Aquatic Macrophytes in Constructed Wetlands: A Fight against Water Pollution
Abstract
:1. Introduction
2. Wetland Systems
2.1. Natural and Constructed Wetlands
2.2. Types of Constructed Wetlands
3. Macrophytes and Wetlands
4. Wetland Removal of Water Contaminants
4.1. Trace Metal Elements
4.2. Pharmaceuticals
4.3. Pesticides
4.4. Sewage
4.5. Cyanotoxins
4.6. Nanoparticles
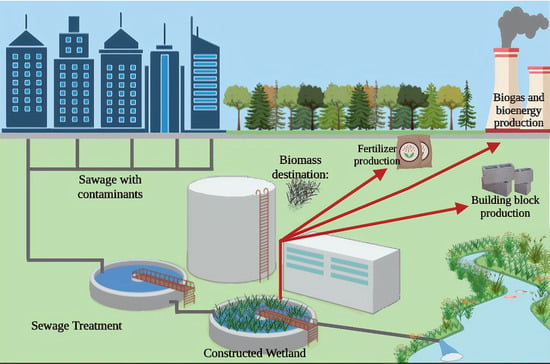
5. Wetland System Biomass Management and Destination
6. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- UNICEF; WHO. Progress on Drinking Water, Sanitation and Hygiene—Joint Monitoring Programme 2017 Update and SDG Baselines; World Health Organization (WHO); The United Nations Childrens’ Fund (UNICEF): Geneva, Switzerland, 2017. [Google Scholar]
- UNDP. Sustainable Development Goals—Goal 6: Clean Water and Sanitation. 2015. Available online: https://www.undp.org/content/undp/en/home/sustainable-development-goals/goal-6-clean-water-and-sanitation.html (accessed on 17 May 2020).
- Rodriguez-Narvaez, O.M.; Peralta-Hernandez, J.M.; Goonetilleke, A.; Bandala, E.R. Treatment Technologies for Emerging Contaminants in Water: A Review. Chem. Eng. J. 2017, 323, 361–380. [Google Scholar] [CrossRef] [Green Version]
- Tran, N.H.; Reinhard, M.; Gin, K.Y.-H. Occurrence and Fate of Emerging Contaminants in Municipal Wastewater Treatment Plants from Different Geographical Regions-a Review. Water Res. 2018, 133, 182–207. [Google Scholar] [CrossRef] [PubMed]
- Gogoi, A.; Mazumder, P.; Tyagi, V.K.; Chaminda, G.G.T.; An, A.K.; Kumar, M. Occurrence and Fate of Emerging Contaminants in Water Environment: A Review. Groundw. Sustain. Dev. 2018, 6, 169–180. [Google Scholar] [CrossRef]
- Gomes, M.P.; Gonçalves, C.A.; de Brito, J.C.M.; Souza, A.M.; da Silva, F.V.C.; Bicalho, E.M.; Figueredo, C.C.; Garcia, Q.S. Ciprofloxacin Induces Oxidative Stress in Duckweed (Lemna Minor L.): Implications for Energy Metabolism and Antibiotic-Uptake Ability. J. Hazard. Mater. 2017, 328, 140–149. [Google Scholar] [CrossRef] [PubMed]
- Sauvé, S.; Desrosiers, M. A Review of What Is an Emerging Contaminant. Chem. Cent. J. 2014, 8, 15. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- EPA. Recommendations for Public Water Systems to Manage Cyanotoxins in Drinking Water; 2015. Available online: https://www.epa.gov/sites/production/files/2018-11/documents/cyanotoxin-management-drinking-water.pdf (accessed on 17 May 2020).
- Baylor University. Environmental Quality Research Questions Identified for Latin American Region: Global Horizon Scanning Project Will Help Scientists Address Pressing Environmental and Health Issues in the Latin American Region. ScienceDaily, 3 May 2020. Available online: https://www.sciencedaily.com/releases/2018/05/180503142935.htm(accessed on 3 November 2020).
