Relationship of Blood and Urinary Manganese Levels with Cognitive Function in Elderly Individuals in the United States by Race/Ethnicity, NHANES 2011–2014
Abstract
:1. Introduction
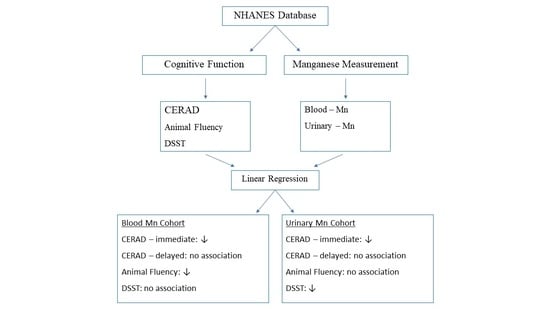
2. Materials and Methods
2.1. Study Deseign and Data Sources
2.2. Cognitive Function Assessments
2.3. Manganese Exposure Assessments
2.4. Covariates
2.5. Statistical Analysis
3. Results
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Li, L.; Yang, X. The Essential Element Manganese, Oxidative Stress, and Metabolic Diseases: Links and Interactions. Oxidative Med. Cell. Longev. 2018, 2018, 7580707. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Oulhote, Y.; Mergler, D.; Bouchard, M.F. Sex-and age-differences in blood manganese levels in the US general population: National health and nutrition examination survey 2011–2012. Environ. Health 2014, 13, 87. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lide, D.R.; Fredrikse, H.P.R. CRC Handbook of Chemistry and Physics; Chemical Composition of the Human Body; CRC Press: Boca Raton, FL, USA, 1994; pp. 7–11. [Google Scholar]
- Dobson, A.W.; Erikson, K.M.; Aschner, M. Manganese neurotoxicity. Ann. N. Y. Acad. Sci. 2004, 1012, 115–128. [Google Scholar] [CrossRef] [PubMed]
- Lucchini, R.G.; Martin, C.J.; Doney, B.C. From Manganism to Manganese-Induced Parkinsonism: A Conceptual Model Based on the Evolution of Exposure. Neuromol. Med. 2009, 11, 311–321. [Google Scholar] [CrossRef] [PubMed]
- Martinez-Finley, E.J.; Gavin, C.E.; Aschner, M.; Gunter, T.E. Manganese neurotoxicity and the role of reactive oxygen species. Free Radic. Biol. Med. 2013, 62, 65–75. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Flynn, M.R.; Susi, P. Neurological risks associated with manganese exposure from welding operations—A literature review. Int. J. Hyg. Environ. Health 2009, 212, 459–469. [Google Scholar] [CrossRef]
- Mohammed, R.S.; Ibrahim, W.; Sabry, D.; El-Jaafary, S.I. Occupational metals exposure and cognitive performance among foundry workers using tau protein as a biomarker. Neurotoxicology 2020, 76, 10–16. [Google Scholar] [CrossRef]
- Rolle-McFarland, D.; Liu, Y.; Mostafaei, F.; Zauber, S.E.; Zhou, Y.; Li, Y.; Fan, Q.; Zheng, W.; Nie, L.H.; Wells, E.M. The association of bone, fingernail and blood manganese with cognitive and olfactory function in Chinese workers. Sci. Total Environ. 2019, 666, 1003–1010. [Google Scholar] [CrossRef]
- Seo, J.; Chang, Y.; Jang, K.E.; Park, J.W.; Kim, Y.-T.; Park, S.-J.; Jeong, K.S.; Kim, A.; Kim, S.H.; Kim, Y. Altered executive function in the welders: A functional magnetic resonance imaging study. Neurotoxicol. Teratol. 2016, 56, 26–34. [Google Scholar] [CrossRef]
- Aschner, M.; Erikson, K.M.; Hernández, E.H.; Tjalkens, R. Manganese and its role in Parkinson’s disease: From transport to neuropathology. Neuromol. Med. 2009, 11, 252–266. [Google Scholar] [CrossRef]
- Chen, P.; Culbreth, M.; Aschner, M. Exposure, epidemiology, and mechanism of the environmental toxicant manganese. Environ. Sci. Pollut. Res. 2016, 23, 13802–13810. [Google Scholar] [CrossRef] [PubMed]
- World Health Organization. No. WHO/HEP/ECH/WSH/2021.5; Manganese in Drinking Water: Background Document for Development of WHO Guidelines for Drinking-Water Quality; World Health Organization: Geneva, Switzerland, 2021. [Google Scholar]
- Mitchell, E.J.; Frisbie, S.H.; Roudeau, S.; Carmona, A.; Ortega, R. How much manganese is safe for infants? A review of the scientific basis of intake guidelines and regulations relevant to the manganese content of infant formulas. J. Trace Elem. Med. Biol. 2021, 65, 126710. [Google Scholar] [CrossRef] [PubMed]
