times |
|---|---|---|
| 50(10)–5 | 110 °C | 1, 3, 5, 7, 9, and 12 h |
| 50(30)–5 | 110 °C | 30 min; 2 h; 3 h 30 min; 5 h; 6 h 30′ min; 7 h; 7 h 30 min; 12 h |
| 50(50)–5 | 110 °C | 30 min; 1 h; 1 h 30 min; 2 h; 2 h 30 min; 3 h 30 min; 4 h 30 min; |
| 5 h 30 min; 6 h 30 min; 7 h; 7 h 30 min; 12 h |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Turioni, C.; Guerrini, G.; Squartini, A.; Morari, F.; Maggini, M.; Gross, S. Biodegradable Hydrogels: Evaluation of Degradation as a Function of Synthesis Parameters and Environmental Conditions. Soil Syst. 2021, 5, 47. https://0-doi-org.brum.beds.ac.uk/10.3390/soilsystems5030047
Turioni C, Guerrini G, Squartini A, Morari F, Maggini M, Gross S. Biodegradable Hydrogels: Evaluation of Degradation as a Function of Synthesis Parameters and Environmental Conditions. Soil Systems. 2021; 5(3):47. https://0-doi-org.brum.beds.ac.uk/10.3390/soilsystems5030047
Chicago/Turabian StyleTurioni, Chiara, Giacomo Guerrini, Andrea Squartini, Francesco Morari, Michele Maggini, and Silvia Gross. 2021. "Biodegradable Hydrogels: Evaluation of Degradation as a Function of Synthesis Parameters and Environmental Conditions" Soil Systems 5, no. 3: 47. https://0-doi-org.brum.beds.ac.uk/10.3390/soilsystems5030047