Possible Roles of Amphiphilic Molecules in the Origin of Biological Homochirality
Abstract
:1. Introduction
2. Amphiphilic Molecules and the Origin of Life
2.1. Theories of Origin of Life
2.2. Lipid World Hypothesis
2.3. Amphiphiles and Definition of Life
3. Prebiotic Condition and Amphiphiles
3.1. Sources of Amphiphiles in Prebiotic Condition
3.2. Chiral Amphiphiles in Prebiotic Condition
4. Amphiphilic Molecule and Homochirality
4.1. Chemical Reactions
4.1.1. Advantage Factors
4.1.2. Reaction Building Blocks
- (1)
- The table provides “reaction building blocks” which can construct complex models for the reaction process in an extremely simple way.
- (2)
- The classification enables one to identify the type of process that efficiently leads to breaking the mirror symmetry.
- (3)
- The table takes g into account to evaluate the contribution of the advantage factors to the process.
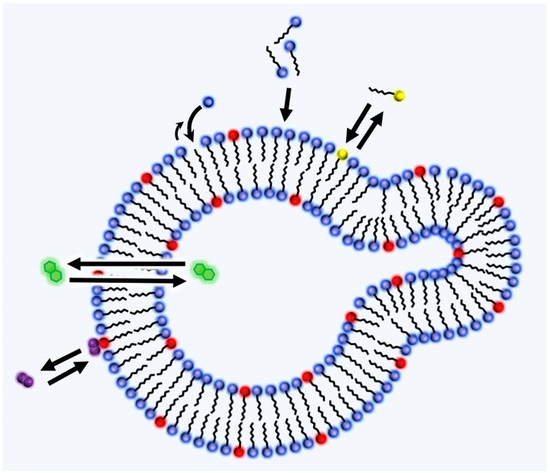
4.2. Spatial Arrangement and Conformation
4.3. Adsorption
4.4. Permeation
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Lombard, J.; López-García, P.; Moreira, D. The early evolution of lipid membranes and the three domains of life. Nat. Rev. Microbiol. 2012, 10, 507–515. [Google Scholar] [CrossRef] [PubMed]
- Koga, Y. Early evolution of membrane lipids: How did the lipid divide occur? J. Mol. Evol. 2011, 72, 274–282. [Google Scholar] [CrossRef] [PubMed]
- Caforio, A.; Siliakus, M.F.; Exterkate, M.; Jain, S.; Jumde, V.R.; Andringa, R.L.H.; Kengen, S.W.M.; Minnaard, A.J.; Driessen, A.J.M.; van der Oost, J. Converting escherichia coli into an archaebacterium with a hybrid heterochiral membrane. Proc. Natl. Acad. Sci. USA 2018, 115, 3704–3709. [Google Scholar] [CrossRef] [PubMed]
- Forterre, P. The two ages of the RNA world, and the transition to the DNA world: A story of viruses and cells. Biochimie 2005, 87, 793–803. [Google Scholar] [CrossRef] [PubMed]
- Orgel, L.E. Molecular replication. Nature 1992, 358, 203–209. [Google Scholar] [CrossRef] [PubMed]
- Leslie, E.O. Prebiotic chemistry and the origin of the RNA world. Crit. Rev. Biochem. Mol. Biol. 2010, 39, 99–123. [Google Scholar] [CrossRef] [PubMed]
- Fox, S.W. Self-sequencing of amino acids and origins of polyfunctional protocells. Orig. Life 1984, 14, 485–488. [Google Scholar] [CrossRef]
- Fox, S.W.; Jungck, J.R.; Nakashima, T. From proteinoid microsphere to contemporary cell: Formation of internucleotide and peptide bonds by proteinoid particles. Orig. Life 1974, 5, 227–237. [Google Scholar] [CrossRef]
- Ikehara, K. Possible steps to the emergence of life: The [gadv]-protein world hypothesis. Chem. Rec. 2005, 5, 107–118. [Google Scholar] [CrossRef]
- Segré, D.; Ben-Eli, D.; Deamer, D.W.; Lancet, D. The lipid world. Orig. Life Evol. Biospheres 2001, 31, 119–145. [Google Scholar] [CrossRef]
- Deamer, D. The role of lipid membranes in life’s origin. Life 2017, 7, 5. [Google Scholar] [CrossRef] [PubMed]
- Walde, P. Surfactant assemblies and their various possible roles for the origin(s) of life. Orig. Life Evol. Biosph. 2006, 36, 109–150. [Google Scholar] [CrossRef] [PubMed]
- Ruiz-Mirazo, K.; Briones, C.; de la Escosura, A. Prebiotic systems chemistry: New perspectives for the origins of life. Chem. Rev. 2014, 114, 285–366. [Google Scholar] [CrossRef] [PubMed]
- Lancet, D.; Zidovetzki, R.; Markovitch, O. Systems protobiology: Origin of life in lipid catalytic networks. J. R. Soc. Interface 2018, 15, 20180159. [Google Scholar] [CrossRef] [PubMed]
- Stano, P.; Luisi, P.L. Achievements and open questions in the self-reproduction of vesicles and synthetic minimal cells. Chem. Commun. 2010, 46, 3639–3653. [Google Scholar] [CrossRef] [PubMed]
