Reduction of Mycotoxins during Fermentation of Whole Grain Sorghum to Whole Grain Ting (a Southern African Food)
Abstract
:1. Introduction
2. Results and Discussion
2.1. Presence of Mycotoxins
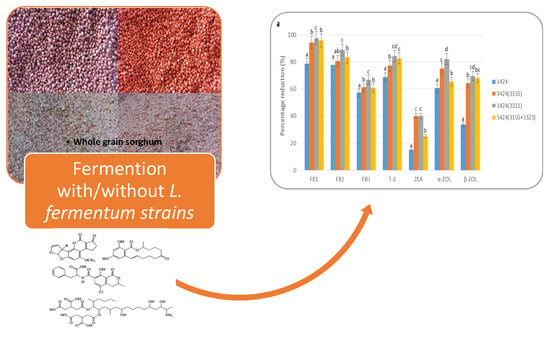
2.2. Mycotoxin Reduction
3. Conclusions
4. Materials and Methods
4.1. Raw Material and Sample Preparation
4.2. Lactobacillus Strains
4.3. Fermentation of Sorghum into WG-Ting
4.4. Mycotoxin Standards
4.5. Mycotoxin Extraction
4.6. Mycotoxin Analysis
4.7. Statistical Analysis
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Udomkun, P.; Wiredu, A.N.; Nagle, M.; Bandyopadhyay, R.; Müller, J.; Vanlauwe, B. Mycotoxins in sub-Saharan Africa: Present situation, socio-economic impact, awareness, and outlook. Food Cont. 2017, 72, 110–122. [Google Scholar] [CrossRef]
- IARC. Improving Public Health through Mycotoxin Control. In IARC Scientific Publication No. 158; Pitt, J.I., Wild, C.P., Baan, R.A., Gelderblom, W.C.A., Miller, J.D., Riley, R.T., Wu, F., Eds.; International Agency for Research on Cancer: Lyon, France, 2012. [Google Scholar]
- Misihairabgwi, J.M.; Ezekiel, C.N.; Sulyok, M.; Shephard, G.S.; Krska, R. Mycotoxin contamination of foods in Southern Africa: A 10-year review (2007–2016). Crit. Rev. Food Sci. Nutr. 2019, 59, 43–58. [Google Scholar] [CrossRef] [PubMed]
- Gbashi, S.; Madala, N.E.; De Saeger, S.; De Boevre, M.; Adekoya, I.; Adebo, O.A.; Njobeh, P.B. The socio-economic impact of mycotoxin contamination in Africa. In Fungi and Mycotoxins—Their Occurrence, Impact on Health and the Economy as well as Pre- and Postharvest Management Strategies; Njobeh, P.B., Stepman, F., Eds.; InTech: Rijeka, Croatia, 2019. [Google Scholar]
- Marasas, W.F.O. Discovery and occurrence of the fumonisins: A historical perspective. Environ. Health Perspect. 2001, 109, 239–243. [Google Scholar] [PubMed]
- Marasas, W.F.; Riley, R.T.; Hendricks, K.A.; Stevens, V.L.; Sadler, T.W.; Gelineau-van Waes, J. Fumonisins disrupt sphingolipid metabolism, folate transport, and neural tube development in embryo culture and in vivo: A potential risk factor for human neural tube defects among populations consuming fumonisin-contaminated maize. J. Nutr. 2004, 134, 711–716. [Google Scholar] [CrossRef] [PubMed]
- Shephard, G.S.; Burger, H.M.; Rheeder, J.P.; Alberts, J.F.; Gelderblom, W.C.A. The effectiveness of regulatory maximum levels for fumonisin mycotoxins in commercial and subsistence maize crops in South Africa. Food Cont. 2019, 97, 77–80. [Google Scholar] [CrossRef]
- Shephard, G.S. Impact of mycotoxins on human health in developing countries. Food Addit. Contam. 2008, 25, 146–151. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chala, A.; Taye, W.; Ayalew, A.; Krska, R.; Sulyok, M.; Logrieco, A. Multimycotoxin analysis of sorghum (Sorghum bicolor L. Moench) and finger millet (Eleusine coracana L. Garten) from Ethiopia. Food Cont. 2014, 45, 29–35. [Google Scholar] [CrossRef]
