Connections among Land Use, Water Quality, Biodiversity of Aquatic Invertebrates, and Fish Behavior in Amazon Rivers
Abstract
:1. Introduction
2. Materials and Methods
2.1. Study Area
2.2. Hydrography, Land Use, and Land Cover
2.3. Water Quality
2.4. Zooplankton Biodiversity Analysis
2.5. Experimental Methods for the Avoidance Tests
2.5.1. Test Organisms
2.5.2. Avoidance Tests
2.6. Statistical Analysis
3. Results
3.1. Hydrography, Land Use, and Land Cover
3.2. Water Quality
3.3. Zooplankton Biodiversity Analysis
3.4. Avoidance Tests
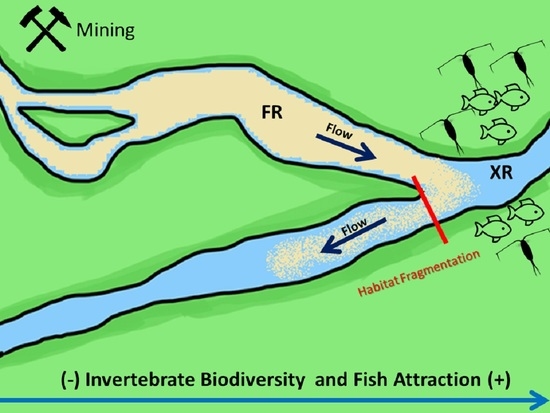
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Velasquez, C.; Queiroz, H.; Bernasconi, P. Fique Por Dentro: A Bacia do Rio Xingu em Mato Grosso, 1st ed.; Instituto Sócio Ambiental: São Paulo, Brazil, 2010; ISBN 9788585994785. [Google Scholar]
- Schwartzman, S.; Boas, A.V.; Ono, K.Y.; Fonseca, M.G.; Doblas, J.; Zimmerman, B.; Junqueira, P.; Jerozolimski, A.; Salazar, M.; Junqueira, R.P.; et al. The natural and social history of the indigenous lands and protected areas corridor of the Xingu River basin. Philos. Trans. R. Soc. B Biol. Sci. 2013, 368, 20120164. [Google Scholar] [CrossRef] [PubMed]
- Jézéquel, C.; Tedesco, P.A.; Bigorne, R.; Maldonado-Ocampo, J.A.; Ortega, H.; Hidalgo, M.; Martens, K.; Torrente-Vilara, G.; Zuanon, J.; Acosta, A.; et al. A database of freshwater fish species of the amazon basin. Sci. Data 2020, 7, 1–9. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ribeiro, D.R.G.; Faccin, H.; Molin, T.R.D.; de Carvalho, L.M.; Amado, L.L. Metal and metalloid distribution in different environmental compartments of the Middle Xingu River in the Amazon, Brazil. Sci. Total Environ. 2017, 605, 66–74. [Google Scholar] [CrossRef] [PubMed]
- Silva-Junior, E.F.; Moulton, T.P.; Boëchat, I.G.; Gücker, B. Leaf decomposition and ecosystem metabolism as functional indicators of land use impacts on tropical streams. Ecol. Indic. 2014, 36, 195–204. [Google Scholar] [CrossRef]
- Vannote, R.L.; Minshall, G.W.; Cummins, K.W.; Sedell, J.R.; Cushing, C.E. The river continuum concept. Can. J. Fish. Aquat. Sci. 1980, 37, 130–137. [Google Scholar] [CrossRef]
- Masese, F.O.; Kitaka, N.; Kipkemboi, J.; Gettel, G.M.; Irvine, K.; McClain, M.E. Litter processing and shredder distribution as indicators of riparian and catchment influences on ecological health of tropical streams. Ecol. Indic. 2014, 46, 23–37. [Google Scholar] [CrossRef]
- Ilha, P.; Rosso, S.; Schiesari, L. Effects of deforestation on headwater stream fish assemblages in the Upper Xingu River Basin, Southeastern Amazonia. Neotrop. Ichthyol. 2019, 17, 1–12. [Google Scholar] [CrossRef]
- Hickin, E.J. Vegetation and river channel dynamics. Can. Geogr. Le Géographe Can. 1984, 28, 111–126. [Google Scholar] [CrossRef]
- Silvério, D.V.; Brando, P.M.; Macedo, M.N.; Beck, P.S.A.; Bustamante, M.; Coe, M.T. Agricultural expansion dominates climate changes in southeastern Amazonia: The overlooked non-ghg forcing. Environ. Res. Lett. 2015, 10, 104015. [Google Scholar] [CrossRef]
