Occurrence and Probabilistic Risk Assessment of Fumonisin B1, Fumonisin B2 and Deoxynivalenol in Nixtamalized Maize in Mexico City
Abstract
:1. Introduction
2. Results
2.1. Occurrence of FB1, FB2 and DON in Nixtamalized Maize Samples
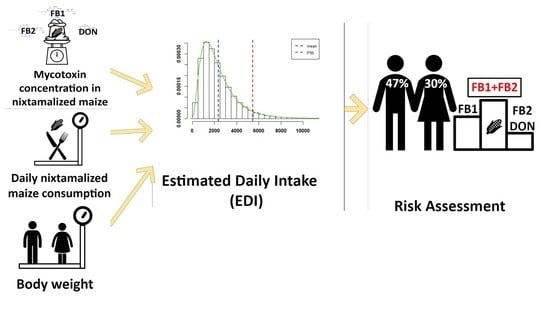
2.2. Probabilistic Exposure Assessment
2.2.1. Exposure Assessment Model
2.2.2. Fitting Distributions to Experimental Data
2.2.3. Modeling Uncertainty Parameters on Each Variable of the Exposure Model
2.2.4. Integrating Uncertainty and Variability in a Two-Dimensional Monte-Carlo Simulation Framework
2.2.5. Obtaining the EDI Values by Performing the Arithmetic Operation Defined for the Exposure Assessment Model
2.3. Risk Assessment
3. Discussion
4. Materials and Methods
4.1. Occurrence of FB1, FB2 and DON in the Nixtamalized Maize Samples
4.1.1. Chemicals and Reagents
4.1.2. Collection of Samples
4.1.3. Extraction
4.1.4. LC-MS/MS Analysis
4.2. Probabilistic Exposure Assessment Resulting from the Mycotoxin Levels in The Nixtamalized Maize
4.2.1. Exposure Assessment Model
4.2.2. Fitting a Distribution to the Experimental Data
Concentration Data
Nixtamalized Maize Consumption
Body Weight
4.2.3. Modeling Uncertainty Parameters on Each Variable of the Exposure Model
4.2.4. Integrating Uncertainty and Variability in a Two-Dimensional Monte-Carlo Simulation Framework
4.2.5. Obtaining the EDI Values by Performing the Arithmetic Operation Defined for the Exposure Assessment Model
4.3. Risk Assessment
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Munkvold, G.P.; Arias, S.; Taschl, I.; Gruber-Dorninger, C. Mycotoxins in corn: Occurrence, impacts, and management. In Corn. Chemistry and Technology, 3rd ed.; Serna-Saldivar, S.O., Ed.; Woodhead Publishing: Duxford, UK, 2019; pp. 235–287. [Google Scholar]
- JECFA. Fumonisins. In Evaluation of Certain Contaminants in Food: Eighty-Third Report of the Joint FAO/WHO Expert Committee on Food Additives; WHO Technical Report Series; FAO, WHO, Eds.; WHO: Geneva, Switzerland, 2017; Volume 1002, pp. 55–73. [Google Scholar]
- Gil-Serna, J.; Vázquez, C.; González-Jaén, M.; Patiño, B. Mycotoxins | Toxicology. In Encyclopedia of Food Microbiology, 2nd ed.; Batt, C.A.T.M.L., Ed.; Academic Press: London, UK, 2014; pp. 887–892. [Google Scholar]
- IARC. Fumonisin b1. In Iarc Monographs on the Evaluation of Carcinogenic Risks to Humans; IARC Press: Lyon, France, 2002; Volume 82, pp. 301–366. [Google Scholar]
- Voss, K.; Ryu, D.; Jackson, L.; Riley, R.; Gelineau-van Waes, J. Reduction of fumonisin toxicity by extrusion and nixtamalization (alkaline cooking). J. Agric. Food Chem. 2017, 65, 7088–7096. [Google Scholar] [CrossRef] [PubMed]
