Cardiac Remodeling in Chronic Kidney Disease
Abstract
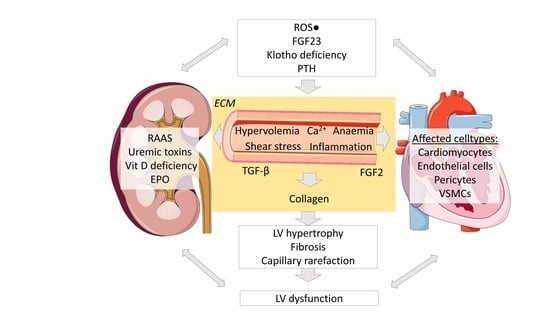
:1. Chronic Kidney Disease
Cellular Crosstalk in the Heart
2. Pathology and Pathophysiology of the Cardiorenal Syndrome
2.1. Left-Ventricular Hypertrophy in CKD
2.2. Cardiac Fibrosis
2.3. Capillary Rarefaction
2.4. Oxidative Stress
2.5. Inflammation
2.6. Advanced Glycation end Products
2.7. Growth Factors
2.8. FGF23
2.9. Klotho
2.10. Uremic Toxins
3. Mouse Models of Cardiac Remodeling in CKD
3.1. Surgically Induced Models
3.2. Chemically Induced Models
3.3. Genetically Induced Models
4. Potential Therapeutic Targets of Cardiac Remodeling in CKD
Author Contributions
Funding
Conflicts of Interest
References
- Luyckx, V.A.; Tonelli, M.; Stanifer, J.W. The global burden of kidney disease and the sustainable development goals. Bull. World Health Organ. 2018, 96, 414–422. [Google Scholar] [CrossRef]
- Wang, H.; Naghavi, M.; Allen, C.; Barber, R.M.; Bhutta, Z.A.; Carter, A.; Coggeshall, M. GBD 2015 Mortality and Causes of Death Collaborators Global, regional, and national life expectancy, all-cause mortality, and cause-specific mortality for 249 causes of death, 1980–2015: A systematic analysis for the Global Burden of Disease Study 2015. Lancet 2016, 388, 1459–1544. [Google Scholar] [CrossRef] [Green Version]
- Bikbov, B.; Perico, N.; Remuzzi, G. On behalf of the GBD Genitourinary Diseases Expert Group Disparities in Chronic Kidney Disease Prevalence among Males and Females in 195 Countries: Analysis of the Global Burden of Disease 2016 Study. Nephron 2018, 139, 313–318. [Google Scholar] [CrossRef]
- Hill, N.R.; Fatoba, S.T.; Oke, J.L.; Hirst, J.A.; O’Callaghan, C.A.; Lasserson, D.S.; Richard Hobbs, F.D. Global Prevalence of Chronic Kidney Disease—A Systematic Review and Meta-Analysis. PLoS ONE 2016, 11, e0158765. [Google Scholar] [CrossRef] [PubMed]
- Shamseddin, M.K.; Khaled Shamseddin, M.; Parfrey, P.S. Sudden cardiac death in chronic kidney disease: Epidemiology and prevention. Nat. Rev. Nephrol. 2011, 7, 145–154. [Google Scholar] [CrossRef] [PubMed]
- Zhou, P.; Pu, W.T. Recounting Cardiac Cellular Composition. Circ. Res. 2016, 118, 368–370. [Google Scholar] [CrossRef] [PubMed]
- Pinto, A.R.; Ilinykh, A.; Ivey, M.J.; Kuwabara, J.T.; D’Antoni, M.L.; Debuque, R.; Chandran, A.; Wang, L.; Arora, K.; Rosenthal, N.A.; et al. Revisiting Cardiac Cellular Composition. Circ. Res. 2016, 118, 400–409. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Massaia, A.; Chaves, P.; Samari, S.; Miragaia, R.J.; Meyer, K.; Teichmann, S.A.; Noseda, M. Single Cell Gene Expression to Understand the Dynamic Architecture of the Heart. Front. Cardiovasc. Med. 2018, 5, 167. [Google Scholar] [CrossRef] [Green Version]
- Wagner, J.U.G.; Dimmeler, S. Cellular cross-talks in the diseased and aging heart. J. Mol. Cell. Cardiol. 2020, 138, 136–146. [Google Scholar] [CrossRef] [Green Version]
- Wang, L.; Yu, P.; Zhou, B.; Song, J.; Li, Z.; Zhang, M.; Guo, G.; Wang, Y.; Chen, X.; Han, L.; et al. Single-cell reconstruction of the adult human heart during heart failure and recovery reveals the cellular landscape underlying cardiac function. Nat. Cell Biol. 2020, 22, 108–119. [Google Scholar] [CrossRef]
- Perbellini, F.; Watson, S.A.; Bardi, I.; Terracciano, C.M. Heterocellularity and Cellular Cross-Talk in the Cardiovascular System. Front. Cardiovasc. Med. 2018, 5, 143. [Google Scholar] [CrossRef] [PubMed]
- Talman, V.; Kivelä, R. Cardiomyocyte-Endothelial Cell Interactions in Cardiac Remodeling and Regeneration. Front. Cardiovasc. Med. 2018, 5, 101. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kramann, R.; Erpenbeck, J.; Schneider, R.K.; Röhl, A.B.; Hein, M.; Brandenburg, V.M.; van Diepen, M.; Dekker, F.; Marx, N.; Floege, J.; et al. Speckle tracking echocardiography detects uremic cardiomyopathy early and predicts cardiovascular mortality in ESRD. J. Am. Soc. Nephrol. 2014, 25, 2351–2365. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rasić, S.; Kulenović, I.; Haracić, A.; Catović, A. Left ventricular hypertrophy and risk factors for its development in uraemic patients. Bosn. J. Basic Med. Sci. 2004, 4, 34–40. [Google Scholar] [CrossRef] [Green Version]