- Gomes, M.P.M.P.; Moura, P.A.S.P.A.S.; Nascentes, C.C.C.C.; Scotti, M.R. Arbuscular Mycorrhizal Fungi and Arsenate Uptake by Brachiaria Grass (Brachiaria Decumbens). Bioremediat. J. 2015, 19, 151–159. [Google Scholar] [CrossRef]
- Zhou, Q.; Zhang, J.; Fu, J.; Shi, J.; Jiang, G. Biomonitoring: An Appealing Tool for Assessment of Metal Pollution in the Aquatic Ecosystem. Anal. Chim. Acta 2008, 606, 135–150. [Google Scholar] [CrossRef]
- Gomes, M.P.; Rocha, D.C.; de Brito, J.C.M.; Tavares, D.S.; Marques, R.Z.; Soffiatti, P.; Sant’Anna-Santos, B.F. Emerging Contaminants in Water Used for Maize Irrigation: Economic and Food Safety Losses Associated with Ciprofloxacin and Glyphosate. Ecotoxicol. Environ. Saf. 2020, 196, 110549. [Google Scholar] [CrossRef]
- Luo, Y.; Guo, W.; Ngo, H.H.; Nghiem, L.D.; Hai, F.I.; Zhang, J.; Liang, S.; Wang, X.C. A Review on the Occurrence of Micropollutants in the Aquatic Environment and Their Fate and Removal during Wastewater Treatment. Sci. Total Environ. 2014, 473–474, 619–641. [Google Scholar] [CrossRef]
- Blum, K.M.; Andersson, P.L.; Ahrens, L.; Wiberg, K.; Haglund, P. Persistence, Mobility and Bioavailability of Emerging Organic Contaminants Discharged from Sewage Treatment Plants. Sci. Total Environ. 2018, 612, 1532–1542. [Google Scholar] [CrossRef]
- Geissen, V.; Mol, H.; Klumpp, E.; Umlauf, G.; Nadal, M.; van der Ploeg, M.; van de Zee, S.E.A.T.M.; Ritsema, C.J. Emerging Pollutants in the Environment: A Challenge for Water Resource Management. Int. Soil Water Conserv. Res. 2015, 3, 57–65. [Google Scholar] [CrossRef]
- Gomes, M.P.; Tavares, D.S.; Richardi, V.S.; Marques, R.Z.; Wistuba, N.; de Brito, J.C.M.; Soffiatti, P.; Sant’Anna-Santos, B.F.; da Silva, M.A.N.; Juneau, P. Enrofloxacin and Roundup® Interactive Effects on the Aquatic Macrophyte Elodea Canadensis Physiology. Environ. Pollut. 2019, 249, 453–462. [Google Scholar] [CrossRef]
- Baken, K.A.; Sjerps, R.M.A.; Schriks, M.; van Wezel, A.P. Toxicological Risk Assessment and Prioritization of Drinking Water Relevant Contaminants of Emerging Concern. Environ. Int. 2018, 118, 293–303. [Google Scholar] [CrossRef]
- Kwach, B.O.; Shikuku, V.O. Microplastics as Emerging Contaminants: Occurrence, Toxicology, and Analysis. In Effects of Emerging Chemical Contaminants on Water Resources and Environmental Health; IGI Global: Hersey, PA, USA, 2020; pp. 31–44. [Google Scholar] [CrossRef]
- Noguera-Oviedo, K.; Aga, D.S. Lessons Learned from More than Two Decades of Research on Emerging Contaminants in the Environment. J. Hazard. Mater. 2016, 316, 242–251. [Google Scholar] [CrossRef] [PubMed]
- UE. Directive 2000/60/EC; 2000; p 73. Available online: https://eur-lex.europa.eu/legal-content/EN/TXT/?uri=celex%3A32000L0060 (accessed on 27 May 2020).
- Sehar, S.; Nasser, H.A.A. Wastewater Treatment of Food Industries through Constructed Wetland: A Review. Int. J. Environ. Sci. Technol. 2019, 16, 6453–6472. [Google Scholar] [CrossRef]
- Ingrao, C.; Failla, S.; Arcidiacono, C. A Comprehensive Review of Environmental and Operational Issues of Constructed Wetland Systems. Curr. Opin. Environ. Sci. Health 2020, 13, 35–45. [Google Scholar] [CrossRef]
- Kumar, S.; Dutta, V. Constructed Wetland Microcosms as Sustainable Technology for Domestic Wastewater Treatment: An Overview. Environ. Sci. Pollut. Res. 2019, 26, 11662–11673. [Google Scholar] [CrossRef]
- Hua, S.C. The Use of Constructed Wetlands for Wastewater Treatment; Wetlands International—Asia Pacific: Selangor, Malaysia, 2003. [Google Scholar]
- Guittonny-Philippe, A.; Masotti, V.; Combroux, I.; Malleret, L.; Boudenne, J.-L.; Petit, M.-E.; Monnier, Y.; Coulomb, B.; Viglione, J.; Laffont-Schwob, I. Proposal of a New Ecotoxicity Evaluation Tool Based on Morphological Responses of Five Helophytes to Mixtures of Pollutants: The Helophyte Development Index. Ecol. Eng. 2015, 77, 180–188. [Google Scholar] [CrossRef]