- JECFA. Fumonisins (addendum). In Safety Evaluation of Certain Contaminants in Food: Prepared by the Eighty-Third Meeting of the Joint FAO/WHO Expert Committee on Food Additives (JECFA). FAO JECFA Monographs 19 bis; WHO Food Additives Series; FAO, WHO, Eds.; WHO: Geneva, Switzerland, 2018; Volume 74, pp. 415–573. [Google Scholar]
- Pestka, J.J. Deoxynivalenol: Mechanisms of action, human exposure, and toxicological relevance. Arch. Toxic. 2010, 84, 663–679. [Google Scholar] [CrossRef] [PubMed]
- EFSA. Risks to human and animal health related to the presence of deoxynivalenol and its acetylated and modified forms in food and feed. EFSA J. 2017, 15, e4718. [Google Scholar]
- JECFA. Deoxynivalenol (addendum). In Safety Evaluation of Certain Contaminants in Food: Prepared by the Seventy-Second Meeting of the Joint FAO/WHO Expert Committee on Food Additives ( JECFA). FAO JECFA Monographs 8; WHO Food Additives Series; FAO, WHO, Eds.; WHO: Geneva, Switzerland, 2011; Volume 63, pp. 317–485. [Google Scholar]
- Abbas, H.; Mirocha, C.; Rosiles, R.; Carvajal, M. Decomposition of zearalenone and deoxynivalenol in the process of making tortillas from corn. Cereal Chem. 1988, 65, 15–19. [Google Scholar]
- Logrieco, A.F.; Miller, J.D.; Eskola, M.; Krska, R.; Ayalew, A.; Bandyopadhyay, R.; Battilani, P.; Bhatnagar, D.; Chulze, S.; De Saeger, S. The Mycotox Charter: Increasing awareness of, and concerted action for, minimizing mycotoxin exposure worldwide. Toxins 2018, 10, 149. [Google Scholar] [CrossRef] [Green Version]
- Wild, C.P.; Gong, Y.Y. Mycotoxins and human disease: A largely ignored global health issue. Carcinogenesis 2009, 31, 71–82. [Google Scholar] [CrossRef]
- Wall-Martínez, H.; Ramírez-Martínez, A.; Wesolek, N.; Brabet, C.; Durand, N.; Rodríguez-Jimenes, G.; García-Alvarado, M.; Salgado-Cervantes, M.; Robles-Olvera, V.; Roudot, A. Risk assessment of exposure to mycotoxins (aflatoxins and fumonisins) through corn tortilla intake in Veracruz City (Mexico). Food Addit. Contam. Part A 2019, 36, 929–939. [Google Scholar] [CrossRef]
- Gilbert-Sandoval, I.; Wesseling, S.; Rietjens, I.M. Aflatoxin b1 in nixtamalized maize in Mexico; occurrence and accompanying risk assessment. Toxicol. Rep. 2019, 6, 1135–1142. [Google Scholar] [CrossRef]
- Paulo, M.J.; van der Voet, H.; Jansen, M.J.; ter Braak, C.J.; van Klaveren, J.D. Risk assessment of dietary exposure to pesticides using a bayesian method. Pest Manag. Sci. 2005, 61, 759–766. [Google Scholar] [CrossRef]
- Wall-Martínez, H.; Ramírez-Martínez, A.; Wesolek, N.; Brabet, C.; Rodríguez-Jimenes, G.; García-Alvarado, M.; Salgado-Cervantes, M.; Robles-Olvera, V.; Roudot, A. Statistical analysis of corn consumption for improved mycotoxin exposure estimates for the population of Veracruz City, Mexico. Food Addit. Contam. Part A 2017, 34, 864–879. [Google Scholar] [CrossRef]
