Valorizing Waste Lignocellulose-Based Furniture Boards by Phosphoric Acid and Hydrogen Peroxide (Php) Pretreatment for Bioethanol Production and High-Value Lignin Recovery
Abstract
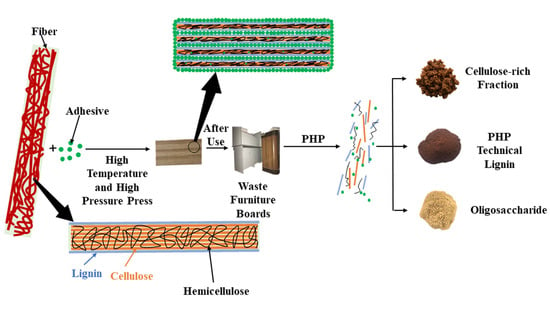
:1. Introduction
2. Materials and Methods
2.1. Feedstocks and Chemicals
2.2. The PHP Pretreatment Process
2.3. Enzymatic Hydrolysis
2.4. Simultaneous Saccharification and Fermentation (SSF) for Ethanol
2.5. Determination of Glucose and Ethanol Concentration
2.6. Scanning Electron Microscopy (SEM)
2.7. X-Ray Diffraction (XRD)
2.8. Water Retention Value (WRV) of Cellulose-Rich Fraction
2.9. Simons’ Stain (SS)
2.10. Lignin Characterization by Nuclear Magnetic Resonance (NMR)
3. Results and Discussion
3.1. The Main Fractions of Furniture Boards before and after PHP Pretreatment
3.2. Performances of the Cellulose-Rich Fraction for Enzymatic Hydrolysis and Ethanol Conversion
3.3. Characteristics of Recovered Lignin from PHP Pretreatment
3.4. Mass Balance of These Three Furniture Boards by PHP Pretreatment
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Özdenkçi, K.; De Blasio, C.; Muddassar, H.R.; Melin, K.; Oinas, P.; Koskinen, J.; Sarwar, G.; Järvinen, M. A novel biorefinery integration concept for lignocellulosic biomass. Energy Convers. Manag. 2017, 149, 974–987. [Google Scholar] [CrossRef] [Green Version]
- Van Osch, D.J.G.P.; Kollau, L.J.B.M.; Van Den Bruinhorst, A.; Asikainen, S.; Rocha, M.A.A.; Kroon, M.C. Ionic liquids and deep eutectic solvents for lignocellulosic biomass fractionation. Phys. Chem. Chem. Phys. 2017, 19, 2636–2665. [Google Scholar] [CrossRef]
- Mattila, H.; Kuuskeri, J.; Lundell, T. Single-step, single-organism bioethanol production and bioconversion of lignocellulose waste materials by phlebioid fungal species. Bioresour. Technol. 2017, 225, 254–261. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Siengchum, T.; Isenberg, M.; Chuang, S.S.C. Fast pyrolysis of coconut biomass-An FTIR study. Fuel 2013, 105, 559–565. [Google Scholar] [CrossRef]
- Blinová, L.; Bartošová, A.; Sirotiak, M. Unconventional type of biomass suitable for the production of Biofuels. Adv. Mater. Res. 2014, 860–863, 514–517. [Google Scholar] [CrossRef]
- Xiong, X.; Guo, W.; Lu, F.; Zhang, M.; Wu, Z.; Lu, R.; Miyakoshi, T. Current state and development trend of Chinese furniture industry. J. Wood Sci. 2017, 63, 433–444. [Google Scholar] [CrossRef]
- Halvarsson, S.; Edlund, H.; Norgren, M. Properties of medium-density fibreboard (MDF) based on wheat straw and melamine modified urea formaldehyde (UMF) resin. Ind. Crop. Prod. 2008, 28, 37–46. [Google Scholar] [CrossRef]
- Merrild, H.; Christensen, T.H. Recycling of wood for particle board production: Accounting of greenhouse gases and global warming contributions. Waste Manag. Res. 2009, 27, 781–788. [Google Scholar] [CrossRef]
