Mycotoxin Co-Occurrence in Milks and Exposure Estimation: A Pilot Study in São Paulo, Brazil
Abstract
:1. Introduction
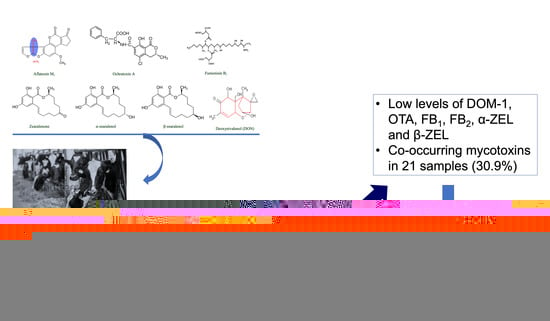
2. Results
3. Discussion
4. Conclusions
5. Materials and Methods
5.1. Sampling Design
5.2. Mycotoxin Analysis of Milk Samples
5.3. Estimation of Probable Daily Intake of Mycotoxins
5.4. Statistical Analysis
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Conflicts of Interest
References
- Buszewska-Forajta, M. Mycotoxins, invisible danger of feedstuff with toxic effect on animals. Toxicon 2020, 182, 34–53. [Google Scholar] [CrossRef] [PubMed]
- Coppa, C.F.S.C.; Khaneghah, A.M.; Alvito, P.; Assunção, R.; Martins, C.; Eş, I.; Gonçalves, B.L.; Neeff, D.V.; Sant’Ana, A.S.; Corassin, C.H.; et al. The occurrence of mycotoxins in breast milk, fruit products and cereal-based infant formula: A review. Trends Food Sci. Technol. 2019, 92, 81–93. [Google Scholar] [CrossRef]
- Ismail, A.; Gonçalves, B.L.; Neeff, D.V.; Ponzilacqua, B.; Coppa, C.F.S.C.; Hintzsche, H.; Sajid, M.; Cruz, A.G.; Corassin, C.H.; Oliveira, C.A.F. Aflatoxin in foodstuffs: Occurrence and recent advances in decontamination. Food Res. Int. 2018, 113, 74–85. [Google Scholar] [CrossRef] [PubMed]
- Yiannikouris, A.; Jouany, J.P. Mycotoxins in feeds and their fate in animals: A review. Animal Res. 2002, 51, 81–99. [Google Scholar] [CrossRef] [Green Version]
- Campagnollo, F.B.; Ganev, K.C.; Mousavi Khaneghah, A.; Portella, J.; Cruz, A.G.; Granato, D.; Corassin, C.H.; Oliveira, C.A.F.; Sant’Ana, A.S. The occurrence and effect of unit operations for dairy products processing on the fate of aflatoxin M1: A review. Food Control 2016, 68, 310–329. [Google Scholar] [CrossRef]
- Oliveira, C.A.F.; Corassin, C.H.; Corrêa, B.; Oswald, I.P. Animal Health: Mycotoxins. In Encyclopedia of Agriculture and Food Systems, 2nd ed.; Van Alfen, N.K., Ed.; Elsevier Ltd.: Oxford, UK, 2014; pp. 358–377. [Google Scholar]
- Gonçalves, B.L.; Gonçalves, J.L.; Rosim, R.E.; Cappato, L.P.; Cruz, A.G.; Oliveira, C.A.F. Effects of different sources of Saccharomyces cerevisiae biomass on milk production, composition, and aflatoxin M1 excretion in milk from dairy cows fed aflatoxin B1. J. Dairy Sci. 2017, 100, 5701–5708. [Google Scholar] [CrossRef] [Green Version]
- International Agency for Research on Cancer. IARC Monograph on the Evaluation of Carcinogenic Risk to Humans. IARC Monographs 2002, 82. Available online: http://monographs.iarc.fr/ENG/Monographs/vol82/mono82.pdf (accessed on 22 March 2021).