- Taye, W.; Ayalew, A.; Denjene, M.; Chala, A. Fungal invasion and mycotoxin contamination of stored sorghum grain as influenced by threshing methods. Int. J. Pest Manag. 2018, 64, 66–76. [Google Scholar] [CrossRef]
- Njobeh, P.B.; Dutton, M.F.; Makun, H.A. Mycotoxins and human health: Significance, prevention and control. In Smart Biomolecules in Medicine; Ajay, K.M., Ashutosh, T., Shivanti, B.M., Eds.; VBRI Press: Allahabad, India, 2010; pp. 132–177. [Google Scholar]
- Odunmbaku, L.A.; Sobowale, S.S.; Adenekan, M.K.; Oloyede, T.; Adebiyi, J.A.; Adebo, O.A. Influence of steeping duration, drying temperature and duration on the chemical composition of sorghum-starch. Food Sci. Nutr. 2018, 6, 348–355. [Google Scholar] [CrossRef] [PubMed]
- Warth, B.; Parich, A.; Atehnkeng, J.; Bandyopadhyay, R.; Schuhmacher, R.; Sulyok, M.; Krska, R. Quantitation of mycotoxins in food and feed from Burkina Faso and Mozambique using a modern LC-MS/MS multitoxin method. J. Agric. Food Chem. 2012, 60, 9352–9936. [Google Scholar] [CrossRef] [PubMed]
- Makun, H.A.; Gbodi, T.A.; Akanya, H.O.; Salako, E.A.; Ogbadu, G.H. Fungi and some mycotoxins found in mouldy sorghum in Niger State, Nigeria. World J. Agric. Sci. 2009, 5, 5–17. [Google Scholar]
- Matumba, L.; Van Poucke, C.; Ediage, E.N.; De Saeger, S. Keeping mycotoxins away from the food: Does the existence of regulations have any impact in Africa? Crit. Rev. Food Sci. Nutr. 2017, 57, 1584–1592. [Google Scholar] [CrossRef] [PubMed]
- Taylor, J.R.N.; Schober, T.J.; Bean, S.R. Novel food and non-food uses for sorghum and millets. J. Cereal Sci. 2006, 44, 252–271. [Google Scholar] [CrossRef]
- Adebo, O.A.; Njobeh, P.B.; Adebiyi, J.A.; Gbashi, S.; Phoku, J.Z.; Kayitesi, E. Fermented pulse-based food products in developing nations as functional foods and ingredients. In Functional Food—Improve Health through Adequate Food; Hueda, M.C., Ed.; InTech: Rijeka, Croatia, 2017; pp. 77–109. [Google Scholar]
- Adebo, O.A.; Njobeh, P.B.; Kayitesi, E. Fermentation by Lactobacillus fermentum strains (singly and in combination) enhances the properties of ting from two whole grain sorghum types. J. Cereal Sci. 2018, 82, 49–56. [Google Scholar] [CrossRef]
- Sekwati-Monang, B.; Gänzle, M.G. Microbiological and chemical characterization of ting, a sorghum-based sourdough product from Botswana. Int. J. Food Microbiol. 2011, 150, 115–121. [Google Scholar] [CrossRef] [PubMed]
- Adebo, O.A.; Njobeh, P.B.; Mulaba-Bafubiandi, A.F.; Adebiyi, J.A.; Desobgo, Z.S.C.; Kayitesi, E. Optimization of fermentation conditions for ting production using response surface methodology. J. Food Proc. Preserv. 2018, 42, e13381. [Google Scholar] [CrossRef]