- Mitsnefes, M.M.; Daniels, S.R.; Schwartz, S.M.; Meyer, R.A.; Khoury, P.; Strife, C.F. Severe left ventricular hypertrophy in pediatric dialysis: Prevalence and predictors. Pediatr. Nephrol. 2000, 14, 898–902. [Google Scholar] [CrossRef]
- Amann, K.; Rychlík, I.; Miltenberger-Milteny, G.; Ritz, E. Left ventricular hypertrophy in renal failure. Kidney Int. Suppl. 1998, 68, S78–S85. [Google Scholar] [CrossRef] [Green Version]
- Izumaru, K.; Hata, J.; Nakano, T.; Nakashima, Y.; Nagata, M.; Fukuhara, M.; Oda, Y.; Kitazono, T.; Ninomiya, T. Reduced Estimated GFR and Cardiac Remodeling: A Population-Based Autopsy Study. Am. J. Kidney Dis. 2019, 74, 373–381. [Google Scholar] [CrossRef] [Green Version]
- Fearnley, C.J.; Roderick, H.L.; Bootman, M.D. Calcium signaling in cardiac myocytes. Cold Spring Harb. Perspect. Biol. 2011, 3, a004242. [Google Scholar] [CrossRef] [Green Version]
- Kisling, A.; Lust, R.M.; Katwa, L.C. What is the role of peptide fragments of collagen I and IV in health and disease? Life Sci. 2019, 228, 30–34. [Google Scholar] [CrossRef]
- Rudenko, T.E.; Kamyshova, E.S.; Vasilyeva, M.P.; Bobkova, I.N.; Solomakhina, N.I.; Shvetsov, M.Y. Risk factors for diastolic left ventricular myocardial dysfunction in patients with chronic kidney disease. Ter. Arkh. 2018, 90, 60–67. [Google Scholar] [CrossRef]
- London, G.M. Left ventricular alterations and end-stage renal disease. Nephrol. Dial. Transplant. 2002, 17, 29–36. [Google Scholar] [CrossRef] [PubMed]
- Speiser, B.; Riess, C.F.; Schaper, J. The extracellular matrix in human myocardium: Part I: Collagens I, III, IV, and VI. Cardioscience 1991, 2, 225–232. [Google Scholar] [PubMed]
- Weber, K.T. Cardiac interstitium in health and disease: The fibrillar collagen network. J. Am. Coll. Cardiol. 1989, 13, 1637–1652. [Google Scholar] [CrossRef] [Green Version]
- Chen, M.; Arcari, L.; Engel, J.; Freiwald, T.; Platschek, S.; Zhou, H.; Zainal, H.; Buettner, S.; Zeiher, A.M.; Geiger, H.; et al. Aortic stiffness is independently associated with interstitial myocardial fibrosis by native T1 and accelerated in the presence of chronic kidney disease. IJC Heart Vasc. 2019, 24, 100389. [Google Scholar] [CrossRef]
- Nitta, K.; Akiba, T.; Uchida, K.; Otsubo, S.; Otsubo, Y.; Takei, T.; Ogawa, T.; Yumura, W.; Kabaya, T.; Nihei, H. Left ventricular hypertrophy is associated with arterial stiffness and vascular calcification in hemodialysis patients. Hypertens. Res. 2004, 27, 47–52. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pluta, A.; Stróżecki, P.; Krintus, M.; Odrowąż-Sypniewska, G.; Manitius, J. Left ventricular remodeling and arterial remodeling in patients with chronic kidney disease stage 1–3. Ren. Fail. 2015, 37, 1105–1110. [Google Scholar] [CrossRef] [Green Version]
- Pateinakis, P.; Papagianni, A. Cardiorenal Syndrome Type 4—Cardiovascular Disease in Patients with Chronic Kidney Disease: Epidemiology, Pathogenesis, and Management. Int. J. Nephrol. 2011, 2011, 938651. [Google Scholar] [CrossRef] [Green Version]
- Vanlandewijck, M.; He, L.; Mäe, M.A.; Andrae, J.; Ando, K.; Del Gaudio, F.; Nahar, K.; Lebouvier, T.; Laviña, B.; Gouveia, L.; et al. A molecular atlas of cell types and zonation in the brain vasculature. Nature 2018, 554, 475–480. [Google Scholar] [CrossRef] [Green Version]
- Gu, W.; Ni, Z.; Tan, Y.-Q.; Deng, J.; Zhang, S.-J.; Lv, Z.-C.; Wang, X.-J.; Chen, T.; Zhang, Z.; Hu, Y.; et al. Adventitial Cell Atlas of wt (Wild Type) and ApoE (Apolipoprotein E)-Deficient Mice Defined by Single-Cell RNA Sequencing. Arterioscler. Thromb. Vasc. Biol. 2019, 39, 1055–1071. [Google Scholar] [CrossRef]
- Kramann, R.; Goettsch, C.; Wongboonsin, J.; Iwata, H.; Schneider, R.K.; Kuppe, C.; Kaesler, N.; Chang-Panesso, M.; Machado, F.G.; Gratwohl, S.; et al. Adventitial MSC-like Cells Are Progenitors of Vascular Smooth Muscle Cells and Drive Vascular Calcification in Chronic Kidney Disease. Cell Stem Cell 2016, 19, 628–642. [Google Scholar] [CrossRef] [Green Version]