- International Agency for Research on Cancer. IARC Monographs on the Evaluation of Carcinogenic Risks to Humans. IARC Monographs 1993, 56. Available online: http://monographs.iarc.fr/ENG/Monographs/vol56/mono56.pdf (accessed on 22 March 2021).
- Boudra, H.; Morgavi, D.P. Development and validation of a HPLC method for the quantitation of ochratoxins in plasma and raw milk. J. Chromatogr. B 2006, 843, 295–301. [Google Scholar] [CrossRef]
- Wu, L.; Liao, P.; He, L.; Ren, W.; Yin, J.; Duan, J.; Li, T. Growth performance, serum biochemical profile, jejunal morphology, and the expression of nutrients transporter genes in deoxynivalenol (DON)-challenged growing pigs. BMC Vet. Res. 2015, 11, 144–154. [Google Scholar] [CrossRef] [Green Version]
- Warth, B.; Sulyok, M.; Berthiller, F.; Schuhmacher, R.; Krska, R. New insights into the human metabolism of the Fusarium mycotoxins deoxynivalenol and zearalenone. Toxicol. Lett. 2013, 220, 88–94. [Google Scholar] [CrossRef] [Green Version]
- Schoevers, E.J.; Santos, R.R.; Colenbrander, B.; Fink-Gremmels, J.; Roelen, B.A. Transgenerational toxicity of zearalenone in pigs. Reprod. Toxicol. 2012, 34, 110–119. [Google Scholar] [CrossRef]
- Franco, L.T.; Petta, T.; Rottinghaus, G.E.; Bordin, K.; Gomes, G.A.; Alvito, P.; Assunção, R.; Oliveira, C.A.F. Assessment of mycotoxin exposure and risk characterization using occurrence data in foods and urinary biomarkers in Brazil. Food Chem. Toxicol. 2019, 128, 21–34. [Google Scholar] [CrossRef] [PubMed]
- Jager, A.V.; Tonin, F.G.; Baptista, G.Z.; Souto, P.C.M.C.; Oliveira, C.A.F. Assessment of aflatoxin exposure using serum and urinary biomarkers in São Paulo, Brazil: A pilot study. Int. J. Hyg. Environ. Health 2016, 219, 294–300. [Google Scholar] [CrossRef]
- Agência Nacional de Vigilância Sanitária. Resolução RDC n 7, de 18 de Fevereiro de 2011. Available online: http://portal.anvisa.gov.br/wps/wcm/connect/bc17db804f45fe2cbd41fdd785749fbd/Resolu%C3%A7%C3%A3o+0-2011-GGALI.pdf?MOD=AJPERES (accessed on 9 February 2021).
- Iha, M.H.; Barbosa, C.B.; Okada, I.A.; Trucksess, M.W. Aflatoxin M1 in milk and distribution and stability of aflatoxin M1 during production and storage of yoghurt and cheese. Food Control 2013, 29, 1–6. [Google Scholar] [CrossRef] [Green Version]
- Silva, M.V.; Janeiro, V.; Bando, E.; Machinski Junior, M. Occurrence and estimative of aflatoxin M1 intake in UHT cow milk in Paraná State, Brazil. Food Control 2015, 53, 222–225. [Google Scholar] [CrossRef]
- Instituto Brasileiro de Geografia e Estatística. Análise do Consumo Alimentar Pessoal no Brasil. Rio de Janeiro, Brazil. 2011. Available online: https://biblioteca.ibge.gov.br/visualizacao/livros/liv50063.pdf (accessed on 15 December 2020).