- Amrose, S.E.; Cherukumilli, K.; Wright, N.C. Chemical Contamination of Drinking Water in Resource-Constrained Settings: Global Prevalence and Piloted Mitigation Strategies. Annu. Rev. Environ. Resour. 2020, 45, 195–226. [Google Scholar] [CrossRef]
- Zoni, S.; Lucchini, R.G. Manganese exposure: Cognitive, motor and behavioral effects on children: A review of recent findings. Curr. Opin. Pediatrics 2013, 25, 255–260. [Google Scholar] [CrossRef] [Green Version]
- Bowler, R.M.; Kornblith, E.S.; Gocheva, V.V.; Colledge, M.; Bollweg, G.; Kim, Y.; Beseler, C.L.; Wright, C.W.; Adams, S.W.; Lobdell, D. Environmental exposure to manganese in air: Associations with cognitive functions. NeuroToxicology 2015, 49, 139–148. [Google Scholar] [CrossRef] [Green Version]
- Racette, B.A.; Nelson, G.; Dlamini, W.W.; Hershey, T.; Prathibha, P.; Turner, J.R.; Checkoway, H.; Sheppard, L.; Nielsen, S.S. Environmental Manganese Exposure and Cognitive Control in a South African Population. NeuroToxicology 2022, 89, 31–40. [Google Scholar] [CrossRef]
- Menezes-Filho, J.A.; Novaes, C.D.O.; Moreira, J.C.; Sarcinelli, P.N.; Mergler, D. Elevated manganese and cognitive performance in school-aged children and their mothers. Environ. Res. 2011, 111, 156–163. [Google Scholar] [CrossRef] [Green Version]
- Bouchard, M.; Laforest, F.; Vandelac, L.; Bellinger, D.; Mergler, D. Hair Manganese and Hyperactive Behaviors: Pilot Study of School-Age Children Exposed through Tap Water. Environ. Health Perspect. 2007, 115, 122–127. [Google Scholar] [CrossRef] [Green Version]
- Claus Henn, B.; Ettinger, A.S.; Schwartz, J.; Téllez-Rojo, M.M.; Lamadrid-Figueroa, H.; Hernández-Avila, M.; Schnaas, L.; Amarasiriwardena, C.; Bellinger, D.C.; Hu, H.; et al. Early postnatal blood manganese levels and children’s neurodevelopment. Epidemiology 2010, 21, 433–439. [Google Scholar] [CrossRef]
- Bauer, J.A.; White, R.F.; Coull, B.A.; Austin, C.; Oppini, M.; Zoni, S.; Fedrighi, C.; Cagna, G.; Placidi, D.; Guazzetti, S.; et al. Critical windows of susceptibility in the association between manganese and neurocognition in Italian adolescents living near ferro-manganese industry. NeuroToxicology 2021, 87, 51–61. [Google Scholar] [CrossRef]
- Díaz-Venegas, C.; Downer, B.; Langa, K.; Wong, R. Racial and ethnic differences in cognitive function among older adults in the USA. Int. J. Geriatr. Psychiatry 2016, 31, 1004–1012. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Avila, J.F.; Vonk, J.M.; Verney, S.P.; Witkiewitz, K.; Rentería, M.A.; Schupf, N.; Mayeux, R.; Manly, J.J. Sex/gender differences in cognitive trajectories vary as a function of race/ethnicity. Alzheimer’s Dement. 2019, 15, 1516–1523. [Google Scholar] [CrossRef] [PubMed]
- Morris, J.C.; Heyman, A.; Mohs, R.C.; Hughes, J.P.; van Belle, G.; Fillenbaum, G.; Mellits, E.D.; Clark, C. The Consortium to Establish a Registry for Alzheimer’s Disease (CERAD). Part I. Clinical and neuropsychological assessment of Alzheimer’s disease. Neurology 1989, 39, 1159–1165. [Google Scholar] [PubMed]
- Strauss, E.; Sherman, E.M.S.; Spreen, O. A compendium of neuropsychological tests: Administration, norms, and commentary. Am. Chem. Soc. 2006, 3, 500–501. [Google Scholar]
- Wechsler, D. WAIS Manual; Psychological Corporation: New York, NY, USA, 1997. [Google Scholar]
- Laboratory Procedure Manual. In Organic Analytical Toxicology Branch; Division of Laboratory Sciences, National Center for Environmental Health: Atlanta, GA, USA, 2013.