- Morigaki, K.; Dallavalle, S.; Walde, P.; Colonna, S.; Luisi, P.L. Autopoietic self-reproduction of chiral fatty acid vesicles. J. Am. Chem. Soc. 1997, 119, 292–301. [Google Scholar] [CrossRef]
- Markvoort, A.J.; ten Eikelder, H.M.M.; Hilbers, P.A.J.; de Greef, T.F.A.; Meijer, E.W. Theoretical models of nonlinear effects in two-component cooperative supramolecular copolymerizations. Nat. Commun. 2011, 2, 509. [Google Scholar] [CrossRef] [PubMed]
- Dalko, P.I.; Moisan, L. In the golden age of organocatalysis. Angew. Chem. Int. Ed. 2004, 43, 5138–5175. [Google Scholar] [CrossRef]
- Deamer, D.W. Boundary structures are formed by organic components of the murchison carbonaceous chondrite. Nature 1985, 317, 792–794. [Google Scholar] [CrossRef]
- Dyson, F.J. A model for the origin of life. J. Mol. Evol. 1982, 18, 344–350. [Google Scholar] [CrossRef]
- Dyson, F. Origins of Life; Cambridge University Press: Cambridge, UK, 1999. [Google Scholar]
- Dyson, F. Origins of life. In Nishina Memorial Lectures: Creators of Modern Physics; Springer: Berlin/Heidelberg, Germany, 2007; Volume 746, pp. 71–97. [Google Scholar]
- Oparin, A.I. Biochemical processes in the simplest structures. In The Origin of Life on the Earth; Elsevier: Amsterdam, The Netherlands, 1959; pp. 428–436. [Google Scholar]
- Eigen, M.; Gardiner, W.; Schuster, P.; Winkler-Oswatitsch, R. The origin of genetic information. Sci. Am. 1981, 244, 88–118. [Google Scholar] [CrossRef]
- Cairns-Smith, A.G. The origin of life and the nature of the primitive gene. J. Theor. Biol. 1965, 10, 53–88. [Google Scholar] [CrossRef]
- Szathmáry, E. The origin of replicators and reproducers. Philos. Trans. Royal. Soc. B 2006, 361, 1761–1776. [Google Scholar] [CrossRef] [Green Version]
- Sagan, L. On the origin of mitosing cells. J. Theor. Biol. 1967, 14, 225–274. [Google Scholar] [CrossRef]
- Von Neumann, J. The general and logical theory of automata. In Cerebral Mechanisms in Behavior; the Hixon Symposium; Wiley: Oxford, UK, 1951; pp. 1–41. [Google Scholar]
- Miller, S.L. A production of amino acids under possible primitive earth conditions. Science 1953, 117, 528–529. [Google Scholar] [CrossRef]
- Schlesinger, G.; Miller, S.L. Prebiotic synthesis in atmospheres containing CH4, CO, and CO2. J. Mol. Evol. 1983, 19, 376–382. [Google Scholar] [CrossRef]
- Cleaves, H.J.; Chalmers, J.H.; Lazcano, A.; Miller, S.L.; Bada, J.L. A reassessment of prebiotic organic synthesis in neutral planetary atmospheres. Orig. Life Evol. Biosph. 2008, 38, 105–115. [Google Scholar] [CrossRef]
- Bernhardt, H.S. The RNA world hypothesis: The worst theory of the early evolution of life (except for all the others)a. Biol. Direct 2012, 7, 23. [Google Scholar] [CrossRef]
- Mallik, S.; Kundu, S. The lipid-RNA world. arXiv 2012, arXiv:1211.0413. [Google Scholar]
- Biscans, A. Exploring the emergence of RNA nucleosides and nucleotides on the early earth. Life 2018, 8, 57. [Google Scholar] [CrossRef]
- Nakashima, S.; Kebukawa, Y.; Kitadai, N.; Igisu, M.; Matsuoka, N. Geochemistry and the origin of life: From extraterrestrial processes, chemical evolution on earth, fossilized life’s records, to natures of the extant life. Life 2018, 8, 39. [Google Scholar] [CrossRef]
- Robertson, M.P.; Joyce, G.F. The origins of the RNA world. Cold Spring Harb. Perspect. Biol. 2010, 4, a003608. [Google Scholar] [CrossRef]
- Hud, N.V.; Cafferty, B.J.; Krishnamurthy, R.; Williams, L.D. The origin of RNA and “my grandfather’s axe”. Chem. Biol. 2013, 20, 466–474. [Google Scholar] [CrossRef]
- D‘Aguanno, E.; Altamura, E.; Mavelli, F.; Fahr, A.; Stano, P.; Luisi, P. Physical routes to primitive cells: An experimental model based on the spontaneous entrapment of enzymes inside micrometer-sized liposomes. Life 2015, 5, 969–996. [Google Scholar] [CrossRef]