- European Communities. Regulation no 1881 of 19 december 2006. Off. J. Eur. Commun. 2006, 364, 5–24. [Google Scholar]
- Jager, A.V.; Tedesco, M.P.; Souto, P.C.M.C.; Oliveira, C.A.F. Assessment of aflatoxin intake in São Paulo, Brazil. Food Control 2013, 33, 87–92. [Google Scholar] [CrossRef] [Green Version]
- Shundo, L.; Navas, S.A.; Lamardo, L.C.A.; Ruvieri, V.; Sabino, M. Estimate of aflatoxin M1 exposure in milk and occurrence in Brazil. Food Control 2009, 20, 655–657. [Google Scholar] [CrossRef]
- Huang, L.C.; Zheng, N.; Zheng, B.Q.; Wen, F.; Cheng, J.B.; Han, R.W.; Xu, X.M.; Li, S.L.; Wang, J.Q. Simultaneous determination of aflatoxin M1, ochratoxin A, zearalenone and α-zearalenol in milk by UHPLC–MS/MS. Food Chem. 2014, 146, 242–249. [Google Scholar] [CrossRef]
- Gazzotti, T.; Lugoboni, B.; Zironi, E.; Barbarossa, A.; Serraino, A.; Pagliuca, G. Determination of fumonisin B1 in bovine milk by LC-MS/MS. Food Control 2009, 20, 1171–1174. [Google Scholar] [CrossRef]
- Souza, M.D.L.M.; Sulyok, M.; Freitas-Silva, O.; Costa, S.S.; Brabet, C.; Machinski, M.; Sekiyama, B.L.; Vargas, E.A.; Krska, R.; Schuhmacher, R. Cooccurrence of mycotoxins in maize and poultry feeds from Brazil by liquid chromatography/tandem mass spectrometry. Sci. World J. 2013, 2013, 427369. [Google Scholar] [CrossRef]
- Franco, L.T.; Petta, T.; Rottinghaus, G.E.; Bordin, K.; Gomes, G.A.; Oliveira, C.A.F. Co-occurrence of mycotoxins in maize food and maize-based feed from small-scale farms in Brazil: A pilot study. Mycotoxin Res. 2019, 35, 65–73. [Google Scholar] [CrossRef] [PubMed]
- Vidnes, A.; Paulsen, B.; Bergsten, C. Report from SCOOP task 3.2.10 “collection of occurrence data of Fusarium toxins in food and assessment of dietary intake by the population of EU member states” 2003. Eur. Comm. 2003, 1, 239–257. [Google Scholar]
- European Food Safety Authority. Appropriateness to set a group health-based guidance value for zearalenone and its modified forms. EFSA J. 2016, 14, e04425. [Google Scholar]
- Food and Agriculture Organization. Evaluation of Certain Food Additives and Contaminants: Sixty-Eight Report of the Joint FAO/WHO Expert Committee on Food Additives. WHO Technical Report Series 2007, 947. Available online: https://apps.who.int/iris/handle/10665/43870 (accessed on 12 April 2021).
- Food and Agriculture Organization. Evaluation of Certain Food Additives and Contaminants: Seventy-Fourth Report of the Joint FAO/WHO Expert Committee on Food Additives. WHO Technical Report Series 2011a, 966. Available online: https://apps.who.int/iris/bitstream/handle/10665/44788/WHO_TRS_966_eng.pdf?sequence=1 (accessed on 12 April 2021).
- Food and Agriculture Organization. Evaluation of certain food additives and contaminants: Seventy-second report of the joint FAO/WHO Expert Committee on Food Additives. WHO Technical Report Series 2011b, 959. Available online: https://apps.who.int/iris/handle/10665/44514 (accessed on 12 April 2021).
- Coppa, C.F.S.C.; Cirelli, A.C.; Gonçalves, B.L.; Barnabe, E.M.B.; Petta, T.; Franco, L.T.; Javanmardi, F.; Mousavi-Khaneghah, A.; Lee, S.H.I.; Corassin, C.H.; et al. Mycotoxin occurrence in breast milk and exposure estimation of lactating mothers using urinary biomarkers in São Paulo, Brazil. Environ. Pollut. 2021, 279, 116938. [Google Scholar] [CrossRef] [PubMed]
- Flores-Flores, M.W.; González-Peñas, E. An LC-MS/MS method for multi-mycotoxin quantification in cow milk. Food Chem. 2017, 218, 378–385. [Google Scholar] [CrossRef]
- Instituto Brasileiro de Geografia e Estatística. Antropometria e Estado Nutricional de Crianças, Adolescentes e Adultos No Brasil. IBGE: Rio de Janeiro, Brazil. 2010. Available online: https://biblioteca.ibge.gov.br/visualizacao/livros/liv45419.pdf (accessed on 15 December 2020).
