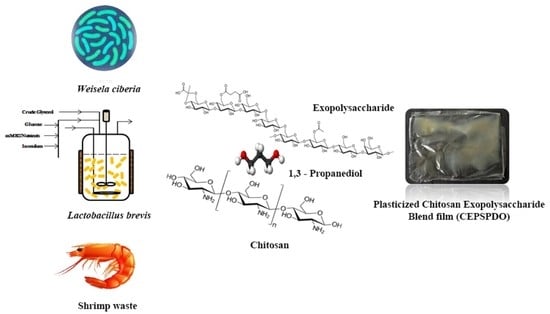
Synthesis and Characterization of Transparent Biodegradable Chitosan: Exopolysaccharide Composite Films Plasticized by Bio-Derived 1,3-Propanediol
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Film Formation
2.3. Characterization of the Films
2.3.1. Film Thickness and Tensile Strength
2.3.2. Scanning Electron Microscopy (SEM)
2.3.3. Fourier Transform Infrared Spectroscopy (FTIR)
2.3.4. Thermogravimetric Analysis (TGA)
2.3.5. UV-Vis Spectroscopy
2.3.6. Wide-Angle X-ray Diffraction (WAXD)
2.3.7. Water Transfer Rate of Blend Films
2.3.8. Antioxidant Activity
2.3.9. Moisture Content
2.3.10. Water Absorption
2.3.11. Film Solubility
2.3.12. Biodegradability of Composite Films
3. Results and Discussion
3.1. Film Casting, as Well as Physical and Mechanical Characteristics
3.2. Fourier Transform Infrared Spectroscopy (FTIR)
3.3. UV-Vis Spectroscopy
3.4. Thermogravimetric Analysis (TGA)
3.5. Wide- and Small-Angle X-ray Scattering (WAXS/SAXS)
3.6. Water Vapor Transfer Rate
3.7. Anti-Oxidant Activity
3.8. Moisture Content, Moisture Absorption, and Film Solubility
3.9. Biodegradability of Composite Films
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Volova, T.; Demidenko, A.; Kiselev, E.; Baranovskiy, S.; Shishatskaya, E.; Zhila, N. Polyhydroxyalkanoate synthesis based on glycerol and implementation of the process under conditions of pilot production. Appl. Microbiol. Biotechnol. 2019, 103, 225–237. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Soto, L.R.; Byrne, E.; van Niel, E.W.; Sayed, M.; Villanueva, C.C.; Hatti-Kaul, R. Hydrogen and polyhydroxybutyrate production from wheat straw hydrolysate using Caldicellulosiruptor species and Ralstonia eutropha in a coupled process. Bioresour. Technol. 2019, 272, 259–266. [Google Scholar] [CrossRef] [PubMed]
- Lunt, J. Large-scale production, properties and commercial applications of polylacticacid polymers. Polym. Degrad. Stab. 1998, 59, 145–152. [Google Scholar] [CrossRef]
- Tajik, S.; Maghsoudlou, Y.; Khodaiyan, F.; Jafari, S.M.; Ghasemlou, M.; Aalami, M. Soluble soybean polysaccharide: A new carbohydrate to make a biodegradable film for sustainable green packaging. Carbohydr. Polym. 2013, 97, 817–824. [Google Scholar] [CrossRef] [PubMed]
- González, K.; Martin, L.; González, A.; Retegi, A.; Eceiza, A.; Gabilondo, N. D-isosorbide and 1,3-propanediol as plasticizers for starch-based films: Characterization and aging study. J. Appl. Polym. Sci. 2017, 134, 44793. [Google Scholar] [CrossRef]
- Crouvisier-Urion, K.; Bodart, P.R.; Winckler, P.; Raya, J.; Gougeon, R.G.D.; Cayot, P.; Domenek, S.; Debeaufort, F.; Karbowiak, T. Biobased composite films from chitosan and lignin: Antioxidant activity related to structure and moisture. ACS Sustain. Chem. Eng. 2016, 4, 6371–6381. [Google Scholar] [CrossRef]
- Yoshida, C.M.; Junior, E.N.O.; Franco, T.T. Chitosan tailor-made films: The effects of additives on barrier and mechanical properties. Packag. Technol. Sci. Int. J. 2009, 22, 161–170. [Google Scholar] [CrossRef]
- Karthik, N.; Akanksha, K.; Binod, P.; Pandey, A. Production, purification and properties of fungal chitinases—A review. Indian J. Exp. Biol. 2014, 52, 1025–1035. [Google Scholar] [PubMed]
- Aider, M. Chitosan application for active bio-based films production and potential in the food industry. Lwt-Food Sci. Technol. 2010, 43, 837–842. [Google Scholar] [CrossRef]
- Leceta, I.; Guerrero, P.; de la Caba, K. Functional properties of chitosan-based films. Carbohydr. Polym. 2013, 93, 339–346. [Google Scholar] [CrossRef]
- Leceta, I.; Guerrero, P.; Ibarburu, I.; Dueñas, M.; de la Caba, K. Characterization and antimicrobial analysis of chitosan-based films. J. Food Eng. 2013, 116, 889–899. [Google Scholar] [CrossRef]