- Kramann, R.; Schneider, R.K.; DiRocco, D.P.; Machado, F.; Fleig, S.; Bondzie, P.A.; Henderson, J.M.; Ebert, B.L.; Humphreys, B.D. Perivascular Gli1+ progenitors are key contributors to injury-induced organ fibrosis. Cell Stem Cell 2015, 16, 51–66. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pannier, B.; Guerin, A.P.; Marchais, S.J.; Metivier, F.; Safar, M.E.; London, G.M. Postischemic vasodilation, endothelial activation, and cardiovascular remodeling in end-stage renal disease. Kidney Int. 2000, 57, 1091–1099. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Prommer, H.-U.; Maurer, J.; von Websky, K.; Freise, C.; Sommer, K.; Nasser, H.; Samapati, R.; Reglin, B.; Guimarães, P.; Pries, A.R.; et al. Chronic kidney disease induces a systemic microangiopathy, tissue hypoxia and dysfunctional angiogenesis. Sci. Rep. 2018, 8, 5317. [Google Scholar] [CrossRef]
- Bonetti, P.O.; Lerman, L.O.; Lerman, A. Endothelial dysfunction: A marker of atherosclerotic risk. Arterioscler. Thromb. Vasc. Biol. 2003, 23, 168–175. [Google Scholar] [CrossRef]
- Duni, A.; Liakopoulos, V.; Rapsomanikis, K.-P.; Dounousi, E. Chronic Kidney Disease and Disproportionally Increased Cardiovascular Damage: Does Oxidative Stress Explain the Burden? Oxid. Med. Cell. Longev. 2017, 2017, 9036450. [Google Scholar] [CrossRef] [PubMed]
- Sárközy, M.; Kovács, Z.Z.A.; Kovács, M.G.; Gáspár, R.; Szűcs, G.; Dux, L. Mechanisms and Modulation of Oxidative/Nitrative Stress in Type 4 Cardio-Renal Syndrome and Renal Sarcopenia. Front. Physiol. 2018, 9, 1648. [Google Scholar] [CrossRef] [PubMed]
- Dounousi, E.; Papavasiliou, E.; Makedou, A.; Ioannou, K.; Katopodis, K.P.; Tselepis, A.; Siamopoulos, K.C.; Tsakiris, D. Oxidative stress is progressively enhanced with advancing stages of CKD. Am. J. Kidney Dis. 2006, 48, 752–760. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- DuPont, J.J.; Ramick, M.G.; Farquhar, W.B.; Townsend, R.R.; Edwards, D.G. NADPH oxidase-derived reactive oxygen species contribute to impaired cutaneous microvascular function in chronic kidney disease. Am. J. Physiol. Renal Physiol. 2014, 306, F1499–F1506. [Google Scholar] [CrossRef] [Green Version]
- Himmelfarb, J.; McMonagle, E. Manifestations of oxidant stress in uremia. Blood Purif. 2001, 19, 200–205. [Google Scholar] [CrossRef]
- Feng, W.; Zhang, K.; Liu, Y.; Chen, J.; Cai, Q.; He, W.; Zhang, Y.; Wang, M.-H.; Wang, J.; Huang, H. Advanced oxidation protein products aggravate cardiac remodeling via cardiomyocyte apoptosis in chronic kidney disease. Am. J. Physiol. Heart Circ. Physiol. 2018, 314, H475–H483. [Google Scholar] [CrossRef]
- Gupta, J.; Dominic, E.A.; Fink, J.C.; Ojo, A.O.; Barrows, I.R.; Reilly, M.P.; Townsend, R.R.; Joffe, M.M.; Rosas, S.E.; Wolman, M.; et al. Association between Inflammation and Cardiac Geometry in Chronic Kidney Disease: Findings from the CRIC Study. PLoS ONE 2015, 10, e0124772. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Freise, C.; Schaefer, B.; Bartosova, M.; Bayazit, A.; Bauer, U.; Pickardt, T.; Berger, F.; Rasmussen, L.M.; Jensen, P.S.; Laube, G.; et al. Arterial tissue transcriptional profiles associate with tissue remodeling and cardiovascular phenotype in children with end-stage kidney disease. Sci. Rep. 2019, 9, 10316. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hassan, M.O.; Duarte, R.; Dix-Peek, T.; Vachiat, A.; Naidoo, S.; Dickens, C.; Grinter, S.; Manga, P.; Naicker, S. Correlation between volume overload, chronic inflammation, and left ventricular dysfunction in chronic kidney disease patients. Clin. Nephrol. 2016, 86, 131–135. [Google Scholar] [CrossRef] [PubMed]
- Dozio, E.; Ambrogi, F.; de Cal, M.; Vianello, E.; Ronco, C.; Corsi Romanelli, M.M. Role of the Soluble Receptor for Advanced Glycation End Products (sRAGE) as a Prognostic Factor for Mortality in Hemodialysis and Peritoneal Dialysis Patients. Mediat. Inflamm. 2018, 2018, 1347432. [Google Scholar] [CrossRef] [Green Version]
- Dozio, E.; Corradi, V.; Vianello, E.; Scalzotto, E.; de Cal, M.; Corsi Romanelli, M.M.; Ronco, C. Increased Levels of sRAGE in Diabetic CKD-G5D Patients: A Potential Protective Mechanism against AGE-Related Upregulation of Fibroblast Growth Factor 23 and Inflammation. Mediat. Inflamm. 2017, 2017, 9845175. [Google Scholar] [CrossRef] [Green Version]
- Leifheit-Nestler, M.; Kirchhoff, F.; Nespor, J.; Richter, B.; Soetje, B.; Klintschar, M.; Heineke, J.; Haffner, D. Fibroblast growth factor 23 is induced by an activated renin-angiotensin-aldosterone system in cardiac myocytes and promotes the pro-fibrotic crosstalk between cardiac myocytes and fibroblasts. Nephrol. Dial. Transplant. 2018, 33, 1722–1734. [Google Scholar] [CrossRef]
