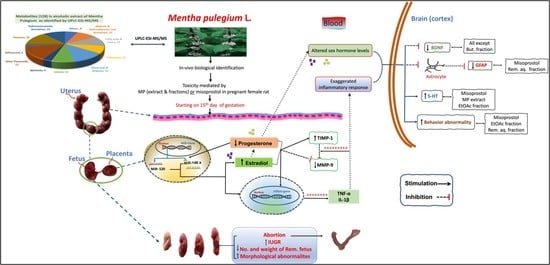
Mentha pulegium L. (Pennyroyal, Lamiaceae) Extracts Impose Abortion or Fetal-Mediated Toxicity in Pregnant Rats; Evidenced by the Modulation of Pregnancy Hormones, MiR-520, MiR-146a, TIMP-1 and MMP-9 Protein Expressions, Inflammatory State, Certain Related Signaling Pathways, and Metabolite Profiling via UPLC-ESI-TOF-MS
Abstract
:- Verification of Mentha pulegium (MP) abortifacient capacity in pregnant rats.
- UPLC-MS revealed MP profile to harbor metabolites with phytoestrogenic potential.
1. Introduction
2. Results
2.1. Dose Selection
2.1.1. Abortifacient and Biochemical Effects of Different Doses of Mentha pulegium L. Body Weight-Related
2.1.2. Histopathological Effects of the Different Doses of Mentha pulegium L. on Uterine Tissues
2.1.3. Acute Toxicity Test
2.2. Identification of the Abortifacient Mechanism of MP Extract and Fractions Thereof
2.2.1. Abortifacient Effects/Activities of 250 mg/kg and Different Fractions of Mentha pulegium L.
2.2.2. Effect of Mentha pulegium L. on the Serum Levels of Progesterone and Estradiol
2.2.3. Effect of Mentha pulegium L. on Placental Protein Expressions of MiR-520 and MiR-146a
2.2.4. Effect of Mentha pulegium L. on Uterine Protein Expressions of MMP-9 and TIMP-1
2.2.5. Effect of Mentha pulegium L. on Serum Inflammatory Markers
2.2.6. Effect of Mentha pulegium L. on Behavioral Changes in Open Field Test
2.2.7. Effect of Mentha pulegium L. on Cortical GFAP, BDNF, and 5HT-3 Levels
2.3. Metabolite Profiling of Mentha pulegium L. (Pennyroyal) Extract Using UPLC-ESI-TOF-MS
2.3.1. Hydroxycinnamic Acids and Derivatives
2.3.2. Flavones and Derivatives
2.3.3. Flavanones and Flavanol Derivatives
2.3.4. Prenyl Flavanones
2.3.5. Biflavanoids
2.3.6. Terpenes
3. Discussion
4. Conclusions
5. Materials and Methods
5.1. Plant Material
5.2. Chemicals and Reagents
5.3. Experimental Design
5.3.1. Part I: Dose Selection
5.3.2. Part II: Identification of the Abortifacient Mechanism of MP Extract and Fractions Thereof
5.4. Tissue and Serum Preparation
5.5. Behavioral Open Field Test (OFT)
5.6. Parameters Assessed by ELISA Technique
5.7. Histopathological Examinations
5.8. Quantitative Real-Time PCR for miR-520 and miR146a Placental Expression
5.9. Western Blotting
5.10. Statistical Analysis
5.11. Metabolite Profiling of Pennyroyal (MP) Extract and Active Fractions by UPLC-ESI-TOF-MS
5.11.1. Sample Preparation
5.11.2. Instrument and Spectral Acquisition
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| BDNF | brain derived neurotrophic factor |
| But | butanol |
| EtOAc | ethyl acetate |
| GFAP | glial fibrillary acidic protein |
| 5-HT | seretonin |
| IL-1β | interlukin-1 beta |
| IUGR | intra-uterine growth retardation |
| MecH | methylene chloride |
| MiR-146a | micro-RNA 146a |
| MiR-520 | micro-RNA 520 |
| MMP-9 | matrix metalloproteinase-9 |
| MP | Mentha pulegium |
| OFT | open field test |
| Rem. aq | remaining aqueous liquor |
| TIMP-1 | tissue inhibitor matrix metalloproteinase-1 |
| TNF-α | tumor necrosis factor-alfa |
| UPLC-ESI-TOF-MS | ultra performance liquid chromatography-electrospray ionisation time-of-flight mass spectrometry |
Appendix A
Appendix B
Appendix C
Appendix D
Appendix E
References
- Ciganda, C.; Laborde, A. Herbal infusions used for induced abortion. J. Toxicol. Clin. Toxicol. 2003, 41, 235–239. [Google Scholar] [CrossRef] [PubMed]
- Anwar, F.; Abbas, A.; Mehmood, T.; Gilani, A.H.; Rehman, N.u. Mentha: A genus rich in vital nutra-pharmaceuticals—A review. Phytother. Res. 2019, 33, 2548–2570. [Google Scholar] [CrossRef] [PubMed]
- Teixeira, B.; Marques, A.; Ramos, C.; Batista, I.; Serrano, C.; Matos, O.; Neng, N.R.; Nogueira, J.M.; Saraiva, J.A.; Nunes, M.L. European pennyroyal (Mentha pulegium) from Portugal: Chemical composition of essential oil and antioxidant and antimicrobial properties of extracts and essential oil. Ind. Crop. Prod. 2012, 36, 81–87. [Google Scholar] [CrossRef]
- Hadi, M.Y.; Hameed, I.H.; Ibraheam, I.A. Mentha pulegium: Medicinal uses, Anti-Hepatic, Antibacterial, Antioxidant effect and Analysis of Bioactive Natural Compounds: A Review. Res. J. Pharm. Technol. 2017, 10, 3580–3584. [Google Scholar] [CrossRef]
- Abdelli, M.; Moghrani, H.; Aboun, A.; Maachi, R. Algerian Mentha pulegium L. leaves essential oil: Chemical composition, antimicrobial, insecticidal and antioxidant activities. Ind. Crop. Prod. 2016, 94, 197–205. [Google Scholar] [CrossRef]
- Riddle, J.M. Eve’s Herbs: A History of Contraception and Abortion in the West; Harvard University Press: Cambridge, MA, USA, 1997. [Google Scholar]
- Schiebinger, L. Plants and Empire; Harvard University Press: Cambridge, MA, USA, 2021. [Google Scholar]
- Marzouk, M.M.; Hussein, S.R.; Elkhateeb, A.; El-shabrawy, M.; Abdel-Hameed, E.-S.S.; Kawashty, S.A. Comparative study of Mentha species growing wild in Egypt: LC-ESI-MS analysis and chemosystematic significance. J. Appl. Pharm. Sci. 2018, 8, 116–122. [Google Scholar]
- Taamalli, A.; Arráez-Román, D.; Abaza, L.; Iswaldi, I.; Fernández-Gutiérrez, A.; Zarrouk, M.; Segura-Carretero, A. LC-MS-based metabolite profiling of methanolic extracts from the medicinal and aromatic species Mentha pulegium and Origanum majorana. Phytochem. Anal. 2015, 26, 320–330. [Google Scholar] [CrossRef]
- Blanchard, K.; Winikoff, B.; Ellertson, C. Misoprostol used alone for the termination of early pregnancy: A review of the evidence. Contraception 1999, 59, 209–217. [Google Scholar] [CrossRef]