- Ma, X.; Qiao, C.; Zhang, J.; Xu, J. Effect of sorbitol content on microstructure and thermal properties of chitosan films. Int. J. Biol. Macromol. 2018, 119, 1294–1297. [Google Scholar] [CrossRef] [PubMed]
- Martínez-Camacho, A.; Cortez-Rocha, M.; Ezquerra-Brauer, J.; Graciano-Verdugo, A.; Rodriguez-Félix, F.; Castillo-Ortega, M.M.; Yepiz-Gomez, M.S.; Plascencia-Jatomea, M. Chitosan composite films: Thermal, structural, mechanical and antifungal properties. Carbohydr. Polym. 2010, 82, 305–315. [Google Scholar] [CrossRef]
- Bocqué, M.; Voirin, C.; Lapinte, V.; Caillol, S.; Robin, J.J. Petro-based and bio-based plasticizers: Chemical structures to plasticizing properties. J. Polym. Sci. Part A Polym. Chem. 2016, 54, 11–33. [Google Scholar] [CrossRef]
- Sasikumar, K.; Vaikkath, D.K.; Devendra, L.; Nampoothiri, K.M. An exopolysaccharide (EPS) from a Lactobacillus plantarum BR2 with potential benefits for making functional foods. Bioresour. Technol. 2017, 241, 1152–1156. [Google Scholar] [CrossRef]
- Vivek, N.; Pandey, A.; Binod, P. Biological valorization of pure and crude glycerol into 1,3-propanediol using a novel isolate Lactobacillus brevis N1E9.3.3. Bioresour. Technol. 2016, 213, 222–230. [Google Scholar] [CrossRef]
- Vivek, N.; Aswathi, T.V.; Sven, P.R.; Pandey, A.; Binod, P. Self-cycling fermentation for 1,3-propanediol production: Comparative evaluation of metabolite flux in cell recycling, simple batch and continuous processes using Lactobacillus brevis N1E9.3.3 strain. J. Biotechnol. 2017, 259, 110–119. [Google Scholar] [CrossRef]
- Vivek, N.; Pandey, A.; Binod, P. An efficient aqueous two phase systems using dual inorganic electrolytes to separate 1,3-propanediol from the fermented broth. Bioresour. Technol. 2018, 254, 239–246. [Google Scholar] [CrossRef]
- Rodríguez-Núñez, J.R.; Madera-Santana, T.J.; Sánchez-Machado, D.I.; López-Cervantes, J.; Valdez, H.S. Chitosan/hydrophilic plasticizer-based films: Preparation, physicochemical and antimicrobial properties. J. Polym. Environ. 2014, 22, 41–51. [Google Scholar] [CrossRef]
- Priyadarshi, R.; Kumar, B.; Deeba, F.; Kulshreshtha, A.; Negi, Y.S. Chitosan films incorporated with Apricot (Prunus armeniaca) kernel essential oil as active food packaging material. Food Hydrocoll. 2018, 85, 158–166. [Google Scholar] [CrossRef]
- Nagendra, B.; Mohan, K.; Gowd, E.B. Polypropylene/layered double hydroxide (LDH) nanocomposites: Influence of LDH particle size on the crystallization behavior of polypropylene. ACS Appl. Mater. Interfaces 2015, 7, 12399–12410. [Google Scholar] [CrossRef] [PubMed]
- Hafsa, J.; ali Smach, M.; Khedher, M.R.B.; Charfeddine, B.; Limem, K.; Majdoub, H.; Rouatbi, S. Physical, antioxidant and antimicrobial properties of chitosan films containing Eucalyptus globulus essential oil. Lwt-Food Sci. Technol. 2016, 68, 356–364. [Google Scholar] [CrossRef]
- Suyatma, N.E.; Tighzert, L.; Copinet, A.; Coma, V. Effects of hydrophilic plasticizers on mechanical, thermal, and surface properties of chitosan films. J. Agric. Food Chem. 2005, 53, 3950–3957. [Google Scholar] [CrossRef] [PubMed]
- Kalaycıoğlu, Z.; Torlak, E.; Akın-Evingür, G.; Özen, İ.; Erim, F.B. Antimicrobial and physical properties of chitosan films incorporated with turmeric extract. Int. J. Biol. Macromol. 2017, 101, 882–888. [Google Scholar] [CrossRef] [PubMed]
- Mobed-Miremadi, M.; Nagendra, R.K.; Ramachandruni, S.L.; Rook, J.J.; Keralapura, M.; Goedert, M. Polystyrene microsphere and 5-fluorouracil release from custom-designed wound dressing films. Prog. Biomater. 2013, 2, 1. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Skornyakov, I.; Komar, V. Infrared spectra of structural modifications of dextran. J. Appl. Spectrosc. 1996, 63, 309–317. [Google Scholar] [CrossRef]