- Böckmann, I.; Lischka, J.; Richter, B.; Deppe, J.; Rahn, A.; Fischer, D.-C.; Heineke, J.; Haffner, D.; Leifheit-Nestler, M. FGF23-Mediated Activation of Local RAAS Promotes Cardiac Hypertrophy and Fibrosis. Int. J. Mol. Sci. 2019, 20, 4634. [Google Scholar] [CrossRef] [Green Version]
- Corda, S.; Mebazaa, A.; Gandolfini, M.P.; Fitting, C.; Marotte, F.; Peynet, J.; Charlemagne, D.; Cavaillon, J.M.; Payen, D.; Rappaport, L.; et al. Trophic effect of human pericardial fluid on adult cardiac myocytes. Differential role of fibroblast growth factor-2 and factors related to ventricular hypertrophy. Circ. Res. 1997, 81, 679–687. [Google Scholar] [CrossRef]
- Scheinowitz, M.; Kotlyar, A.; Zimand, S.; Ohad, D.; Leibovitz, I.; Bloom, N.; Goldberg, I.; Nass, D.; Engelberg, S.; Savion, N.; et al. Basic fibroblast growth factor induces myocardial hypertrophy following acute infarction in rats. Exp. Physiol. 1998, 83, 585–593. [Google Scholar] [CrossRef] [Green Version]
- Liu, Q.; Zhu, L.-J.; Waaga-Gasser, A.M.; Ding, Y.; Cao, M.; Jadhav, S.J.; Kirollos, S.; Shekar, P.S.; Padera, R.F.; Chang, Y.-C.; et al. The axis of local cardiac endogenous Klotho-TGF-β1-Wnt signaling mediates cardiac fibrosis in human. J. Mol. Cell. Cardiol. 2019, 136, 113–124. [Google Scholar] [CrossRef] [Green Version]
- Isakova, T.; Wahl, P.; Vargas, G.S.; Gutiérrez, O.M.; Scialla, J.; Xie, H.; Appleby, D.; Nessel, L.; Bellovich, K.; Chen, J.; et al. Fibroblast growth factor 23 is elevated before parathyroid hormone and phosphate in chronic kidney disease. Kidney Int. 2011, 79, 1370–1378. [Google Scholar] [CrossRef] [Green Version]
- Xue, C.; Yang, B.; Zhou, C.; Dai, B.; Liu, Y.; Mao, Z.; Yu, S.; Mei, C. Fibroblast Growth Factor 23 Predicts All-Cause Mortality in a Dose-Response Fashion in Pre-Dialysis Patients with Chronic Kidney Disease. Am. J. Nephrol. 2017, 45, 149–159. [Google Scholar] [CrossRef]
- Mirza, M.A.I.; Larsson, A.; Melhus, H.; Lind, L.; Larsson, T.E. Serum intact FGF23 associate with left ventricular mass, hypertrophy and geometry in an elderly population. Atherosclerosis 2009, 207, 546–551. [Google Scholar] [CrossRef]
- Nakano, C.; Hamano, T.; Fujii, N.; Obi, Y.; Matsui, I.; Tomida, K.; Mikami, S.; Inoue, K.; Shimomura, A.; Nagasawa, Y.; et al. Intact fibroblast growth factor 23 levels predict incident cardiovascular event before but not after the start of dialysis. Bone 2012, 50, 1266–1274. [Google Scholar] [CrossRef] [PubMed]
- Faul, C.; Amaral, A.P.; Oskouei, B.; Hu, M.-C.; Sloan, A.; Isakova, T.; Gutiérrez, O.M.; Aguillon-Prada, R.; Lincoln, J.; Hare, J.M.; et al. FGF23 induces left ventricular hypertrophy. J. Clin. Investig. 2011, 121, 4393–4408. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jovanovich, A.; Ix, J.H.; Gottdiener, J.; McFann, K.; Katz, R.; Kestenbaum, B.; de Boer, I.H.; Sarnak, M.; Shlipak, M.G.; Mukamal, K.J.; et al. Fibroblast growth factor 23, left ventricular mass, and left ventricular hypertrophy in community-dwelling older adults. Atherosclerosis 2013, 231, 114–119. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Leifheit-Nestler, M.; Große Siemer, R.; Flasbart, K.; Richter, B.; Kirchhoff, F.; Ziegler, W.H.; Klintschar, M.; Becker, J.U.; Erbersdobler, A.; Aufricht, C.; et al. Induction of cardiac FGF23/FGFR4 expression is associated with left ventricular hypertrophy in patients with chronic kidney disease. Nephrol. Dial. Transplant. 2016, 31, 1088–1099. [Google Scholar] [CrossRef] [Green Version]
- Mitsnefes, M.M.; Betoko, A.; Schneider, M.F.; Salusky, I.B.; Wolf, M.S.; Jüppner, H.; Warady, B.A.; Furth, S.L.; Portale, A.A. FGF23 and Left Ventricular Hypertrophy in Children with CKD. Clin. J. Am. Soc. Nephrol. 2018, 13, 45–52. [Google Scholar] [CrossRef]
- Di Marco, G.S.; Reuter, S.; Kentrup, D.; Grabner, A.; Amaral, A.P.; Fobker, M.; Stypmann, J.; Pavenstädt, H.; Wolf, M.; Faul, C.; et al. Treatment of established left ventricular hypertrophy with fibroblast growth factor receptor blockade in an animal model of CKD. Nephrol. Dial. Transplant. 2014, 29, 2028–2035. [Google Scholar] [CrossRef] [Green Version]
