Influence of Two Depuration Periods on the Activity and Transcription of Antioxidant Enzymes in Tilapia Exposed to Repeated Doses of Cylindrospermopsin under Laboratory Conditions
Abstract
:1. Introduction
2. Results and Discussion
2.1. Results
2.1.1. Gluthatione Peroxidase and Gluthatione-S-Transferase Activities


2.1.2. Glutathione Peroxidase and Glutathione-S-Transferase Gene Expression


2.1.3. Glutathione-S-Transferase Protein Expression (Western Blotting)

2.1.4. Determination of CYN in Water Samples from Aquaria
2.2. Discussion
3. Experimental Section
3.1. Chemicals and Reagents
3.2. Aphanizomenon Ovalisporum Culture
3.3. CYN Extraction and Analysis
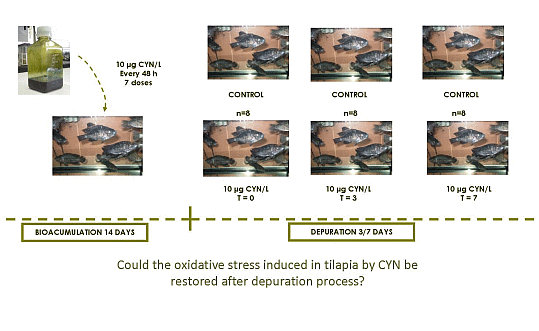
3.4. Experimental Set up and Fish Acclimation
3.5. Experimental Exposure
| Groups of Tilapia fish | |||||||
|---|---|---|---|---|---|---|---|
| Treatment condition | Period of treatment | 1 | 2 | 3 | 4 | 5 | 6 |
| Exposure to A. ovalisporum (CYN+) culture | 14 days | - | + | - | + | - | + |
| Depuration period | 0 day | + | + | - | - | - | - |
| 3 days | - | - | + | + | - | - | |
| 7 days | - | - | - | - | + | + | |
3.6. Preparation of Postmitochondrial Supernatant (PMS)
3.7. Protein Estimation
3.8. Glutathione Peroxidase and Glutathione-S-Transferase Activities
3.9. RNA Preparation and Reverse Transcription
3.10. Real-Time Polymerase Chain Reaction (RT-PCR)
| Gene | Forward primer | Reverse primer |
|---|---|---|
| Beta-actine | CAATGAGAGGTTCCGTTGC | AGGATTCCATACCAAGGAAGG |
| GPx | CCAAGAGAACTGCAAGAACGA | CAGGACACGTCATTCCTACAC |
| GST | TAATGGGAGAGGGAAGATGG | CTCTGCGATGTAATTCAGGA |
3.11. Western Blot Analysis for sGST
3.12. Statistical Analysis
4. Conclusions
Acknowledgments
Conflicts of Interest
References
- Carmichael, W.W.; Azevedo, S.M.; An, J.S.; Molica, R.J.; Jochimsen, E.M.; Lau, S.; Rinehart, K.L.; Shaw, G.R.; Eaglesham, G.K. Human fatalities from cyanobacteria: Chemical and biological evidence for cyanotoxins. Environ. Health Persp. 2001, 109, 663–668. [Google Scholar] [CrossRef]
- Falconer, I.R.; Humpage, A.R. Health risk assessment of cyanobacterial (blue-green algal) toxins in drinking water. Int. J. Environ. Res. Public Health 2005, 2, 43–50. [Google Scholar] [CrossRef]
- Poniedziałek, B.; Rzymski, P.; Kokociński, M. Cylindrospermopsin: Water-linked potential threat to human health in Europe. Environ. Toxicol. Phar. 2012, 34, 651–660. [Google Scholar] [CrossRef]
- Merel, S.; Walker, D.; Chicana, R.; Snyder, S.; Baurès, E.; Thomas, O. State of knowledge and concerns on cyanobacterial blooms and cyanotoxins. Environ. Int. 2013, 59, 303–327. [Google Scholar] [CrossRef] [Green Version]
- Neilan, B.A.; Pearson, L.A.; Muenchhoff, J.; Moffitt, M.C.; Dittmann, E. Environmental conditions that influence toxin biosynthesis in cyanobacteria. Environ. Microbiol. 2013, 15, 1239–1253. [Google Scholar] [CrossRef]
- Banker, R.; Carmeli, S.; Werman, M.; Teltsch, B.; Poral, R.; Sukenik, A. Uracil moiety is required for toxicity of the cyanobacterial hepatotoxin cylindrospermopsin. J. Toxicol. Environ. Health Part A 2001, 62, 281–288. [Google Scholar] [CrossRef]