- Marín-Muñiz, J.L.; Hernández, M.E.; Gallegos-Pérez, M.P.; Amaya-Tejeda, S.I. Plant Growth and Pollutant Removal from Wastewater in Domiciliary Constructed Wetland Microcosms with Monoculture and Polyculture of Tropical Ornamental Plants. Ecol. Eng. 2020, 147, 105658. [Google Scholar] [CrossRef]
- Wang, R.; Tai, Y.; Wan, X.; Ruan, W.; Man, Y.; Wang, J.; Yang, Y.; Yang, Y. Enhanced Removal of Microcystis Bloom and Microcystin-LR Using Microcosm Constructed Wetlands with Bioaugmentation of Degrading Bacteria. Chemosphere 2018, 210, 29–37. [Google Scholar] [CrossRef]
- Gopal, B. Natural and Constructed Wetlands for Wastewater Treatment: Potentials and Problems. Water Sci. Technol. 1994, 40, 27–35. [Google Scholar] [CrossRef]
- Stefanakis, A.I. Introduction to Constructed Wetland Technology. In Constructed Wetlands for Industrial Wastewater Treatment; Wiley: Chichester, UK, 2018; pp. 1–21. [Google Scholar] [CrossRef]
- Cole, S. The Emergence of Treatment Wetlands. Environ. Sci. Technol. 1998, 32, 218A–223A. [Google Scholar] [CrossRef]
- Luederitz, V.; Eckert, E.; Lange-Weber, M.; Lange, A.; Gersberg, R.M. Nutrient Removal Efficiency and Resource Economics of Vertical Flow and Horizontal Flow Constructed Wetlands. Ecol. Eng. 2001, 18, 157–171. [Google Scholar] [CrossRef]
- Nikolic, V.; Milicevic, D.; Milenkovic, S. Wetlands, Constructed Wetlands and Theirs Role in Wastewater Treatment with Principles and Examples of Using It in Serbia. Facta Univ. Ser. Archit. Civ. Eng. 2009, 7, 65–82. [Google Scholar] [CrossRef]
- Ciria, M.P.; Solano, M.L.; Soriano, P. Role of Macrophyte Typha Latifolia in a Constructed Wetland for Wastewater Treatment and Assessment of Its Potential as a Biomass Fuel. Biosyst. Eng. 2005, 92, 535–544. [Google Scholar] [CrossRef]
- Herath, I.; Vithanage, M. Phytoremediation in Constructed Wetlands. In Phytoremediation; Springer International Publishing: Cham, Switzerland, 2015; pp. 243–263. [Google Scholar] [CrossRef]
- Pandey, J.; Chauhan, A.; Jain, R.K. Integrative Approaches for Assessing the Ecological Sustainability of in Situ Bioremediation. FEMS Microbiol. Rev. 2009, 33, 324–375. [Google Scholar] [CrossRef] [Green Version]
- Rahman, K.Z.; Wiessner, A.; Kuschk, P.; van Afferden, M.; Mattusch, J.; Müller, R.A. Removal and Fate of Arsenic in the Rhizosphere of Juncus Effusus Treating Artificial Wastewater in Laboratory-Scale Constructed Wetlands. Ecol. Eng. 2014, 69, 93–105. [Google Scholar] [CrossRef]
- Doty, S.L. Enhancing Phytoremediation through the Use of Transgenics and Endophytes. New Phytol. 2008, 179, 318–333. [Google Scholar] [CrossRef]
- Ahn, C.; Gillevet, P.M.; Sikaroodi, M. Molecular Characterization of Microbial Communities in Treatment Microcosm Wetlands as Influenced by Macrophytes and Phosphorus Loading. Ecol. Indic. 2007, 7, 852–863. [Google Scholar] [CrossRef]
- Fester, T.; Giebler, J.; Wick, L.Y.; Schlosser, D.; Kästner, M. Plant–Microbe Interactions as Drivers of Ecosystem Functions Relevant for the Biodegradation of Organic Contaminants. Curr. Opin. Biotechnol. 2014, 27, 168–175. [Google Scholar] [CrossRef]
- Stout, L.; Nüsslein, K. Biotechnological Potential of Aquatic Plant–Microbe Interactions. Curr. Opin. Biotechnol. 2010, 21, 339–345. [Google Scholar] [CrossRef] [PubMed]
- Prashar, P.; Kapoor, N.; Sachdeva, S. Rhizosphere: Its Structure, Bacterial Diversity and Significance. Rev. Environ. Sci. Bio/Technol. 2014, 13, 63–77. [Google Scholar] [CrossRef]
- Kadlec, R.H.; Wallace, S. Treatment Wetlands; CRC Press: Boca Raton, FL, USA, 2008. [Google Scholar] [CrossRef]
- A Review of the Design and Performance of Vertical-Flow and Hybrid Reed Bed Treatment Systems. Water Sci. Technol. 1999, 40, 1–9. [CrossRef]
- Gikas, G.D.; Tsihrintzis, V.A. Municipal Wastewater Treatment Using Constructed Wetlands. Water Util. J. 2014, 8, 57–65. [Google Scholar]