- Adebo, O.A.; Njobeh, P.B.; Gbashi, S.; Nwinyi, O.C.; Mavumengwana, V. Review on microbial degradation of aflatoxins. Crit. Rev. Food Sci. Nutr. 2017, 57, 3208–3217. [Google Scholar] [CrossRef] [PubMed]
- Alberts, J.F.; Gelderblom, W.C.A.; Botha, A.; van Zyl, W.H. Degradation of aflatoxin B1 by fungal laccase enzymes. Int. J. Food Microbiol. 2009, 135, 47–52. [Google Scholar] [CrossRef]
- Samuel, M.S.; Sivaramakrishna, A.; Mehta, A. Degradation and detoxification of aflatoxin B1 by Pseudomonas putida. Int. Biodeter. Biodegr. 2014, 86, 202–209. [Google Scholar] [CrossRef]
- Adebo, O.A.; Njobeh, P.B.; Mavumengwana, V. Degradation and detoxification of AFB1 by Staphylocococcus warneri, Sporosarcina sp. and Lysinibacillus fusiformis. Food Cont. 2016, 68, 92–96. [Google Scholar] [CrossRef]
- Karlovsky, P.; Suman, M.; Berthiller, F.; De Meester, J.; Eisenbrand, G.; Perrin, I.; Oswald, I.P.; Speijers, G.; Chiodini, A.; Recker, T.; et al. Impact of food processing and detoxification treatments on mycotoxin contamination. Mycotoxin Res. 2016, 32, 179–205. [Google Scholar] [CrossRef] [Green Version]
- Adebo, O.A.; Njobeh, P.B.; Sidu, S.; Tlou, M.G.; Mavumengwana, V. Aflatoxin B1 degradation by liquid cultures and lysates of three bacterial strains. Int. J. Food Microbiol. 2016, 233, 11–19. [Google Scholar] [CrossRef]
- Ezekiel, C.N.; Abia, W.A.; Ogara, I.M.; Sulyok, M.; Warth, B.; Krska, R. Fate of mycotoxins in two popular traditional cereal-based beverages (kunu-zaki and pito) from rural Nigeria. LWT-Food Sci. Technol. 2015, 60, 137–141. [Google Scholar] [CrossRef]
- Nyamete, F.A.; Bennink, M.; Mugula, J.K. Potential of lactic acid fermentation in reducing aflatoxin B1 in Tanzania maize-based gruel. Afric. J. Food Agric. Nutr. Dev. 2016, 16, 11139–11151. [Google Scholar] [CrossRef]
- Okeke, C.A.; Ezekiel, C.N.; Sulyok, M.; Ogunremi, O.R.; Ezeamagu, C.O.; Sarkanj, B.; Warth, B.; Krska, R. Traditional processing impacts mycotoxin levels and nutritional value of ogi—A maize-based complementary food. Food Cont. 2018, 86, 224–233. [Google Scholar] [CrossRef]
- Adebo, O.A.; Njobeh, P.B.; Adebiyi, J.A.; Kayitesi, E. Co-influence of fermentation time and temperature on physicochemical properties, bioactive components and microstructure of ting (a Southern African food) from whole grain sorghum. Food Biosci. 2018, 25, 118–127. [Google Scholar] [CrossRef]
- European Commission Regulation No 1881/2006 of 19 December 2006 Setting Maximum Levels for Certain Contaminants in Foodstuffs. Available online: http://eur-lex.europa.eu/LexUriServ/LexUriServ.do?uri=CONSLEG:2006R1881:20100701:EN:PDF (accessed on 23 December 2017).