- Luisi, P.L. Chemistry constraints on the origin of life. Isr. J. Chem. 2015, 55, 906–918. [Google Scholar] [CrossRef]
- Luisi, P.L.; Stano, P.; de Souza, T. Spontaneous overcrowding in liposomes as possible origin of metabolism. Orig. Life Evol. Biosph. 2015, 44, 313–317. [Google Scholar] [CrossRef]
- Luisi, P.L.; Allegretti, M.; Pereira de Souza, T.; Steiniger, F.; Fahr, A.; Stano, P. Spontaneous protein crowding in liposomes: A new vista for the origin of cellular metabolism. ChemBioChem 2010, 11, 1989–1992. [Google Scholar] [CrossRef]
- Dwars, T.; Paetzold, E.; Oehme, G. Reactions in micellar systems. Angew. Chem. Int. Ed. 2005, 44, 7174–7199. [Google Scholar] [CrossRef]
- Fendler, J.H. Atomic and molecular clusters in membrane mimetic chemistry. Chem. Rev. 1987, 87, 877–899. [Google Scholar] [CrossRef]
- Lancet, D.; Sadovsky, E.; Seidemann, E. Probability model for molecular recognition in biological receptor repertoires: Significance to the olfactory system. Proc. Natl. Acad. Sci. USA 1993, 90, 3715–3719. [Google Scholar] [CrossRef]
- Rosenwald, S.; Kafri, R.A.N.; Lancet, D. Test of a statistical model for molecular recognition in biological repertoires. J. Theor. Biol. 2002, 216, 327–336. [Google Scholar] [CrossRef]
- Armstrong, D.L.; Markovitch, O.; Zidovetzki, R.; Lancet, D. Replication of simulated prebiotic amphiphile vesicles controlled by experimental lipid physicochemical properties. Phys. Biol. 2011, 8, 066001. [Google Scholar] [CrossRef]
- Armstrong, D.L.; Lancet, D.; Zidovetzki, R. Replication of simulated prebiotic amphiphilic vesicles in a finite environment exhibits complex behavior that includes high progeny variability and competition. Astrobiology 2018, 18, 419–430. [Google Scholar] [CrossRef]
- Farmer, J.D.; Kauffman, S.A.; Packard, N.H. Autocatalytic replication of polymers. Physica D 1986, 22, 50–67. [Google Scholar] [CrossRef]
- Hordijk, W. Autocatalytic confusion clarified. J. Theor. Biol. 2017, 435, 22–28. [Google Scholar] [CrossRef]
- Segré, D. A statistical chemistry approach to the origin of life. Chemtracts–Biochem. Mol. Biol. 1999, 12, 382–397. [Google Scholar]
- Solé, R.V. Evolution and self-assembly of protocells. Int. J. Biochem. Cell Biol. 2009, 41, 274–284. [Google Scholar] [CrossRef] [Green Version]
- Shapiro, R. A replicator was not involved in the origin of life. IUBMB Life 2000, 49, 173–176. [Google Scholar] [CrossRef]
- Anet, F.A.L. The place of metabolism in the origin of life. Curr. Opin. Chem. Biol. 2004, 8, 654–659. [Google Scholar] [CrossRef]
- Segré, D.; Lancet, D.; Kedem, O.; Pilpel, Y. Graded autocatalysis replication domain (gard): Kinetic analysis of self-replication in mutually catalytic sets. Orig. Life Evol. Biospheres 1998, 28, 501–514. [Google Scholar] [CrossRef]
- Luisi, P.L. The minimal autopoietic unit. Orig. Life Evol. Biospheres 2015, 44, 335–338. [Google Scholar] [CrossRef]
- Stano, P.; Luisi, P.L. Semi-synthetic minimal cells: Origin and recent developments. Curr. Opin. Biotechnol. 2013, 24, 633–638. [Google Scholar] [CrossRef]
- Stano, P.; Luigi Luisi, P. Self-reproduction of micelles, reverse micelles, and vesicles: Compartments disclose a general transformation pattern. In Advances in Planar Lipid Bilayers and Liposomes; LiuLiu, A.L., Ed.; Elsevier Inc.: Amsterdam, The Netherlands, 2008; Volume 7, pp. 221–263. [Google Scholar]
- Luisi, P.L.; Varela, F.J. Self-replicating micelles—A chemical version of a minimal autopoietic system. Orig. Life Evol. Biospheres 1989, 19, 633–643. [Google Scholar] [CrossRef]