- Trumbo, P.; Yates, A.A.; Schlicker, S.; Poos, M. Dietary reference intakes: Vitamin A, vitamin K, arsenic, boron, chromium, copper, iodine, iron, manganese, molybdenum, nickel, silicon, vanadium, and zinc. J. Am. Diet Assoc. 2001, 101, 294–301. [Google Scholar] [CrossRef]
- Aschner, J.L.; Aschner, M. Nutritional aspects of manganese homeostasis. Mol. Asp. Med. 2005, 26, 353–362. [Google Scholar] [CrossRef]
- Hope, S.J.; Daniel, K.; Gleason, K.L.; Comber, S.; Nelson, M.; Powell, J.J. Influence of tea drinking on manganese intake, manganese status and leucocyte expression of MnSOD and cytosolic aminopeptidase P. Eur. J. Clin. Nutr. 2006, 60, 1–8. [Google Scholar] [CrossRef] [Green Version]
- Wu, C.; Woo, J.G.; Zhang, N. Association between urinary manganese and blood pressure: Results from National Health and Nutrition Examination Survey (NHANES), 2011–2014. PLoS ONE 2017, 12, e0188145. [Google Scholar] [CrossRef]
- Liu, W.; Xin, Y.; Li, Q.; Shang, Y.; Ping, Z.; Min, J.; Cahill, C.M.; Rogers, J.T.; Wang, F. Biomarkers of environmental manganese exposure and associations with childhood neurodevelopment: A systematic review and meta-analysis. Environ. Health 2020, 19, 104. [Google Scholar] [CrossRef]
- Sanders, A.P.; Desrosiers, T.A.; Warren, J.L.; Herring, A.H.; Enright, D.; Olshan, A.F.; Meyer, R.E.; Fry, R.C. Association between arsenic, cadmium, manganese, and lead levels in private wells and birth defects prevalence in North Carolina: A semi-ecologic study. BMC Public Health 2014, 14, 955. [Google Scholar] [CrossRef] [Green Version]
- Balachandran, R.C.; Mukhopadhyay, S.; McBride, D.; Veevers, J.; Harrison, F.E.; Aschner, M.; Haynes, E.N.; Bowman, A.B. Brain manganese and the balance between essential roles and neurotoxicity. J. Biol. Chem. 2020, 295, 6312–6329. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Carmona, A.; Zogzas, C.E.; Roudeau, S.; Porcaro, F.; Garrevoet, J.; Spiers, K.M.; Salomé, M.; Cloetens, P.; Mukhopadhyay, S.; Ortega, R. SLC30A10 Mutation Involved in Parkinsonism Results in Manganese Accumulation within Nanovesicles of the Golgi Apparatus. ACS Chem. Neurosci. 2019, 10, 599–609. [Google Scholar] [CrossRef] [PubMed]
- Pfalzer, A.C.; Bowman, A.B. Relationships Between Essential Manganese Biology and Manganese Toxicity in Neurological Disease. Curr. Environ. Health Rep. 2017, 4, 223–228. [Google Scholar] [CrossRef] [PubMed]
- Roels, H.; Lauwerys, R.; Genet, P.; Sarhan, M.J.; de Fays, M.; Hanotiau, I.; Buchet, J.-P. Relationship between external and internal parameters of exposure to manganese in workers from a manganese oxide and salt producing plant. Am. J. Ind. Med. 1987, 11, 297–305. [Google Scholar] [CrossRef]
- Zheng, W.; Kim, H.; Zhao, Q. Comparative toxicokinetics of manganese chloride and methylcyclopentadienyl manganese tricarbonyl (MMT) in Sprague-Dawley rats. Toxicol. Sci. 2000, 54, 295–301. [Google Scholar] [CrossRef] [Green Version]
- Gandhi, D.; Rudrashetti, A.P.; Rajasekaran, S. The impact of environmental and occupational exposures of manganese on pulmonary, hepatic, and renal functions. J. Appl. Toxicol. 2022, 42, 103–129. [Google Scholar] [CrossRef]
- Takeda, A.; Sawashita, J.; Okada, S. Biological half-lives of zinc and manganese in rat brain. Brain Res. 1995, 695, 53–58. [Google Scholar] [CrossRef]
- Toxicological Profile for Manganese. In Agency for Toxic Substances and Disease Registry (ATSDR); US Department of Health and Human Services: Atlanta, GA, USA, 2012.