- Kinnear, S. Cylindrospermopsin: A decade of progress on bioaccumulation research. Mar. Drugs 2010, 8, 542–564. [Google Scholar] [CrossRef]
- Chiswell, R.K.; Shaw, G.R.; Eaglesham, G.; Smith, M.J.; Norris, R.L.; Seawright, A.A.; Moore, M.R. Stability of cylindrospermopsin, the toxin from cyanobacterium, Cylindropsermopsis raciborskii: Effect of pH, temperature, and sunlight on decomposition. Environ. Toxicol. 1999, 14, 155–161. [Google Scholar] [CrossRef]
- Rücker, J.; Stüken, A.; Nixdorf, B.; Fastner, J.; Chorus, I.; Wiedner, C. Concentrations of particulate and dissolved cylindrospermopsin in 21 Aphanizomenon-dominated temperate lakes. Toxicon 2007, 50, 800–809. [Google Scholar] [CrossRef]
- De La Cruz, A.A.; Hiskia, A.; Kaloudis, T.; Chernoff, N.; Hill, D.; Antoniou, M.G.; He, X.; Loftin, K.; O’Shea, K.; Zhao, C.; et al. A review on cylindrospermopsin: the global occurrence, detection, toxicity and degradation of a potent cyanotoxin. Environ. Sci. 2013, 15, 1979–2003. [Google Scholar]
- Kinnear, S.H.W.; Fabbro, L.D.; Duivenvoorden, L.J.; Hibberd, E.M.A. Multiple-organ toxicity resulting from cylindrospermopsin exposure in tadpoles of the cane toad (Bufo marinus). Environ. Toxicol. 2007, 22, 550–558. [Google Scholar] [CrossRef]
- White, S.H.; Duivenvoorden, L.J.; Fabbro, L.D.; Eaglesham, G.K. Influence of intracellular toxin concentration on cylindrospermopsin bioaccumulation in freshwater gastropod (Melanoides tuberculata). Toxicon 2006, 47, 497–509. [Google Scholar] [CrossRef]
- Puerto, M.; Jos, A.; Pichardo, S.; Gutiérrez-Praena, D.; Cameán, A.M. Acute effects of pure Cylindrospermopsin on the activity and transcription of antioxidant enzymes in Tilapia (Oreochromis niloticus) exposed by gavage. Ecotoxicology 2011, 20, 1852–1860. [Google Scholar] [CrossRef]
- Gutiérrez-Praena, D.; Jos, A.; Pichardo, S.; Moyano, R.; Blanco, A.; Monterde, J.; Cameán, A.M. Time-dependent histopathological changes induced in Tilapia (Oreochromis niloticus) after acute exposure to pure cylindrospermopsin by oral and intraperitoneal route. Ecotoxicol. Environ. Saf. 2012, 76, 102–113. [Google Scholar] [CrossRef]
- Metcalf, J.S.; Barakate, A.; Codd, G.A. Inhibition of plant protein synthesis by the cyanobacterial hepatotoxin, cylindrospermopsin. FEMS Microbiol. Lett. 2004, 235, 125–129. [Google Scholar] [CrossRef]
- Kinnear, S.H.W.; Fabbro, L.D.; Duivenvoorden, L.J. Variable growth responses of water thyme (Hydrilla verticillata) to whole-cell extracts of Cylindrospermopsis raciborskii. Arch. Environ. Contam. Toxicol. 2008, 54, 187–194. [Google Scholar] [CrossRef]
- Rasmussen, J.P.; Cursaro, M.; Froscio, S.M.; Saint, C.P. An examination of the antibiotic effects of cylindrospermopsin on common gram-positive and gram-negative bacteria and the protozoan Naegleria lovaniensis. Environ. Toxicol. 2008, 23, 36–43. [Google Scholar] [CrossRef]
- Žegura, B.; Štraser, A.; Filipic, M. Genotoxicity and potential carcinogenicity of cyanobacterial toxins—A review. Mutat. Res. 2011, 727, 16–41. [Google Scholar] [CrossRef]