- Lathuillière, M.J.; Johnson, M.S.; Galford, G.L.; Couto, E.G. Environmental footprints show China and Europe’s evolving resource appropriation for soybean production in Mato Grosso, Brazil. Environ. Res. Lett. 2014, 9, 074001. [Google Scholar] [CrossRef]
- Gerwing, J.J. Degradation of forests through logging and fire in the Eastern Brazilian Amazon. For. Ecol. Manag. 2002, 157, 131–141. [Google Scholar] [CrossRef]
- Oliveira, R.S.; Dawson, T.E.; Burgess, S.S.O.; Nepstad, D.C. Hydraulic redistribution in three amazonian trees. Oecologia 2005, 145, 354–363. [Google Scholar] [CrossRef] [PubMed]
- Aksnes, D.L.; Nejstgaard, J.; Sædberg, E.; Sørnes, T. Optical control of fish and zooplankton populations. Limnol. Oceanogr. 2004, 49, 233–238. [Google Scholar] [CrossRef] [Green Version]
- Mol, J.H.; Ouboter, P.E. Downstream effects of erosion from small-scale gold mining on the instream habitat and fish community of a small neotropical rainforest stream. Conserv. Biol. 2004, 18, 201–214. [Google Scholar] [CrossRef]
- Kemp, P.; Sear, D.; Collins, A.; Naden, P.; Jones, I. The impacts of fine sediment on riverine fish. Hydrol. Process. 2011, 25, 1800–1821. [Google Scholar] [CrossRef]
- Andrian, I.F.; Silva, H.B.R.; Peretti, D. Dieta de Astyanax bimaculatus (Linnaeus, 1758) (Characiformes, Characidae), da área de influência do reservatório de Corumbá, Estado de Goiás, Brasil. Acta Sci. 2001, 23, 435–440. [Google Scholar] [CrossRef]
- Cordeiro, J.G.; Maximino, C.; Siqueira-Silva, D.H. Histological staining of fish gonadal tissue. Protocols 2019. [Google Scholar] [CrossRef]
- Santos, D.C.M.; Cupertino, M.C.; Matta, S.L.P.; Oliveira, J.A.; Santos, J.A.D. Histological alterations in liver and testis of Astyanax aff. bimaculatus caused by acute exposition to zinc. Rev. Ceres. 2015, 62, 133–141. [Google Scholar] [CrossRef]
- Marcon, L.; Thomé, R.G.; Mounteer, A.H.; Bazzoli, N.; Rizzo, E.; Benjamin, L.A. Immunohistochemical, morphological and histometrical analyses of follicular development in Astyanax bimaculatus (Teleostei: Characidae) exposed to an organochlorine insecticide. Ecotoxicol. Environ. 2017, 143, 249–258. [Google Scholar] [CrossRef]
- Mereles, M.A.; Pineyro, J.I.G.; Marshall, B.G.; Sousa, R.G.C. Impacts of fish farm dams on temporal and spatial distribution of Astyanax cf. bimaculatus in microbasins of Machado River (Rondônia, Brazil). Biota Amaz. 2017, 7, 4–7. [Google Scholar] [CrossRef]
- Normandom, F.T.; Santiago, K.B.; Gomes, M.V.T.; Rizzo, E.; Bazzoli, N. Impact of the Três Marias dam on the reproduction of the forage fish Astyanax bimaculatus and A. fasciatus from the São Francisco River, downstream from the dam, southeastern Brazil. Environ. Biol. Fishes 2013, 97, 309–319. [Google Scholar] [CrossRef]
- Fentem, J.; Balls, M. Replacement of Fish in Ecotoxicology Testing: Use of bacteria, other lower organisms and fish cells in vitro. In Ecotoxicology Monitoring; Richardson, M., Ed.; VCH: Weinheim, Germany, 1993; pp. 71–81. ISBN 978-3527285600. [Google Scholar]