- Bachmann, P.A.; Luisi, P.L.; Lang, J. Autocatalytic self-replicating micelles as models for prebiotic structures. Nature 1992, 357, 57–59. [Google Scholar] [CrossRef]
- Buhse, T.; Nagarajan, R.; Lavabre, D.; Micheau, J.C. Phase-transfer model for the dynamics of “micellar autocatalysis”. J. Phys. Chem. A 1997, 101, 3910–3917. [Google Scholar] [CrossRef]
- Buhse, T.; Pimienta, V.; Lavabre, D.; Micheau, J.C. Experimental evidence of kinetic bistability in a biphasic surfactant system. J. Phys. Chem. A 1997, 101, 5215–5217. [Google Scholar] [CrossRef]
- Buhse, T.; Lavabre, D.; Nagarajan, R.; Micheau, J.C. Origin of autocatalysis in the biphasic alkaline hydrolysis of c-4 to c-8 ethyl alkanoates. J. Phys. Chem. A 1998, 102, 10552–10559. [Google Scholar] [CrossRef]
- Pizzarello, S.; Shock, E. The organic composition of carbonaceous meteorites: The evolutionary story ahead of biochemistry. Cold Spring Harb. Perspect. Biol. 2010, 2, a002105. [Google Scholar] [CrossRef]
- Huang, Y.; Wang, Y.; Alexandre, M.R.; Lee, T.; Rose-Petruck, C.; Fuller, M.; Pizzarello, S. Molecular and compound-specific isotopic characterization of monocarboxylic acids in carbonaceous meteorites. Geochim. Cosmochim. Acta 2005, 69, 1073–1084. [Google Scholar] [CrossRef]
- Sephton, M.A. Organic compounds in carbonaceous meteorites. Nat. Prod. Rep. 2002, 19, 292–311. [Google Scholar] [CrossRef]
- Olah, G.A.; Mathew, T.; Prakash, G.K.S. Chemical formation of methanol and hydrocarbon (“organic”) derivatives from co2 and h2—carbon sources for subsequent biological cell evolution and life’s origin. J. Am. Chem. Soc. 2016, 139, 566–570. [Google Scholar] [CrossRef]
- Rushdi, A.I.; Simoneit, B.R.T. Lipid formation by aqueous fischer-tropsch-type synthesis over a temperature range of 100 to 400 degrees c. Orig. Life Evol. Biospheres 2001, 31, 103–118. [Google Scholar] [CrossRef]
- McCollom, T.M.; Ritter, G.; Simoneit, B.R.T. Lipid synthesis under hydrothermal conditions by fischer- tropsch-type reactions. Orig. Life Evol. Biospheres 1999, 29, 153–166. [Google Scholar] [CrossRef]
- Rushdi, A.I.; Simoneit, B.R.T. Abiotic condensation synthesis of glyceride lipids and wax esters under simulated hydrothermal conditions. Orig. Life Evol. Biospheres 2006, 36, 93–108. [Google Scholar] [CrossRef]
- Mayer, C.; Schreiber, U.; Dávila, M. Selection of prebiotic molecules in amphiphilic environments. Life 2017, 7, 3. [Google Scholar] [CrossRef]
- Lowell, R.P.; Rona, P.A.; Von Herzen, R.P. Seafloor hydrothermal systems. J. Geophys. Res. Solid Earth 1995, 100, 327–352. [Google Scholar] [CrossRef]
- Ehrenfreund, P.; Irvine, W.; Becker, L.; Blank, J.; Brucato, J.R.; Colangeli, L.; Derenne, S.; Despois, D.; Dutrey, A.; Fraaije, H.; et al. Astrophysical and astrochemical insights into the origin of life. Rep. Prog. Phys. 2002, 65, 1427–1487. [Google Scholar] [CrossRef] [Green Version]
- Chyba, C.; Sagan, C. Endogenous production, exogenous delivery and impact-shock synthesis of organic molecules: An inventory for the origins of life. Nature 1992, 355, 125–132. [Google Scholar] [CrossRef]
- Kadko, D.; Baker, E.; Alt, J.; Baross, J. Global impact of submarine hydrothermal processes: Ridge. In Vents Workshop, NSF RIDGE Initiative and NOAA Vents Program; Durham Ridge Office: Durham, NH, USA, 1995. [Google Scholar]
- Elderfield, H.; Schultz, A. Mid-ocean ridge hydrothermal fluxes and the chemical composition of the ocean. Annu. Rev. Earth Planet. Sci. 1996, 24, 191–224. [Google Scholar] [CrossRef]
- Delsemme, A.H. Cometary origin of carbon, nitrogen and water on the earth. Orig. Life Evol. Biospheres 1991, 21, 279–298. [Google Scholar] [CrossRef]