- Smith, D.; Gwiazda, R.; Bowler, R.; Roels, H.; Park, R.; Bs, C.T.; Lucchini, R. Biomarkers of Mn exposure in humans. Am. J. Ind. Med. 2007, 50, 801–811. [Google Scholar] [CrossRef]
- Ellingsen, D.G.; Hetland, S.M.; Thomassen, Y. Manganese air exposure assessment and biological monitoring in the manganese alloy production industry. J. Environ. Monit. 2003, 5, 84–90. [Google Scholar] [CrossRef]
- Laohaudomchok, W.; Lin, X.; Herrick, R.F.; Fang, S.C.; Cavallari, J.; Christiani, D.C.; Weisskopf, M.G. Toenail, Blood, and Urine as Biomarkers of Manganese Exposure. J. Occup. Environ. Med. 2011, 53, 506–510. [Google Scholar] [CrossRef] [Green Version]
- Levin-Schwartz, Y.; Gennings, C.; Henn, B.C.; Coull, B.A.; Placidi, D.; Lucchini, R.; Smith, D.R.; Wright, R.O. Multi-media biomarkers: Integrating information to improve lead exposure assessment. Environ. Res. 2020, 183, 109148. [Google Scholar] [CrossRef] [PubMed]
- Brown, R.C.; Lockwood, A.H.; Sonawane, B.R. Neurodegenerative Diseases: An Overview of Environmental Risk Factors. Environ. Health Perspect. 2005, 113, 1250–1256. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cannon, J.; Greenamyre, J.T. The Role of Environmental Exposures in Neurodegeneration and Neurodegenerative Diseases. Toxicol. Sci. 2011, 124, 225–250. [Google Scholar] [CrossRef]
- Charlet, L.; Chapron, Y.; Faller, P.; Kirsch, R.; Stone, A.T.; Baveye, P.C. Neurodegenerative diseases and exposure to the environmental metals Mn, Pb, and Hg. Coord. Chem. Rev. 2012, 256, 2147–2163. [Google Scholar] [CrossRef]
- Fujishiro, H.; Kambe, T. Manganese transport in mammals by zinc transporter family proteins, ZNT and ZIP. J. Pharmacol. Sci. 2022, 148, 125–133. [Google Scholar] [CrossRef]


| Variables | N | Weighted Sample | (%) * | Variables | N | Weighted Sample | Mean (SE) | Median (IQR) |
|---|---|---|---|---|---|---|---|---|
| Gender | ||||||||
| Male | 1011 | 1,7508,127 | 46 | Age (year) | 2068 | 38,452,145 | 69.1 (0.24) | 67.7 (11) |
| Female | 1057 | 20,944,017 | 54 | Blood Mn (µg/L) | 2068 | 38,452,145 | 9.4 (0.08) | 8.8 (3.8) |
| Race/Ethnicity | CERAD learning | 2068 | 38,452,145 | 6.5 (0.1) | 6.5 (2) | |||
| Hispanic | 385 | 2,618,094 | 7 | CERAD recall | 2068 | 38,452,145 | 6.1 (0.1) | 5.8 (3.2) |
| NH White | 988 | 30,935,957 | 80 | Animal Fluency | 2068 | 38,452,145 | 18 (0.2) | 17 (7.5) |
| NH Black | 491 | 2,980,219 | 8 | DSST | 2068 | 38,452,145 | 52 (0.7) | 53 (24) |
| Other Race | 204 | 1,917,874 | 5 | z-score | 2068 | 38,452,145 | 0.24 (0.04) | 0.28 (1.1) |
| Education | ||||||||
| <High School | 531 | 6,133,440 | 16 | |||||
| High School | 490 | 8,783,404 | 23 | |||||
| >High School | 1046 | 23,530,632 | 61 | |||||
| Missing | 1 | |||||||
| PIR | ||||||||
| ≤0.99 | 333 | 3,298,332 | 9 | |||||
| ≥1 | 1550 | 32,672,780 | 91 | |||||
| Missing | 185 | |||||||
| Marital Status | ||||||||
| Married/Living with partner | 1201 | 25,353,317 | 69 | |||||
| Widowed/Divorced/ Separated | 742 | 11,403,761 | 31 | |||||
| Missing | 125 | |||||||
| Alcohol Consumption | ||||||||
| >12 drinks/year | 1397 | 27,847,784 | 73 | |||||
| <12 drinks/year | 640 | 10,098,855 | 27 | |||||
| Missing | 31 | |||||||