- Rudén, C.; Adams, J.; Ågerstrand, M.; Brock, T.C.; Poulsen, V.; Schleka, C.E.; Wheeler, J.R.; Henry, T.R. Assessing the relevance of ecotoxicological studies for regulatory decision making. Integr. Environ. Assess. Manag. 2017, 13, 652–663. [Google Scholar] [CrossRef] [PubMed]
- Alcívar, M.A.; Sendra, M.; Silva, D.C.V.R.; González-Ortegón, E.; Blasco, J.; Moreno-Garrido, I.; Araújo, C.V.M. Could Contamination avoidance be an endpoint that protects the environment? An overview on how species respond to copper, glyphosate, and silver nanoparticles. Toxics 2021, 9, 301. [Google Scholar] [CrossRef] [PubMed]
- Lopes, I.; Baird, D.J.; Ribeiro, R. Avoidance of copper contamination by field populations of Daphnia longispina. Environ. Toxicol. Chem. 2004, 23, 1702–1708. [Google Scholar] [CrossRef] [Green Version]
- Rosa, R.; Materatski, P.; Moreira-Santos, M.; Sousa, J.P.; Ribeiro, R. A scaled-up system to evaluate zooplankton spatial avoidance and the population immediate decline concentration. Environ. Toxicol. Chem. 2012, 31, 1301–1305. [Google Scholar] [CrossRef]
- Silva, D.C.V.R.; Araújo, C.V.M.; López-Doval, J.C.; Neto, M.B.; Silva, F.T.; Paiva, T.C.B.; Pompêo, M.L.M. Potential effects of triclosan on spatial displacement and local population decline of the fish Poecilia Reticulata sing a non-forced system. Chemosphere 2017, 184, 329–336. [Google Scholar] [CrossRef] [PubMed]
- Silva, D.C.V.R.; Araújo, C.V.M.; Marassi, R.J.; Cardoso-Silva, S.; Neto, M.B.; Silva, G.C.; Ribeiro, R.; Silva, F.T.; Paiva, T.C.B.; Pompêo, M.L.M. Influence of interspecific interactions on avoidance response to contamination. Sci. Total Environ. 2018, 642, 824–831. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Silva, D.C.V.R.; Queiroz, L.G.; Marassi, R.J.; Araújo, C.V.M.; Bazzan, T.; Cardoso-Silva, S.; Silva, G.C.; Müller, M.; Silva, F.T.; Montagner, C.C.; et al. Predicting zebrafish spatial avoidance triggered by discharges of dairy wastewater: An experimental approach based on self-purification in a model river. Environ. Pollut. 2020, 266, 115325. [Google Scholar] [CrossRef] [PubMed]
- Araújo, C.V.M.; Laissaoui, A.; Silva, D.C.V.R.; Ramos-Rodríguez, E.; González-Ortegón, E.; Espíndola, E.L.G.; Baldó, F.; Mena, F.; Parra, G.; Blasco, J.; et al. Not only toxic but repellent: What can organisms’ responses tell us about contamination and what are the ecological consequences when they flee from an environment? Toxics 2020, 8, 118. [Google Scholar] [CrossRef] [PubMed]
- Souza, C.M.; Shimbo, J.Z.; Rosa, M.R.; Parente, L.L.; Alencar, A.A.; Rudorff, B.F.T.; Hasenack, H.; Matsumoto, M.; Ferreira, L.G.; Souza-Filho, P.W.M.; et al. Reconstructing three decades of land use and land cover changes in brazilian biomes with landsat archive and earth engine. Remote Sens. 2020, 12, 2735. [Google Scholar] [CrossRef]
- QGIS Development Team. QGIS Geographic Information System. Open Source Geospatial Foundation Project. Qgisorg. 2014. Available online: http://Qgis.Osgeo.Org (accessed on 31 January 2022).