- Gutiérrez-Praena, D.; Jos, A.; Pichardo, S.; Cameán, A.M. Oxidative stress responses in tilapia (Oreochromis niloticus) exposed to a single dose of pure cylindrospermopsin under laboratory conditions: influence of the exposure way and the time of sacrifice. Aquat. Toxicol. 2011, 105, 100–106. [Google Scholar] [CrossRef]
- Gutiérrez-Praena, D.; Pichardo, S.; Jos, M.A.; Cameán, A.M. Toxicity and glutathione implication in effects observed by exposure of the liver fish cell line PLHC-1 to pure cylindrospermopsin. Ecotoxicol. Environ. Saf. 2011, 74, 1567–1572. [Google Scholar]
- Gutiérrez-Praena, D.; Jos, A.; Pichardo, S.; Puerto, M.; Cameán, A.M. Influence of the exposure way and the time of sacrifice on the effects induced by a single dose of pure Cylindrospermopsin on the activity and transcription of glutathione peroxidase and glutathione-S-transferase enzymes in Tilapia (Oreochromis niloticus). Chemosphere 2013, 90, 986–992. [Google Scholar] [CrossRef]
- Masten, S.; Carson, B. Cylindrospermopsin—Review of Toxicological Literature. Toxicological Summary For Cylindrospermopsin. Final Report 2000; CASRN 143545-90-8; National Institute of Environmental Health Sciences: Research Triangle Park, NC, USA, 2001. [Google Scholar]
- Guzmán-Guillén, R.; Prieto, A.I.; Vasconcelos, V.M.; Cameán, A.M. Cyanobacterium producing cylindrospermopsin cause oxidative stress at environmentally relevant concentrations in sub-chronically exposed tilapia (Oreochromis niloticus). Chemosphere 2013, 90, 1184–1194. [Google Scholar] [CrossRef]
- Guzmán-Guillén, R.; Prieto, A.I.; Moreno, I.; Vasconcelos, V.M.; Moyano, R.; Blanco, A.; Cameán Fernandez, A.M. Cyanobacterium producing cylindrospermopsin cause histopathological changes at environmentally relevant concentrations in subchronically exposed tilapia (Oreochromis niloticus). Environ. Toxicol. 2013. [Google Scholar] [CrossRef]
- Ferreira, M.; Moradas-Ferreira, P.; Reis-Henriques, M.A. Oxidative stress biomarkers in two resident species, mullet (Mugil cephalus) and flounder (Platichthys flesus), from a polluted site in River Douro Estuary, Portugal. Aquat. Toxicol. 2005, 71, 39–48. [Google Scholar] [CrossRef]
- Ferreira, M.; Moradas-Ferreira, P.; Reis-Henriques, M.A. The effect of long-term depuration on levels of oxidative stress biomarkers in mullets (Mugil cephalus) chronically exposed to contaminants. Mar. Environ. Res. 2007, 64, 181–190. [Google Scholar] [CrossRef]
- Özcan Oruç, E. Oxidative stress, steroid hormone concentrations and acetylcholinesterase activity in Oreochromis niloticus exposed to chlorpyrifos. Pestic. Biochem. Phys. 2010, 96, 160–166. [Google Scholar] [CrossRef]
- Freitas, R.; Ramos Pinto, L.; Sampaioc, M.; Costac, A.; Silva, M.; Rodrigues, A.M.; Quintino, V.; Figueira, E. Effects of depuration on the element concentration in bivalves: Comparison between sympatric Ruditapes decussatus and Ruditapes philippinarum. Estuar. Coast. Shelf Sci. 2012, 110, 43–53. [Google Scholar] [CrossRef]