| HTN | ||||||||
| Yes | 1262 | 21,869,127 | 57 | |||||
| No | 804 | 16,514,849 | 43 | |||||
| Missing | 2 | |||||||
| DM | ||||||||
| Yes | 472 | 7,293,920 | 20 | |||||
| No | 1508 | 29,747,613 | 80 | |||||
| Missing | 88 | |||||||
| CAD | ||||||||
| Yes | 172 | 3,086,811 | 8 | |||||
| No | 1886 | 35,263,550 | 92 | |||||
| Missing | 10 | |||||||
| Stroke | ||||||||
| Yes | 138 | 2,341,705 | 6 | |||||
| No | 1926 | 36,057,144 | 94 | |||||
| Missing | 4 |
| Model 1 (n = 2068) * | Model 2 (n = 1772) † | Model 3 (n = 1744) ‡ | Model 4 (n = 1650) § | |||||
|---|---|---|---|---|---|---|---|---|
| Variables | β (95% CI) ¶ | p-Value | β (95% CI) ¶ | p-Value | β (95% CI) ¶ | p-Value | β (95% CI) ¶ | p-Value |
| Mn | −0.03 (−0.2 to 0.2) | 0.714 | −0.2 (−0.3 to −0.01) | 0.04 | −0.2 (−0.3 to 0.01) | 0.07 | −0.2 (−0.3 to 0.03) | 0.09 |
| Gender | ||||||||
| Male | referent | referent | referent | referent | referent | referent | ||
| Female | 6 (4 to 7) | <0.0001 | 6 (4 to 8) | <0.0001 | 6 (4 to 8) | <0.0001 | ||
| Age (year) | −0.8 (−0.9 to −0.7) | <0.0001 | −0.8 (−0.9 to −0.6) | <0.0001 | −0.8 (−0.9 to −0.6) | <0.0001 | ||
| Race/Ethnicity | ||||||||
| NH White | referent | referent | referent | referent | referent | referent | ||
| Hispanic | −5 (−8 to −2) | 0.005 | −4 (−8 to −1) | 0.01 | −4 (−8 to −1) | 0.01 | ||
| NH Black | −2 (−4 to 1) | 0.18 | −1 (−4 to 1) | 0.31 | −0.5 (−3 to 2) | 0.65 | ||
| Other Race | −2 (−5 to 1) | 0.21 | −2 (−5 to 1) | 0.23 | −1 (−4 to 2) | 0.32 | ||
| Education | ||||||||
| >High School | referent | referent | referent | referent | referent | referent | ||
| High School | −4 (−6 to −2) | 0.0004 | −4 (−6 to −2) | 0.001 | −4 (−6 to −2) | 0.001 | ||
| <High School | −6 (−10 to −2) | 0.004 | −6 (−10 to −2) | 0.01 | −5 (−10 to −1) | 0.01 | ||
| PIR | ||||||||
| ≤0.99 | referent | referent | referent | referent | referent | referent | ||
| ≥1 | 5 (3 to 7) | <0.0001 | 5 (3 to 7) | <0.0001 | 5 (3 to 7) | <0.0001 | ||
| Marital Status | ||||||||
| Married/Living with partner | referent | referent | referent | referent | referent | referent | ||
| Widowed/Divorced/ Separated | −1 (−4 to 1) | 0.31 | −1 (−4 to 1) | 0.34 | 0.8 (−3 to 2) | 0.48 | ||
| Alcohol Consumption | ||||||||
| <12 drinks/year | referent | referent | referent | referent | ||||
| >12 drinks/year | 1 (−1 to 4) | 0.17 | 1 (−1 to 4) | 0.19 | ||||
| HTN | ||||||||
| No | referent | referent | ||||||
| Yes | −1 (−3 to 1) | 0.33 | ||||||
| DM | ||||||||
| No | referent | referent | ||||||
| Yes | −2 (−4 to −1) | 0.002 | ||||||
| Stroke | ||||||||
| No | referent | referent | ||||||
| Yes | −0.1 (−3 to 3) | 0.97 | ||||||
| CAD | ||||||||
| No | referent | referent | ||||||
| Yes | −2 (−6 to 3) | 0.43 | ||||||
| Model 1 (n = 2068) * | Model 2 (n = 1772) † | Model 3 (n = 1744) ‡ | Model 4 (n = 1650) § | |||||
|---|---|---|---|---|---|---|---|---|
| Variables | β (95% CI) ¶ | p-Value | β (95% CI) ¶ | p-Value | β (95% CI) ¶ | p-Value | β (95% CI) ¶ | p-Value |
| Mn | 0.4 ( −1 to 0.2) | 0.21 | −0.8 (−1 to −0.1) | 0.02 | −0.7 (−1 to −0.1) | 0.02 | −0.8 (−1 to −0.1) | 0.03 |
| Gender | ||||||||