- Atanasova-Penichon, V.; Barreau, C.; Richard-Forget, F. Antioxidant secondary metabolites in cereals: Potential involvement in resistance to Fusarium and mycotoxin accumulation. Front. Microbiol. 2016, 7, 1–16. [Google Scholar] [CrossRef]
- Telles, A.C.; Kupski, L.; Furlong, E.B. Phenolic compound in beans as protection against mycotoxins. Food Chem. 2017, 214, 293–299. [Google Scholar] [CrossRef] [PubMed]
- South African Government Foodstuffs, Cosmetics and Disinfectants Act (Act 54 of 1972) and Regulations Published under Government Notice No. R. 1145, Dated 8 October 2004. Available online: http://www.sagl.co.za/Portals/0/Maize%20Crop%202015%202016/Page%2073.pdf (accessed on 3 March 2019).
- European Commission Recommendation of 27 March 2013 on the Presence of T-2 and HT-2 Toxin in Cereals and Cereal Products. Available online: http://eur-lex.europa.eu/LexUriServ/LexUriServ.do?uri=OJ:L:2013:091:0012:0015:EN:PDF (accessed on 23 December 2017).
- Oueslati, S.; Romero-González, R.; Lasram, S.; Frenich, A.G.; Martínez Vidal, J.L. Multi-mycotoxin determination in cereals and derived products marketed in Tunisia using ultra-high performance liquid chromatography coupled to triple quadrupole mass spectrometry. Food Chem. Toxicol. 2012, 50, 2376–2381. [Google Scholar] [CrossRef]
- Nyamete, F.A.; Mourice, B.; Mugula, J.K. Fumonisin B1 reduction in lactic acid bacteria fermentation of maize porridges. Tanz. J. Agric. Sci. 2016, 15, 13–20. [Google Scholar]
- Dawlal, P.; Brabet, C.; Thantsha, M.S.; Buys, E.M. Potential of lactic acid bacteria for the reduction of fumonisin exposure in African fermented maize based foods. World Mycotoxin J. 2017, 10, 309–318. [Google Scholar] [CrossRef] [Green Version]
- Dawlal, P.; Brabet, C.; Thantsha, M.S.; Buys, E.M. Visualisation and quantification of fumonisins bound by lactic acid bacteria isolates from traditional African maize-based fermented cereals, ogi and mahewu. Food Addit. Contam. Part A 2019, 36, 296–307. [Google Scholar] [CrossRef]
- Zhao, H.; Wang, X.; Zhang, J.; Zhang, J.; Zhang, B. The mechanism of Lactobacillus strains for their ability to remove fumonisins B1 and B2. Food Chem. Toxicol. 2016, 97, 40–46. [Google Scholar] [CrossRef]
- Garda, J.; Macedo, R.M.; Faria, R.; Bernd, L.; Dors, G.C.; Badiale-Furlong, E. Alcoholic fermentation effects on malt spiked with trichothecenes. Food Cont. 2005, 16, 423–428. [Google Scholar] [CrossRef]
- Shetty, P.H.; Jespersen, L. Saccharomyces cerevisiae and lactic acid bacteria as potential mycotoxin decontaminating agents. Trends Food Sci. Technol. 2006, 17, 48–55. [Google Scholar] [CrossRef]