- Gagnaire, B.; Cavalie, I.; Camilleri, V.; Adam-Guillermin, C. Effects of depleted uranium on oxidative stress, detoxification, and defence parameters of zebrafish Danio rerio. Arch. Environ. Contam. Toxicol. 2013, 64, 140–150. [Google Scholar] [CrossRef]
- Ozawa, K.; Yokoyama, A.; Ishikawa, K.; Kumagai, M.; Watanabe, M.F.; Park, H.D. Accumulation and depuration of microcystin produced by the cyanobacterium Microcystis in a freshwater snail. Limnology 2003, 4, 131–138. [Google Scholar] [CrossRef]
- Vasconcelos, V.; Oliveira, S.; Teles, F.O. Impact of a toxic and a non-toxic strain of Microcystis aeruginosa on the crayfish Procambarus clarkii. Toxicon 2001, 39, 1461–1470. [Google Scholar] [CrossRef]
- Tricarico, E.; Bertocchi, S.; Brusconi, S.; Casalone, E.; Gherardi, F.; Giorgi, G.; Mastromei, G.; Parisi, G. Depuration of microcystin-LR from the red swamp crayfish Procambarus clarkii with assessment of its food quality. Aquaculture 2008, 285, 90–95. [Google Scholar] [CrossRef]
- Galanti, L.N.; Ame, M.V.; Wunderlin, D.A. Accumulation and detoxification dynamic of cyanotoxins in the freshwater shrimp Palaemonetes argentinus. Harmful Algae 2013, 27, 88–97. [Google Scholar] [CrossRef]
- Kankaanpää, H.; Leinio, S.; Olin, M.; Sjövall, O.; Meriluoto, J.; Lehtonen, K.K. Accumulation and depuration of cyanobacterial toxin nodularin and biomarker responses in the mussel Mytilus edulis. Chemosphere 2007, 68, 1210–1217. [Google Scholar] [CrossRef]
- Saker, M.L.; Metcalf, J.S.; Codd, G.A.; Vasconcelos, V.M. Accumulation and depuration of the cyanobacterial toxin cylindrospermopsin in the freshwater mussel Anodonta cygnea. Toxicon 2004, 42, 185–194. [Google Scholar]
- Humpage, A.R; Falconer, I.R. Oral toxicity of the cyanobacterial toxin cylindrospermopsin in male swiss albino mice: determination of no observed adverse effect level for deriving a drinking water guideline value. Environ. Toxicol. 2003, 18, 94–103. [Google Scholar] [CrossRef]
- Norris, R.L.; Seawright, A.A.; Shaw, G.R.; Smith, M.J.; Chiswell, R.K.; Moore, M.R. Distribution of 14C-cylindrospermopsin in vivo in the mouse. Environ. Toxicol. 2001, 16, 498–505. [Google Scholar] [CrossRef]
- Guzmán-Guillén, R.; Prieto, A.I.; Moreno, I.; Ríos, V.; Vasconcelos, V.M.; Cameán, A.M. Effects of depuration on oxidative biomarkers in tilapia (Oreochromis niloticus) after subchronic exposure to cyanobacterium producing cylindrospermopsin. Aquatic Toxicol. 2014, 149, 40–49. [Google Scholar] [CrossRef]
- Vernet, P.; Rock, E.; Mazur, A.; Rayssiguier, Y.; Dufaure, J.P.; Drevet, J.R. Selenium-independent epididymis-restricted glutathione peroxidase 5 protein (GPX5) can back up failing Se-dependent GPXs in mice subjected to selenium deficiency. Mol. Reprod. Dev. 1999, 54, 362–370. [Google Scholar] [CrossRef]
- Wang, L.; Liang, X.F.; Liao, W.Q.; Lei, L.M.; Han, B.P. Structural and functional characterization of microcystin detoxification-related liver genes in a phytoplanktivorous fish, Nile tilapia (Oreochromis niloticus). Comp. Biochem. Physiol. C Toxicol. Pharmacol. 2006, 144, 216–227. [Google Scholar] [CrossRef]