- Taher, E.; Swelam, M.; Mansy, A.; Elgammal, M. Methotrexate and Misoprostol against Misoprostol Alone for Early Medical Abortion: Comparative Study. J. High Inst. Public Health 2014, 44, 21–24. [Google Scholar] [CrossRef] [Green Version]
- Moreno-Ruiz, N.; Borgatta, L.; Yanow, S.; Kapp, N.; Wiebe, E.; Winikoff, B. Alternatives to mifepristone for early medical abortion. Int. J. Gynecol. Obstet. 2007, 96, 212–218. [Google Scholar] [CrossRef]
- Fouche-Camargo, J.S. Uterotonics and tocolytics. In Clinical Pharmacology during Pregnancy; Elsevier: Amsterdam, The Netherlands, 2022; pp. 323–338. [Google Scholar]
- Shokrzadeh, M.; Dashti, A.; Aghajanshakeri, S.; Pourabbas, B.; Ghassemi Barghi, N.; Ogunkunle, A. Prevention effects of Foeniculum vulgare (Fennel) hydroalcoholic extract for threatened abortion by misoprostol induction in experimental mice. Int. J. Trad. Nat. Med. 2019, 9, 1–16. [Google Scholar]
- Auffret, M.; Bernard-Phalippon, N.; Dekemp, J.; Carlier, P.; Boyer, M.G.; Vial, T.; Gautier, S. Misoprostol exposure during the first trimester of pregnancy: Is the malformation risk varying depending on the indication? Eur. J. Obstet. Gynecol. Reprod. Biol. 2016, 207, 188–192. [Google Scholar] [CrossRef] [PubMed]
- Barbero, P.; Liascovich, R.; Valdez, R.; Moresco, A. Misoprostol teratogenicity: A prospective study in Argentina. Arch. Argent. Pediatr. 2011, 109, 226–231. [Google Scholar]
- Kazuno, S.; Yanagida, M.; Shindo, N.; Murayama, K. Mass spectrometric identification and quantification of glycosyl flavonoids, including dihydrochalcones with neutral loss scan mode. Anal. Biochem. 2005, 347, 182–192. [Google Scholar] [CrossRef]
- Jiang, R.-W.; Lau, K.-M.; Hon, P.-M.; Mak, T.C.; Woo, K.-S.; Fung, K.-P. Chemistry and biological activities of caffeic acid derivatives from Salvia miltiorrhiza. Curr. Med. Chem. 2005, 12, 237–246. [Google Scholar] [CrossRef]
- Farag, M.A.; Rasheed, D.M.; Kropf, M.; Heiss, A.G. Metabolite profiling in Trigonella seeds via UPLC-MS and GC-MS analyzed using multivariate data analyses. Anal. Bioanal. Chem. 2016, 408, 8065–8078. [Google Scholar] [CrossRef]
- Otify, A.M.; El-Sayed, A.M.; Michel, C.G.; Farag, M.A. Metabolites profiling of date palm (Phoenix dactylifera L.) commercial by-products (pits and pollen) in relation to its antioxidant effect: A multiplex approach of MS and NMR metabolomics. Metabolomics 2019, 15, 119. [Google Scholar] [CrossRef]
- Jebali, J.; Ghazghazi, H.; Aouadhi, C.; ELBini-Dhouib, I.; Ben Salem, R.; Srairi-Abid, N.; Marrakchi, N.; Rigane, G. Tunisian Native Mentha pulegium L. Extracts: Phytochemical Composition and Biological Activities. Molecules 2022, 27, 314. [Google Scholar] [CrossRef]
- Zhang, A.; Sun, H.; Yan, G.; Wang, X. Recent developments and emerging trends of mass spectrometry for herbal ingredients analysis. TrAC Trends Anal. Chem. 2017, 94, 70–76. [Google Scholar] [CrossRef]
- Hajlaouia, H.; Mighrib, H.; Hamdauic, G.; Aounia, M. Antioxidant activities and RP-HPLC identification of polyphenols in the methanolic extract of Mentha genus. Tunis. J. Med. Plants Nat. Prod. (TJMPNP) 2015, 14, 1–11. [Google Scholar]
- Sellami, I.H.; Maamouri, E.; Chahed, T.; Wannes, W.A.; Kchouk, M.E.; Marzouk, B. Effect of growth stage on the content and composition of the essential oil and phenolic fraction of sweet marjoram (Origanum majorana L.). Ind. Crops Prod. 2009, 30, 395–402. [Google Scholar] [CrossRef]
- Buckingham, J. Dictionary of Natural Products, Supplement 4; CRC Press: Boca Raton, FL, USA, 1997; Volume 11. [Google Scholar]
- Loussouarn, M.; Krieger-Liszkay, A.; Svilar, L.; Bily, A.; Birtić, S.; Havaux, M. Carnosic acid and carnosol, two major antioxidants of rosemary, act through different mechanisms. Plant Physiol. 2017, 175, 1381–1394. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Guerrero, I.C.; Andrés, L.S.; León, L.G.; Machín, R.P.; Padrón, J.M.; Luis, J.G.; Delgadillo, J. Abietane diterpenoids from Salvia pachyphylla and S. clevelandii with cytotoxic activity against human cancer cell lines. J. Nat. Prod. 2006, 69, 1803–1805. [Google Scholar] [CrossRef] [PubMed]
- Wang, D.; Yu, W.; Cao, L.; Xu, C.; Tan, G.; Zhao, Z.; Huang, M.; Jin, J. Comparative pharmacokinetics and tissue distribution of cryptotanshinone, tanshinone IIA, dihydrotanshinone I, and tanshinone I after oral administration of pure tanshinones and liposoluble extract of Salvia miltiorrhiza to rats. Biopharm. Drug Dispos. 2020, 41, 54–63. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Nair, S.N.; Menon, S.; Shailajan, S. A liquid chromatography/electrospray ionization tandem mass spectrometric method for quantification of asiatic acid from plasma: Application to pharmacokinetic study in rats. Rapid Commun. Mass Spectrom. 2012, 26, 1899–1908. [Google Scholar] [CrossRef]
- Shen, D.; Pan, M.-H.; Wu, Q.-L.; Park, C.-H.; Juliani, H.R.; Ho, C.-T.; Simon, J.E. LC-MS method for the simultaneous quantitation of the anti-inflammatory constituents in oregano (Origanum species). J. Agric. Food Chem. 2010, 58, 7119–7125. [Google Scholar] [CrossRef]
- Basal, W.T.; Ahmed, A.R.T.; Mahmoud, A.A.; Omar, A.R. Lufenuron induces reproductive toxicity and genotoxic effects in pregnant albino rats and their fetuses. Sci. Rep. 2020, 10, 19544. [Google Scholar] [CrossRef]
- Mesiano, S. Roles of estrogen and progesterone in human parturition. Endocrinol. Parturition 2001, 27, 86–104. [Google Scholar]