- Pitt, J.I.; Wild, C.P.; Baan, R.A.; Gelderblom, W.C.; Miller, J.; Riley, R.; Wu, F. Improving Public Health Through Mycotoxin Control; International Agency for Research on Cancer: Lyon, France, 2012; Volume No.158. [Google Scholar]
- Schaarschmidt, S.; Fauhl-Hassek, C. Mycotoxins during the Processes of Nixtamalization and Tortilla Production. Toxins 2019, 11, 227. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Palencia, E.; Torres, O.; Hagler, W.; Meredith, F.I.; Williams, L.D.; Riley, R.T. Total fumonisins are reduced in tortillas using the traditional nixtamalization method of Mayan communities. J. Nutr. 2003, 133, 3200–3203. [Google Scholar] [CrossRef]
- Shephard, G.S.; Leggott, N.; Stockenstrom, S.; Somdyala, N.; Marasas, W. Preparation of South African maize porridge: Effect on fumonisin mycotoxin levels. S. Afr. J. Sci. 2002, 98, 393–396. [Google Scholar]
- De Girolamo, A.; Pascale, M.; Visconti, A. Comparison of methods and optimisation of the analysis of fumonisins B(1) and B(2) in masa flour, an alkaline cooked corn product. Food Addit. Contam. Part A Chem. Anal. Control Expo. Risk Assess 2011, 28, 667–675. [Google Scholar] [CrossRef] [PubMed]
- Cortez-Rocha, M.; Gil-León, M.; Suárez-Jiménez, G.; Rosas-Burgos, E.; Sánchez-Mariñez, R.; Burgos-Hernández, A.; Lozano-Taylor, J.; Cinco-Moroyoqui, F. Occurrence of fumonisin B 1 and hydrolyzed fumonisin B 1 in Mexican nixtamalized cornmeal. Bull. Environ. Contam. Toxicol. 2005, 74, 73–77. [Google Scholar] [CrossRef]
- Dombrink-Kurtzman, M.A.; Dvorak, T.J.; Barron, M.E.; Rooney, L.W. Effect of nixtamalization (alkaline cooking) on fumonisin-contaminated corn for production of masa and tortillas. J. Agric. Chem. 2000, 48, 5781–5786. [Google Scholar] [CrossRef]
- Rivera, J.A.; Barquera, S.; Campirano, F.; Campos, I.; Safdie, M.; Tovar, V. Epidemiological and nutritional transition in mexico: Rapid increase of non-communicable chronic diseases and obesity. Public Health Nutr. 2002, 5, 113–122. [Google Scholar] [CrossRef] [PubMed]
- Allen, L.H. The mexican food system: Traditional and modern. Ecol. Food and Nutr. 1992, 27, 219–234. [Google Scholar] [CrossRef]
- INSP; FUNSALUD; NESTLÉ. Qué y cómo comemos los Mexicanos. Consumo de alimentos en la población urbana, 1st ed.; mayo de 2015; Arvizú-Martínez, O., Polo-Oteyza, E., Shamah-Levy, T., Eds.; Instituto Nacional de Salud Pública: Cuernavaca, Mexico, 2015.
- CONEVAL. Construcción de las líneas de bienestar. Documento metodológico. Metodología para la medición multidimensional de la pobreza, Primera edición ed.; CONEVAL, Ed.; CONEVAL: Mexico City, DF, Mexico, 2012.
- FAOSTAT. Faostat. Food Balance Sheet. Region: Europe (total). Element: Food Supply Quantity (kg/capita/yr). Year: 2017. Item: Maize and Products. 2020. Available online: http://www.fao.org/faostat/en/#data/FBS (accessed on 4 October 2020).