| Male | referent | referent | referent | referent | referent | referent | ||
| Female | −0.5 (−9 to 8) | 0.91 | 0.9 (−8 to 10) | 0.83 | 2 (−7 to 10) | 0.64 | ||
| Age (year) | −3 (−3 to −2) | <0.0001 | −3 (−3 to −2) | <0.0001 | −2 (−3 to −2) | <0.0001 | ||
| Race/Ethnicity | ||||||||
| NH White | referent | referent | referent | referent | referent | referent | ||
| Hispanic | −23 (−30 to −20) | <0.0001 | −23 (−30 to −20) | <0.0001 | −23 (−30 to −20) | <0.0001 | ||
| NH Black | −33 (−40 to −30) | <0.0001 | −32 (−40 to −20) | <0.0001 | −31 (−40 to −20) | <0.0001 | ||
| Other Race | −24 (−30 to −10) | 0.0005 | −23 (−40 to −10) | 0.0007 | −21 (−30 to −10) | 0.003 | ||
| Education | ||||||||
| >High School | referent | referent | referent | referent | referent | referent | ||
| High School | −29 (−40 to −20) | <0.0001 | −28 (−40 to −20) | <0.0001 | −28 (−40 to −20) | <0.0001 | ||
| <High School | −33 (−40 to −30) | <0.0001 | −32 (−40 to −30) | <0.0001 | −31 (−40 to −20) | <0.0001 | ||
| PIR | ||||||||
| ≤0.99 | referent | referent | referent | referent | referent | referent | ||
| ≥1 | 12 (2 to 20) | 0.02 | 11 (2 to 20) | 0.02 | 11 (1 to 20) | 0.03 | ||
| Marital Status | ||||||||
| Married/Living with partner | referent | referent | referent | referent | referent | referent | ||
| Widowed/Divorced/Separated | −0.4 (−9 to 8) | 0.93 | −0.7 (−10 to 8) | 0.88 | −1 (−10 to 8) | 0.82 | ||
| Alcohol Consumption | ||||||||
| <12 drinks/year | referent | referent | referent | referent | ||||
| >12 drinks/year | 7 (0.8 to 10) | 0.03 | 7 (0.8 to 10) | 0.03 | ||||
| HTN | ||||||||
| No | referent | referent | ||||||
| Yes | −6 (−10 to 0.2) | 0.06 | ||||||
| DM | ||||||||
| No | referent | referent | ||||||
| Yes | −8 (−10 to −1) | 0.03 | ||||||
| Stroke | ||||||||
| No | referent | referent | ||||||
| Yes | −6 (−20 to 5) | 0.27 | ||||||
| CAD | ||||||||
| No | referent | referent | ||||||
| Yes | −9 (−20 to 4) | 0.16 | ||||||
| Variables | N | Weighted Sample | (%) * | Variables | N | Weighted Sample | Mean (SE) | Median (IQR) |
|---|---|---|---|---|---|---|---|---|
| Gender | ||||||||
| Male | 464 | 7,702,844 | 46 | Age (year) | 950 | 16,795,626 | 69.3 (0.3) | 67.9 (10.7) |
| Female | 486 | 9,092,783 | 54 | Urinary Mn (µg/L) | 950 | 16,795,626 | 0.19 (0.03) | 0.09 (0.07) |
| Race/Ethnicity | CERAD learning | 950 | 16,795,626 | 6.6 (0.01) | 6.6 (2.1) | |||
| Hispanic | 176 | 1,216,571 | 7 | CERAD recall | 950 | 16,795,626 | 6.3 (0.1) | 6.1 (3.3) |
| NH White | 451 | 13,351,140 | 79 | Animal Fluency | 950 | 16,795,626 | 18 (0.3) | 16 (8) |
| NH Black | 225 | 1,438,019 | 9 | DSST | 950 | 16,795,626 | 52 (0.8) | 51 (25) |
| Other Race | 98 | 789,895 | 5 | z-score | 950 | 16,795,626 | 0.23 (0.04) | 0.29 (1.1) |
| Education | ||||||||
| <High School | 259 | 2,847,794 | 17 | |||||
| High School | 209 | 3,422,549 | 20 | |||||
| >High School | 480 | 10,513,695 | 63 | |||||
| Missing | 2 | |||||||
| PIR | ||||||||
| ≤0.99 | 166 | 1,539,528 | 10 | |||||
| ≥1 | 693 | 13,934,209 | 90 | |||||
| Missing | 91 | |||||||
| Marital Status | ||||||||
| Married/Living with partner | 557 | 10,964,908 | 65 | |||||
| Widowed/Divorced/Separated | 336 | 5,008,167 | 30 | |||||
