Mycotoxin Zearalenone Attenuates Innate Immune Responses and Suppresses NLRP3 Inflammasome Activation in LPS-Activated Macrophages
Abstract
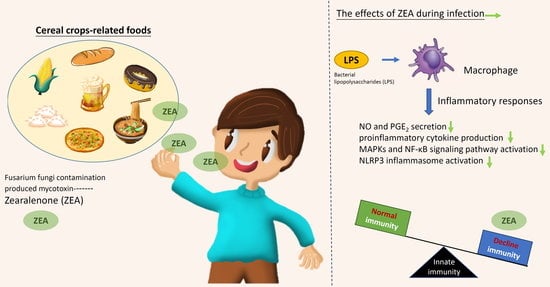
:1. Introduction
2. Results
2.1. ZEA Attenuates Inducible Nitric Oxide Synthase (iNOS) and COX-2 Expressions of and Inhibits NO and Prostaglandin E2 (PGE2) Productions by LPS-Activated Macrophages
2.2. ZEA Suppresses the Expression and Production of TNF-α and IL-6 by LPS-Activated Macrophages
2.3. ZEA Inhibits the Activation of MAPKs and NF-κB Signaling Pathways by LPS-Activated Macrophages
2.4. ZEA Inhibits IL-1β Secretion and Suppresses NLRP3 Inflammasome Activation by LPS/ATP- and LPS/nigericin-Activated Macrophages
2.5. ZEA Suppresses Proinflammatory Cytokine Secretions by LPS-Activated Human Macrophages
2.6. ZEA Reduces the Secretion of Proinflammatory Mediator and Cytokines by LPS-Activated Murine Peritoneal and Bone Marrow-Derived Macrophages (BMDMs)
3. Discussion
4. Conclusions
5. Materials and Methods
5.1. Animals
5.2. Cell Culture
5.3. Peritoneal Macrophags and BMDMs Preparation
5.4. Cell Viability Assay
5.5. NO Production Assay
5.6. Western Blot Analysis
5.7. qPCR
5.8. ELISA
5.9. NF-κB Promoter Reporter Assay
5.10. Immunofluorescence Staining
5.11. Statistical Analysis
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Zheng, W.; Feng, N.; Wang, Y.; Noll, L.; Xu, S.; Liu, X.; Lu, N.; Zou, H.; Gu, J.; Yuan, Y.; et al. Effects of zearalenone and its derivatives on the synthesis and secretion of mammalian sex steroid hormones: A review. Food Chem. Toxicol. 2019, 126, 262–276. [Google Scholar] [CrossRef]
- Mally, A.; Solfrizzo, M.; Degen, G.H. Biomonitoring of the mycotoxin Zearalenone: Current state-of-the art and application to human exposure assessment. Arch. Toxicol. 2016, 90, 1281–1292. [Google Scholar] [CrossRef] [PubMed]
- Ropejko, K.; Twaruzek, M. Zearalenone and Its Metabolites-General Overview, Occurrence, and Toxicity. Toxins 2021, 13, 35. [Google Scholar] [CrossRef] [PubMed]
- Hueza, I.M.; Raspantini, P.C.; Raspantini, L.E.; Latorre, A.O.; Gorniak, S.L. Zearalenone, an estrogenic mycotoxin, is an immunotoxic compound. Toxins 2014, 6, 1080–1095. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sun, L.H.; Lei, M.Y.; Zhang, N.Y.; Zhao, L.; Krumm, C.S.; Qi, D.S. Hepatotoxic effects of mycotoxin combinations in mice. Food Chem. Toxicol. 2014, 74, 289–293. [Google Scholar] [CrossRef] [PubMed]
- El-Makawy, A.; Hassanane, M.S.; Abd Alla, E.S. Genotoxic evaluation for the estrogenic mycotoxin zearalenone. Reprod. Nutr. Dev. 2001, 41, 79–89. [Google Scholar] [CrossRef] [PubMed]
- Hirayama, D.; Iida, T.; Nakase, H. The Phagocytic Function of Macrophage-Enforcing Innate Immunity and Tissue Homeostasis. Int. J. Mol. Sci. 2017, 19, 92. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Nyati, K.K.; Masuda, K.; Zaman, M.M.; Dubey, P.K.; Millrine, D.; Chalise, J.P.; Higa, M.; Li, S.; Standley, D.M.; Saito, K.; et al. TLR4-induced NF-kappaB and MAPK signaling regulate the IL-6 mRNA stabilizing protein Arid5a. Nucleic Acids Res. 2017, 45, 2687–2703. [Google Scholar] [CrossRef]
- Peroval, M.Y.; Boyd, A.C.; Young, J.R.; Smith, A.L. A critical role for MAPK signalling pathways in the transcriptional regulation of toll like receptors. PLoS ONE 2013, 8, e51243. [Google Scholar] [CrossRef] [Green Version]
- Franchi, L.; Eigenbrod, T.; Munoz-Planillo, R.; Nunez, G. The inflammasome: A caspase-1-activation platform that regulates immune responses and disease pathogenesis. Nat. Immunol. 2009, 10, 241–247. [Google Scholar] [CrossRef]
- Kelley, N.; Jeltema, D.; Duan, Y.; He, Y. The NLRP3 Inflammasome: An Overview of Mechanisms of Activation and Regulation. Int. J. Mol. Sci. 2019, 20, 3328. [Google Scholar] [CrossRef] [Green Version]