- Mokoena, M.P.; Chelule, P.K.; Ggaleni, N. Reduction of fumonisin B1 and zearalenone by lactic acid bacteria in fermented maize meal. J. Food Prot. 2005, 68, 2095–2099. [Google Scholar] [CrossRef]
- Adhikari, M.; Negi, B.; Kaushik, N.; Adhikari, A.; Al-Khedhairy, A.A.; Kaushik, N.K.; Choi, E.H. T-2 mycotoxin: Toxicological effects and decontamination strategies. Oncotarget 2017, 8, 33933–33952. [Google Scholar] [CrossRef] [PubMed]
- Amezqueta, S.; Gonzales Peñas, E.; Murillo-Arbizu, M.; Lopez de Cerain, A. Ochratoxin A decontamination: A review. Food Cont. 2009, 20, 326–333. [Google Scholar] [CrossRef]
- Meca, G.; Blaiotta, G.; Ritieni, A. Reduction of ochratoxin A during the fermentation of Italian red wine Moscato. Food Control 2010, 21, 579–583. [Google Scholar] [CrossRef]
- Motloung, L.; De Saeger, S.; De Boevre, M.; Detavernier, C.; Audenaert, K.; Adebo, O.A.; Njobeh, P.B. Study on mycotoxin contamination in South African food spices. World Mycotoxin J. 2019, 11, 401–409. [Google Scholar] [CrossRef]
- Njobeh, P.B.; Dutton, M.F.; Aberg, A.T.; Haggblom, P. Estimation of multi-mycotoxin contamination in South African compound feeds. Toxins 2012, 4, 836–848. [Google Scholar] [CrossRef] [PubMed]
- Wenzl, T.; Johannes, H.; Schaechtele, A.; Robouch, P.; Storka, J. Guidance Document on the Estimation of LOD and LOQ for Measurements in the Field of Contaminants in Feed and Food. Available online: http://publications.jrc.ec.europa.eu/repository/bitstream/JRC102946/eur%2028099%20en_lod%20loq%20guidance%20document.pdf (accessed on 18 May 2018).
- Adebo, O.A.; Njobeh, P.B.; Sidu, S.; Adebiyi, J.A.; Mavumengwana, V. Aflatoxin B1 degradation by culture and lysate of a Pontibacter specie. Food Cont. 2017, 80, 99–103. [Google Scholar] [CrossRef]

| Rt (min) | Mycotoxin Standard | MW | Parent Ion m/z (Precursor) | MS/MS Fragments | CE | R2 (Neat Solvent) | R2 (Sorghum Mix) | ARR (%) | LOD (µg/kg) | LOQ (µg/kg) |
|---|---|---|---|---|---|---|---|---|---|---|
| 2.78 | DON | 296 | 297.10 | 231, 249 | 12 | 0.989 | 0.981 | 89 | 0.11 | 1.74 |
| 6.87 | AFG2 | 330 | 331.00 | 245, 313 | 32 | 0.999 | 0.992 | 82 | 0.01 | 0.04 |
| 7.12 | AFG1 | 328 | 329.00 | 243, 311 | 28 | 0.999 | 0.990 | 85 | 0.01 | 0.04 |
| 7.30 | AFB2 | 314 | 315.00 | 259, 287 | 31 | 0.999 | 0.989 | 91 | 0.59 | 1.98 |
| 7.48 | AFB1 | 312 | 313.00 | 241, 285 | 24 | 0.999 | 0.997 | 90 | 0.01 | 0.04 |
| 7.58 | FB1 | 721 | 722.40 | 352, 334 | 42 | 0.997 | 0.996 | 98 | 0.34 | 0.87 |
| 8.03 | β-ZOL | 322 | 323.00 | 277, 305 | 11 | 0.998 | 0.998 | 90 | 0.21 | 0.71 |
| 8.20 | FB2 | 705 | 706.10 | 336, 318 | 38 | 0.999 | 0.996 | 99 | 0.41 | 0.93 |