- Monroe, A.A.; Pizzarello, S. The soluble organic compounds of the bells meteorite: Not a unique or unusual composition. Geochim. Cosmochim. Acta 2011, 75, 7585–7595. [Google Scholar] [CrossRef]
- Pizzarello, S. The chemistry of life’s origin: A carbonaceous meteorite perspective. Acc. Chem. Res. 2006, 39, 231–237. [Google Scholar] [CrossRef]
- Ehrenfreund, P.; Glavin, D.P.; Botta, O.; Cooper, G.; Bada, J.L. Extraterrestrial amino acids in orgueil and ivuna: Tracing the parent body of ci type carbonaceous chondrites. Proc. Natl. Acad. Sci. USA 2001, 98, 2138–2141. [Google Scholar] [CrossRef]
- Pizzarello, S.; Holmes, W. Nitrogen-containing compounds in two CR2 meteorites: 15N composition, molecular distribution and precursor molecules. Geochim. Cosmochim. Acta 2009, 73, 2150–2162. [Google Scholar] [CrossRef]
- Pizzarello, S.; Wang, Y.; Chaban, G.M. A comparative study of the hydroxy acids from the murchison, gra 95229 and lap 02342 meteorites. Geochim. Cosmochim. Acta 2010, 74, 6206–6217. [Google Scholar] [CrossRef]
- Fishkis, M. Steps towards the formation of a protocell: The possible role of short peptides. Orig. Life Evol. Biosph. 2007, 37, 537–553. [Google Scholar] [CrossRef]
- Adamala, K.; Szostak, J.W. Competition between model protocells driven by an encapsulated catalyst. Nat. Chem. 2013, 5, 495–501. [Google Scholar] [CrossRef] [Green Version]
- Murillo-Sánchez, S.; Beaufils, D.; González Mañas, J.M.; Pascal, R.; Ruiz-Mirazo, K. Fatty acids‘ double role in the prebiotic formation of a hydrophobic dipeptide. Chem. Sci. 2016, 7, 3406–3413. [Google Scholar] [CrossRef]
- Pizzarello, S.; Schrader, D.L.; Monroe, A.A.; Lauretta, D.S. Large enantiomeric excesses in primitive meteorites and the diverse effects of water in cosmochemical evolution. Proc. Natl. Acad. Sci. USA 2012, 109, 11949–11954. [Google Scholar] [CrossRef] [Green Version]
- Kricheldorf, H.R. Polypeptides and 100 years of chemistry of α-amino acidn-carboxyanhydrides. Angew. Chem. Int. Ed. 2006, 45, 5752–5784. [Google Scholar] [CrossRef]
- Ehler, K.W.; Orgel, L.E. N, N‘-carbonyldiimidazole-induced peptide formation in aqueous solution. Biochim. Biophys. Acta Protein Struct. Mol. Enzymol. 1976, 434, 233–243. [Google Scholar] [CrossRef]
- Liu, R.; Orgel, L.E. Polymerization of β-amino acids in aqueous solution. Orig. Life Evol. Biospheres 1998, 28, 47–60. [Google Scholar] [CrossRef]
- Monnard, P.A.; Deamer, D.W. Membrane self-assembly processes: Steps toward the first cellular life. Anat. Rec. 2002, 268, 196–207. [Google Scholar] [CrossRef]
- Namani, T.; Deamer, D.W. Stability of model membranes in extreme environments. Orig. Life Evol. Biospheres 2008, 38, 329–341. [Google Scholar] [CrossRef]
- Budin, I.; Szostak, J.W. Physical effects underlying the transition from primitive to modern cell membranes. Proc. Natl. Acad. Sci. USA 2011, 108, 5249–5254. [Google Scholar] [CrossRef] [Green Version]
- Szostak, J.W.; Bartel, D.P.; Luisi, P.L. Synthesizing life. Nature 2001, 409, 387–390. [Google Scholar] [CrossRef]
- Anella, F.; Danelon, C. Reconciling ligase ribozyme activity with fatty acid vesicle stability. Life 2014, 4, 929–943. [Google Scholar] [CrossRef]
- Anella, F.; Danelon, C. Prebiotic factors influencing the activity of a ligase ribozyme. Life 2017, 7, 17. [Google Scholar] [CrossRef]
- Olasagasti, F.; Kim, H.J.; Pourmand, N.; Deamer, D.W. Non-enzymatic transfer of sequence information under plausible prebiotic conditions. Biochimie 2011, 93, 556–561. [Google Scholar] [CrossRef]
- Wilson, M.A.; Wei, C.; Pohorille, A. Towards co-evolution of membrane proteins and metabolism. Orig. Life Evol. Biospheres 2014, 44, 357–361. [Google Scholar] [CrossRef]