- Brix, H. Do Macrophytes Play a Role in Constructed Treatment Wetlands? Water Sci. Technol. 1997, 35, 11–17. [Google Scholar] [CrossRef]
- Pompêo, M. Monitoramento e Manejo de Macrófitas Aquáticas Em Reservatórios Tropicais Brasileiros; Instituto de Biociências, Universidade de São Paulo: São Paulo, Brasil, 2017. [Google Scholar] [CrossRef]
- Machado, A.I.; Beretta, M.; Fragoso, R.; Duarte, E. Overview of the State of the Art of Constructed Wetlands for Decentralized Wastewater Management in Brazil. J. Environ. Manag. 2017, 187, 560–570. [Google Scholar] [CrossRef]
- Zurita, F.; De Anda, J.; Belmont, M.A. Treatment of Domestic Wastewater and Production of Commercial Flowers in Vertical and Horizontal Subsurface-Flow Constructed Wetlands. Ecol. Eng. 2009, 35, 861–869. [Google Scholar] [CrossRef]
- Marrugo-Negrete, J.; Enamorado-Montes, G.; Durango-Hernández, J.; Pinedo-Hernández, J.; Díez, S. Removal of Mercury from Gold Mine Effluents Using Limnocharis Flava in Constructed Wetlands. Chemosphere 2017, 167, 188–192. [Google Scholar] [CrossRef]
- Said, N.S.M.; Abdullah, S.R.S.; Ismail, N.I.; Hasan, H.A.; Othman, A.R. Phytoremediation of Real Coffee Industry Effluent through a Continuous Two-Stage Constructed Wetland System. Environ. Technol. Innov. 2020, 17, 100502. [Google Scholar] [CrossRef]
- Mant, C.; Costa, S.; Williams, J.; Tambourgi, E. Phytoremediation of Chromium by Model Constructed Wetland. Bioresour. Technol. 2006, 97, 1767–1772. [Google Scholar] [CrossRef]
- Dornelas, F.L.; Machado, M.B.; Von Sperling, M. Performance Evaluation of Planted and Unplanted Subsurface-Flow Constructed Wetlands for the Post-Treatment of UASB Reactor Effluents. Water Sci. Technol. 2009, 60, 3025–3033. [Google Scholar] [CrossRef]
- Tadesse, A.T.; Seyoum, L.A. Evaluation of Selected Wetland Plants for Removal of Chromium from Tannery Wastewater in Constructed Wetlands, Ethiopia. Afr. J. Environ. Sci. Technol. 2015, 9, 420–427. [Google Scholar] [CrossRef] [Green Version]
- Cardinal, P.; Anderson, J.C.; Carlson, J.C.; Low, J.E.; Challis, J.K.; Beattie, S.A.; Bartel, C.N.; Elliott, A.D.; Montero, O.F.; Lokesh, S.; et al. Macrophytes May Not Contribute Significantly to Removal of Nutrients, Pharmaceuticals, and Antibiotic Resistance in Model Surface Constructed Wetlands. Sci. Total Environ. 2014, 482–483, 294–304. [Google Scholar] [CrossRef]
- De Souza, T.D.; Borges, A.C.; de Matos, A.T.; Mounteer, A.H.; de Queiroz, M.E.L.R. Removal of Chlorpyrifos Insecticide in Constructed Wetlands with Different Plant Species. Rev. Bras. Eng. Agrícola Ambient. 2017, 21, 878–883. [Google Scholar] [CrossRef] [Green Version]
- Wang, Q.; Xie, H.; Ngo, H.H.; Guo, W.; Zhang, J.; Liu, C.; Liang, S.; Hu, Z.; Yang, Z.; Zhao, C. Microbial Abundance and Community in Subsurface Flow Constructed Wetland Microcosms: Role of Plant Presence. Environ. Sci. Pollut. Res. 2016, 23, 4036–4045. [Google Scholar] [CrossRef]
- Licata, M.; Gennaro, M.C.; Tuttolomondo, T.; Leto, C.; La Bella, S. Research Focusing on Plant Performance in Constructed Wetlands and Agronomic Application of Treated Wastewater—A Set of Experimental Studies in Sicily (Italy). PLoS ONE 2019, 14, e0219445. [Google Scholar] [CrossRef]
- Gill, L.W.; Ring, P.; Casey, B.; Higgins, N.M.P.; Johnston, P.M. Long Term Heavy Metal Removal by a Constructed Wetland Treating Rainfall Runoff from a Motorway. Sci. Total Environ. 2017, 601–602, 32–44. [Google Scholar] [CrossRef]
- De Oliveira, M.; Atalla, A.A.; Frihling, B.E.F.; Cavalheri, P.S.; Migliolo, L.; Filho, F.J.C.M. Ibuprofen and Caffeine Removal in Vertical Flow and Free-Floating Macrophyte Constructed Wetlands with Heliconia Rostrata and Eichornia Crassipes. Chem. Eng. J. 2019, 373, 458–467. [Google Scholar] [CrossRef]