- Safarian, S.; Unnthorsson, R. An assessment of the sustainability of lignocellulosic bioethanol production from wastes in Iceland. Energies 2018, 11, 1493. [Google Scholar] [CrossRef]
- Moreno, A.D.; Ibarra, D.; Alvira, P.; Tomás-Pejó, E.; Ballesteros, M. A review of biological delignification and detoxification methods for lignocellulosic bioethanol production. Crit. Rev. Biotechnol. 2015, 35, 342–354. [Google Scholar] [CrossRef]
- Khosravi, S.; Khabbaz, F.; Nordqvist, P.; Johansson, M. Wheat-gluten-based adhesives for particle boards: Effect of crosslinking agents. Macromol. Mater. Eng. 2014, 299, 116–124. [Google Scholar] [CrossRef]
- Ravindran, R.; Jaiswal, A.K. A comprehensive review on pre-treatment strategy for lignocellulosic food industry waste: Challenges and opportunities. Bioresour. Technol. 2016, 199, 92–102. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wang, Q.; Wang, Z.; Shen, F.; Hu, J.; Sun, F.; Lin, L.; Yang, G.; Zhang, Y.; Deng, S. Pretreating lignocellulosic biomass by the concentrated phosphoric acid plus hydrogen peroxide (PHP) for enzymatic hydrolysis: Evaluating the pretreatment flexibility on feedstocks and particle sizes. Bioresour. Technol. 2014, 166, 420–428. [Google Scholar] [CrossRef] [PubMed]
- Wang, Q.; Hu, J.; Shen, F.; Mei, Z.; Yang, G.; Zhang, Y.; Hu, Y.; Zhang, J.; Deng, S. Pretreating wheat straw by the concentrated phosphoric acid plus hydrogen peroxide (PHP): Investigations on pretreatment conditions and structure changes. Bioresour. Technol. 2016, 199, 245–257. [Google Scholar] [CrossRef]
- Qiu, J.; Ma, L.; Shen, F.; Yang, G.; Zhang, Y.; Deng, S.; Zhang, J.; Zeng, Y.; Hu, Y. Pretreating wheat straw by phosphoric acid plus hydrogen peroxide for enzymatic saccharification and ethanol production at high solid loading. Bioresour. Technol. 2017, 238, 174–181. [Google Scholar] [CrossRef]
- Wang, Q.; Tian, D.; Hu, J.; Shen, F.; Yang, G.; Zhang, Y.; Deng, S.; Zhang, J.; Zeng, Y.; Hu, Y. Fates of hemicellulose, lignin and cellulose in concentrated phosphoric acid with hydrogen peroxide (PHP) pretreatment. RSC Adv. 2018, 8, 12714–12723. [Google Scholar] [CrossRef] [Green Version]
- Qiu, J.; Wang, Q.; Shen, F.; Yang, G.; Zhang, Y.; Deng, S.; Zhang, J.; Zeng, Y.; Song, C. Optimizing phosphoric acid plus hydrogen peroxide (PHP) pretreatment on wheat straw by response surface method for enzymatic saccharification. Appl. Biochem. Biotechnol. 2017, 181, 1123–1139. [Google Scholar] [CrossRef]
- Wan, X.; Tian, D.; Shen, F.; Hu, J.; Yang, G.; Zhang, Y.; Deng, S.; Zhang, J.; Zeng, Y. Fractionating wheat straw via phosphoric acid with hydrogen peroxide pretreatment and structural elucidation of the derived lignin. Energy Fuels 2018, 32, 5218–5225. [Google Scholar] [CrossRef]