| Never married | 55 | 810,401 | 5 | |||||
| Missing | 2 | |||||||
| Alcohol Consumption | ||||||||
| >12 drinks/year | 644 | 12,105,591 | 73 | |||||
| <12 drinks/year | 288 | 4,409,341 | 27 | |||||
| Missing | 18 | |||||||
| HTN | ||||||||
| Yes | 589 | 96,29,710 | 57 | |||||
| No | 361 | 7,165,916 | 43 | |||||
| DM | ||||||||
| Yes | 214 | 3,081,562 | 19 | |||||
| No | 694 | 12,955,019 | 81 | |||||
| Missing | 42 | |||||||
| CAD | ||||||||
| Yes | 76 | 1,380,980 | 8 | |||||
| No | 869 | 15,348,084 | 92 | |||||
| Missing | 5 | |||||||
| Stroke | ||||||||
| Yes | 49 | 760,890 | 5 | |||||
| No | 900 | 16,002,161 | 95 | |||||
| Missing | 1 |
| Model 1 (n = 2068) * | Model 2 (n = 1772) † | Model 3 (n = 1744) ‡ | Model 4 (n = 1650) § | |||||
|---|---|---|---|---|---|---|---|---|
| Variables | β (95% CI) ¶ | p-Value | β (95% CI) ¶ | p-Value | β (95% CI) ¶ | p-Value | β (95% CI) ¶ | p-Value |
| Mn | −0.7 (−2 to 0.2) | 0.16 | −1 (−2 to 0.3) | 0.12 | −1 (−2 to 0.2) | 0.09 | −2 (−4 to −0.7) | 0.01 |
| Gender | ||||||||
| Male | referent | referent | referent | referent | referent | referent | ||
| Female | 7 (5 to 9) | <0.0001 | 7 (5 to 9) | <0.0001 | 7 (5 to 9) | <0.0001 | ||
| Age (year) | −0.8 (−1 to −0.6) | <0.0001 | −0.8 (−1 to −0.5) | <0.0001 | −0.7 (−1 to −0.5) | <0.0001 | ||
| Race/Ethnicity | ||||||||
| NH White | referent | referent | referent | referent | referent | referent | ||
| Hispanic | −7 (−10 to −4) | <0.0001 | −7 (−10 to −4) | <0.0001 | −6 (−10 to −4) | 0.0001 | ||
| NH Black | 0.2 (−2 to 3) | 0.89 | 0.5 (−2 to 3) | 0.67 | 0.7 (−2 to 4) | 0.6 | ||
| Other Race | −5 (−10 to 1) | 0.1 | −4 (−10 to 11) | 0.12 | −4 (−10 to 1) | 0.13 | ||
| Education | ||||||||
| >High School | referent | referent | referent | referent | referent | referent | ||
| High School | −4 (−10 to −4) | 0.0003 | −4 (−6 to −2) | 0.001 | −3 (−6 to −1) | 0.003 | ||
| <High School | −7 (−10 to −4) | 0.0002 | −7 (−10 to 3) | 0.0004 | −6 (−10 to −3) | 0.001 | ||
| PIR | ||||||||
| ≤0.99 | referent | referent | referent | referent | referent | referent | ||
| ≥1 | 1 (−2 to 5) | 0.4 | 1 (−2 to −5) | 0.51 | 0.9 (−3 to 5) | 0.59 | ||
| Marital Status | ||||||||
| Married/Living with partner | referent | referent | referent | referent | referent | referent | ||
| Widowed/Divorced/Separated | −2 (−5 to 0.6) | 0.12 | −2 (−5 to 0.5) | 0.11 | −2 (−5 to 0.6) | 0.13 | ||
| Alcohol Consumption | ||||||||
| <12 drinks/year | referent | referent | referent | referent | ||||
| >12 drinks/year | 2 (−1 to 5) | 0.19 | 2 (−1 to 5) | 0.24 | ||||
| HTN | ||||||||
| No | referent | referent | ||||||
| Yes | −2 (−5 to 0.5) | 0.1 | ||||||
| DM | ||||||||
| No | referent | referent | ||||||
| Yes | −1 (−4 to 1) | 0.27 | ||||||
| Stroke | ||||||||
| No | referent | referent | ||||||
| Yes | −3 (−7 to 2) | 0.27 | ||||||
| CAD | ||||||||
| No | referent | referent | ||||||
| Yes | 0.3 (−6 to 7) | 0.93 | ||||||
| Model 1 (n = 2068) * | Model 2 (n = 1772) † | Model 3 (n = 1744) ‡ | Model 4 (n = 1650) § | |||||
|---|---|---|---|---|---|---|---|---|
| Variables | β (95% CI) ¶ | p-Value | β (95% CI) ¶ | p-Value | β (95% CI) ¶ | p-Value | β (95% CI) ¶ | p-Value |