- Bridges, R.S. A quantitative analysis of the roles of dosage, sequence, and duration of estradiol and progesterone exposure in the regulation of maternal behavior in the rat. Endocrinology 1984, 114, 930–940. [Google Scholar] [CrossRef]
- Amadi, C.N.; Igweze, Z.N.; Orisakwe, O.E. Heavy metals in miscarriages and stillbirths in developing nations. Middle East Fertil. Soc. J. 2017, 22, 91–100. [Google Scholar] [CrossRef]
- Wang, X.; Chen, X.; Feng, X.; Chang, F.; Chen, M.; Xia, Y.; Chen, L. Triclosan causes spontaneous abortion accompanied by decline of estrogen sulfotransferase activity in humans and mice. Sci. Rep. 2015, 5, 18252. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Obernosterer, G.; Leuschner, P.J.; Alenius, M.; Martinez, J. Post-transcriptional regulation of microRNA expression. RNA 2006, 12, 1161–1167. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cai, M.; Kolluru, G.K.; Ahmed, A. Small molecule, big prospects: microRNA in pregnancy and its complications. J. Pregnancy 2017, 2017, 6972732. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Timofeeva, A.V.; Gusar, V.A.; Kan, N.E.; Prozorovskaya, K.N.; Karapetyan, A.O.; Bayev, O.R.; Chagovets, V.V.; Kliver, S.F.; Iakovishina, D.Y.; Frankevich, V.E. Identification of potential early biomarkers of preeclampsia. Placenta 2018, 61, 61–71. [Google Scholar] [CrossRef] [PubMed]
- Ye, H.-x.; Li, L.; Dong, Y.-j.; Li, P.-h.; Su, Q.; Guo, Y.-h.; Lu, Y.-r.; Zhong, Y.; Jia, Y.; Cheng, J.-q. miR-146a-5p improves the decidual cytokine microenvironment by regulating the toll-like receptor signaling pathway in unexplained spontaneous abortion. Int. Immunopharmacol. 2020, 89, 107066. [Google Scholar] [CrossRef]
- Sang, Q.; Yao, Z.; Wang, H.; Feng, R.; Wang, H.; Zhao, X.; Xing, Q.; Jin, L.; He, L.; Wu, L. Identification of microRNAs in human follicular fluid: Characterization of microRNAs that govern steroidogenesis in vitro and are associated with polycystic ovary syndrome in vivo. J. Clin. Endocrinol. Metab. 2013, 98, 3068–3079. [Google Scholar] [CrossRef] [Green Version]
- Yuan, D.-z.; Yu, L.-l.; Qu, T.; Zhang, S.-m.; Zhao, Y.-b.; Pan, J.-l.; Xu, Q.; He, Y.-p.; Zhang, J.-h.; Yue, L.-m. Identification and characterization of progesterone-and estrogen-regulated MicroRNAs in mouse endometrial epithelial cells. Reprod. Sci. 2015, 22, 223–234. [Google Scholar] [CrossRef] [Green Version]
- Zhang, Y.; Li, P.; Guo, Y.; Liu, X.; Zhang, Y. MMP-9 and TIMP-1 in placenta of hypertensive disorder complicating pregnancy. Exp. Ther. Med. 2019, 18, 637–641. [Google Scholar] [CrossRef] [Green Version]
- Chen, J.; Khalil, R.A. Matrix metalloproteinases in normal pregnancy and preeclampsia. Prog. Mol. Biol. Transl. Sci. 2017, 148, 87–165. [Google Scholar]
- Laskowska, M. Altered maternal serum matrix metalloproteinases MMP-2, MMP-3, MMP-9, and MMP-13 in severe early-and late-onset preeclampsia. BioMed Res. Int. 2017, 2017, 6432426. [Google Scholar] [CrossRef] [Green Version]
- Dang, Y.; Li, W.; Tran, V.; Khalil, R.A. EMMPRIN-mediated induction of uterine and vascular matrix metalloproteinases during pregnancy and in response to estrogen and progesterone. Biochem. Pharmacol. 2013, 86, 734–747. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mönckedieck, V.; Sannecke, C.; Husen, B.; Kumbartski, M.; Kimmig, R.; Tötsch, M.; Winterhager, E.; Grümmer, R. Progestins inhibit expression of MMPs and of angiogenic factors in human ectopic endometrial lesions in a mouse model. Mol. Hum. Reprod. 2009, 15, 633–643. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kalagiri, R.R.; Carder, T.; Choudhury, S.; Vora, N.; Ballard, A.R.; Govande, V.; Drever, N.; Beeram, M.R.; Uddin, M.N. Inflammation in complicated pregnancy and its outcome. Am. J. Perinatol. 2016, 33, 1337–1356. [Google Scholar] [CrossRef] [PubMed]
- Christiansen, O.B.; Nielsen, H.S.; Kolte, A.M. Seminars in Fetal and Neonatal Medicine. In Inflammation and Miscarriage; Elsevier: Amsterdam, The Netherlands, 2006; pp. 302–308. [Google Scholar]
- Ginsberg, Y.; Khatib, N.; Weiner, Z.; Beloosesky, R. Maternal inflammation, fetal brain implications and suggested neuroprotection: A summary of 10 years of research in animal models. Rambam Maimonides Med. J. 2017, 8, e0028. [Google Scholar] [CrossRef]
- Schreiber, K.; Sciascia, S.; De Groot, P.G.; Devreese, K.; Jacobsen, S.; Ruiz-Irastorza, G.; Salmon, J.E.; Shoenfeld, Y.; Shovman, O.; Hunt, B.J. Antiphospholipid syndrome. Nat. Rev. Dis. Primers 2018, 4, 17103. [Google Scholar] [CrossRef]
- Cotechini, T.; Komisarenko, M.; Sperou, A.; Macdonald-Goodfellow, S.; Adams, M.A.; Graham, C.H. Inflammation in rat pregnancy inhibits spiral artery remodeling leading to fetal growth restriction and features of preeclampsia. J. Exp. Med. 2014, 211, 165–179. [Google Scholar] [CrossRef]
- Romero, R.; Durum, S.; Dinarello, C.; Hobbins, J.; Mitchell, M. Interleukin-1: A Signal for the Initiation of Labor in Chorioamnionitis. In Proceedings of the 33rd Annual Meeting for the Society for Gynecologic Investigation, Toronto, ON, Canada, 19–22 March 1986; pp. 19–22. [Google Scholar]
- Pařízek, A.; Koucký, M.; Dušková, M. Progesterone, inflammation and preterm labor. J. Steroid Biochem. Mol. Biol. 2014, 139, 159–165. [Google Scholar] [CrossRef]
- He, Y.; Sun, Q. IFN-γ induces upregulation of TNF-α, downregulation of MMP-2 and MMP-9 expressions in abortion rat. Eur. Rev. Med. Pharm. Sci. 2018, 22, 4762–4767. [Google Scholar]