- Hitz, T.; Blocher, M.; Walde, P.; Luisi, P.L. Stereoselectivity aspects in the condensation of racemic nca−amino acids in the presence and absence of liposomes. Macromolecules 2001, 34, 2443–2449. [Google Scholar] [CrossRef]
- Holland, P.M.; Rubingh, D.N. Nonideal multicomponent mixed micelle model. J. Phys. Chem. 1983, 87, 1984–1990. [Google Scholar] [CrossRef]
- Vora, S.; George, A.; Desai, H.; Bahadur, P. Mixed micelles of some anionic-anionic, cationic-cationic, and ionic-nonionic surfactants in aqueous media. J. Surfactants Deterg. 1999, 2, 213–221. [Google Scholar] [CrossRef]
- Budin, I.; Prywes, N.; Zhang, N.; Szostak, J.W. Chain-length heterogeneity allows for the assembly of fatty acid vesicles in dilute solutions. Biophys. J. 2014, 107, 1582–1590. [Google Scholar] [CrossRef]
- Burton, A.; Berger, E. Insights into abiotically-generated amino acid enantiomeric excesses found in meteorites. Life 2018, 8, 14. [Google Scholar] [CrossRef]
- Cooper, G.; Rios, A.; Nuevo, M. Monosaccharides and their derivatives in carbonaceous meteorites: A scenario for their synthesis and onset of enantiomeric excesses. Life 2018, 8, 36. [Google Scholar] [CrossRef]
- Kafri, R.; Markovitch, O.; Lancet, D. Spontaneous chiral symmetry breaking in early molecular networks. Biol. Direct 2010, 5, 38. [Google Scholar] [CrossRef]
- Morozov, L.L.; Kuz Min, V.V.; Goldanskii, V.I. Comparative analysis of the role of statistical fluctuations and factor of advantage (parity nonconservation) in the origins of optical activity. Orig. Life 1983, 13, 119–138. [Google Scholar] [CrossRef]
- Gol’danskiĭ, V.I.; Kuz’min, V.V. Spontaneous breaking of mirror symmetry in nature and the origin of life. Sov. Phys. Usp. 1989, 32, 1–29. [Google Scholar] [CrossRef]
- Avetisov, V.A.; Kuz’min, V.V.; Goldanskii, V.I. Handedness, origin of life and evolution. Phys. Today 1991, 44, 33–41. [Google Scholar] [CrossRef]
- Frank, F.C. On spontaneous asymmetric synthesis. Biochim. Biophys. Acta 1953, 11, 459–463. [Google Scholar] [CrossRef]
- Morozov, L. Mirror symmetry breaking in biochemical evolution. Orig. Life 1979, 9, 187–217. [Google Scholar] [CrossRef]
- Pino, P.; Lorenzi, G.P. Optically active vinyl polymers. Ii. The optical activity of isotactic and block polymers of optically active α-olefins in dilute hydrocarbon solution. J. Am. Chem. Soc. 1960, 82, 4745–4747. [Google Scholar] [CrossRef]
- Pino, P.; Ciardelli, F.; Lorenzi, G.P.; Montagnoli, G. Optically active vinyl polymers. Ix. Optical activity and conformation in dilute solution of isotactic poly-α-olefins. Makromol. Chem. 1963, 61, 207–224. [Google Scholar] [CrossRef]
- Luisi, P.L.; Pino, P. Conformational properties of optically active poly-Alpha-olefins in solution. J. Phys. Chem. 1968, 72, 2400–2405. [Google Scholar] [CrossRef]
- Green, M.M.; Peterson, N.C.; Sato, T.; Teramoto, A.; Cook, R.; Lifson, S. A helical polymer with a cooperative response to chiral information. Science 1995, 268, 1860–1866. [Google Scholar] [CrossRef]
- Green, M.M.; Park, J.W.; Sato, T.; Teramoto, A.; Lifson, S.; Selinger, R.L.B.; Selinger, J.V. The macromolecular route to chiral amplification. Angew. Chem. Int. Ed. 1999, 38, 3138–3154. [Google Scholar] [CrossRef]
- Lifson, S.; Green, M.M.; Andreola, C.; Peterson, N.C. Macromolecular stereochemistry: Helical sense preference in optically active polyisocyanates. Amplification of a conformational equilibrium deuterium isotope effect. J. Am. Chem. Soc. 1989, 111, 8850–8858. [Google Scholar] [CrossRef]
- Selinger, J.V.; Selinger, R.L.B. Theory of chiral order in random copolymers. Phys. Rev. Lett. 1996, 76, 58–61. [Google Scholar] [CrossRef]
- Selinger, J.V.; Selinger, R.L.B. Cooperative chiral order in copolymers of chiral and achiral units. Phys. Rev. E 1997, 55, 1728–1731. [Google Scholar] [CrossRef] [Green Version]