- Chen, J.; Ying, G.G.; Wei, X.D.; Liu, Y.S.; Liu, S.S.; Hu, L.X.; He, L.Y.; Chen, Z.F.; Chen, F.R.; Yang, Y.Q. Removal of Antibiotics and Antibiotic Resistance Genes from Domestic Sewage by Constructed Wetlands: Effect of Flow Configuration and Plant Species. Sci. Total Environ. 2016, 571, 974–982. [Google Scholar] [CrossRef]
- Mahabali, S.; Spanoghe, P. Mitigation of Two Insecticides by Wetland Plants: Feasibility Study for the Treatment of Agricultural Runoff in Suriname (South America). Water Air Soil Pollut. 2014, 225, 1771. [Google Scholar] [CrossRef]
- Saggaï, M.M.; Ainouche, A.; Nelson, M.; Cattin, F.; El Amrani, A. Long-Term Investigation of Constructed Wetland Wastewater Treatment and Reuse: Selection of Adapted Plant Species for Metaremediation. J. Environ. Manag. 2017, 201, 120–128. [Google Scholar] [CrossRef]
- Bao, S.; Liang, L.; Huang, J.; Liu, X.; Tang, W.; Yi, J.; Fang, T. Removal and Fate of Silver Nanoparticles in Lab-Scale Vertical Flow Constructed Wetland. Chemosphere 2019, 214, 203–209. [Google Scholar] [CrossRef]
- Hu, X.; Liu, X.; Yang, X.; Guo, F.; Su, X.; Chen, Y. Acute and Chronic Responses of Macrophyte and Microorganisms in Constructed Wetlands to Cerium Dioxide Nanoparticles: Implications for Wastewater Treatment. Chem. Eng. J. 2018, 348, 35–45. [Google Scholar] [CrossRef]
- Huang, J.; Yan, C.; Liu, J.; Guan, W.; Singh, R.P.; Cao, C.; Xiao, J. Feasibility Study of Vertical Flow Constructed Wetland for Tertiary Treatment of Nanosilver Wastewater and Temporal-Spatial Distribution of Pollutants and Microbial Community. J. Environ. Manag. 2019, 245, 28–36. [Google Scholar] [CrossRef] [PubMed]
- Hwang, J.-I.; Li, Z.; Andreacchio, N.; Ordonez Hinz, F.; Wilson, P.C. Potential Use of Floating Treatment Wetlands Established with Canna Flaccida for Removing Organic Contaminants from Surface Water. Int. J. Phytoremediat. 2020, 22, 1304–1312. [Google Scholar] [CrossRef] [PubMed]
- Ezemonye, L.I.; Adebayo, P.O.; Enuneku, A.A.; Tongo, I.; Ogbomida, E. Potential Health Risk Consequences of Heavy Metal Concentrations in Surface Water, Shrimp (Macrobrachium Macrobrachion) and Fish (Brycinus Longipinnis) from Benin River, Nigeria. Toxicol. Rep. 2019, 6, 1–9. [Google Scholar] [CrossRef]
- Kröpfelová, L.; Vymazal, J.; Švehla, J.; Štíchová, J. Removal of Trace Elements in Three Horizontal Sub-Surface Flow Constructed Wetlands in the Czech Republic. Environ. Pollut. 2009, 157, 1186–1194. [Google Scholar] [CrossRef]
- Zheng, S.; Gu, B.; Zhou, Q.; Li, Y. Variations of Mercury in the Inflow and Outflow of a Constructed Treatment Wetland in South Florida, USA. Ecol. Eng. 2013, 61, 419–425. [Google Scholar] [CrossRef]
- Gomes, M.V.T.; de Souza, R.R.; Teles, V.S.; Mendes, É.A. Phytoremediation of Water Contaminated with Mercury Using Typha Domingensis in Constructed Wetland. Chemosphere 2014, 103, 228–233. [Google Scholar] [CrossRef] [PubMed]
- Prasetya, A.; Prihutami, P.; Warisaura, A.D.; Fahrurrozi, M.; Petrus, H.T.B.M. Characteristic of Hg Removal Using Zeolite Adsorption and Echinodorus Palaefolius Phytoremediation in Subsurface Flow Constructed Wetland (SSF-CW) Model. J. Environ. Chem. Eng. 2020, 8, 103781. [Google Scholar] [CrossRef]
- Basile, A.; Sorbo, S.; Conte, B.; Cobianchi, R.C.; Trinchella, F.; Capasso, C.; Carginale, V. Toxicity, Accumulation, and Removal of Heavy Metals by Three Aquatic Macrophytes. Int. J. Phytoremediat. 2012, 14, 374–387. [Google Scholar] [CrossRef]
- Yang, B.; Lan, C.Y.; Yang, C.S.; Liao, W.B.; Chang, H.; Shu, W.S. Long-Term Efficiency and Stability of Wetlands for Treating Wastewater of a Lead/Zinc Mine and the Concurrent Ecosystem Development. Environ. Pollut. 2006, 143, 499–512. [Google Scholar] [CrossRef]
- ASM. Plants may be transmitting superbugs to people. ScienceDaily, 23 June 2019. Available online: https://www.sciencedaily.com/releases/2019/06/190623122530.htm(accessed on 3 November 2020).