- Zhong, Y.; Lu, Y.; Yang, X.; Tang, Y.; Zhao, K.; Yuan, C.; Zhong, X. The roles of NLRP3 inflammasome in bacterial infection. Mol. Immunol. 2020, 122, 80–88. [Google Scholar] [CrossRef] [PubMed]
- Gross, O.; Poeck, H.; Bscheider, M.; Dostert, C.; Hannesschlager, N.; Endres, S.; Hartmann, G.; Tardivel, A.; Schweighoffer, E.; Tybulewicz, V.; et al. Syk kinase signalling couples to the Nlrp3 inflammasome for anti-fungal host defence. Nature 2009, 459, 433–436. [Google Scholar] [CrossRef] [PubMed]
- Allen, I.C.; Scull, M.A.; Moore, C.B.; Holl, E.K.; McElvania-TeKippe, E.; Taxman, D.J.; Guthrie, E.H.; Pickles, R.J.; Ting, J.P. The NLRP3 inflammasome mediates in vivo innate immunity to influenza A virus through recognition of viral RNA. Immunity 2009, 30, 556–565. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kanneganti, T.D.; Body-Malapel, M.; Amer, A.; Park, J.H.; Whitfield, J.; Franchi, L.; Taraporewala, Z.F.; Miller, D.; Patton, J.T.; Inohara, N.; et al. Critical role for Cryopyrin/Nalp3 in activation of caspase-1 in response to viral infection and double-stranded RNA. J. Biol. Chem. 2006, 281, 36560–36568. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Broz, P.; Monack, D.M. Molecular mechanisms of inflammasome activation during microbial infections. Immunol. Rev. 2011, 243, 174–190. [Google Scholar] [CrossRef]
- Bulgaru, C.V.; Marin, D.E.; Pistol, G.C.; Taranu, I. Zearalenone and the Immune Response. Toxins 2021, 13, 248. [Google Scholar] [CrossRef] [PubMed]
- Pierron, A.; Alassane-Kpembi, I.; Oswald, I.P. Impact of mycotoxin on immune response and consequences for pig health. Anim. Nutr. 2016, 2, 63–68. [Google Scholar] [CrossRef]
- Dahiya, Y.; Pandey, R.K.; Bhatt, K.H.; Sodhi, A. Role of prostaglandin E2 in peptidoglycan mediated iNOS expression in mouse peritoneal macrophages in vitro. FEBS Lett. 2010, 584, 4227–4232. [Google Scholar] [CrossRef] [Green Version]
- Cuzzocrea, S.; Salvemini, D. Molecular mechanisms involved in the reciprocal regulation of cyclooxygenase and nitric oxide synthase enzymes. Kidney Int. 2007, 71, 290–297. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Paduch, R.; Kandefer-Szerszen, M. Nitric Oxide (NO) and Cyclooxygenase-2 (COX-2) Cross-Talk in Co-Cultures of Tumor Spheroids with Normal Cells. Cancer Microenviron. 2011, 4, 187–198. [Google Scholar] [CrossRef] [Green Version]
- Albrecht, L.J.; Tauber, S.C.; Merres, J.; Kress, E.; Stope, M.B.; Jansen, S.; Pufe, T.; Brandenburg, L.O. Lack of Proinflammatory Cytokine Interleukin-6 or Tumor Necrosis Factor Receptor-1 Results in a Failure of the Innate Immune Response after Bacterial Meningitis. Mediators Inflamm. 2016, 2016, 7678542. [Google Scholar] [CrossRef] [Green Version]
- Wellmer, A.; Gerber, J.; Ragheb, J.; Zysk, G.; Kunst, T.; Smirnov, A.; Bruck, W.; Nau, R. Effect of deficiency of tumor necrosis factor alpha or both of its receptors on Streptococcus pneumoniae central nervous system infection and peritonitis. Infect. Immun. 2001, 69, 6881–6886. [Google Scholar] [CrossRef] [Green Version]
- Lane, K.; Andres-Terre, M.; Kudo, T.; Monack, D.M.; Covert, M.W. Escalating Threat Levels of Bacterial Infection Can Be Discriminated by Distinct MAPK and NF-kappaB Signaling Dynamics in Single Host Cells. Cell Syst. 2019, 8, 183–196.e4. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Anand, P.K.; Malireddi, R.K.; Kanneganti, T.D. Role of the nlrp3 inflammasome in microbial infection. Front. Microbiol. 2011, 2, 12. [Google Scholar] [CrossRef] [Green Version]
- Mosser, D.M.; Hamidzadeh, K.; Goncalves, R. Macrophages and the maintenance of homeostasis. Cell. Mol. Immunol. 2021, 18, 579–587. [Google Scholar] [CrossRef]
- Katona, P.; Katona-Apte, J. The interaction between nutrition and infection. Clin. Infect. Dis. 2008, 46, 1582–1588. [Google Scholar] [CrossRef]