- SAS. User’s Guide: Statistics; SAS Institute Inc.: Cary, NC, USA, 2004. [Google Scholar]
| Type A Milk (n = 32) | Pasteurized Milk (n = 4) | UHT Milk (n = 32) | Total (n = 68) | |||||
|---|---|---|---|---|---|---|---|---|
| n (%) | Mean (Range) (ng/L) | n (%) | Mean (Range) (ng/L) | n (%) | Mean (Range) (ng/L) | n (%) | Mean (Range) (ng/L) | |
| AFM1 | 4 (12.5) | 19 (15–21) | 1 (25) | 32 | 1 (3.1) | 227 | 6 (8.8) | 93 (15–227) |
| DOM-1 | 3 (9.4) | 386 (313–459) | 0 | <LOQ | 3 (9.4) | 448 (327–682) | 6 (8.8) | 417 (313–682) |
| OTA | 6 (18.7) | 78 (43–201) | 1 (25) | 73 | 10 (31.2) | 68 (43–148) | 17 (25) | 72 (43–201) |
| FB1 | 4 (12.5) | 1609 (1465–1734) | 2 (50) | 1775 (1595–1955) | 4 (12.5) | 1536 (1393–1705) | 10 (14.7) | 1613 (1393–1955) |
| FB2 | 2 (6.3) | 481 (464–499) | 1 (25) | 1297 | 0 | <LOQ | 3 (4.4) | 753 (464–1297) |
| α-ZEL | 15 (46.9) | 608 (416–966) | 1 (25) | 679 | 23 (71.9) | 612 (334–936) | 39 (57.4) | 612 (334–966) |
| β-ZEL | 16 (50) | 1514 (445–3146) | 1 (25) | 2326 | 11 (34.4) | 0.982 (332–1949) | 28 (41.2) | 1334 (332–3146) |
| Types of Co-Occurring Mycotoxins | Number of Samples Positive for Mycotoxins (n = 68) a |
|---|---|
| AFM1, ZEL | 4 |
| OTA, ZEL | 5 |
| FB, OTA | 2 |
| DOM-1, ZEL | 2 |
| FB, ZEL | 1 |
| AFM1, DOM-1, ZEL | 1 |
| AFM1, FB, ZEL | 1 |
| FB, DOM-1, ZEL | 1 |
| FB, OTA, ZEL | 1 |
| AFM1, FB, DOM-1, ZEL | 1 |
| FB, OTA, DOM-1, ZEL | 2 |
| Total (%) | 21 (30.9) |
| Mycotoxins | Mean PDI (ng/kg Body Weight /Day) 1 | |||||||
|---|---|---|---|---|---|---|---|---|
| October/2019 | January/2020 | May/2020 | June/2020 | |||||
| Adults | Children | Adults | Children | Adults | Children | Adults | Children | |
| AFM1 | 0.043 b | 0.128 a | 0.010 b | 0.029 b | 0 | 0 | 0 | 0 |
| DOM-1 | 0.284 b | 0.852 a | 0.224 b | 0.673 a | 0.185 b | 0.555 a | 0 | 0 |
| OTA | 0.058 c | 0.173 a | 0.061 c | 0.184 a | 0.030 c | 0.090 b | 0.035 c | 0.104 b |
| FB1 | 0.974 b | 2.921 a | 0.953 b | 2.860 a | 0.876 b | 2.629 a | 0.915 b | 2.746 a |
| FB2 | 0.750 b | 2.250 a | 0.268 c | 0.805 b | 0 | 0 | 0.289 c | 0.866 b |
| α-ZEL | 0.468 c | 1.403 a | 0.340 c | 1.020 b | 0.317 c | 0.951 b | 0.319 c | 0.957 b |
| β-ZEL | 0.982 c | 2.947 a | 0.957 c | 2.871 a | 0.509 d | 1.526 b | 0.267 d | 0.800 c |