- Trentini, A.; Maritati, M.; Cervellati, C.; Manfrinato, M.C.; Gonelli, A.; Volta, C.A.; Vesce, F.; Greco, P.; Dallocchio, F.; Bellini, T. Vaginal lactoferrin modulates PGE2, MMP-9, MMP-2, and TIMP-1 amniotic fluid concentrations. Mediat. Inflamm. 2016, 2016, 3648719. [Google Scholar] [CrossRef] [Green Version]
- Marinescu, I.P.; Foarfa, M.C.; Pirlog, M.-C.; Turculeanu, A. Prenatal depression and stress-risk factors for placental pathology and spontaneous abortion. Romanian J. Morphol. Embryol. 2014, 55 (Suppl. S3), 1155–1160. [Google Scholar]
- Fergusson, D.M.; Horwood, L.J.; Boden, J.M. Abortion and mental health disorders: Evidence from a 30-year longitudinal study. Br. J. Psychiatry 2008, 193, 444–451. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Luo, M.; Jiang, X.; Wang, Y.; Wang, Z.; Shen, Q.; Li, R.; Cai, Y. Association between induced abortion and suicidal ideation among unmarried female migrant workers in three metropolitan cities in China: A cross-sectional study. BMC Public Health 2018, 18, 625. [Google Scholar] [CrossRef] [PubMed]
- Reardon, D.C. The abortion and mental health controversy: A comprehensive literature review of common ground agreements, disagreements, actionable recommendations, and research opportunities. SAGE Open Med. 2018, 6. [Google Scholar] [CrossRef] [PubMed]
- Gould, T.D.; Dao, D.; Kovacsics, C. Mood and Anxiety Related Phenotypes in Mice: Characterization Using Behavioral Tests; Springer: Berlin/Heidelberg, Germany, 2009; Volume 2. [Google Scholar]
- Zhang, T.; Zheng, X.; Wang, X.; Zhao, H.; Wang, T.; Zhang, H.; Li, W.; Shen, H.; Yu, L. Maternal exposure to PM2. 5 during pregnancy induces impaired development of cerebral cortex in mice offspring. Int. J. Mol. Sci. 2018, 19, 257. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhang, X.; Li, H.; Sun, H.; Jiang, Y.; Wang, A.; Kong, Y.; Sun, X.; Zhu, G.; Li, Q.; Du, Z. Effects of BDNF signaling on anxiety-related behavior and spatial memory of adolescent rats in different length of maternal separation. Front. Psychiatry 2020, 11, 709. [Google Scholar] [CrossRef] [PubMed]
- Zimcikova, E.; Simko, J.; Karesova, I.; Kremlacek, J.; Malakova, J. Behavioral effects of antiepileptic drugs in rats: Are the effects on mood and behavior detectable in open-field test? Seizure 2017, 52, 35–40. [Google Scholar] [CrossRef] [Green Version]
- Sestakova, N.; Puzserova, A.; Kluknavsky, M.; Bernatova, I. Determination of motor activity and anxiety-related behaviour in rodents: Methodological aspects and role of nitric oxide. Interdiscip. Toxicol. 2013, 6, 126–135. [Google Scholar] [CrossRef] [Green Version]
- Christakis, D.A.; Ramirez, J.S.; Ramirez, J.-M. Overstimulation of newborn mice leads to behavioral differences and deficits in cognitive performance. Sci. Rep. 2012, 2, 546. [Google Scholar] [CrossRef]
- Lopatina, O.; Yoshihara, T.; Nishimura, T.; Zhong, J.; Akther, S.; Fakhrul, A.A.; Liang, M.; Higashida, C.; Sumi, K.; Furuhara, K. Anxiety-and depression-like behavior in mice lacking the CD157/BST1 gene, a risk factor for Parkinson’s disease. Front. Behav. Neurosci. 2014, 8, 133. [Google Scholar] [CrossRef]
- Nynas, J.; Narang, P.; Kolikonda, M.K.; Lippmann, S. Depression and anxiety following early pregnancy loss: Recommendations for primary care providers. Prim. Care Companion CNS Disord. 2015, 17, 26225. [Google Scholar] [CrossRef] [Green Version]
- Gómora-Arrati, P.; González-Arenas, A.; Balandrán-Ruiz, M.A.; Mendoza-Magaña, M.L.; González-Flores, O.; Camacho-Arroyo, I. Changes in the content of GFAP in the rat brain during pregnancy and the beginning of lactation. Neurosci. Lett. 2010, 484, 197–200. [Google Scholar] [CrossRef] [PubMed]
- Kinsley, C.H.; Lambert, K.G. Reproduction-induced neuroplasticity: Natural behavioural and neuronal alterations associated with the production and care of offspring. J. Neuroendocrinol. 2008, 20, 515–525. [Google Scholar] [CrossRef] [PubMed]
- Scott, H.; Phillips, T.J.; Stuart, G.C.; Rogers, M.F.; Steinkraus, B.R.; Grant, S.; Case, C.P. Preeclamptic placentae release factors that damage neurons: Implications for foetal programming of disease. Neuronal Signal. 2018, 2, NS20180139. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Dingsdale, H.; Nan, X.; Garay, S.M.; Mueller, A.; Sumption, L.A.; Chacón-Fernández, P.; Martinez-Garay, I.; Ghevaert, C.; Barde, Y.-A.; John, R.M. The placenta protects the fetal circulation from anxiety-driven elevations in maternal serum levels of brain-derived neurotrophic factor. Transl. Psychiatry 2021, 11, 62. [Google Scholar] [CrossRef] [PubMed]
- Begliuomini, S.; Casarosa, E.; Pluchino, N.; Lenzi, E.; Centofanti, M.; Freschi, L.; Pieri, M.; Genazzani, A.; Luisi, S.; Genazzani, A.R. Influence of endogenous and exogenous sex hormones on plasma brain-derived neurotrophic factor. Hum. Reprod. 2007, 22, 995–1002. [Google Scholar] [CrossRef] [Green Version]
- Wessels, J.M.; Leyland, N.A.; Agarwal, S.K.; Foster, W.G. Estrogen induced changes in uterine brain-derived neurotrophic factor and its receptors. Hum. Reprod. 2015, 30, 925–936. [Google Scholar] [CrossRef] [Green Version]
- Blakeley, P.M.; Capron, L.E.; Jensen, A.B.; O'Donnell, K.J.; Glover, V. Maternal prenatal symptoms of depression and down regulation of placental monoamine oxidase A expression. J. Psychosom. Res. 2013, 75, 341–345. [Google Scholar] [CrossRef]
- Del Río, J.P.; Alliende, M.I.; Molina, N.; Serrano, F.G.; Molina, S.; Vigil, P. Steroid hormones and their action in women’s brains: The importance of hormonal balance. Front. Public Health 2018, 6, 141. [Google Scholar] [CrossRef] [Green Version]