- Zinedine, A.; Soriano, J.M.; Molto, J.C.; Manes, J. Review on the toxicity, occurrence, metabolism, detoxification, regulations and intake of zearalenone: An oestrogenic mycotoxin. Food Chem. Toxicol. 2007, 45, 1–18. [Google Scholar] [CrossRef]
- Lee, H.J.; Park, J.H.; Oh, S.Y.; Cho, D.H.; Kim, S.; Jo, I. Zearalenone-Induced Interaction between PXR and Sp1 Increases Binding of Sp1 to a Promoter Site of the eNOS, Decreasing Its Transcription and NO Production in BAECs. Toxins 2020, 12, 421. [Google Scholar] [CrossRef]
- Marin, D.E.; Pistol, G.C.; Bulgaru, C.V.; Taranu, I. Cytotoxic and inflammatory effects of individual and combined exposure of HepG2 cells to zearalenone and its metabolites. Naunyn Schmiedebergs Arch. Pharmacol. 2019, 392, 937–947. [Google Scholar] [CrossRef]
- Islam, M.R.; Kim, J.W.; Roh, Y.S.; Kim, J.H.; Han, K.M.; Kwon, H.J.; Lim, C.W.; Kim, B. Evaluation of immunomodulatory effects of zearalenone in mice. J. Immunotoxicol. 2017, 14, 125–136. [Google Scholar] [CrossRef] [Green Version]
- Pistol, G.C.; Braicu, C.; Motiu, M.; Gras, M.A.; Marin, D.E.; Stancu, M.; Calin, L.; Israel-Roming, F.; Berindan-Neagoe, I.; Taranu, I. Zearalenone mycotoxin affects immune mediators, MAPK signalling molecules, nuclear receptors and genome-wide gene expression in pig spleen. PLoS ONE 2015, 10, e0127503. [Google Scholar] [CrossRef] [Green Version]
- Pistol, G.C.; Gras, M.A.; Marin, D.E.; Israel-Roming, F.; Stancu, M.; Taranu, I. Natural feed contaminant zearalenone decreases the expressions of important pro- and anti-inflammatory mediators and mitogen-activated protein kinase/NF-kappaB signalling molecules in pigs. Br. J. Nutr. 2014, 111, 452–464. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hung, Y.L.; Wang, S.C.; Suzuki, K.; Fang, S.H.; Chen, C.S.; Cheng, W.C.; Su, C.C.; Yeh, H.C.; Tu, H.P.; Liu, P.L.; et al. Bavachin attenuates LPS-induced inflammatory response and inhibits the activation of NLRP3 inflammasome in macrophages. Phytomedicine 2019, 59, 152785. [Google Scholar] [CrossRef]
- Liu, X.; Quan, N. Immune Cell Isolation from Mouse Femur Bone Marrow. Bio Protoc. 2015, 5, e1631. [Google Scholar] [CrossRef]
- Tang, R.; Zhang, G.; Chen, S.Y. Response gene to complement 32 protein promotes macrophage phagocytosis via activation of protein kinase C pathway. J. Biol. Chem. 2014, 289, 22715–22722. [Google Scholar] [CrossRef] [Green Version]
- Trouplin, V.; Boucherit, N.; Gorvel, L.; Conti, F.; Mottola, G.; Ghigo, E. Bone marrow-derived macrophage production. J. Vis. Exp. 2013, 81, e50966. [Google Scholar] [CrossRef] [Green Version]








Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lee, P.-Y.; Liu, C.-C.; Wang, S.-C.; Chen, K.-Y.; Lin, T.-C.; Liu, P.-L.; Chiu, C.-C.; Chen, I.-C.; Lai, Y.-H.; Cheng, W.-C.; et al. Mycotoxin Zearalenone Attenuates Innate Immune Responses and Suppresses NLRP3 Inflammasome Activation in LPS-Activated Macrophages. Toxins 2021, 13, 593. https://0-doi-org.brum.beds.ac.uk/10.3390/toxins13090593
Lee P-Y, Liu C-C, Wang S-C, Chen K-Y, Lin T-C, Liu P-L, Chiu C-C, Chen I-C, Lai Y-H, Cheng W-C, et al. Mycotoxin Zearalenone Attenuates Innate Immune Responses and Suppresses NLRP3 Inflammasome Activation in LPS-Activated Macrophages. Toxins. 2021; 13(9):593. https://0-doi-org.brum.beds.ac.uk/10.3390/toxins13090593
Chicago/Turabian StyleLee, Po-Yen, Ching-Chih Liu, Shu-Chi Wang, Kai-Yin Chen, Tzu-Chieh Lin, Po-Len Liu, Chien-Chih Chiu, I-Chen Chen, Yu-Hung Lai, Wei-Chung Cheng, and et al. 2021. "Mycotoxin Zearalenone Attenuates Innate Immune Responses and Suppresses NLRP3 Inflammasome Activation in LPS-Activated Macrophages" Toxins 13, no. 9: 593. https://0-doi-org.brum.beds.ac.uk/10.3390/toxins13090593