- Torres, O.A.; Palencia, E.; de Pratdesaba, L.L.; Grajeda, R.; Fuentes, M.; Speer, M.C.; Merrill Jr, A.H.; O’Donnell, K.; Bacon, C.W.; Glenn, A.E. Estimated fumonisin exposure in guatemala is greatest in consumers of lowland maize. J. Nutr. 2007, 137, 2723–2729. [Google Scholar] [CrossRef] [Green Version]
- Torres, O.; Matute, J.; Gelineau-van Waes, J.; Maddox, J.R.; Gregory, S.G.; Ashley-Koch, A.E.; Showker, J.L.; Zitomer, N.C.; Voss, K.A.; Riley, R.T. Urinary fumonisin B 1 and estimated fumonisin intake in women from high-and low-exposure communities in Guatemala. Mol. Nutr. Food Res. 2014, 58, 973–983. [Google Scholar] [CrossRef]
- Missmer, S.A.; Suarez, L.; Felkner, M.; Wang, E.; Merrill Jr, A.H.; Rothman, K.J.; Hendricks, K.A. Exposure to fumonisins and the occurrence of neural tube defects along the Texas–Mexico border. Environ. Health Perspect. 2006, 114, 237–241. [Google Scholar] [CrossRef] [PubMed]
- Wall-Martínez, H.A.; Pascari, X.; Ramos, A.J.; Marin, S.; Sanchis, V. Frequency and levels of mycotoxins in beer from the mexican market and exposure estimate for deoxynivalenol mycotoxins. Mycotoxin Res. 2019, 35, 207–216. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gonzalez-Osnaya, L.; Farres, A. Deoxynivalenol and zearalenone in Fusarium-contaminated wheat in Mexico City. Food Addit. Contam. 2011, 4, 71–78. [Google Scholar] [CrossRef]
- Lopez, P.; De Rijk, T.; Sprong, R.; Mengelers, M.; Castenmiller, J.; Alewijn, M. A mycotoxin-dedicated total diet study in The Netherlands in 2013: Part ii–occurrence. World Mycotoxin J. 2016, 9, 89–108. [Google Scholar] [CrossRef]
- Pouillot, R.; Delignette-Muller, M.; Kelly, D.; Denis, J. Mc2d: Tools for Two-Dimensional Monte-Carlo Simulations. Available online: https://cran.r-project.org/web/packages/mc2d/index.html (accessed on 4 October 2020).
- Cullen, A.C.; Frey, C.H. Probabilistic Techniques in Exposure Assessment: A Handbook for Dealing with Variability and Uncertainty in Models and Inputs; Springer Science & Business Media: New York, NJ, USA, 1999; p. XVI, 336. [Google Scholar]
- Delignette-Muller, M.L.; Dutang, C. Fitdistrplus: An R package for fitting distributions. J. Stat. Softw. 2015, 64, 1–34. [Google Scholar] [CrossRef] [Green Version]
- R:Core Team. R: A Language and Environment for Statistical Computing. R Foundation for Statistical Computing. Available online: http://www.R-project.org/ (accessed on 4 October 2020).
- INEGI. Instituto nacional de estadística y geografía. México en cifras, consulta nacional, estatal y municipal. Available online: https://inegi.org.mx/app/areasgeograficas/default.aspx (accessed on 4 October 2020).
- Serna-Saldivar, S.O. History of corn and wheat tortillas. In Tortillas: Wheat Flour and Corn Products; Rooney, L.W., Serna-Saldivar, S.O., Eds.; AACC International, Inc.: St. Paul, MN, USA, 2015; pp. 1–28. [Google Scholar]
- Hu, C.; Pozdnyakov, V.; Yun, J. Coga: Convolution of Gamma Distributions. Version: 1.1.0. Available online: https://cran.r-project.org/web/packages/coga/index.html (accessed on 4 October 2020).