| 8.25 | FB3 | 705 | 706.30 | 336, 354 | 35 | 0.999 | 0.997 | 94 | 1.02 | 4.11 |
| 8.28 | OTB | 369 | 370.10 | 205, 324 | 14 | 0.998 | 0.991 | 94 | 0.06 | 0.19 |
| 8.38 | α-ZOL | 322 | 323.10 | 277, 305 | 9 | 0.999 | 0.993 | 87 | 0.65 | 2.17 |
| 8.53 | T-2 | 466 | 467.20 | 245, 305 | 11 | 0.999 | 0.997 | 96 | 0.39 | 0.90 |
| 8.74 | ZEA | 318 | 319.10 | 185, 187 | 21 | 0.999 | 0.991 | 92 | 0.03 | 0.11 |
| 8.78 | OTA | 403 | 404.00 | 239, 221 | 38 | 0.999 | 0.993 | 86 | 0.04 | 0.14 |
| FB1 | FB2 | FB3 | T-2 | ZEA | α-ZOL | β-ZOL | |
|---|---|---|---|---|---|---|---|
| LT-sorghum | |||||||
| Raw LT-sorghum | 162.67 d ± 3.90 | 12.00 b ± 0.99 | 400.00 d ± 2.98 | 7.39 b ± 1.20 | 6.67 c ± 0.50 | 28.00 d ± 0.45 | 37.33 d ± 0.53 |
| 3424 | 34.68 c ± 5.58 | 2.67 a ± 0.45 | 170.67 c ± 2.35 | 2.32 a ± 0.74 | 5.67 b ± 0.28 | 11.00 c ± 0.99 | 24.67 c ± 0.49 |
| 3424 + 3165 | 9.33 b ± 1.40 | 2.31 a ± 0.98 | 155.33 b ± 1.45 | 1.68 a ± 0.78 | 4.00 a ± 0.82 | 7.00 b ± 0.97 | 13.33 b ± 0.75 |
| 3424 + 3321 | 4.00 a ± 2.63 | 1.33 a ± 1.06 | 133.33 a ± 2.19 | 1.17 a ± 0.63 | 4.00 a ± 0.05 | 5.00 a ± 0.92 | 11.83 a ± 0.63 |
| 3424 (3165 + 3321) | 6.67 ab ± 2.10 | 2.00 a ± 0.92 | 156.67 b ± 2.09 | 1.28 a ± 0.75 | 5.00 b ± 0.06 | 9.67 c ± 0.81 | 11.94 a ± 0.07 |
| HT-sorghum | |||||||
| Raw HT-sorghum | – | – | 148.00 d ± 1.93 | 6.67 b ± 1.00 | 6.04 b ± 0.13 | 20.89 c ± 0.82 | 25.33 c ± 0.44 |
| 2872 | – | – | 105.33 c ± 1.80 | 4.06 a ± 0.06 | 5.33 ab ± 0.43 | 10.33 b ± 0.44 | 19.31 b ± 0.44 |
| 2872 + 3165 | – | – | 91.07 b ± 1.74 | 3.94 a ± 0.85 | 4.82 a ± 0.1 | 8.69 a ± 0.21 | 12.00 a ± 0.87 |
| 2872 + 3321 | – | – | 84.06 a ± 2.29 | 3.17 a ± 0.17 | 4.67 a ± 0.3 | 8.67 a ± 0.51 | 10.67 a ± 0.46 |
| 2872 (3165 + 3321) | – | – | 93.38 b ± 1.82 | 3.85 a ± 0.33 | 5.00 a ± 0.71 | 9.00 a ± 0.23 | 18.33 b ± 1.21 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Adebo, O.A.; Kayitesi, E.; Njobeh, P.B. Reduction of Mycotoxins during Fermentation of Whole Grain Sorghum to Whole Grain Ting (a Southern African Food). Toxins 2019, 11, 180. https://0-doi-org.brum.beds.ac.uk/10.3390/toxins11030180
Adebo OA, Kayitesi E, Njobeh PB. Reduction of Mycotoxins during Fermentation of Whole Grain Sorghum to Whole Grain Ting (a Southern African Food). Toxins. 2019; 11(3):180. https://0-doi-org.brum.beds.ac.uk/10.3390/toxins11030180
Chicago/Turabian StyleAdebo, Oluwafemi Ayodeji, Eugenie Kayitesi, and Patrick Berka Njobeh. 2019. "Reduction of Mycotoxins during Fermentation of Whole Grain Sorghum to Whole Grain Ting (a Southern African Food)" Toxins 11, no. 3: 180. https://0-doi-org.brum.beds.ac.uk/10.3390/toxins11030180