- Selinger, J.V.; Selinger, R.L.B. Cooperative chiral order in polyisocyanates: New statistical problems. Macromolecules 1998, 31, 2488–2492. [Google Scholar] [CrossRef]
- Gu, H.; Sato, T.; Teramoto, A.; Varichon, L.; Green, M.M. Molecular mechanisms for the optical activities of polyisocyanates induced by intramolecular chiral perturbations. Polym. J. 1997, 29, 77–84. [Google Scholar] [CrossRef]
- Ke, Y.Z.; Nagata, Y.; Yamada, T.; Suginome, M. Majority-rules-type helical poly(quinoxaline-2,3-diyl)s as highly efficient chirality-amplification systems for asymmetric catalysis. Angew. Chem. Int. Ed. 2015, 54, 9333–9337. [Google Scholar] [CrossRef]
- Yamamoto, T.; Murakami, R.; Komatsu, S.; Suginome, M. Chirality-amplifying, dynamic induction of single-handed helix by chiral guests to macromolecular chiral catalysts bearing boronyl pendants as receptor sites. J. Am. Chem. Soc. 2018, 140, 3867–3870. [Google Scholar] [CrossRef]
- Nagata, Y.; Nishikawa, T.; Suginome, M. Exerting control over the helical chirality in the main chain of sergeants-and-soldiers-type poly(quinoxaline-2,3-diyl)s by changing from random to block copolymerization protocols. J. Am. Chem. Soc. 2015, 137, 4070–4073. [Google Scholar] [CrossRef]
- Green, M.M.; Cheon, K.S.; Yang, S.Y.; Park, J.W.; Swansburg, S.; Liu, W. Chiral studies across the spectrum of polymer science. Acc. Chem. Res. 2001, 34, 672–680. [Google Scholar] [CrossRef]
- Yashima, E.; Maeda, K.; Nishimura, T. Detection and amplification of chirality by helical polymers. Chem. Eur. J. 2004, 10, 42–51. [Google Scholar] [CrossRef]
- Fujiki, M. Helix generation, amplification, switching, and memory of chromophoric polymers. Top. Curr. Chem. 2008, 284, 119–186. [Google Scholar]
- Yashima, E.; Maeda, K.; Furusho, Y. Single and double-stranded helical polymers: Synthesis, structures, and functions. Acc. Chem. Res. 2008, 41, 1166–1180. [Google Scholar] [CrossRef]
- Yashima, E.; Maeda, K.; Iida, H.; Furusho, Y.; Nagai, K. Helical polymers: Synthesis, structures, and functions. Chem. Rev. 2009, 109, 6102–6211. [Google Scholar] [CrossRef]
- Maeda, K.; Yashima, E. Helical polyacetylenes induced via noncovalent chiral interactions and their applications as chiral materials. Top. Curr. Chem. 2017, 375, 72. [Google Scholar] [CrossRef]
- Yashima, E.; Ousaka, N.; Taura, D.; Shimomura, K.; Ikai, T.; Maeda, K. Supramolecular helical systems: Helical assemblies of small molecules, foldamers, and polymers with chiral amplification and their functions. Chem. Rev. 2016, 116, 13752–13990. [Google Scholar] [CrossRef]
- Brunsveld, L.; Folmer, B.J.B.; Meijer, E.W.; Sijbesma, R.P. Supramolecular polymers. Chem. Rev. 2001, 101, 4071–4097. [Google Scholar] [CrossRef]
- Palmans, A.R.A.; Meijer, E.W. Amplification of chirality in dynamic supramolecular aggregates. Angew. Chem. Int. Ed. 2007, 46, 8948–8968. [Google Scholar] [CrossRef]
- De Greef, T.F.A.; Smulders, M.M.J.; Wolffs, M.; Schenning, A.P.H.J.; Sijbesma, R.P.; Meijer, E.W. Supramolecular polymerization. Chem. Rev. 2009, 109, 5687–5754. [Google Scholar] [CrossRef]
- Blocher, M.; Liu, D.; Walde, P.; Luisi, P.L. Liposome-assisted selective polycondensation of α-amino acids and peptides. Macromolecules 1999, 32, 7332–7334. [Google Scholar] [CrossRef]
- Ishigami, T.; Suga, K.; Umakoshi, H. Chiral recognition of l-amino acids on liposomes prepared with l-phospholipid. ACS Appl. Mater. Interfaces 2015, 7, 21065–21072. [Google Scholar] [CrossRef]
- Okamoto, Y.; Kishi, Y.; Ishigami, T.; Suga, K.; Umakoshi, H. Chiral selective adsorption of ibuprofen on a liposome membrane. J. Phys. Chem. B 2016, 120, 2790–2795. [Google Scholar] [CrossRef]