- Leifheit-Nestler, M.; Haffner, D. Paracrine Effects of FGF23 on the Heart. Front. Endocrinol. 2018, 9, 278. [Google Scholar] [CrossRef] [Green Version]
- Andrukhova, O.; Slavic, S.; Smorodchenko, A.; Zeitz, U.; Shalhoub, V.; Lanske, B.; Pohl, E.E.; Erben, R.G. FGF23 regulates renal sodium handling and blood pressure. EMBO Mol. Med. 2014, 6, 744–759. [Google Scholar] [CrossRef]
- David, V.; Martin, A.; Isakova, T.; Spaulding, C.; Qi, L.; Ramirez, V.; Zumbrennen-Bullough, K.B.; Sun, C.C.; Lin, H.Y.; Babitt, J.L.; et al. Inflammation and functional iron deficiency regulate fibroblast growth factor 23 production. Kidney Int. 2016, 89, 135–146. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Santamaría, R.; Díaz-Tocados, J.M.; Pendón-Ruiz de Mier, M.V.; Robles, A.; Salmerón-Rodríguez, M.D.; Ruiz, E.; Vergara, N.; Aguilera-Tejero, E.; Raya, A.; Ortega, R.; et al. Increased Phosphaturia Accelerates The Decline in Renal Function: A Search for Mechanisms. Sci. Rep. 2018, 8, 13701. [Google Scholar] [CrossRef] [PubMed]
- Rodelo-Haad, C.; Santamaria, R.; Muñoz-Castañeda, J.R.; Pendón-Ruiz de Mier, M.V.; Martin-Malo, A.; Rodriguez, M. FGF23, Biomarker or Target? Toxins 2019, 11, 175. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zou, D.; Wu, W.; He, Y.; Ma, S.; Gao, J. The role of klotho in chronic kidney disease. BMC Nephrol. 2018, 19, 285. [Google Scholar] [CrossRef] [Green Version]
- Hu, M.C.; Shi, M.; Cho, H.J.; Adams-Huet, B.; Paek, J.; Hill, K.; Shelton, J.; Amaral, A.P.; Faul, C.; Taniguchi, M.; et al. Klotho and phosphate are modulators of pathologic uremic cardiac remodeling. J. Am. Soc. Nephrol. 2015, 26, 1290–1302. [Google Scholar] [CrossRef] [Green Version]
- Tranæus Lindblad, Y.; Olauson, H.; Vavilis, G.; Hammar, U.; Herthelius, M.; Axelsson, J.; Bárány, P. The FGF23-Klotho axis and cardiac tissue Doppler imaging in pediatric chronic kidney disease—A prospective cohort study. Pediatr. Nephrol. 2018, 33, 147–157. [Google Scholar] [CrossRef] [Green Version]
- Grabner, A.; Schramm, K.; Silswal, N.; Hendrix, M.; Yanucil, C.; Czaya, B.; Singh, S.; Wolf, M.; Hermann, S.; Stypmann, J.; et al. FGF23/FGFR4-mediated left ventricular hypertrophy is reversible. Sci. Rep. 2017, 7, 1993. [Google Scholar] [CrossRef]
- Taguchi, K.; Elias, B.C.; Brooks, C.R.; Ueda, S.; Fukami, K. Uremic Toxin-Targeting as a Therapeutic Strategy for Preventing Cardiorenal Syndrome. Circ. J. 2019, 84, 2–8. [Google Scholar] [CrossRef] [Green Version]
- Yokoro, M.; Nakayama, Y.; Yamagishi, S.-I.; Ando, R.; Sugiyama, M.; Ito, S.; Yano, J.; Taguchi, K.; Kaida, Y.; Saigusa, D.; et al. Asymmetric Dimethylarginine Contributes to the Impaired Response to Erythropoietin in CKD-Anemia. J. Am. Soc. Nephrol. 2017, 28, 2670–2680. [Google Scholar] [CrossRef]
- Tang, W.H.W.; Wang, Z.; Shrestha, K.; Borowski, A.G.; Wu, Y.; Troughton, R.W.; Klein, A.L.; Hazen, S.L. Intestinal microbiota-dependent phosphatidylcholine metabolites, diastolic dysfunction, and adverse clinical outcomes in chronic systolic heart failure. J. Card. Fail. 2015, 21, 91–96. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lekawanvijit, S. Cardiotoxicity of Uremic Toxins: A Driver of Cardiorenal Syndrome. Toxins 2018, 10, 352. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- For the European Uremic Toxin Work Group (EUTox); Vanholder, R.; De Smet, R.; Glorieux, G.; Argilés, A.; Baurmeister, U.; Brunet, P.; Clark, W.; Cohen, G.; De Deyn, P.P.; et al. Review on uremic toxins: Classification, concentration, and interindividual variability. Kidney Int. 2003, 63, 1934–1943. [Google Scholar] [CrossRef] [Green Version]
- Kim, H.Y.; Yoo, T.-H.; Hwang, Y.; Lee, G.H.; Kim, B.; Jang, J.; Yu, H.T.; Kim, M.C.; Cho, J.-Y.; Lee, C.J.; et al. Indoxyl sulfate (IS)-mediated immune dysfunction provokes endothelial damage in patients with end-stage renal disease (ESRD). Sci. Rep. 2017, 7, 3057. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lin, C.-Y.; Hsu, Y.-J.; Hsu, S.-C.; Chen, Y.; Lee, H.-S.; Lin, S.-H.; Huang, S.-M.; Tsai, C.-S.; Shih, C.-C. CB1 cannabinoid receptor antagonist attenuates left ventricular hypertrophy and Akt-mediated cardiac fibrosis in experimental uremia. J. Mol. Cell. Cardiol. 2015, 85, 249–261. [Google Scholar] [CrossRef] [PubMed]