| Mn | −20 (−40 to −10) | 0.003 | −30 (−40 to −10) | 0.002 | −30 (−40 to −10) | 0.0002 | −30 (−70 to 10) | 0.13 |
| Gender | ||||||||
| Male | referent | referent | referent | referent | referent | referent | ||
| Female | 70 (40 to 100) | <0.0001 | 80 (50 to 100) | <0.0001 | 70 (50 to 100) | <0.0001 | ||
| Age (year) | −10 (−12 to −9) | <0.0001 | −10 (−12 to −9) | <0.0001 | −10 (−2 to −9) | <0.0001 | ||
| Race/Ethnicity | ||||||||
| NH White | referent | referent | referent | referent | referent | referent | ||
| Hispanic | −120 (−150 to −90) | <0.0001 | −120 (−150 to −90) | <0.0001 | −120 (−150 to −90) | <0.0001 | ||
| NH Black | −110 (−130 to −80) | <0.0001 | −100 (−120 to −80) | <0.0001 | −100 (−130 to −70) | <0.0001 | ||
| Other Race | −20 (−60 to 20) | 0.29 | −10 (−50 to 20) | 0.45 | −20 (−60 to 20) | 0.38 | ||
| Education | ||||||||
| >High School | referent | referent | referent | referent | referent | referent | ||
| High School | −80 (−110 to −40) | <0.0001 | −70 (−110 to −40) | 0.0003 | −60 (−100 to −80) | 0.002 | ||
| <High School | −130 (−160 to −100) | <0.0001 | −120 (−150 to −90) | <0.0001 | −110 (−140 to −80) | <0.0001 | ||
| PIR | ||||||||
| ≤0.99 | referent | referent | referent | referent | referent | referent | ||
| ≥1 | 80 (40 to 110) | <0.0001 | 70 (40 to 110) | 0.0002 | 70 (40 to 110) | 0.0002 | ||
| Marital Status | ||||||||
| Married/Living with partner | referent | referent | referent | referent | referent | referent | ||
| Widowed/Divorced/Separated | −20 (−50 to 10) | 0.09 | −30 (−50 to 10) | 0.06 | −30 (−60 to 10) | 0.04 | ||
| Alcohol Consumption | ||||||||
| <12 drinks/year | referent | referent | referent | referent | ||||
| >12 drinks/year | 40 (10 to 70) | 0.01 | 30 (10 to 60) | 0.03 | ||||
| HTN | ||||||||
| No | referent | referent | ||||||
| Yes | −2 (−30 to 20) | 0.88 | ||||||
| DM | ||||||||
| No | referent | referent | ||||||
| Yes | −60 (−100 to −30) | 0.0003 | ||||||
| Stroke | ||||||||
| No | referent | referent | ||||||
| Yes | −20 (−70 to 40) | 0.5 | ||||||
| CAD | ||||||||
| No | referent | referent | ||||||
| Yes | −50 (−100 to 10) | 0.1 | ||||||
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Barahona, A.J.; Bursac, Z.; Veledar, E.; Lucchini, R.; Tieu, K.; Richardson, J.R. Relationship of Blood and Urinary Manganese Levels with Cognitive Function in Elderly Individuals in the United States by Race/Ethnicity, NHANES 2011–2014. Toxics 2022, 10, 191. https://0-doi-org.brum.beds.ac.uk/10.3390/toxics10040191
Barahona AJ, Bursac Z, Veledar E, Lucchini R, Tieu K, Richardson JR. Relationship of Blood and Urinary Manganese Levels with Cognitive Function in Elderly Individuals in the United States by Race/Ethnicity, NHANES 2011–2014. Toxics. 2022; 10(4):191. https://0-doi-org.brum.beds.ac.uk/10.3390/toxics10040191
Chicago/Turabian StyleBarahona, Arturo J., Zoran Bursac, Emir Veledar, Roberto Lucchini, Kim Tieu, and Jason R. Richardson. 2022. "Relationship of Blood and Urinary Manganese Levels with Cognitive Function in Elderly Individuals in the United States by Race/Ethnicity, NHANES 2011–2014" Toxics 10, no. 4: 191. https://0-doi-org.brum.beds.ac.uk/10.3390/toxics10040191