- Ishigami, T.; Kaneko, Y.; Suga, K.; Okamoto, Y.; Umakoshi, H. Homochiral oligomerization of l-histidine in the presence of liposome membranes. Colloid. Polym. Sci. 2015, 293, 3649–3653. [Google Scholar] [CrossRef]
- Arranz-Gibert, P.; Guixer, B.; Malakoutikhah, M.; Muttenthaler, M.; Guzmán, F.; Teixidó, M.; Giralt, E. Lipid bilayer crossing—The gate of symmetry. Water-soluble phenylproline-based blood-brain barrier shuttles. J. Am. Chem. Soc. 2015, 137, 7357–7364. [Google Scholar] [CrossRef]
- Valentová, J.; Bauerová, K.; Farah, L.; Devínsky, F. Does stereochemistry influence transdermal permeation of flurbiprofen through the rat skin? Arch. Dermatol. Res. 2010, 302, 635–638. [Google Scholar] [CrossRef]
- Touitou, E.; Godin, B.; Kommuru, T.; Afouna, M.; Reddy, I. Transport of chiral molecules across the skin. In Chirality in Drug Design and Development; Marcel Dekker: New York, NY, USA, 2004. [Google Scholar]







| Source | Amount a/kg yr−1 |
|---|---|
| Terrestrial Sources | |
| UV photolysis b | 3 × 108 |
| Electric discharge c | 3 × 107 |
| Shocks from impacts d | 4 × 102 |
| Hydrothermal vents e | 1 × 108 |
| Extraterrestrial Sources f | |
| Interplanetary dust particles | 2 × 108 |
| Comets | 1 × 1011 |
| Total | 1011 |
| Compound(s) | Murchison a nmol/g b | nc | Bells nmol/g | n | Ivuna nmol/g | n |
|---|---|---|---|---|---|---|
| Ammonia | 1100 | 280 | 5300 | |||
| Amines | 130 | 20 | nf d | 38 | 5 | |
| Amino acids | 600 | >85 | 93 | 13 | 156 e | 12 |
| Aldehydes/ketones | 200 f | 18 | 134 | 14 | 1369 | 23 |
| Hydroxy acids | 455 g | 17 | 1231 | 11 | 2136 | 10 |
| Di-carboxylic acids | 300 | 26 | 43 | 15 | 857 | 15 |
| Carboxylic acids | 3000 | 48 | 495 | 11 | 937 | 14 |
| Hydrocarbons | 1850 | 237 | 265 | 82 | 221 | 30 |
| Alkanes | 350 | 140 | 32 | 32 | 221 | 30 |
| Aromatic | 300 | 87 | 250 | 27 | 489 h | 34 |
| Polar | 1200 | 10 | ne i | ne |
| Type of Advantage Factors | True (+) or Imaginary (−) | a |
|---|---|---|
| Local advantage factors | ||
| Circularly polarized light | + | |
| Static magnetic field (SMF) | − | |
| Static electric field (SEF) | − | |
| Gravitational field (GF) | − | |
| SMF + SEF | − | |
| Rotation (Coriolis force) + GF | − | |
| SMF + GF | − | |
| Rotation + SMF + SEF | + | |
| Rotation + SMF + GF | + | |
| SMF + Linearly polarized light | + | |
| Global advantage factors | ||
| Weak neutral currents | + | |
| Longitudinally polarized β particles | + |
| Block | Name of the Reaction a | Reaction Formula b | Type of Process | ||||
|---|---|---|---|---|---|---|---|
| I | Synthesis | Racemizing | 0 | ||||
| II | Racemization | ″ | 0 | ||||
| III | Accidental autocatalysis | ″ | 0 | - | |||
| IV | Binary racemization | ″ | 0 | - | |||
| V | Binary destruction | ″ | 0 | - | |||
| VI | Accidental superautocatalysis | ″ | 0 | - | |||
| VII | Destruction | Neutral | |1| | ||||
| VIII | Autocatalysis | ″ | |1| | ||||
| IX | Cross-inversion | ″ | |1| | ||||
| X | Annihilation | Deracemizing | - | |1| | - | ||
| XI | Superautocatalysis | ″ | |1| | |1| | |||
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Suzuki, N.; Itabashi, Y. Possible Roles of Amphiphilic Molecules in the Origin of Biological Homochirality. Symmetry 2019, 11, 966. https://0-doi-org.brum.beds.ac.uk/10.3390/sym11080966
Suzuki N, Itabashi Y. Possible Roles of Amphiphilic Molecules in the Origin of Biological Homochirality. Symmetry. 2019; 11(8):966. https://0-doi-org.brum.beds.ac.uk/10.3390/sym11080966
Chicago/Turabian StyleSuzuki, Nozomu, and Yutaka Itabashi. 2019. "Possible Roles of Amphiphilic Molecules in the Origin of Biological Homochirality" Symmetry 11, no. 8: 966. https://0-doi-org.brum.beds.ac.uk/10.3390/sym11080966