- Xie, J.; Yoon, J.; An, S.-W.; Kuro-o, M.; Huang, C.-L. Soluble Klotho Protects against Uremic Cardiomyopathy Independently of Fibroblast Growth Factor 23 and Phosphate. J. Am. Soc. Nephrol. 2015, 26, 1150–1160. [Google Scholar] [CrossRef]
- Panizo, S.; Barrio-Vázquez, S.; Naves-Díaz, M.; Carrillo-López, N.; Rodríguez, I.; Fernández-Vázquez, A.; Valdivielso, J.M.; Thadhani, R.; Cannata-Andía, J.B. Vitamin D receptor activation, left ventricular hypertrophy and myocardial fibrosis. Nephrol. Dial. Transplant. 2013, 28, 2735–2744. [Google Scholar] [CrossRef] [Green Version]
- Gluba-Brzózka, A.; Franczyk, B.; Ciałkowska-Rysz, A.; Olszewski, R.; Rysz, J. Impact of Vitamin D on the Cardiovascular System in Advanced Chronic Kidney Disease (CKD) and Dialysis Patients. Nutrients 2018, 10, 709. [Google Scholar] [CrossRef] [Green Version]
- Ogino, A.; Takemura, G.; Kawasaki, M.; Tsujimoto, A.; Kanamori, H.; Li, L.; Goto, K.; Maruyama, R.; Kawamura, I.; Takeyama, T.; et al. Erythropoietin receptor signaling mitigates renal dysfunction-associated heart failure by mechanisms unrelated to relief of anemia. J. Am. Coll. Cardiol. 2010, 56, 1949–1958. [Google Scholar] [CrossRef] [Green Version]
- Gut, N.; Piecha, G.; Aldebssi, F.; Schaefer, S.; Bekeredjian, R.; Schirmacher, P.; Ritz, E.; Gross-Weissmann, M.-L. Erythropoietin combined with ACE inhibitor prevents heart remodeling in 5/6 nephrectomized rats independently of blood pressure and kidney function. Am. J. Nephrol. 2013, 38, 124–135. [Google Scholar] [CrossRef]
- Kennedy, D.J.; Malhotra, D.; Shapiro, J.I. Molecular insights into uremic cardiomyopathy: Cardiotonic steroids and Na/K ATPase signaling. Cell. Mol. Biol. 2006, 52, 3–14. [Google Scholar]
- Liu, J.; Tian, J.; Chaudhry, M.; Maxwell, K.; Yan, Y.; Wang, X.; Shah, P.T.; Khawaja, A.A.; Martin, R.; Robinette, T.J.; et al. Attenuation of Na/K-ATPase Mediated Oxidant Amplification with pNaKtide Ameliorates Experimental Uremic Cardiomyopathy. Sci. Rep. 2016, 6, 34592. [Google Scholar] [CrossRef]
- Drummond, C.A.; Hill, M.C.; Shi, H.; Fan, X.; Xie, J.X.; Haller, S.T.; Kennedy, D.J.; Liu, J.; Garrett, M.R.; Xie, Z.; et al. Na/K-ATPase signaling regulates collagen synthesis through microRNA-29b-3p in cardiac fibroblasts. Physiol. Genom. 2016, 48, 220–229. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chuppa, S.; Liang, M.; Liu, P.; Liu, Y.; Casati, M.C.; Cowley, A.W.; Patullo, L.; Kriegel, A.J. MicroRNA-21 regulates peroxisome proliferator-activated receptor alpha, a molecular mechanism of cardiac pathology in Cardiorenal Syndrome Type 4. Kidney Int. 2018, 93, 375–389. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yan, L.; Mathew, L.; Chellan, B.; Gardner, B.; Earley, J.; Puri, T.S.; Hofmann Bowman, M.A. S100/Calgranulin-mediated inflammation accelerates left ventricular hypertrophy and aortic valve sclerosis in chronic kidney disease in a receptor for advanced glycation end products-dependent manner. Arterioscler. Thromb. Vasc. Biol. 2014, 34, 1399–1411. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yan, L.; Bowman, M.A.H. Chronic sustained inflammation links to left ventricular hypertrophy and aortic valve sclerosis: A new link between S100/RAGE and FGF23. Inflamm. Cell Signal. 2014, 1, e279. [Google Scholar]
- Li, Y.; Takemura, G.; Okada, H.; Miyata, S.; Maruyama, R.; Esaki, M.; Kanamori, H.; Li, L.; Ogino, A.; Ohno, T.; et al. Molecular signaling mediated by angiotensin II type 1A receptor blockade leading to attenuation of renal dysfunction-associated heart failure. J. Card. Fail. 2007, 13, 155–162. [Google Scholar] [CrossRef]
- Winterberg, P.D.; Jiang, R.; Maxwell, J.T.; Wang, B.; Wagner, M.B. Myocardial dysfunction occurs prior to changes in ventricular geometry in mice with chronic kidney disease (CKD). Physiol. Rep. 2016, 4, e12732. [Google Scholar] [CrossRef] [Green Version]
- Wang, X.; Chaudhry, M.A.; Nie, Y.; Xie, Z.; Shapiro, J.I.; Liu, J. A Mouse 5/6th Nephrectomy Model That Induces Experimental Uremic Cardiomyopathy. J. Vis. Exp. 2017, e55825. [Google Scholar] [CrossRef]
- Baumann, M.; Leineweber, K.; Tewiele, M.; Wu, K.; Türk, T.R.; Su, S.; Gössl, M.; Buck, T.; Wilde, B.; Heemann, U.; et al. Imatinib ameliorates fibrosis in uraemic cardiac disease in BALB/c without improving cardiac function. Nephrol. Dial. Transplant. 2010, 25, 1817–1824. [Google Scholar] [CrossRef] [Green Version]