- Water Environment Federation. Standard Methods for the Examination of Water and Wastewater; American Public Health Association: Alexandria, VA, USA, 1999; Volume 51, ISBN 0875532357. [Google Scholar]
- Wetzel, R.G.; Likens, G.E. Limnological Analysis, 2nd ed.; Springer Verlag: New York, NY, USA, 1991. [Google Scholar]
- EMBRAPA (Empresa Brasileira de Pesquisa Agropecuária). Sistema de Cultivo de Pirarucu, 1st ed.; Embrapa: Belém, Brazil, 2021. [Google Scholar]
- CETESB (Companhia Ambiental do Estado de São Paulo). Norma Técnica L5.304—Zooplâncton de Água Doce: Métodos Qualitativo e Quantitativo; CETESB: São Paulo, Brazil, 2012. [Google Scholar]
- Harris, R.P.; Wiebe, P.H.; Lenz, J.; Skjoldal, H.R.; Huntley, M. Zooplankton Methodology Manual, 1st ed.; Academic Press: London, UK, 2000. [Google Scholar]
- OECD (Organisation for Economic Co-operation and Deveplopment). Guidelines for Testing of Chemicals: Fish Acute Toxicity Test; Politics & International Relations: London, UK, 2000; Volume 16. [Google Scholar]
- Trancoso, R.; Carneiro Filho, A.; Tomasella, J.; Schietti, J.; Forsberg, B.; Miller, R. Deforestation and conservation in major watersheds of the Brazilian Amazon. Environ. Conserv. 2009, 36, 277–288. [Google Scholar] [CrossRef] [Green Version]
- Rizzo, R.; Garcia, A.S.; Vilela, V.M.D.F.N.; Ballester, M.V.R.; Neill, C.; Victoria, D.C.; da Rocha, H.R.; Coe, M. Land use changes in Southeastern Amazon and trends in rainfall and water yield of the Xingu River during 1976–2015. Clim. Chang. 2020, 162, 1419–1436. [Google Scholar] [CrossRef]
- Nobre, C.A.; Sampaio, G.; Borma, L.S.; Castilla-Rubio, J.C.; Silva, J.S.; Cardoso, M. Land-Use and climate change risks in the Amazon and the need of a novel sustainable development paradigm. Proc. Natl. Acad. Sci. USA 2016, 113, 10759–10768. [Google Scholar] [CrossRef] [Green Version]
- Amigo, I. When will the amazon hit a tipping point? Nature 2020, 578, 505–507. [Google Scholar] [CrossRef] [Green Version]
- Horbe, A.M.C.; Queiroz, M.M.D.A.; Moura, C.A.V.; Toro, M.A.G. Geoquímica das águas do médio e baixo Rio Madeira e seus principais tributários—Amazonas—Brasil. Acta Amaz. 2013, 43, 489–504. [Google Scholar] [CrossRef] [Green Version]
- Sioli, H. Hydrochemistry and geology in the brazilian amazon region. Amazoniana 1968, 1, 267–277. [Google Scholar]
- Do Nascimento, C.W.A.; Lima, L.H.V.; da Silva, F.L.; Biondi, C.M.; Campos, M.C.C. Natural concentrations and reference values of heavy metals in sedimentary soils in the brazilian amazon. Environ. Monit. Assess. 2018, 190, 606. [Google Scholar] [CrossRef] [PubMed]
- Chua, E.M.; Wilson, S.P.; Vink, S.; Flint, N. The influence of riparian vegetation on water quality in a mixed land use river basin. River Res. Appl. 2019, 35, 259–267. [Google Scholar] [CrossRef]
- Rakotondrabe, F.; Ndam Ngoupayou, J.R.; Mfonka, Z.; Rasolomanana, E.H.; Nyangono Abolo, A.J.; Ako Ako, A. Water quality assessment in the Bétaré-Oya gold mining area (East-Cameroon): Multivariate statistical analysis approach. Sci. Total Environ. 2018, 610–611, 831–844. [Google Scholar] [CrossRef]
- Khan, M.Y.A.; Hu, H.; Tian, F.; Wen, J. Monitoring the spatio-temporal impact of small tributaries on the hydrochemical characteristics of Ramganga River, Ganges Basin, India. Int. J. River Basin Manag. 2020, 18, 231–241. [Google Scholar] [CrossRef]
- Cajado, R.A.; Silva, F.K.S.; Oliveira, L.S.; Zacardi, D.M. Limnological characteristics effect of the Tapajós and Amazon Rivers about variability in the composition and abundance of fish larvae (Pará-Brazil). J. Appl. Hydro-Environment Clim. 2020, 2, 1–17. [Google Scholar]
- Guenther, M.; Bozelli, R. Effects of inorganic turbidity on the phytoplankton of an amazonian lake impacted by bauxite tailings. Hydrobiologia 2004, 511, 151–159. [Google Scholar] [CrossRef]