- Almar, M.; Otero, L.; Santos, C.; González Gallego, J. Liver glutathione content and glutathione-dependent enzymes of two species of freshwater fish as bioindicators of chemical pollution. J. Environ. Sci. Health Part B 1998, 33, 769–783. [Google Scholar] [CrossRef]
- Paris-Palacios, S.; Biagianti-Risbourg, S.; Vernet, G. Biochemical and (ultra)structural hepatic perturbations of Brachydanio rerio (Teleostei Cyprinidae) exposed to two sublethal concentrations of copper sulfate. Aquat. Toxicol. 2000, 50, 109–124. [Google Scholar] [CrossRef]
- Norris, R.L.G.; Seawright, A.A.; Shaw, G.R.; Senogles, P.; Eaglesham, G.K.; Smith, M.J.; Chiswell, R.K.; Moore, M.R. Hepatic xenobiotic metabolism of cylindrospermopsin in vivo in the mouse. Toxicon 2002, 40, 471–476. [Google Scholar] [CrossRef]
- Wiegand, C.; Pflugmacher, S.; Oberemm, A.; Meems, N.; Beattie, K.A.; Steinberg, C.E.W.; Codd, G.A. Uptake and effects of microcystin-LR on detoxification enzymes of early life stages of the zebrafish (Danio rerio). Environ. Toxicol. 1999, 14, 89–95. [Google Scholar] [CrossRef]
- Burmester, V.; Nimptsch, J.; Wiegand, C. Adaptation of freshwater mussels to cyanobacterial toxins: Response of the biotransformation and antioxidant enzymes. Ecotox. Environ. Saf. 2012, 78, 296–309. [Google Scholar] [CrossRef]
- Li, G.; Xie, P.; Fu, J.; Hao, L.; Xiong, Q.; Li, H. Microcystin-induced variations in transcription of GSTs in an omnivorous freshwater fish, goldfish. Aquat. Toxicol. 2008, 88, 75–80. [Google Scholar] [CrossRef]
- He, S.; Liang, X.; Sun, J.; Shen, D. Induction of liver GST transcriptions by tert-butylhydroquinone reduced microcystin-LR accumulation in Nile tilapia (Oreochromis niloticus). Ecotox. Environ. Saf. 2013, 90, 128–135. [Google Scholar] [CrossRef]
- Terao, K.; Ohmori, S.; Igarashi, K.; Ohtani, I.; Watanabe, M.F.; Harada, K.I.; Ito, E.; Watanabe, M. Electron microscopic studies on experimental poisoning in mice induced by cylindrospermopsin isolated from blue-green alga. Umezakia Natans. Toxicon 1994, 32, 833–843. [Google Scholar]
- Runnegar, M.T.; Kong, S.M.; Zhong, Y.Z.; Lu, S.C. Inhibition of reduced glutathione synthesis by cyanobacterial alkaloid cylindrospermopsin in cultured rat hepatocytes. Biochem. Pharmacol. 1995, 49, 219–225. [Google Scholar] [CrossRef]
- Humpage, A.R.; Fontaine, F.; Froscio, S.M.; Burcham, P.C.; Falconer, I.R. Cylindrospermopsin genotoxicity and cytotoxicity: role of cytochrome P-450 and oxidative stress. J. Toxicol. Environ. Health Part A 2005, 68, 739–753. [Google Scholar]
- Sun, Y.; Yu, H.; Zhang, J.; Yin, Y.; Shi, H.; Wang, X. Bioaccumulation, depuration and oxidative stress in fish Carassius auratus under phenanthrene exposure. Chemosphere 2006, 63, 1319–1327. [Google Scholar] [CrossRef]
- Banker, R.; Carmeli, S.; Hadas, O.; Teltsch, B.; Poral, R.; Sukenik, A. Identification of cylindrospermopsin in Aphanizomenon ovalisporum (cyanophyceae) isolated from Lake Kinneret, Israel. J. Phycol. 1997, 33, 613–616. [Google Scholar]