- Lawrence, J.W.; Claire, D.C.; Weissig, V.; Rowe, T.C. Delayed Cytotoxicity and Cleavage of Mitochondrial DNA in Ciprofloxacin-Treated Mammalian Cells. Mol. Pharmacol. 1996, 50, 1178–1188. [Google Scholar]
- Lowes, D.A.; Wallace, C.; Murphy, M.P.; Webster, N.R.; Galley, H.F. The Mitochondria Targeted Antioxidant MitoQ Protects against Fluoroquinolone-Induced Oxidative Stress and Mitochondrial Membrane Damage in Human Achilles Tendon Cells. Free Radic. Res. 2009, 43, 323–328. [Google Scholar] [CrossRef] [PubMed]
- Gomes, M.P.; Richardi, V.S.; Bicalho, E.M.; da Rocha, D.C.; Navarro-Silva, M.A.; Soffiatti, P.; Garcia, Q.S.; Sant’Anna-Santos, B.F. Effects of Ciprofloxacin and Roundup on Seed Germination and Root Development of Maize. Sci. Total Environ. 2019, 651, 2671–2678. [Google Scholar] [CrossRef] [PubMed]
- De Morais, L.C.S.; Esterhuizen-Londt, M.; de Assis, C.S.H.; Pflugmacher, S. Phytoremediation: Green Technology for the Removal of Mixed Contaminants of a Water Supply Reservoir. Int. J. Phytoremediat. 2019, 21, 372–379. [Google Scholar] [CrossRef] [Green Version]
- Pei, M.; Zhang, B.; He, Y.; Su, J.; Gin, K.; Lev, O.; Shen, G.; Hu, S. State of the Art of Tertiary Treatment Technologies for Controlling Antibiotic Resistance in Wastewater Treatment Plants. Environ. Int. 2019, 131, 105026. [Google Scholar] [CrossRef]
- García, J.; García-Galán, M.J.; Day, J.W.; Boopathy, R.; White, J.R.; Wallace, S.; Hunter, R.G. A Review of Emerging Organic Contaminants (EOCs), Antibiotic Resistant Bacteria (ARB), and Antibiotic Resistance Genes (ARGs) in the Environment: Increasing Removal with Wetlands and Reducing Environmental Impacts. Bioresour. Technol. 2020, 307, 123228. [Google Scholar] [CrossRef]
- Van Bruggen, A.H.C.; He, M.M.; Shin, K.; Mai, V.; Jeong, K.C.; Finckh, M.R.; Morris, J.G. Environmental and Health Effects of the Herbicide Glyphosate. Sci. Total Environ. 2018, 616–617, 255–268. [Google Scholar] [CrossRef]
- Dhir, B. Green Technologies for the Removal of Agrochemicals by Aquatic Plants. In Agrochemicals Detection, Treatment and Remediation: Pesticides and Chemical Fertilizers; Butterworth Heinemann, Elsevier: Oxford, UK, 2020. [Google Scholar] [CrossRef]
- Gomes, M.P.; Juneau, P. Oxidative Stress in Duckweed (Lemna Minor L.) Induced by Glyphosate: Is the Mitochondrial Electron Transport Chain a Target of This Herbicide? Environ. Pollut. 2016, 218, 402–409. [Google Scholar] [CrossRef]
- Gomes, M.P.; Maccario, S.; Lucotte, M.; Labrecque, M.; Juneau, P. Consequences of Phosphate Application on Glyphosate Uptake by Roots: Impacts for Environmental Management Practices. Sci. Total Environ. 2015, 537, 115–119. [Google Scholar] [CrossRef]
- Gomes, M.P.; Le Manac’h, S.G.; Moingt, M.; Smedbol, E.; Paquet, S.; Labrecque, M.; Lucotte, M.; Juneau, P. Impact of Phosphate on Glyphosate Uptake and Toxicity in Willow. J. Hazard. Mater. 2016, 304, 269–279. [Google Scholar] [CrossRef] [Green Version]
- Vymazal, J.; Březinová, T. The Use of Constructed Wetlands for Removal of Pesticides from Agricultural Runoff and Drainage: A Review. Environ. Int. 2015, 75, 11–20. [Google Scholar] [CrossRef]
- Liu, X.; Guo, X.; Liu, Y.; Lu, S.; Xi, B.; Zhang, J.; Wang, Z.; Bi, B. A Review on Removing Antibiotics and Antibiotic Resistance Genes from Wastewater by Constructed Wetlands: Performance and Microbial Response. Environ. Pollut. 2019, 254, 112996. [Google Scholar] [CrossRef]
- Zhang, D.Q.; Jinadasa, K.B.S.N.; Gersberg, R.M.; Liu, Y.; Ng, W.J.; Tan, S.K. Application of Constructed Wetlands for Wastewater Treatment in Developing Countries—A Review of Recent Developments (2000–2013). J. Environ. Manag. 2014, 141, 116–131. [Google Scholar] [CrossRef]
- Kasak, K.; Truu, J.; Ostonen, I.; Sarjas, J.; Oopkaup, K.; Paiste, P.; Kõiv-Vainik, M.; Mander, Ü.; Truu, M. Biochar Enhances Plant Growth and Nutrient Removal in Horizontal Subsurface Flow Constructed Wetlands. Sci. Total Environ. 2018, 639, 67–74. [Google Scholar] [CrossRef] [PubMed]