- Bennetts, H.; Underwood, E.; Shier, F.L. A specific breeding problem of sheep on subterranean clover pastures in Western Australia. Vet. J. 1946, 102, 348–352. [Google Scholar]
- Cos, P.; De Bruyne, T.; Apers, S.; Berghe, D.V.; Pieters, L.; Vlietinck, A.J. Phytoestrogens: Recent developments. Planta Med. 2003, 69, 589–599. [Google Scholar]
- Basly, J.-P.; Lavier, M.-C.C. Dietary phytoestrogens: Potential selective estrogen enzyme modulators? Planta Med. 2005, 71, 287–294. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Al-Fartosi, K.G.; Al-Rekabi, E.A. Effect of phenolic compounds of leaves extractes from Mentha spicata and Mentha longifolia on sex hormones level of female rats. J. Coll. Educ. Pure Sci. 2012, 2, 118–126. [Google Scholar]
- Jung, B.I.; Kim, M.S.; Kim, H.A.; Kim, D.; Yang, J.; Her, S.; Song, Y.S. Caffeic acid phenethyl ester, a component of beehive propolis, is a novel selective estrogen receptor modulator. Phytother. Res. Int. J. Devoted Pharmacol. Toxicol. Eval. Nat. Prod. Deriv. 2010, 24, 295–300. [Google Scholar] [CrossRef] [PubMed]
- Ekeuku, S.O.; Pang, K.-L.; Chin, K.-Y. Effects of caffeic acid and its derivatives on bone: A systematic review. Drug Des. Dev. Ther. 2021, 15, 259. [Google Scholar] [CrossRef]
- Zych, M.; Kaczmarczyk-Sedlak, I.; Wojnar, W.; Folwarczna, J. Effect of rosmarinic acid on the serum parameters of glucose and lipid metabolism and oxidative stress in estrogen-deficient rats. Nutrients 2019, 11, 267. [Google Scholar] [CrossRef] [Green Version]
- Ghasemzadeh, A.; Khaki, A.; Farzadi, L.; Khaki, A.; Marjani, M.; Ashteani, H.A.; Hamdi, B.A.; Ghadamkheir, E.; Naeimikararoudi, M.; Ouladsahebmadarek, E. Effect of rosmarinic acid on estrogen, FSH and LH in female diabetic rats. Afr. J. Pharm. Pharmacol. 2011, 5, 1427–1431. [Google Scholar] [CrossRef] [Green Version]
- Aliyev, A.; Ozcan-Sezer, S.; Akdemir, A.; Gurer-Orhan, H. In vitro evaluation of estrogenic, antiestrogenic and antitumor effects of amentoflavone. Hum. Exp. Toxicol. 2021, 40, 1510–1518. [Google Scholar] [CrossRef]
- Sanderson, J.T.; Hordijk, J.; Denison, M.S.; Springsteel, M.F.; Nantz, M.H.; Van Den Berg, M. Induction and inhibition of aromatase (CYP19) activity by natural and synthetic flavonoid compounds in H295R human adrenocortical carcinoma cells. Toxicol. Sci. 2004, 82, 70–79. [Google Scholar] [CrossRef] [Green Version]
- Wei, Y.; Yuan, P.; Zhang, Q.; Fu, Y.; Hou, Y.; Gao, L.; Zheng, X.; Feng, W. Acacetin improves endothelial dysfunction and aortic fibrosis in insulin-resistant SHR rats by estrogen receptors. Mol. Biol. Rep. 2020, 47, 6899–6918. [Google Scholar] [CrossRef]
- Vitale, D.C.; Piazza, C.; Melilli, B.; Drago, F.; Salomone, S. Isoflavones: Estrogenic activity, biological effect and bioavailability. Eur. J. Drug Metab. Pharmacokinet. 2013, 38, 15–25. [Google Scholar] [CrossRef]
- Nynca, A.; Słonina, D.; Jablońska, O.; Kamińska, B.; Ciereszko, R. Daidzein affects steroidogenesis and oestrogen receptor expression in medium ovarian follicles of pigs. Acta Vet. Hung. 2013, 61, 85–98. [Google Scholar] [CrossRef] [PubMed]
- Yang, X.; Jiang, Y.; Yang, J.; He, J.; Sun, J.; Chen, F.; Zhang, M.; Yang, B. Prenylated flavonoids, promising nutraceuticals with impressive biological activities. Trends Food Sci. Technol. 2015, 44, 93–104. [Google Scholar] [CrossRef]
- Botta, B.; Vitali, A.; Menendez, P.; Misiti, D.; Monache, G.D. Prenylated flavonoids: Pharmacology and biotechnology. Curr. Med. Chem. 2005, 12, 713–739. [Google Scholar] [CrossRef] [PubMed]
- Milligan, S.R.; Kalita, J.C.; Pocock, V.; Van De Kauter, V.; Stevens, J.F.; Deinzer, M.L.; Rong, H.; De Keukeleire, D. The endocrine activities of 8-prenylnaringenin and related hop (Humulus lupulus L.) flavonoids. J. Clin. Endocrinol. Metab. 2000, 85, 4912–4915. [Google Scholar] [CrossRef]
- Weng, X.C.; Gordon, M.H. Antioxidant activity of quinones extracted from tanshen (Salvia miltiorrhiza Bunge). J. Agric. Food Chem. 1992, 40, 1331–1336. [Google Scholar] [CrossRef]
- Xu, S.; Liu, P. Tanshinone II-A: New perspectives for old remedies. Expert Opin. Ther. Pat. 2013, 23, 149–153. [Google Scholar] [CrossRef] [PubMed]
- Nwankudu, N.; Ndibe, N.; Ijioma, S. Oxytocic effect of Ananas comosus fruit juice on isolated pregnant rats uteri. Niger. Vet. J. 2015, 36, 1318–1326. [Google Scholar]
- Ochiogu, I.S.; Uchendu, C.N.; Ihedioha, J.I. A new and simple method of confirmatory detection of mating in albino rats (Rattus norvegicus). Anim. Res. Int. 2006, 3, 527–530. [Google Scholar] [CrossRef] [Green Version]
- Shibeshi, W.; Makonnen, E.; Zerihun, L.; Debella, A. Effect of Achyranthes aspera L. on fetal abortion, uterine and pituitary weights, serum lipids and hormones. Afr. Health Sci. 2006, 6, 108–112. [Google Scholar]
- Li, L.; Huang, Q.; Duan, X.; Han, L.; Peng, D. Protective effect of Clinopodium chinense (Benth.) O. Kuntze against abnormal uterine bleeding in female rats. J. Pharmacol. Sci. 2020, 143, 1–8. [Google Scholar] [CrossRef]
- Marcondes, F.; Bianchi, F.; Tanno, A. Determination of the estrous cycle phases of rats: Some helpful considerations. Braz. J. Biol. 2002, 62, 609–614. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Camilleri, C.; Beiter, R.M.; Puentes, L.; Aracena-Sherck, P.; Sammut, S. Biological, behavioral and physiological consequences of drug-induced pregnancy termination at first-trimester human equivalent in an animal model. Front. Neurosci. 2019, 13, 544. [Google Scholar] [CrossRef] [PubMed]