- Kennedy, D.J.; Elkareh, J.; Shidyak, A.; Shapiro, A.P.; Smaili, S.; Mutgi, K.; Gupta, S.; Tian, J.; Morgan, E.; Khouri, S.; et al. Partial nephrectomy as a model for uremic cardiomyopathy in the mouse. Am. J. Physiol. Renal Physiol. 2008, 294, F450–F454. [Google Scholar] [CrossRef] [PubMed]
- Ham, O.; Jin, W.; Lei, L.; Huang, H.H.; Tsuji, K.; Huang, M.; Roh, J.; Rosenzweig, A.; Lu, H.A.J. Pathological cardiac remodeling occurs early in CKD mice from unilateral urinary obstruction, and is attenuated by Enalapril. Sci. Rep. 2018, 8, 16087. [Google Scholar] [CrossRef] [PubMed]
- Shi, M.; McMillan, K.L.; Wu, J.; Gillings, N.; Flores, B.; Moe, O.W.; Hu, M.C. Cisplatin nephrotoxicity as a model of chronic kidney disease. Lab. Investig. 2018, 98, 1105–1121. [Google Scholar] [CrossRef] [PubMed]
- Neuburg, S.; Dussold, C.; Gerber, C.; Wang, X.; Francis, C.; Qi, L.; David, V.; Wolf, M.; Martin, A. Genetic background influences cardiac phenotype in murine chronic kidney disease. Nephrol. Dial. Transplant. 2018, 33, 1129–1137. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rizzi, E.; Guimaraes, D.A.; Ceron, C.S.; Prado, C.M.; Pinheiro, L.C.; Martins-Oliveira, A.; Gerlach, R.F.; Tanus-Santos, J.E. β1-Adrenergic blockers exert antioxidant effects, reduce matrix metalloproteinase activity, and improve renovascular hypertension-induced cardiac hypertrophy. Free Radic. Biol. Med. 2014, 73, 308–317. [Google Scholar] [CrossRef] [Green Version]
- Fuellen, G.; Nacken, W.; Sorg, C.; Kerkhoff, C. Computational Searches for Missing Orthologs: The Case of S100A12 in Mice. OMICS A J. Integr. Biol. 2004, 8, 334–340. [Google Scholar] [CrossRef]
- Kieswich, J.E.; Chen, J.; Alliouachene, S.; Caton, P.W.; McCafferty, K.; Thiemermann, C.; Yaqoob, M.M. A novel model of reno-cardiac syndrome in the C57BL/ 6 mouse strain. BMC Nephrol. 2018, 19, 346. [Google Scholar] [CrossRef]
- Schneider, M.P.; Scheppach, J.B.; Raff, U.; Toncar, S.; Ritter, C.; Klink, T.; Störk, S.; Wanner, C.; Schlieper, G.; Saritas, T.; et al. Left Ventricular Structure in Patients With Mild-to-Moderate CKD-a Magnetic Resonance Imaging Study. Kidney Int. Rep. 2019, 4, 267–274. [Google Scholar] [CrossRef] [Green Version]
- Thadhani, R.; Appelbaum, E.; Pritchett, Y.; Chang, Y.; Wenger, J.; Tamez, H.; Bhan, I.; Agarwal, R.; Zoccali, C.; Wanner, C.; et al. Vitamin D therapy and cardiac structure and function in patients with chronic kidney disease: The PRIMO randomized controlled trial. JAMA 2012, 307, 674–684. [Google Scholar] [CrossRef] [Green Version]
- J-DAVID Investigators; Shoji, T.; Inaba, M.; Fukagawa, M.; Ando, R.; Emoto, M.; Fujii, H.; Fujimori, A.; Fukui, M.; Hase, H.; et al. Effect of Oral Alfacalcidol on Clinical Outcomes in Patients Without Secondary Hyperparathyroidism Receiving Maintenance Hemodialysis: The J-DAVID Randomized Clinical Trial. JAMA 2018, 320, 2325–2334. [Google Scholar]
- Vera, M.; Torramade-Moix, S.; Martin-Rodriguez, S.; Cases, A.; Cruzado, J.M.; Rivera, J.; Escolar, G.; Palomo, M.; Diaz-Ricart, M. Antioxidant and Anti-Inflammatory Strategies Based on the Potentiation of Glutathione Peroxidase Activity Prevent Endothelial Dysfunction in Chronic Kidney Disease. Cell. Physiol. Biochem. 2018, 51, 1287–1300. [Google Scholar] [CrossRef] [PubMed]
- Haller, S.T.; Yan, Y.; Drummond, C.A.; Xie, J.; Tian, J.; Kennedy, D.J.; Shilova, V.Y.; Xie, Z.; Liu, J.; Cooper, C.J.; et al. Rapamycin Attenuates Cardiac Fibrosis in Experimental Uremic Cardiomyopathy by Reducing Marinobufagenin Levels and Inhibiting Downstream Pro-Fibrotic Signaling. J. Am. Heart Assoc. 2016, 5, e004106. [Google Scholar] [CrossRef] [Green Version]
- Zapolski, T.; Furmaga, J.; Wysokiński, A.P.; Wysocka, A.; Rudzki, S.; Jaroszyński, A. The atrial uremic cardiomyopathy regression in patients after kidney transplantation—The prospective echocardiographic study. BMC Nephrol. 2019, 20, 152. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Nazzal, L.; Roberts, J.; Singh, P.; Jhawar, S.; Matalon, A.; Gao, Z.; Holzman, R.; Liebes, L.; Blaser, M.J.; Lowenstein, J. Microbiome perturbation by oral vancomycin reduces plasma concentration of two gut-derived uremic solutes, indoxyl sulfate and p-cresyl sulfate, in end-stage renal disease. Nephrol. Dial. Transplant. 2017, 32, 1809–1817. [Google Scholar] [CrossRef] [PubMed]