| Mycotoxin | RT (min.) | Mass (g/mol) | Molecularion | Transition (m/z) | Calibration Range (ng/L) | LOD (ng/L) | LOQ (ng/L) |
|---|---|---|---|---|---|---|---|
| AFM1 | 4.09 | 328.3 | [M+H]+ | 329.0 > 273.1 a | 31–500 | 5 | 15 |
| 329.0 > 229.0 b | |||||||
| AFB1 | 4.80 | 312.3 | [M+H]+ | 312.7 > 284.9 a | 31–500 | 40 | 120 |
| 312.7 > 241.1 b | |||||||
| AFB2 | 4.59 | 314.3 | [M+H]+ | 314.7 > 259.0 a | 31–500 | 40 | 120 |
| 314.7 > 287.0 b | |||||||
| AFG1 | 4.46 | 328.3 | [M+H]+ | 328.9 > 243.0 a | 63–1000 | 40 | 120 |
| 328.9 > 199.5 b | |||||||
| AFG2 | 4.18 | 330.3 | [M+H]+ | 330.9 > 245.0 a | 94–1500 | 60 | 180 |
| 330.9 > 188.9 b | |||||||
| DON | 2.04 | 296.3 | [M+H]+ | 297.3 > 249.1 a | 313–5000 | 333 | 1223 |
| 297.3 > 231.1 b | |||||||
| DOM-1 | 2.62 | 282.1 | [M+Ac]− | 339.2 > 249.1 a | 625–10,000 | 170 | 310 |
| 339.2 > 59.1 b | |||||||
| OTA | 5.70 | 403.1 | [M+H]+ | 404.0 > 238.9 a | 63–1000 | 5 | 17 |
| 404.0 > 357.9 b | |||||||
| FB1 | 5.34 | 721.8 | [M+H]+ | 722.5 > 334.0 a | 625–10,000 | 300 | 620 |
| 722.5 > 352.1 b | |||||||
| FB2 | 5.74 | 705.8 | [M+H]+ | 706.5 > 336.2 a | 625–10,000 | 100 | 330 |
| 706.5 > 318.3 b | |||||||
| ZEN | 6.01 | 318.1 | [M−H]− | 317.1 > 175.1 a | 313–5000 | 100 | 300 |
| 317.1 > 130.9 b | |||||||
| α-ZEL | 5.53 | 320.2 | [M–H]− | 319.1 > 275.2 a | 313–5000 | 73 | 283 |
| 319.1 > 160.2 b | |||||||
| β-ZEL | 5.76 | 320.2 | [M–H]− | 319.1 > 275.2 a | 313–5000 | 60 | 200 |
| 319.1 > 160.2 b |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Frey, M.; Rosim, R.; Oliveira, C. Mycotoxin Co-Occurrence in Milks and Exposure Estimation: A Pilot Study in São Paulo, Brazil. Toxins 2021, 13, 507. https://0-doi-org.brum.beds.ac.uk/10.3390/toxins13080507
Frey M, Rosim R, Oliveira C. Mycotoxin Co-Occurrence in Milks and Exposure Estimation: A Pilot Study in São Paulo, Brazil. Toxins. 2021; 13(8):507. https://0-doi-org.brum.beds.ac.uk/10.3390/toxins13080507
Chicago/Turabian StyleFrey, Matheus, Roice Rosim, and Carlos Oliveira. 2021. "Mycotoxin Co-Occurrence in Milks and Exposure Estimation: A Pilot Study in São Paulo, Brazil" Toxins 13, no. 8: 507. https://0-doi-org.brum.beds.ac.uk/10.3390/toxins13080507