- Yao, F.; Tian, D.; Shen, F.; Hu, J.; Zeng, Y.; Yang, G.; Zhang, Y.; Deng, S.; Zhang, J. Recycling solvent system in phosphoric acid plus hydrogen peroxide pretreatment towards a more sustainable lignocellulose biorefinery for bioethanol. Bioresour. Technol. 2019, 275, 19–26. [Google Scholar] [CrossRef]
- Tian, D.; Han, Y.; Lu, C.; Zhang, X.; Yuan, G. Acidic ionic liquid as “quasi-homogeneous” catalyst for controllable synthesis of cellulose acetate. Carbohydr. Polym. 2014, 113, 83–90. [Google Scholar] [CrossRef]
- Cheng, Q.; Wang, J.; McNeel, J.F.; Jacobson, P.M. Water retention value measurements of cellulosic materials using a centrifuge technique. BioResources 2010, 5, 1945–1954. [Google Scholar]
- Chandra, R.; Ewanick, S.; Hsieh, C.; Saddler, J.N. The characterization of pretreated lignocellulosic substrates prior to enzymatic hydrolysis, Part 1: A modified Simons’ staining technique. Biotechnol. Prog. 2008, 24, 1178–1185. [Google Scholar] [CrossRef] [PubMed]
- Wen, J.; Xue, B.; Sun, S.; Sun, R. Quantitative structural characterization and thermal properties of birch lignins after auto-catalyzed organosolv pretreatment and enzymatic hydrolysis. J. Chem. Technol. Biotechnol. 2013, 88, 1663–1771. [Google Scholar] [CrossRef]
- Castellano, J.M.; Gómez, M.; Fernández, M.; Esteban, L.S.; Carrasco, J.E. Study on the effects of raw materials composition and pelletization conditions on the quality and properties of pellets obtained from different woody and non woody biomasses. Fuel 2015, 139, 629–636. [Google Scholar] [CrossRef]
- Andersen, N.; Johansen, K.S.; Michelsen, M.; Stenby, E.H.; Krogh, K.B.R.M.; Olsson, L. Hydrolysis of cellulose using mono-component enzymes shows synergy during hydrolysis of phosphoric acid swollen cellulose (PASC), but competition on Avicel. Enzyme Microb. Technol. 2008, 42, 362–370. [Google Scholar] [CrossRef]
- Sun, S.; Sun, S.; Cao, X.; Sun, R. The role of pretreatment in improving the enzymatic hydrolysis of lignocellulosic materials. Bioresour. Technol. 2016, 199, 49–58. [Google Scholar] [CrossRef]
- Katsimpouras, C.; Kalogiannis, K.G.; Kalogianni, A.; Lappas, A.A.; Topakas, E. Production of high concentrated cellulosic ethanol by acetone/water oxidized pretreated beech wood. Biotechnol. Biofuels 2017, 10, 54. [Google Scholar] [CrossRef] [Green Version]
- Imman, S.; Arnthong, J.; Burapatana, V.; Champreda, V.; Laosiripojana, N. Effects of acid and alkali promoters on compressed liquid hot water pretreatment of rice straw. Bioresour. Technol. 2014, 171, 29–36. [Google Scholar] [CrossRef]
- Liu, H.; Pang, B.; Zhao, Y.; Lu, J.; Han, Y.; Wang, H. Comparative study of two different alkali-mechanical pretreatments of corn stover for bioethanol production. Fuel 2018, 221, 21–27. [Google Scholar] [CrossRef]
- Shimizu, F.L.; Monteiro, P.Q.; Ghiraldi, P.H.C.; Melati, R.B.; Pagnocca, F.C.; de Souza, W.; Sant’Anna, C.; Brienzo, M. Acid, alkali and peroxide pretreatments increase the cellulose accessibility and glucose yield of banana pseudostem. Ind. Crop. Prod. 2018, 115, 62–68. [Google Scholar] [CrossRef] [Green Version]