- Stramek, A.K.; Johnson, M.L.; Taylor, V.J. Improved timed-mating, non-invasive method using fewer unproven female rats with pregnancy validation via early body mass increases. Lab. Anim. 2019, 53, 148–159. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Namulindwa, A.; Nkwangu, D.; Oloro, J. Determination of the abortifacient activity of the aqueous extract of Phytolacca dodecandra (LHer) leaf in Wistar rats. Afr. J. Pharm. Pharmacol. 2015, 9, 43–47. [Google Scholar]
- Paylor, R.; Spencer, C.M.; Yuva-Paylor, L.A.; Pieke-Dahl, S. The use of behavioral test batteries, II: Effect of test interval. Physiol. Behav. 2006, 87, 95–102. [Google Scholar] [CrossRef]
- Elfouly, A.; Awny, M.; Ibrahim, M.; Aboelsaad, M.; Tian, J.; Sayed, M. Effects of Long-Acting Testosterone Undecanoate on Behavioral Parameters and Na+, K+-ATPase mRNA Expression in Mice with Alzheimers Disease. Neurochem. Res. 2021, 46, 2238–2248. [Google Scholar] [CrossRef]
- Rojas-Carvajal, M.; Fornaguera, J.; Mora-Gallegos, A.; Brenes, J.C. Testing experience and environmental enrichment potentiated open-field habituation and grooming behaviour in rats. Anim. Behav. 2018, 137, 225–235. [Google Scholar] [CrossRef]
- Emad, A.M.; Rasheed, D.M.; El-Kased, R.F.; El-Kersh, D.M. Antioxidant, Antimicrobial Activities and Characterization of Polyphenol-Enriched Extract of Egyptian Celery (Apium graveolens L., Apiaceae) Aerial Parts via UPLC/ESI/TOF-MS. Molecules 2022, 27, 698. [Google Scholar] [CrossRef]
- Rasheed, D.M.; Emad, A.M.; Ali, S.F.; Ali, S.S.; Farag, M.A.; Meselhy, M.R.; Sattar, E.A. UPLC-PDA-ESI/MS metabolic profiling of dill shoots bioactive fraction; evidence of its antioxidant and hepatoprotective effects in vitro and in vivo. J. Food Biochem. 2021, 45, e13741. [Google Scholar] [CrossRef]
- Bancroft, J.D.; Stevens, A. Theory and Practice of Histological Techniques, 4th ed.; Churchill Livingstone: New York, NY, USA, 1996. [Google Scholar]
- Soubh, A.A.; El-Gazar, A.A.; Mohamed, E.A.; Awad, A.S.; El-Abhar, H.S. Further insights for the role of Morin in mRTBI: Implication of non-canonical Wnt/PKC-α and JAK-2/STAT-3 signaling pathways. Int. Immunopharmacol. 2021, 100, 108123. [Google Scholar] [CrossRef]
- Ji, J.; Zhang, R.; Li, H.; Zhu, J.; Pan, Y.; Guo, Q. Analgesic and anti-inflammatory effects and mechanism of action of borneol on photodynamic therapy of acne. Environ. Toxicol. Pharmacol. 2020, 75, 103329. [Google Scholar] [CrossRef] [PubMed]






| Group | No. of Completely Aborted Rats/Group | Abortion % | No. of Fetus Remaining | Serum Progesterone | |
|---|---|---|---|---|---|
| Pregnant | 0/6 | 0 | 11–12 | 22.5 | |
| Misoprostol 100 ug/kg | 3/6 | 50% | 7–8 | 9.8 a | |
| Plant Extract dose | 125 mg/kg | 0/6 | 0 | 8–9 | 18.7 a,b |
| 250 mg/kg | 2/6 | 33% | 7–8 | 11.7 a | |
| 500 mg/kg | 2/6 | 33% | 7–8 | 10.5 a | |
| Group | No. of Completely Aborted Rats/gp | Abortion % | No. of Fetus Remaining |
|---|---|---|---|
| Pregnant | 0/6 | 0 | 12–13 |
| Misoprostol (ST) | 2/6 | 33% | 6–8 |
| MP (250 mg/kg) | 2/6 | 33% | 6–9 |
| MecH (125 mg/kg) | 0/6 | 0 | 7–11 |
| EtOAc (125 mg/kg) | 0/6 | 0 | 7–11 |
| But. (125 mg/kg) | 0/6 | 0 | 3–8 |
| Rem. aq. (125 mg/kg) | 0/6 | 0 | 2–6 |
| Peak # | RT. (min.) | Metabolite Name | Mol. Ion m/z | Mass (ppm) | Elemental Composition | MS2 Ions m/z (−)/(+) | Fractions | |
|---|---|---|---|---|---|---|---|---|
| [M − H]− | [M + H]+ | |||||||
| Hydroxycinnamic acids and derivatives | ||||||||
| 1 | 1.31 | Caffeic acid-O-glucuronide | 355.0852 | −5.2 | C15H16O10 | 193, 161 | ||
| 2 | 1.33 | Caffeic acid | 179.0561 | 0.3 | C9H8O4 | 161 | EtOAc, Rem. aq. | |
| 3 | 1.49 | Ferulic acid | 195.0856 | 0.8 | C10H10O4 | 177, 109 | MecH, Rem. aq | |
| 4 | 1.58 | Caffeic acid dimer | 341.1071 | 343.0823 | −2.2/3.2 | C18H14O7 | 179, 161/181, 163 | EtOAc |
| 5 | 1.87 | Sinapoyl-O-hexoside | 387.1408 | 1.9 | C17H22O10 | 255, 169 | Rem. aq | |
| 6 | 1.89 | Cinnamic acid | 149.0949 | 4.1 | C9H8O2 | 121, 65 | ||
| 7 | 5.17 | Ethyl caffeate | 209.0811 | 1.4 | C11H12O4 | 191, 166 | ||
| 8 | 7.26 | Salvianolic acid K | 557.1252 | −6 | C27H24O13 | 363, 345 | But. | |
| 9 | 8.82 | Caffeoyl-O-sinapoylquinic acid | 559.1422 | −4.3 | C27H28O13 | 490, 354, 287 | MecH, EtOAc | |
| 10 | 8.98 | Salvianolic acid F | 315.0836 | −7.8 | C17H14O6 | 209, 179, 167 | Rem. aq | |
| 11 | 12.55 | Rosmarinic acid | 361.0907 | −2.9 | C18H16O8 | 331, 313 | MecH, EtOAc | |
| 12 | 13.30 | Caftaric acid | 313.2372 | 7.8 | C13H12O9 | 295, 272, 259, 137 | EtOAc | |
| 13 | 14.42 | Methyl rosmarinate | 375.1082 | 1.9 | C19H18O8 | 360, 345, 197 | EtOAc | |
| 14 | 18.03 | Rosmarinic acid derivative | 379.2811 | −2 | C23H38O4 | 361, 319, 165 | EtOAc | |
| Flavones and derivatives | ||||||||
| 15 | 4.87 | Apigenin-6,8-di-C-hexoside | 593.1489 | 3.5 | C27H30O15 | 473, 353 | Rem. aq, EtOAc | |
| 16 | 5.18 | Penduletin-4′-O- glucuronide | 521.1812 | −1.5 | C24H24O13 | 503, 345, 327, 253 | EtOAc | |
| 17 | 7.15 | 5-Hydroxy-6,7,4′-trimethoxyflavone (Salvigenin) | 327.2178 | 3.6 | C18H16O6 | 190, 171 | MecH | |
| 18 | 7.36 | Apigenin-7-O-rutinoside (Isorhoifolin) | 577.1605 | 9.1 | C27H30O14 | 464, 269 | But. | |
| 19 | 7.67 | Unknown apigenin glycoside | 563.1752 | −1.3 | C27H30O13 | 401, 383, 271 | But. | |
| 20 | 7.79 | Diosmetin-7-O-rutinoside (Diosmin) | 607.1656 | 609.1782 | −2.4/−0.3 | C28H32O15 | 299/463, 301 | But. |
| 21 | 7.85 | Luteolin-7-O-glucuronide | 463.0899 | 6.1 | C21H18O12 | 446, 287 | ||