- Pouillot, R.; Delignette-Muller, M.L. Evaluating variability and uncertainty separately in microbial quantitative risk assessment using two r packages. Int. J. Food Microbiol. 2010, 142, 330–340. [Google Scholar] [CrossRef]
- Efron, B.; Tibshirani, R.J. An Introduction to the Bootstrap; Chapman & Hall/CRC:: Boca Raton, FL, USA, 1993; p. 436. [Google Scholar]



| Dataset | Parameters | Bootstrap Parameters (Median (95CI a)) | ||
|---|---|---|---|---|
| Shape | Rate | Shape | Rate | |
| FB1 | 2.8 | 0.006 | 2.9 (2.1–4.0) | 0.006 (0.004–0.009) |
| FB2 | 4.4 | 0.019 | 4.6 (3.4–6.5) | 0.02 (0.01–0.03) |
| FB1 + FB2 | 3.9 | 0.006 | 4.0 (2.9–5.9) | 0.006 (0.004–0.008) |
| DON | 0.98 | 0.008 | 1.0 (0.6–1.5) | 0.009 (0.005–0.01) |
| Dataset | Parameters | Bootstrap Parameters (Median (95CI a)) | ||||||
|---|---|---|---|---|---|---|---|---|
| Men | Women | Men | Women | |||||
| Shape | Rate | Shape | Rate | Shape | Rate | Shape | Rate | |
| NMC | 4.8 | 0.02 | 4.6 | 0.030 | 4.8 (4.4–5.2) | 0.020 (0.019–0.022) | 4.7 (4.3–5.1) | 0.030 (0.028–0.033) |
| Weight | 49.9 | 0.68 | 47.7 | 0.72 | 49.9 (45.4–55.3) | 0.67 (0.62–0.75) | 47.8 (43.5–52.6) | 0.72 (0.66–0.80) |
| Mycotoxin Concentration | Parameter | Mean | sd | Median | 95% |
|---|---|---|---|---|---|
| FB1 (ng/g) | median | 489 | 287 | 432 | 1036 |
| mean | 489 | 289 | 432 | 1040 | |
| 2.5% | 419 | 229 | 370 | 867 | |
| 97.5% | 559 | 352 | 500 | 1221 | |
| FB2 (ng/g) | median | 224 | 106 | 208 | 421 |
| mean | 225 | 106 | 209 | 422 | |
| 2.5% | 199 | 85 | 183 | 365 | |
| 97.5% | 254 | 128 | 236 | 486 | |
| FB1 + FB2 (ng/g) | median | 714 | 354 | 655 | 1377 |
| mean | 714 | 354 | 656 | 1379 | |
| 2.5% | 628 | 280 | 574 | 1171 | |
| 97.5% | 806 | 433 | 744 | 1594 | |
| DON (ng/g) | median | 117 | 118 | 81 | 353 |
| mean | 118 | 119 | 81 | 354 | |
| 2.5% | 90 | 85 | 56 | 265 | |
| 97.5% | 149 | 159 | 108 | 464 |
| Data | Men | Women | |||||||
|---|---|---|---|---|---|---|---|---|---|
| Parameter | Mean | sd | Median | P95 | Mean | sd | Median | P95 | |
| NM consumption (g/day) | median | 237 | 108 | 220 | 438 | 154 | 71 | 143 | 286 |
| mean | 237 | 108 | 220 | 438 | 154 | 71 | 143 | 286 | |
| 2.5% | 230 | 102 | 213 | 422 | 149 | 68 | 138 | 275 | |
| 97.5% | 244 | 114 | 228 | 455 | 158 | 75 | 147 | 297 | |
| Body weight (kg) | median | 74 | 10 | 73 | 92 | 66 | 10 | 66 | 83 |
| mean | 74 | 10 | 73 | 92 | 66 | 10 | 66 | 83 | |
| 2.5% | 73 | 10 | 72 | 90 | 65 | 9 | 65 | 81 | |
| 97.5% | 75 | 11 | 74 | 93 | 67 | 10 | 66 | 84 | |
| EDI (µg/kg bw/day) | Men | Women | |||||||
|---|---|---|---|---|---|---|---|---|---|
| Parameter | Mean | sd | Median | P95 | Mean | sd | Median | P95 | |
| FB1 | median | 1.60 | 1.30 | 1.24 | 4.10 | 1.16 | 0.95 | 0.90 | 3.00 |
| mean | 1.60 | 1.31 | 1.25 | 4.12 | 1.16 | 0.95 | 0.90 | 3.00 | |