- Luo, M.; Tian, D.; Shen, F.; Hu, J.; Zhang, Y.; Yang, G.; Zeng, Y.; Deng, S.; Hu, Y. A comparative investigation of H2O2-involved pretreatments on lignocellulosic biomass for enzymatic hydrolysis. Biomass Convers. Biorefin. 2019, 9, 321–331. [Google Scholar] [CrossRef]
- Alvira, P.; Tomás-Pejó, E.; Ballesteros, M.; Negro, M.J. Pretreatment technologies for an efficient bioethanol production process based on enzymatic hydrolysis: A review. Bioresour. Technol. 2010, 101, 4851–4861. [Google Scholar] [CrossRef] [PubMed]
- Sun, S.; Cao, X.; Sun, S.; Xu, F.; Song, X.; Sun, R.; Jones, G.L. Improving the enzymatic hydrolysis of thermo-mechanical fiber from Eucalyptus urophylla by a combination of hydrothermal pretreatment and alkali fractionation. Biotechnol. Biofuels 2014, 7, 116. [Google Scholar] [CrossRef] [PubMed]
- Boonsombuti, A.; Luengnaruemitchai, A.; Wongkasemjit, S. Enhancement of enzymatic hydrolysis of corncob by microwave-assisted alkali pretreatment and its effect in morphology. Cellulose 2013, 20, 1957–1966. [Google Scholar] [CrossRef]
- Weiss, N.D.; Thygesen, L.G.; Roslander, C.; Gourlay, K.; Felby, C. Biomass-water interactions correlate to recalcitrance and are intensified by pretreatment: An investigation of water constraint and retention in pretreated spruce using low field NMR and water retention value techniques. Biotechnol. Prog. 2016, 33, 146–153. [Google Scholar] [CrossRef]
- Huang, F.; Singh, P.M.; Ragauskas, A.J. Characterization of milled wood lignin (MWL) in loblolly pine stem wood, residue, and bark. J. Agric. Food Chem. 2011, 59, 12910–12916. [Google Scholar] [CrossRef]
- Sun, S.; Huang, Y.; Sun, R.; Tu, M. Strong association of condensed phenolic moieties in isolated lignins with their inhibition of enzymatic hydrolysis. Green Chem. 2016, 18, 4276–4286. [Google Scholar] [CrossRef]
- Su, Y.; Du, R.; Guo, H.; Cao, M.; Wu, Q.; Su, R.; Qi, W.; He, Z. Fractional pretreatment of lignocellulose by alkaline hydrogen peroxide: Characterization of its major components. Food Bioprod. Process. 2014, 94, 322–330. [Google Scholar] [CrossRef]
- Nagy, M.; David, K.; Britovsek, G.J.P.; Ragauskas, A.J. Catalytic hydrogenolysis of ethanol organosolv lignin. Holzforschung 2009, 63, 513–520. [Google Scholar] [CrossRef]
- Pu, Y.; Cao, S.; Ragauskas, A.J. Application of quantitative 31P NMR in biomass lignin and biofuel precursors characterization. Energy Environ. Sci. 2011, 4, 3154–3166. [Google Scholar] [CrossRef]
- Qiao, H.; Zhou, Y.; Yu, F.; Wang, E.; Min, Y.; Huang, Q.; Pang, L.; Ma, T. Effective removal of cationic dyes using carboxylate-functionalized cellulose nanocrystals. Chemosphere 2015, 141, 297–303. [Google Scholar] [CrossRef] [PubMed]