- Di Pofi, G.; Favero, G.; Di Gregorio, F.N.; Ferretti, E.; Viaggiu, E.; Lucentini, L. Multi-Residue Ultra Performance Liquid Chromatography-High Resolution Mass Spectrometric Method for the Analysis of 21 Cyanotoxins in Surface Water for Human Consumption. Talanta 2020, 211, 120738. [Google Scholar] [CrossRef]
- Bittencourt-Oliveira, M.D.C. Detection of Potential Microcystin-Producing Cyanobacteria in Brazilian Reservoirs with a McyB Molecular Marker. Harmful Algae 2003, 2, 51–60. [Google Scholar] [CrossRef]
- Chan, W.C.W. Bionanotechnology Progress and Advances. Biol. Blood Marrow Transplant. 2006, 12 (Suppl. 1), 87–91. [Google Scholar] [CrossRef] [Green Version]
- Khan, I.; Saeed, K.; Khan, I. Nanoparticles: Properties, Applications and Toxicities. Arab. J. Chem. 2019, 12, 908–931. [Google Scholar] [CrossRef]
- Nowack, B.; Bucheli, T.D. Occurrence, Behavior and Effects of Nanoparticles in the Environment. Environ. Pollut. 2007, 150, 5–22. [Google Scholar] [CrossRef] [PubMed]
- Turan, N.B.; Erkan, H.S.; Engin, G.O.; Bilgili, M.S. Nanoparticles in the Aquatic Environment: Usage, Properties, Transformation and Toxicity—A Review. Process Saf. Environ. Prot. 2019, 130, 238–249. [Google Scholar] [CrossRef]
- Ebrahimbabaie, P.; Meeinkuirt, W.; Pichtel, J. Phytoremediation of Engineered Nanoparticles Using Aquatic Plants: Mechanisms and Practical Feasibility. J. Environ. Sci. 2020, 93, 151–163. [Google Scholar] [CrossRef]
- Kasak, K.; Valach, A.C.; Rey-Sanchez, C.; Kill, K.; Shortt, R.; Liu, J.; Dronova, I.; Mander, Ü.; Szutu, D.; Verfaillie, J.; et al. Experimental Harvesting of Wetland Plants to Evaluate Trade-Offs between Reducing Methane Emissions and Removing Nutrients Accumulated to the Biomass in Constructed Wetlands. Sci. Total Environ. 2020, 715, 136960. [Google Scholar] [CrossRef]
- Avellan, C.T.; Ardakanian, R.; Gremillion, P. The Role of Constructed Wetlands for Biomass Production within the Water-Soil-Waste Nexus. Water Sci. Technol. 2017, 75, 2237–2245. [Google Scholar] [CrossRef]
- Roj-Rojewski, S.; Wysocka-Czubaszek, A.; Czubaszek, R.; Kamocki, A.; Banaszuk, P. Anaerobic Digestion of Wetland Biomass from Conservation Management for Biogas Production. Biomass Bioenergy 2019, 122, 126–132. [Google Scholar] [CrossRef]
- Patel, A.G.; Pauli, M.K.; Lima, M.X.; Carvalho, K.Q.; Passig, F.H.; Macioski, G. Destinação final de resíduos da macrófita Eichhornia crassipes na incorporação de blocos de concreto. Specialist Conference on Wetland Systems for Water Pollution Control, Valencia, Spain. 2018. Available online: https://www.researchgate.net/publication/339054547_Final_destination_of_residues_of_the_Eichhornia_crassipes_macrophyte_in_concrete_block_incorporation (accessed on 27 March 2020).
- Maddison, M.; Mauring, T.; Remm, K.; Lesta, M.; Mander, Ü. Dynamics of Typha Latifolia L. Populations in Treatment Wetlands in Estonia. Ecol. Eng. 2009, 35, 258–264. [Google Scholar] [CrossRef]
- Yang, Y.; Wang, J.; Wang, Y.; He, Z. Biomass Decay Rate and Influencing Factors of Four Submerged Aquatic Vegetation in Everglades Wetland. Int. J. Phytoremediat. 2020, 22, 963–971. [Google Scholar] [CrossRef]
- Bedford, B.L.; Leopold, D.J.; Gibbs, J.P. Wetlands Ecosystems. In Encyclopedia of Biodiversity; Levin, S., Ed.; Academic Press: Amsterdam, The Netherlands, 2013; pp. 384–402. [Google Scholar]
- Morrison, J.A. Wetland Vegetation before and after Experimental Purple Loosestrife Removal. Wetlands 2020, 22, 156–169. [Google Scholar] [CrossRef]
- Gomes, M.P.; de Brito, J.C.M.; Bicalho, E.M.; Silva, J.G.; de Fátima, G.M.; Garcia, Q.S.; Figueredo, C.C.; Brito, J.C.M.; Bicalho, E.M.; Silva, J.G.; et al. Ciprofloxacin vs. Temperature: Antibiotic Toxicity in the Free-Floating Liverwort Ricciocarpus natans from a Climate Change Perspective. Chemosphere 2018, 202, 410–419. [Google Scholar] [CrossRef]
- Søvik, A.K.; Augustin, J.; Heikkinen, K.; Huttunen, J.T.; Necki, J.M.; Karjalainen, S.M.; Kløve, B.; Liikanen, A.; Mander, Ü.; Puustinen, M.; et al. Emission of the Greenhouse Gases Nitrous Oxide and Methane from Constructed Wetlands in Europe. J. Environ. Qual. 2006, 35, 2360–2373. [Google Scholar] [CrossRef] [PubMed]