- Guzmán-Guillén, R.; Prieto Ortega, A.I.; Moreno, I.; González, A.G.; Soria-Díaz, M.E.; Vasconcelos, V.; Cameán, A.M. Development and optimization of a method for the determination of Cylindrospermopsin from strains of Aphanizomenon cultures: intra-laboratory assessment of its accuracy by using validation standards. Talanta 2012, 100, 356–363. [Google Scholar] [CrossRef]
- Guzmán-Guillén, R.; Prieto, A.I.; González, A.G.; Soria-Díaz, M.E.; Cameán, A.M. Cylindrospermopsin determination in water by LC-MS/MS: Optimization and validation of the method and application to real samples. Environ. Toxicol. Chem. 2012, 31, 2233–2238. [Google Scholar] [CrossRef]
- Puerto, M.; Prieto, A.I.; Pichardo, S.; Moreno, I.; Jos, A.; Moyano, R.; Cameán, A.M. Effects of dietary N-acetylcysteine (NAC) on the oxidative stress induced in tilapia (Oreochromis niloticus) exposed to a microcystin-producing cyanobacterial water bloom. Environ. Toxicol. Chem. 2009, 28, 1679–1686. [Google Scholar] [CrossRef]
- Bradford, M. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal. Biochem. 1976, 72, 248–254. [Google Scholar] [CrossRef]
- Habig, W.H.; Pabst, M.J.; Jakoby, W.B. Glutathione S-transferases: The first enzymatic step in mercapturic acid formation. Biol. Chem. 1974, 249, 7130–7139. [Google Scholar]
- Puerto, M.; Gutiérrez-Praena, D.; Prieto, A.I.; Pichardo, S.; Jos, A.; Miguel-Carrasco, J.L.; Vázquez, C.M.; Cameán, A. Subchronic effects of cyanobacterial cells on the transcription of antioxidant enzyme genes in tilapia (Oreochromis sp.). Ecotoxicology 2010, 20, 479–490. [Google Scholar]
- Livak, K.J.; Schmittgen, T.D. Analysis of relative gene expression data using Real-Time quantitative PCR and the 2−ΔΔCT method. Methods 2001, 25, 402–408. [Google Scholar] [CrossRef]
- Mate, A.; Barfull, A.; Hermosa, A.M.; Gomez-Amores, L.; Vazquez, C.M.; Planas, J.M. Regulation of sodium-glucose cotransporter SGLT1 in the intestine of hypertensive rats. Am. J. Physiol. Regul. Integr. Comp. Physiol. 2006, 291, 760–767. [Google Scholar] [CrossRef]
© 2014 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/3.0/).
Share and Cite
Ríos, V.; Guzmán-Guillén, R.; Moreno, I.M.; Prieto, A.I.; Puerto, M.; Jos, A.; Cameán, A.M. Influence of Two Depuration Periods on the Activity and Transcription of Antioxidant Enzymes in Tilapia Exposed to Repeated Doses of Cylindrospermopsin under Laboratory Conditions. Toxins 2014, 6, 1062-1079. https://0-doi-org.brum.beds.ac.uk/10.3390/toxins6031062
Ríos V, Guzmán-Guillén R, Moreno IM, Prieto AI, Puerto M, Jos A, Cameán AM. Influence of Two Depuration Periods on the Activity and Transcription of Antioxidant Enzymes in Tilapia Exposed to Repeated Doses of Cylindrospermopsin under Laboratory Conditions. Toxins. 2014; 6(3):1062-1079. https://0-doi-org.brum.beds.ac.uk/10.3390/toxins6031062
Chicago/Turabian StyleRíos, Victoria, Remedios Guzmán-Guillén, Isabel M. Moreno, Ana I. Prieto, María Puerto, Angeles Jos, and Ana M. Cameán. 2014. "Influence of Two Depuration Periods on the Activity and Transcription of Antioxidant Enzymes in Tilapia Exposed to Repeated Doses of Cylindrospermopsin under Laboratory Conditions" Toxins 6, no. 3: 1062-1079. https://0-doi-org.brum.beds.ac.uk/10.3390/toxins6031062