- Abrial, E.; Rabuffetti, A.P.; Espínola, L.A.; Amsler, M.L.; Blettler, M.C.M.; Paira, A.R. Influence of hydrological changes on the fish community in two lotic environments of the Middle Paraná Floodplain, Argentina. Aquat. Ecol. 2014, 48, 337–349. [Google Scholar] [CrossRef]
- Blettler, M.C.M.; Amsler, M.L. Erratum to hydraulic factors controlling the benthic invertebrate distribution within and among dunes of the Middle Paraná River (Argentina) and sampling techniques. J. South Am. Earth Sci. 2012, 35, 27–37. [Google Scholar] [CrossRef]
- Matsumura-Tundisi, T.; Tundisi, J.G.; Souza-Soares, F.; Tundisi, J.E.M. Estrutura da comunidade do zooplancton do Baixo Rio Xingu (PA), em relação ao ciclo hidrológico. Brazilian J. Biol. 2015, 75, S47–S54. [Google Scholar] [CrossRef] [Green Version]
- Ali, H.; Khan, E.; Ilahi, I. Environmental chemistry and ecotoxicology of hazardous heavy metals: Environmental persistence, toxicity, and bioaccumulation. J. Chem. 2019, 2019, 6730305. [Google Scholar] [CrossRef] [Green Version]
- Fuller, M.R.; Doyle, M.W.; Strayer, D.L. Causes and consequences of habitat fragmentation in river networks. Ann. N. Y. Acad. Sci. 2015, 1355, 31–51. [Google Scholar] [CrossRef] [PubMed]
- Zwick, P. Stream habitat fragmentation—A threat to biodiversity. Biodivers. Conserv. 1992, 1, 80–97. [Google Scholar] [CrossRef]
- Araújo, C.V.M.; Silva, D.C.V.R.; Gomes, L.E.T.; Acayaba, R.D.; Montagner, C.C.; Moreira-Santos, M.; Ribeiro, R.; Pompêo, M.L.M. Habitat fragmentation caused by contaminants: Atrazine as a chemical barrier isolating fish populations. Chemosphere 2018, 193, 24–31. [Google Scholar] [CrossRef]
- Maes, J.; Stevens, M.; Breine, J. Poor water quality constrains the distribution and movements of Twaite Shad Alosa Fallax Fallax (Lacépède, 1803) in the watershed of river Scheldt. Fish Diadromy Eur. 2008, 200, 129–143. [Google Scholar] [CrossRef]




| Class | P1XR 1985 | P1XR 2020 | P2FR 1985 | P2FR 2020 | P3XFR 1985 | P3XFR 2020 |
|---|---|---|---|---|---|---|
| Forest | 88.9 | 57.6 | 86.5 | 60.8 | 91.8 | 63.9 |
| Non-forest natural formation | 5.0 | 4.2 | 4.0 | 3.6 | 2.9 | 2.6 |
| Farming | 5.6 | 37.7 | 8.2 | 33.9 | 5.1 | 33.3 |
| Non-vegetated Area | 0.5 | 0.6 | 1.4 | 1.7 | 0.1 | 0.1 |
| Total | 100 | 100 | 100 | 100 | 100 | 100 |
| Chambers | Chamber (%) | Treatment (%) |
|---|---|---|
| C1 (P1XR) | 27.78 | 51.85 |
| C2 (P1XR) | 24.07 | |
| C3 (P3XFR) | 12.96 | 20.37 |
| C4 (P3XFR) | 7.40 | |
| C5 (P2FR) | 16.66 | 27.77 |
| C6 (P2FR) | 11.11 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
de Sousa, R.S.; Silva, G.C.; Bazzan, T.; de la Torre, F.; Nebo, C.; Siqueira-Silva, D.H.; Cardoso-Silva, S.; Pompêo, M.L.M.; de Paiva, T.C.B.; da Silva, F.T.; et al. Connections among Land Use, Water Quality, Biodiversity of Aquatic Invertebrates, and Fish Behavior in Amazon Rivers. Toxics 2022, 10, 182. https://0-doi-org.brum.beds.ac.uk/10.3390/toxics10040182
de Sousa RS, Silva GC, Bazzan T, de la Torre F, Nebo C, Siqueira-Silva DH, Cardoso-Silva S, Pompêo MLM, de Paiva TCB, da Silva FT, et al. Connections among Land Use, Water Quality, Biodiversity of Aquatic Invertebrates, and Fish Behavior in Amazon Rivers. Toxics. 2022; 10(4):182. https://0-doi-org.brum.beds.ac.uk/10.3390/toxics10040182
Chicago/Turabian Stylede Sousa, Rodrigo Silva, Gilmar Clemente Silva, Thiago Bazzan, Fernando de la Torre, Caroline Nebo, Diógenes Henrique Siqueira-Silva, Sheila Cardoso-Silva, Marcelo Luiz Martins Pompêo, Teresa Cristina Brazil de Paiva, Flávio Teixeira da Silva, and et al. 2022. "Connections among Land Use, Water Quality, Biodiversity of Aquatic Invertebrates, and Fish Behavior in Amazon Rivers" Toxics 10, no. 4: 182. https://0-doi-org.brum.beds.ac.uk/10.3390/toxics10040182