| 22 | 8.52 | Apigenin-7-O-glucuronide | 445.0776 | 447.0827 | −7.3/1.1 | C21H18O11 | 269, 175/271 | MecH, But. |
| 23 | 9.35 | Acacetin-7-O-rutinoside (Linarin) | 593.1876 | 1.9 | C28H32O14 | 447, 285 | But., Rem. aq | |
| 24 | 10.11 | 5,6,7-Trihydroxyflavone (Baicalein) | 269.0448 | 1.1 | C15H10O5 | But., MecH | ||
| 25 | 10.32 | Pedalitin tetraacetate | 483.0909 | −2.6 | C24H20O11 | 336, 309 | EtOAc | |
| 26 | 10.42 | 5,7,3′-trihydroxy-4′-methoxyflavone (Diosmetin, 4′-Methylluteolin) | 299.0927 | 301.1382 | −1.5/2.1 | C16H12O6 | 284, 151/283 | But. |
| 27 | 13.11 | 5,7-Dihydroxy-4′,6-dimethoxyflavone (Pectolinarigenin) | 315.0857 | −2.1 | C17H14O6 | 300, 282, 254 | Rem. aq | |
| 28 | 14.02 | 5,4′-Dihydroxy-3,6,7-trimethoxyflavone (Penduletin) | 345.0946 | −6.6 | C18H16O7 | 330, 315, 197 | MecH | |
| 29 | 14.23 | 5,7-Dihydroxy-4′-methoxyflavone (Acacetin) | 283.0611 | 285.0753 | 3.5/−1.5 | C16H12O5 | 268, 151/242, 153 | MecH |
| 30 | 15.33 | 5-hydroxy-3,7,3′,4′-tetramethoxyflavone (Retusin) | 359.1128 | 0.8 | C19H18O7 | 326, 162 | EtOAc, Rem. aq | |
| 31 | 16.41 | 5-Hydroxy-3,3′,4′,6,7-pentamethoxyflavone (Artemetin) | 387.1095 | 389.1230 | 5.2/−0.3 | C20H20O8 | 340, 319/359, 341 | EtOAc, But., Rem. aq |
| 32 | 16.88 | 5-hydroxy-7,4′-dimethoxy-6,8-dimethylflavone (Eucalyptin) | 327.1252 | 8.0 | C19H18O5 | 277, 137 | But., Rem. aq | |
| Flavanone and Flavanol derivatives | ||||||||
| 33 | 5.07 | Naringenin-7-O-glucuronide | 449.1112 | 7.6 | C21H20O11 | 357, 273, 181 | ||
| 34 | 5.37 | Isosakuranetin-O-rutinoside (Didymin) | 595.2831 | 3.6 | C28H34O14 | 577, 457 | ||
| 35 | 5.93 | Hesperetin | 301.2005 | −1.5 | C16H14O6 | 283, 255 | But. | |
| 36 | 8.16 | Eriodictyol-7-O-glucuronide | 463.0990 | 3.2 | C21H20O12 | 354, 286, 218 | But. | |
| 37 | 13.09 | Sakuranetin | 285.0781 | 8 | C16H14O5 | 267, 164 | But. | |
| 38 | 15.45 | Taxifolin | 305.0845 | 8.3 | C15H12O7 | |||
| 39 | 21.51 | Hesperetin-7-O-rutinoside (hesperidin) | 611.2864 | −7.5 | C28H34O15 | 567, 538 | ||
| Prenyl flavones | ||||||||
| 40 | 5.66 | Prenyl pinocembrin | 325.1398 | 1.4 | C20H20O4 | 307, 191 | ||
| 41 | 7.24 | Prenyl kaempferol | 353.2329 | 1.9 | C20H18O6 | 285 | ||
| 42 | 7.93 | 7-O-methyl isoxanthohumol | 369.1311 | −5.9 | C22H24O5 | |||
| 43 | 8.21 | 7,4′-Di-O-methyl isoxanthohumol | 383.184 | −3.4 | C23H26O5 | 365, 233 | ||
| 44 | 10.88 | Isoxanthohumol | 355.1521 | −5.3 | C21H22O5 | 267, 163 | ||
| 45 | 14.99 | Prenyl naringenin | 339.216 | 341.1379 | 6.5/−1.3 | C20H20O5 | 309, 265 | |
| 46 | 21.76 | Dorsmanin F | 441.1897 | −2.5 | C25H28O7 | |||
| Isoflavone | ||||||||
| 47 | 4.35 | Daidzein-8-C-hexoside | 417.1302 | −4.6 | C21H20O9 | 267, 255 | But., Rem. aq | |
| Flavonols | ||||||||
| 48 | 7.35 | Myricetin-3-O-glucuronide | 495.2094 | 4.1 | C21H18O14 | 319, 301, 283 | EtOAc | |
| 49 | 11.44 | 3,5,7,3′,4′,5′-Hexahydroxyflavone (Myricetin) | 317.0562 | 319.0758 | −2.7/0.6 | C15H10O8 | 225, 164/151 | |
| Biflavonoid | ||||||||
| 50 | 18.88 | Di-O-methylamentoflavone (Ginkgetin) | 565.1129 | 567.1288 | 0.3/0.3 | C32H22O10 | 297, 283, 165 | Rem. aq |
| 51 | 21.59 | Amentoflavone-7,4′,4‴-trimethyl ether (Sciadopitysin) | 579.1275 | 581.1424 | −1.8/−3.2 | C33H24O10 | 297 | MecH |
| 52 | 22.62 | 4′-Monomethylamentoflavone (Bilobetin) | 553.2685 | −3.6 | C31H20O10 | 335, 473 | Rem. aq | |
| 53 | 24.06 | Unknown biflavonoid | 647.2296 | 3.2 | C39H34O9 | 629 | ||
| 54 | 24.98 | Isochamaejasmin | 543.1318 | 4.9 | C30H22O10 | 381, 322, 122 | ||
| Quinones | ||||||||
| 55 | 11.49 | Przewaquinone C | 297.1134 | 4.1 | C18H16O4 | 279, 261 | Rem. aq | |
| 56 | 12.76 | Przewaquinone A | 311.1283 | 1.7 | C19H18O4 | EtOAc, Rem. aq | ||
| 57 | 12.78 | Tanshinone IIA | 295.1339 | 3.5 | C19H18O3 | 277, 149 | MecH, Rem. aq | |
| 58 | 15.07 | Przewaquinone F | 313.1075 | 1.5 | C18H16O5 | 277, 259, 149 | Rem. aq | |
| Iridoids | ||||||||
| 59 | 5.54 | Loganic acid | 377.1466 | 6.4 | C16H24O10 | MecH | ||
| 60 | 5.66 | Aucubin | 347.1309 | −6.6 | C15H22O9 | 329, 193 | ||
| 61 | 9.09 | Nepetalactone (Epi-nepetalactone) | 165.0916 | 167.1074 | 3.8/4.5 | C10H14O2 | 147, 107/149, 121 | But. |
| 62 | 10.61 | Dihydronepetalactone | 169.1218 | −2.9 | C10H16O2 | 151, 123, 83 | ||
| 63 | 14.97 | Kanokoside A | 475.1817 | 1.6 | C21H32O12 | 339, 271 | Rem. aq | |
| 64 | 15.80 | Loganin | 391.1634 | 8.9 | C17H26O10 | |||
| 65 | 17.77 | Patriscabroside I | 361.1489 | −1.1 | C16H26O9 | 196, 165 | But., Rem. aq | |
| 66 | 21.56 | Kanokoside C | 639.2484 | −1.7 | C27H42O17 | 621, 579, 562 | ||
| 67 | 22.64 | Deoxyloganic acid tetraacetate | 527.1747 | −2.3 | C24H32O13 | 459, 391, 323 | But. | |
| 68 | 23.67 | Kanokoside D | 625.2671 | −4.9 | C27H44O16 | 607, 581, 521 | ||
| Mono and diterpenes | ||||||||
| 69 | 13.33 | Carnosic acid | 333.2042 | −5.4 | C20H28O4 | 315, 297 | ||
| 70 | 13.53 | Abienol | 291.0687 | −0.2 | C20H34O | 273, 217 | ||
| 71 | 13.88 | Carnosol | 331.1913 | 2.7 | C20H26O4 | 278, 203 | Rem. aq, MecH | |
| 72 | 17.08 | Ethyl abietic acid | 331.1690 | −0.8 | C23H22O2 | 183 | ||
| 73 | 18.28 | 12-O-Methylcarnosic acid | 345.2070 | 2.9 | C21H30O4 | 299, 277 | ||
| 74 | 18.93 | Taxodione | 315.1958 | 0.9 | C20H26O3 | 177, 123 | ||
| 75 | 18.94 | Coleonol (Forskolin) | 409.2562 | −5.5 | C22H34O7 | 351, 341 | ||
| 76 | 19.01 | Pachyphyllone | 315.1958 | 317.2122 | 3.4 | C20H28O3 | 149 | |
| 77 | 19.60 | Picrocrocin | 331.1741 | −3.2 | C16H26O7 | 183, 149 | ||
| 78 | 21.03 | Neoandrographolide | 481.2781 | −3.1 | C26H40O8 | 441, 401 | ||
| 79 | 21.61 | Abietic (Sylvic) acid | 303.2223 | −3.2 | C20H30O2 | 165 | ||