- Shalhoub, V.; Shatzen, E.M.; Ward, S.C.; Davis, J.; Stevens, J.; Bi, V.; Renshaw, L.; Hawkins, N.; Wang, W.; Chen, C.; et al. FGF23 neutralization improves chronic kidney disease-associated hyperparathyroidism yet increases mortality. J. Clin. Investig. 2012, 122, 2543–2553. [Google Scholar] [CrossRef] [Green Version]
- Hu, M.C.; Shi, M.; Gillings, N.; Flores, B.; Takahashi, M.; Kuro, O.M.; Moe, O.W. Recombinant α-Klotho may be prophylactic and therapeutic for acute to chronic kidney disease progression and uremic cardiomyopathy. Kidney Int. 2017, 91, 1104–1114. [Google Scholar] [CrossRef] [Green Version]
- Coyne, D.W.; Goldsmith, D.; Macdougall, I.C. New options for the anemia of chronic kidney disease. Kidney Int. Suppl. 2017, 7, 157–163. [Google Scholar] [CrossRef] [Green Version]
- Calò, L.A.; Vertolli, U.; Pagnin, E.; Ravarotto, V.; Davis, P.A.; Lupia, M.; Naso, E.; Maiolino, G.; Naso, A. Increased rho kinase activity in mononuclear cells of dialysis and stage 3–4 chronic kidney disease patients with left ventricular hypertrophy: Cardiovascular risk implications. Life Sci. 2016, 148, 80–85. [Google Scholar] [CrossRef]
| Factor | References | |
|---|---|---|
| Phosphate Homeostasis | ||
| FGF23/Klotho | [46,47,50,52,53,54,55,56,57,58,59,60,67,68,76] | |
| Vit D receptor agonists | [77,78] | |
| Uremic Toxins | ||
| p-Cresylsulfate | [72] | |
| Indoxylsulfate | [72,75] | |
| ADMA | [69,70] | |
| Growth Factors | ||
| TGF-β | [50] | |
| FGF2 | [48,49] | |
| EPO | [64,79,80] | |
| Metabolic Stress | ||
| AGE | [44,45,69] | |
| ROS | [36,38,40,81,82,83] | |
| PPARα | [84] | |
| TMAO | [69,71] | |
| Inflammation | ||
| S100/calgranulin | [85,86] | |
| Interleukin 6 | [43] | |
| Interleukin 10 | [42] | |
| CRP | [41] | |
| TNF | [42] |
| . | Kidney | Heart (Functional) | Heart (Structural) | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Model | Mouse Strain | Duration (Weeks) | GFR | sCr | BUN | EF/FS | SV/CO | BP | LVH | Fibrosis | Capillary Loss | References |
| surgically induced | ||||||||||||
| 5/6 Nx (2-step) | C57BL/6 | 8 | n.d. | ↑ | ↑ | - | ↓ | n.d. | ↑ | ↑ | n.d. | [75] |
| 5/6 Nx (2-step) | C57BL/6 | 12 | n.d. | ↑ | ↑ | ↓ | n.d. | n.d. | ↑ | ↑ | ↑ | [87] |
| AT1 knockout, 5/6 Nx (2-step) | C57BL/7 | 12 | n.d. | ↑ | ↑ | ↓ | n.d. | n.d. | ↑ | ↑ | - | [87] |
| 5/6 Nx (2-step) | C57BL/6 | 12 | n.d. | ↑ | ↑ | ↓ | n.d. | - | ↑ | ↑ | ↑ | [79] |
| 5/6 Nx (2-step) | 129X1/SvJ | 16 | n.d. | n.d. | ↑ | - | n.d. | ↑ | ↑ | ↑ | n.d. | [88] |
| 5/6 Nx (2-step, pole ligation) | C57BL/6 | 4 | n.d. | ↑ | ↑ | n.d. | n.d. | n.d. | ↑ | ↑ | n.d. | [89] |
| 5/6Nx (1-step) | BALB/c | 8, 16 and 24 | n.d. | n.d. | ↑ (8, 16, 24 wk) | ↓ (24 wk) | n.d. | ↑ (16, 24 wk) | n.d. | ↑ (24 wk) | n.d. | [90] |
| 5/6Nx (1-step) | CD1 | 4, 6 and 8 | n.d. | n.d. | n.d. | ↑ | n.d. | ↑ | ↑ | ↑ | n.d. | [91] |
| UUO | C57BL/6 | 3 | n.d. | ↑ | ↑ | - | n.d. | ↑ | ↑ | ↑ | - | [92] |
| hBAC-S100, UO | C57BL/6 | 10 | n.d. | n.d. | ↑ | - | n.d. | ↑ | ↑ | n.d. | n.d. | [85] |
| 129Sv | 10 | ↓ | ↑ | ↑ | ↓ | ↓ | ↑ | - | ↑ | n.d. | ||
| chemically induced | ||||||||||||
| 0.15% adenine | C57BL/6 | 20 | ↓ | ↑ | ↑ | ↓ | n.d. | n.d. | ↑ | ↑ | n.d. | [86] |
| 10 mg/kg cisplatin + high phosphate diet | 129Sv | 20 | ↓ | ↑ | ↑ | n.d. | n.d. | n.d. | ↑ | ↑ | n.d. | [93] |
| genetically induced | ||||||||||||
| Col4a3 knockout | C57BL/6 | 10 and 20 | ↓ | ↑ | ↑ | - | ↓ (only 20 wk) | ↑ (only 10 wk) | ↑ (only 20 wk) | ↑ (only 20 wk) | n.d. | [94] |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kaesler, N.; Babler, A.; Floege, J.; Kramann, R. Cardiac Remodeling in Chronic Kidney Disease. Toxins 2020, 12, 161. https://0-doi-org.brum.beds.ac.uk/10.3390/toxins12030161
Kaesler N, Babler A, Floege J, Kramann R. Cardiac Remodeling in Chronic Kidney Disease. Toxins. 2020; 12(3):161. https://0-doi-org.brum.beds.ac.uk/10.3390/toxins12030161
Chicago/Turabian StyleKaesler, Nadine, Anne Babler, Jürgen Floege, and Rafael Kramann. 2020. "Cardiac Remodeling in Chronic Kidney Disease" Toxins 12, no. 3: 161. https://0-doi-org.brum.beds.ac.uk/10.3390/toxins12030161