- Wu, Q.; Li, W.; Liu, S. Carboxyl-rich carbon microspheres prepared from pentosan with high adsorption capacity for heavy metal ions. Mater. Res. Bull. 2014, 60, 516–523. [Google Scholar] [CrossRef]





| Feedstocks | Chemical Composition (%) | |||||
|---|---|---|---|---|---|---|
| Cellulose a | Hemicellulose b | Acid-Insoluble Lignin c | Acid-Soluble Lignin | Extractives | Ash | |
| Fiberboard | 31.6 ± 0.30 | 10.0 ± 0.35 | 28.8 ± 0.08 | 2.6 ± 0.04 | 9.5 ± 0.01 | 2.4 ± 0.01 |
| Chipboard | 28.2 ± 0.23 | 8.7 ± 0.42 | 30.3 ± 0.31 | 1.6 ± 0.05 | 5.4 ± 0.01 | 3.8 ± 0.09 |
| Blockboard | 29.5 ± 0.29 | 10.1 ± 0.42 | 24.0 ± 0.34 | 2.4 ± 0.01 | 5.9 ± 0.47 | 5.4 ± 0.13 |
| Pretreated Solids | Solid Yield (%) | Cellulose (%) | Hemicellulose (%) | Lignin (%) | Cellulose Recovery (%) | Hemicellulose Removal (%) | Lignin Removal (%) |
|---|---|---|---|---|---|---|---|
| PHP-Fiberboard | 41.7 | 59.8 ± 0.79 | 4.0 ± 0.09 | 18.3 ± 0.01 | 78.9 | 83.5 | 75.7 |
| PHP-Chipboard | 39.1 | 57.1 ± 0.95 | 5.1 ± 0.17 | 16.6 ± 0.28 | 79.3 | 77.0 | 79.7 |
| PHP-Blockboard | 50.0 | 53.8 ± 0.12 | 5.9 ± 0.04 | 13.4 ± 0.38 | 91.2 | 70.4 | 74.6 |
| Samples | Crystallinity (%) | Crystal Size (nm) | WRV | Value of SS (DO/DB) |
|---|---|---|---|---|
| Fiberboard | 43.56 | 4.64 | 1.04 | 0.10 |
| PHP-Fiberboard | 36.73 | 3.90 | 2.67 | 0.86 |
| Chipboard | 46.19 | 3.74 | 1.03 | 0.38 |
| PHP-Chipboard | 37.97 | 3.63 | 3.43 | 0.86 |
| Blockboard | 47.13 | 3.88 | 1.44 | 0.40 |
| PHP-Blockboard | 42.16 | 3.61 | 3.47 | 1.16 |
| Samples | Lignin-Fiberboard | Lignin-Chipboard | Lignin-Blockboard |
|---|---|---|---|
| Aliphatic OH | 1.51 | 1.52 | 1.68 |
| C-5 substitution | 1.38 | 1.01 | 1.15 |
| Guaiacyl phenolic OH | 0.49 | 0.47 | 0.49 |
| p-Hydroxyphenyl OH | 0.29 | 0.27 | 0.30 |
| Carboxylic OH | 0.67 | 0.61 | 0.65 |
| Total phenolic OH | 2.17 | 1.75 | 1.94 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhao, J.; Tian, D.; Shen, F.; Hu, J.; Zeng, Y.; Huang, C. Valorizing Waste Lignocellulose-Based Furniture Boards by Phosphoric Acid and Hydrogen Peroxide (Php) Pretreatment for Bioethanol Production and High-Value Lignin Recovery. Sustainability 2019, 11, 6175. https://0-doi-org.brum.beds.ac.uk/10.3390/su11216175
Zhao J, Tian D, Shen F, Hu J, Zeng Y, Huang C. Valorizing Waste Lignocellulose-Based Furniture Boards by Phosphoric Acid and Hydrogen Peroxide (Php) Pretreatment for Bioethanol Production and High-Value Lignin Recovery. Sustainability. 2019; 11(21):6175. https://0-doi-org.brum.beds.ac.uk/10.3390/su11216175
Chicago/Turabian StyleZhao, Jingwen, Dong Tian, Fei Shen, Jinguang Hu, Yongmei Zeng, and Churui Huang. 2019. "Valorizing Waste Lignocellulose-Based Furniture Boards by Phosphoric Acid and Hydrogen Peroxide (Php) Pretreatment for Bioethanol Production and High-Value Lignin Recovery" Sustainability 11, no. 21: 6175. https://0-doi-org.brum.beds.ac.uk/10.3390/su11216175