| 80 | 22.85 | Casearborin E | 597.2730 | 2.8 | C33H40O10 | 579, 553 | ||
| 81 | 23.82 | Casearborin C/D | 555.2575 | −2.4 | C31H38O9 | 527 | ||
| 82 | 24.25 | Casearborin A | 539.2663 | 4.4 | C31H38O8 | EtOAc, But., Rem. aq | ||
| 83 | 24.91 | Dihydrotanshinone I | 279.1027 | 4.2 | C18H14O3 | 149 | MecH | |
| Triterpenes | ||||||||
| 84 | 16.05 | Corosolic acid | 473.2323 | 0.1 | C30H48O4 | 455 | MecH | |
| 85 | 17.22 | Asiatic acid | 489.3607 | 6.7 | C30H48O5 | 453, 407, 201 | MecH, EtOAc | |
| 86 | 19.21 | Ursolic acid methyl ester | 471.3478 | 1.8 | C31H50O3 | 425, 407 | ||
| 87 | 20.87 | Melilotoside A | 591.4307 | 8.2 | C35H58O7 | 574, 292, 133 | ||
| 88 | 21.31 | Platanic acid | 459.3481 | 2.7 | C29H46O4 | 442, 316 | But. | |
| 89 | 21.92 | Orthosiphol D | 553.2685 | −0.7 | C31H36O9 | 525 | ||
| 90 | 23.53 | Unknown triterpene (Swietmanin I) | 567.2609 | 3.6 | C32H38O9 | |||
| 91 | 22.71 | Micromeric acid | 455.3526 | 1.3 | C30H46O3 | 437, 247, 203 | ||
| 92 | 23.25 | Ursolic acid | 457.3684 | 1.6 | C30H48O3 | 439, 411, 393 | ||
| 93 | 25.26 | Conrauidienol | 467.3876 | −1.7 | C32H50O2 | 450 | ||
| Fatty acids and esters | ||||||||
| 94 | 7.83 | Pinellic acid | 329.2337 | 4.2 | C18H34O5 | 211, 171 | MecH | |
| 95 | 14.89 | 16-Hydroxyhexadecanoic acid (Juniperic acid) | 271.2267 | −0.3 | C16H32O3 | 225 | MecH | |
| 96 | 17.56 | Myrestic acid | 227.2007 | 0.6 | C14H28O2 | Rem. aq | ||
| 97 | 19.19 | Palmitoleic acid | 253.2173 | 4.3 | C16H30O2 | |||
| 98 | 19.31 | Hydroxyoctadecatrienoic acid | 295.2268 | −6.7 | C18H30O3 | 277, 179 | ||
| 99 | 19.58 | Linolenic acid | 277.2182 | −0.3 | C18H30O2 | |||
| 100 | 22.73 | Palmitic acid | 255.2318 | −0.1 | C16H32O2 | 237 | ||
| 101 | 23.18 | Tetracosanoic (Lignoceric) acid | 367.3574 | 1.0 | C24H48O2 | |||
| 102 | 23.46 | Methyl 12,13-epoxystearate | 313.2753 | 5.0 | C19H36O3 | 257, 239, 97 | MecH, But. | |
| 103 | 23.52 | Glyceryl palmitate | 331.2858 | 4.7 | C19H38O4 | 239 | ||
| 104 | 23.77 | Oleic acid | 281.2485 | 3.6 | C18H34O2 | |||
| 105 | 25.55 | Methyl oleate | 295.2628 | −1.1 | C19H36O2 | |||
| 106 | 26.48 | 3-Hydroxypropyl oleate | 341.3038 | −3.6 | C21H40O3 | 95 | ||
| 107 | 26.66 | Eicosadienoic acid | 309.2762 | −8.2 | C20H36O2 | 291, 109 | ||
| Aliphatic and Hydroxybenzoic acid derivatives | ||||||||
| 108 | 1.18 | Tartaric acid | 149.0098 | −2.0 | C4H6O6 | |||
| 109 | 1.21 | Quinic acid | 191.0549 | −0.5 | C7H12O6 | 111 | Rem. aq | |
| 110 | 1.35 | 3,4-Dihydroxyphenylacetic acid | 167.0007 | −0.9 | C8H8O4 | 148, 78 | ||
| 111 | 1.37 | 2-Isopropylmalic acid | 175.0588 | 8.6 | C7H12O5 | But. | ||
| 112 | 1.38 | Galacturonic acid | 193.0709 | 2.0 | C6H10O7 | MecH | ||
| 113 | 1.39 | Malic acid | 133.0500 | −0.9 | C4H6O5 | Rem. aq, But. | ||
| 114 | 1.40 | Protocatechuic acid hexoside | 315.0716 | 1.8 | C13H16O9 | 195, 153, 109 | But., Rem. aq | |
| 115 | 1.41 | Hydroquinone glucuronide | 285.0592 | −4.5 | C12H14O8 | 165, 152 | But. | |
| 116 | 1.48 | Hydroxyphenyllactic acid | 181.0486 | −5.0 | C9H9O4 | 166, 112 | ||
| 117 | 4.57 | Suberic acid | 173.1190 | 0.1 | C8H14O4 | |||
| 118 | 6.39 | Tuberonic acid (12-hydroxy-7-isojasmonic acid) | 227.1286 | 3.6 | C12H18O4 | 209, 191, 131 | ||
| 119 | 6.61 | Pinonic acid | 183.1021 | 2.7 | C10H16O3 | 137 | But. | |
| 120 | 16.93 | Menthyl salicylate | 277.1786 | −4.3 | C17H24O3 | 231, 137 | ||
| Others | ||||||||
| 121 | 1.26 | Valine | 118.0862 | −3.9 | C5H11NO2 | 58 | ||
| 122 | 1.68 | Niacin (nicotinic acid) | 124.0393 | 1.0 | C6H5NO2 | 106, 80 | ||
| 123 | 2.12 | Proline | 116.0706 | −7.2 | C5H9NO2 | 84, 70 | ||
| 124 | 4.25 | Oleacein | 321.1337 | 1.2 | C17H20O6 | 149 | MecH, EtOAc, But. | |
| 125 | 4.34 | Hydroxyquinoline | 146.0600 | −1.1 | C9H7NO | 118, 91 | ||
| 126 | 8.05 | Loliolide | 197.1166 | −3.3 | C11H16O3 | 179, 105 | ||
| 127 | 10.58 | Tryptophol | 162.0913 | 5.8 | C10H11NO | 146, 118 | ||
| 128 | 26.89 | 7-hydroxy-4-methyl-coumarin (Hymecromone) | 177.0543 | −1.6 | C10H8O3 | 159, 149 | But. | |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
El-Gazar, A.A.; Emad, A.M.; Ragab, G.M.; Rasheed, D.M. Mentha pulegium L. (Pennyroyal, Lamiaceae) Extracts Impose Abortion or Fetal-Mediated Toxicity in Pregnant Rats; Evidenced by the Modulation of Pregnancy Hormones, MiR-520, MiR-146a, TIMP-1 and MMP-9 Protein Expressions, Inflammatory State, Certain Related Signaling Pathways, and Metabolite Profiling via UPLC-ESI-TOF-MS. Toxins 2022, 14, 347. https://0-doi-org.brum.beds.ac.uk/10.3390/toxins14050347
El-Gazar AA, Emad AM, Ragab GM, Rasheed DM. Mentha pulegium L. (Pennyroyal, Lamiaceae) Extracts Impose Abortion or Fetal-Mediated Toxicity in Pregnant Rats; Evidenced by the Modulation of Pregnancy Hormones, MiR-520, MiR-146a, TIMP-1 and MMP-9 Protein Expressions, Inflammatory State, Certain Related Signaling Pathways, and Metabolite Profiling via UPLC-ESI-TOF-MS. Toxins. 2022; 14(5):347. https://0-doi-org.brum.beds.ac.uk/10.3390/toxins14050347
Chicago/Turabian StyleEl-Gazar, Amira A., Ayat M. Emad, Ghada M. Ragab, and Dalia M. Rasheed. 2022. "Mentha pulegium L. (Pennyroyal, Lamiaceae) Extracts Impose Abortion or Fetal-Mediated Toxicity in Pregnant Rats; Evidenced by the Modulation of Pregnancy Hormones, MiR-520, MiR-146a, TIMP-1 and MMP-9 Protein Expressions, Inflammatory State, Certain Related Signaling Pathways, and Metabolite Profiling via UPLC-ESI-TOF-MS" Toxins 14, no. 5: 347. https://0-doi-org.brum.beds.ac.uk/10.3390/toxins14050347