| 2.5% | 1.38 | 1.07 | 1.07 | 3.47 | 0.99 | 0.78 | 0.77 | 2.51 | |
| 97.5% | 1.83 | 1.55 | 1.45 | 4.76 | 1.33 | 1.14 | 1.05 | 3.51 | |
| FB2 | median | 0.74 | 0.52 | 0.60 | 1.74 | 0.53 | 0.38 | 0.44 | 1.27 |
| mean | 0.74 | 0.53 | 0.60 | 1.75 | 0.54 | 0.38 | 0.44 | 1.27 | |
| 2.5% | 0.65 | 0.45 | 0.53 | 1.53 | 0.47 | 0.33 | 0.38 | 1.11 | |
| 97.5% | 0.84 | 0.61 | 0.69 | 1.99 | 0.60 | 0.45 | 0.50 | 1.45 | |
| FB1 + FB2 | median | 2.34 | 1.72 | 1.90 | 5.64 | 1.70 | 1.26 | 1.37 | 4.11 |
| mean | 2.34 | 1.72 | 1.90 | 5.64 | 1.70 | 1.26 | 1.37 | 4.11 | |
| 2.5% | 2.05 | 1.44 | 1.65 | 4.86 | 1.49 | 1.06 | 1.20 | 3.53 | |
| 97.5% | 2.65 | 2.03 | 2.16 | 6.45 | 1.92 | 1.48 | 1.56 | 4.71 | |
| DON | median | 0.39 | 0.47 | 0.23 | 1.28 | 0.28 | 0.34 | 0.17 | 0.93 |
| mean | 0.39 | 0.47 | 0.23 | 1.28 | 0.28 | 0.34 | 0.17 | 0.93 | |
| 2.5% | 0.29 | 0.34 | 0.16 | 0.96 | 0.21 | 0.25 | 0.12 | 0.70 | |
| 97.5% | 0.49 | 0.62 | 0.31 | 1.63 | 0.36 | 0.46 | 0.22 | 1.20 | |
| Mycotoxin | % EDI exceeding the PMTDI 1 (Median [95 CI%] 2) | |
|---|---|---|
| Men | Women | |
| FB1 FB2 FB1 + FB2 DON | 26.9 [21.0–33.6] 3.0 [1.9–5.2] 47.0 [40.0–54.4] 8.6 [5.1–14.2] | 14.6 [10.5–20.1] 0.8 [0.4–1.7] 29.7 [24.5–35.3] 4.3 [2.2–8.2] |
| Analyte | Precursor Ion (m/z) | Declustering Potential (V) | Potential Ion (m/z) | Collision Energy (V) | Cell Exit Potential (V) |
|---|---|---|---|---|---|
| FB1 | 722.5 | 40 | 334.4/352.3 | 57/55 | 4/12 |
| FB2 | 706.4 | 40 | 336.3/318.5 | 53/51 | 8/2 |
| DON | 297.1 | 30 | 249.0/231.0 | 15/17 | 15/15 |
| Analyte | LOQ (ng/g) | LOD (ng/g) | Linearity (ng/g) | Recovery (%) ± RSDr 1 (n = 3) |
| FB1 | 60 | 20 | 100–800 | 118 ± 4.1 |
| FB2 | 60 | 20 | 100–800 | 110 ± 0.2 |
| DON | 120 | 40 | 200-1600 | 75 ± 3.8 |
| Group | Average (kg bw) | SD | Distribution | Shape 1 | Scale 1 |
|---|---|---|---|---|---|
| Men | 73.3 | 10.31 | gamma | 50.64 | 1.44 |
| Women | 65.8 | 9.6 | gamma | 46.97 | 1.40 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gilbert-Sandoval, I.; Wesseling, S.; Rietjens, I.M.C.M. Occurrence and Probabilistic Risk Assessment of Fumonisin B1, Fumonisin B2 and Deoxynivalenol in Nixtamalized Maize in Mexico City. Toxins 2020, 12, 644. https://0-doi-org.brum.beds.ac.uk/10.3390/toxins12100644
Gilbert-Sandoval I, Wesseling S, Rietjens IMCM. Occurrence and Probabilistic Risk Assessment of Fumonisin B1, Fumonisin B2 and Deoxynivalenol in Nixtamalized Maize in Mexico City. Toxins. 2020; 12(10):644. https://0-doi-org.brum.beds.ac.uk/10.3390/toxins12100644
Chicago/Turabian StyleGilbert-Sandoval, Ixchel, Sebastiaan Wesseling, and Ivonne M. C. M. Rietjens. 2020. "Occurrence and Probabilistic Risk Assessment of Fumonisin B1, Fumonisin B2 and Deoxynivalenol in Nixtamalized Maize in Mexico City" Toxins 12, no. 10: 644. https://0-doi-org.brum.beds.ac.uk/10.3390/toxins12100644