- Muthulakshmi, L.; Annaraj, J.; Ramakrishna, S.; Ranjan, S.; Dasgupta, N.; Rangappa, S.M.; Siengchin, S. A sustainable solution for enhanced food packaging via a science-based composite blend of natural-sourced chitosan and microbial extracellular polymerics ubstances. J. Food Process. Preserv. 2021, 45, e15031. [Google Scholar] [CrossRef]
- Kumirska, J.; Czerwicka, M.; Kaczyński, Z.; Bychowska, A.; Brzozowski, K.; Thöming, J.; Stepnowski, P. Application of spectroscopic methods for structural analysis of chitin and chitosan. Mar. Drugs 2010, 8, 1567–1636. [Google Scholar] [CrossRef] [Green Version]
- Bhat, V.T.; James, N.R.; Jayakrishnan, A. A photochemical method for immobilization of azidated dextran onto aminated poly(ethyleneterephthalate) surfaces. Polym. Int. 2008, 57, 124–132. [Google Scholar] [CrossRef]
- Ma, X.; Qiao, C.; Wang, X.; Yao, J.; Xu, J. Structural characterization and properties of polyolsplasticized chitosan films. Int. J. Biol. Macromol. 2019, 135, 240–245. [Google Scholar] [CrossRef]
- Priyadarshi, R.; Kumar, B.; Negi, Y.S. Chitosan film incorporated with citric acid and glycerol as an active packaging material for extension of green chilli shelf life. Carbohydr. Polym. 2018, 195, 329–338. [Google Scholar] [CrossRef] [PubMed]
- Nechita, P. Review on polysaccharides used in coatings for food packaging papers. Coatings 2020, 10, 566. [Google Scholar] [CrossRef]
- Nešić, A.; Cabrera-Barjas, G.; Dimitrijević-Branković, S.; Davidović, S.; Radovanović, N.; Delattre, C. Prospect of Polysaccharide-Based Materials as Advanced Food Packaging. Molecules 2020, 25, 135. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Epure, V.; Griffon, M.; Pollet, E.; Avérous, L. Structure and properties of glycerol-plasticized chitosan obtained by mechanical kneading. Carbohydr. Polym. 2011, 83, 947–952. [Google Scholar] [CrossRef]
- Liu, H.; Adhikari, R.; Guo, Q.; Adhikari, B. Preparation and characterization of glycerol plasticized (high-amylose) starch–chitosan films. J. Food Eng. 2013, 116, 588–597. [Google Scholar] [CrossRef]
- Lavorgna, M.; Piscitelli, F.; Mangiacapra, P.; Buonocore, G.G. Study of the combined effect of both clay and glycerol plasticizer on the properties of chitosan films. Carbohydr. Polym. 2010, 82, 291–298. [Google Scholar] [CrossRef]







| Film | Tensile MPa | Max Force N | Elongation % | Thickness mm | Width mm |
|---|---|---|---|---|---|
| CS | 31.13 | 39.85 | 10.64 | 0.05 | 20 |
| CEPS | 20.08 | 12.85 | 10.00 | 0.05 | 20 |
| CEPSPDO | 43.33 | 13.00 | 20.73 | 0.05 | 20 |
| Film Type | Physiochemical Properties | Antioxidant Activity (%) | Water Vapor Transfer Rate (g/m2/d) | ||
|---|---|---|---|---|---|
| Moisture Content (%) | Moisture Absorption (%) | Film Solubility (%) | |||
| CS | 8.42 | 137.17 | 19.15 | 40.1 | 429.6 |
| CEPSPDO | 10.30 | 193.91 | 51.48 | 28.6 | 424.8 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Vivek, N.; Gopalan, N.; Das, S.; Sasikumar, K.; Sindhu, R.; Nampoothiri, K.M.; Pandey, A.; Binod, P. Synthesis and Characterization of Transparent Biodegradable Chitosan: Exopolysaccharide Composite Films Plasticized by Bio-Derived 1,3-Propanediol. Sustain. Chem. 2021, 2, 49-62. https://0-doi-org.brum.beds.ac.uk/10.3390/suschem2010004
Vivek N, Gopalan N, Das S, Sasikumar K, Sindhu R, Nampoothiri KM, Pandey A, Binod P. Synthesis and Characterization of Transparent Biodegradable Chitosan: Exopolysaccharide Composite Films Plasticized by Bio-Derived 1,3-Propanediol. Sustainable Chemistry. 2021; 2(1):49-62. https://0-doi-org.brum.beds.ac.uk/10.3390/suschem2010004
Chicago/Turabian StyleVivek, Narisetty, Nishant Gopalan, Satyajit Das, Keerthi Sasikumar, Raveendran Sindhu, Kesavan Madhavan Nampoothiri, Ashok Pandey, and Parameswaran Binod. 2021. "Synthesis and Characterization of Transparent Biodegradable Chitosan: Exopolysaccharide Composite Films Plasticized by Bio-Derived 1,3-Propanediol" Sustainable Chemistry 2, no. 1: 49-62. https://0-doi-org.brum.beds.ac.uk/10.3390/suschem2010004