- Chiudioni, F.; Marcheggiani, S.; Puccinelli, C.; Mancini, L. Interaction between Bacterial Enteric Pathogens and Aquatic Macrophytes. Can Salmonella Be Internalized in the Plants Used in Phytoremediation Processes? Int. J. Phytoremediat. 2020, 1–8. [Google Scholar] [CrossRef]




| Contaminant(s) | Species Used | Wetland System Type | Planted System Removal Efficiency | Non-Planted System Removal Efficiency | Plant Removal Efficiency | Study |
|---|---|---|---|---|---|---|
| Trace Element | ||||||
| Hg | Limnocharis flava | HF | 90% | 21% | 69% | Marrugo-Negrete et al. [49] |
| Zn, Cu and Pb | Phragmites australis Typha latifolia | SF | Cu: 60% Zn: 86% Pb: 31% | - | - | Gill et al. [58] |
| Cr | Phragmites karka Cyperus alternifolius Typha domingensis Borassus aethiopum | HF | P. karka: 97.7% C. alternifolius: 98% T. domingensis: 99% B. aethiopium: 99.3% | 97.4% | There were no significant differences between planted and non-planted systems | Tadesse and Seyoum [53] |
| Drugs | ||||||
| Ibuprofen and Caffeine | Heliconia rostrata Eichornia crassipes | VF SF | Ibuprofen: 95.5% Caffeine: 89% | - | - | De Oliveira et al. [59] |
| Antibiotics and ARG | Thalia dealbata Iris tectorum | VF HF SF | Antibiotics: 75.7–98.6% ARG: 63.9–84.0% | Antibiotics: 85% ARG: 85.8% | Antibiotics: –9.3 to 13.6% ARG: 1.8 to 21.9% | Chen et al. [60] |
| Pesticides | ||||||
| Imidacloprid Cyhalothrin | Nymphaea amazonum Eleocharis mutata | SF | Imidacloprid: N. amazonum: 75% E. mutata: 15% Cyhalothrin: N. amazonum and E. mutata: <1% | - | - | Mahabali and Spanoghe [61] |
| Chlorpyrifos (Organophos- phate) | Polygonum punctatum, Cynodon spp. Mentha aquatica | HF | Overall average: 98.6% | 99% | There were no significant differences planted and non-planted systems | Souza et al. [55] |
| Sewage | ||||||
| BOD, COD, TS, TP, TC and EF. | Juncus effusus Lolium perenne Washingtonia robusta Nerium oleander Typha latifolia Cyperus papyrus Canna indica | HF | TS: 91–96% TM: 60% BOD: 80–95% COD: 80% Pathogenic bacteria (TC and EF): 99% | - | - | Saggaï et al. [62] |
| TS, TP, fluorides, chloride and ammonia. | Canna hibrid Alpinia purpurata Hedychium coronarium | HF Polyculture and Monoculture | There were no differences in removal between monoculture and polyculture systems | - | - | Marín-Muniz et al. [26] |
| Cyanotoxins | ||||||
| Microcystin-LR and algal blooms | Iris pseudacorus L. | VF | ≥90% | ≥90% | There were no significant differences between planted and non-planted systems | Wang et al. [27] |
| Nanoparticles | ||||||
| Ag | Phragmites australis | VF | 78.53% | 40.96% | 37.57% | Bao et al. [63] |
| Cerium | Phragmites australis | VF | 17.9% | - | - | Hu et al. [64] |
| Ag | Iris pseudacorus | VF | 96% | - | - | Huang et al. [65] |
| Pharmaceuticals & Pesticides | ||||||
| Acetaminophen, Carbamazepine (pharmaceuticals) and Atrazine (herbicide) | Canna flaccida | SF | Acetaminophen: 100% Atrazine: 100% Carbamazepine: 73–81.8% | Acetaminophen: 100% Atrazine: 21% Carbamazepine: 51.8% | Acetaminophen: 0% Atrazine: 89% Carbamazepine: 21–30% | Hwang et al. [66] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kochi, L.Y.; Freitas, P.L.; Maranho, L.T.; Juneau, P.; Gomes, M.P. Aquatic Macrophytes in Constructed Wetlands: A Fight against Water Pollution. Sustainability 2020, 12, 9202. https://0-doi-org.brum.beds.ac.uk/10.3390/su12219202
Kochi LY, Freitas PL, Maranho LT, Juneau P, Gomes MP. Aquatic Macrophytes in Constructed Wetlands: A Fight against Water Pollution. Sustainability. 2020; 12(21):9202. https://0-doi-org.brum.beds.ac.uk/10.3390/su12219202
Chicago/Turabian StyleKochi, Leticia Y., Patricia L. Freitas, Leila T. Maranho, Philippe Juneau, and Marcelo P. Gomes. 2020. "Aquatic Macrophytes in Constructed Wetlands: A Fight against Water Pollution" Sustainability 12, no. 21: 9202. https://0-doi-org.brum.beds.ac.uk/10.3390/su12219202