White Adipose Tissue as a Site for Islet Transplantation
Abstract
:1. Introduction
2. Previous Trials Using White Adipose Tissue as a Transplant Site for Islet Transplantation
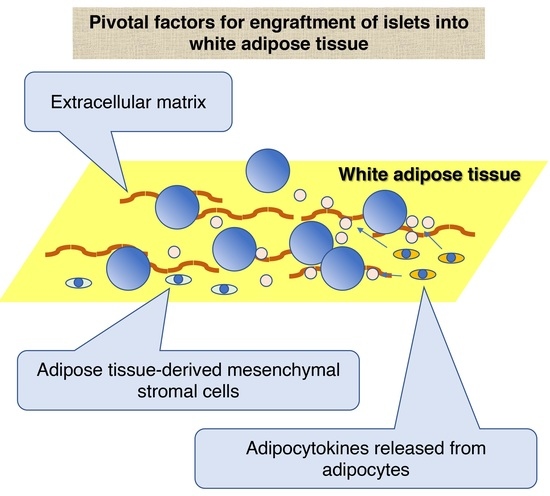
3. Characteristics of White Adipose Tissue as a Site for Islet Transplantation
3.1. ECM of White Adipose Tissue
3.2. Cellular Components of White Adipose Tissue
3.2.1. ADMSCs
3.2.2. Adipocytes
3.2.3. Adipose Tissue Macrophage
4. Clinical Trial of Islet Transplantation into White Adipose Tissue and Conclusion
5. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Bellin, M.D.; Kandaswamy, R.; Parkey, J.; Zhang, H.J.; Liu, B.; Ihm, S.H.; Ansite, J.D.; Witson, J.; Bansal-Pakala, P.; Balamurugan, A.N.; et al. Prolonged insulin independence after islet allotransplants in recipients with type 1 diabetes. Am. J. Transplant. 2008, 8, 2463–2470. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Barton, F.B.; Rickels, M.R.; Alejandro, R.; Hering, B.J.; Wease, S.; Naziruddin, B.; Oberholzer, J.; Odorico, J.S.; Garfinkel, M.R.; Levy, M.; et al. Improvement in outcomes of clinical islet transplantation: 1999–2010. Diabetes Care 2012, 35, 1436–1445. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Balamurugan, A.N.; Naziruddin, B.; Lockridge, A.; Tiwari, M.; Loganathan, G.; Takita, M.; Matsumoto, S.; Papas, K.; Trieger, M.; Rainis, H.; et al. Islet product characteristics and factors related to successful human islet transplantation from the Collaborative Islet Transplant Registry (CITR) 1999–2010. Am. J. Transplant. 2014, 14, 2595–2606. [Google Scholar] [CrossRef] [PubMed]
- Hering, B.J.; Clarke, W.R.; Bridges, N.D.; Eggerman, T.L.; Alejandro, R.; Bellin, M.D.; Chaloner, K.; Czarniecki, C.W.; Goldstein, J.S.; Hunsicker, L.G.; et al. Phase 3 Trial of Transplantation of Human Islets in Type 1 Diabetes Complicated by Severe Hypoglycemia. Diabetes Care 2016, 39, 1230–1240. [Google Scholar] [CrossRef] [Green Version]
- Sakata, N.; Hayes, P.; Tan, A.; Chan, N.K.; Mace, J.; Peverini, R.; Sowers, L.; Pearce, W.J.; Chinnock, R.; Obenaus, A.; et al. MRI assessment of ischemic liver after intraportal islet transplantation. Transplantation 2009, 87, 825–830. [Google Scholar] [CrossRef] [Green Version]
- Sakata, N.; Yoshimatsu, G.; Kodama, S. The Spleen as an Optimal Site for Islet Transplantation and a Source of Mesenchymal Stem Cells. Int. J. Mol. Sci. 2018, 19, 1391. [Google Scholar] [CrossRef] [Green Version]
- Naziruddin, B.; Kanak, M.A.; Chang, C.A.; Takita, M.; Lawrence, M.C.; Dennison, A.R.; Onaca, N.; Levy, M.F. Improved outcomes of islet autotransplant after total pancreatectomy by combined blockade of IL-1beta and TNFalpha. Am. J. Transplant. 2018, 18, 2322–2329. [Google Scholar] [CrossRef] [Green Version]
- Ishiyama, K.; Rawson, J.; Omori, K.; Mullen, Y. Liver natural killer cells play a role in the destruction of islets after intraportal transplantation. Transplantation 2011, 91, 952–960. [Google Scholar] [CrossRef]
- Saeki, Y.; Ishiyama, K.; Ishida, N.; Tanaka, Y.; Ohdan, H. Memory-like Liver Natural Killer Cells are Responsible for Islet Destruction in Secondary Islet Transplantation. Sci. Rep. 2019, 9, 1022. [Google Scholar] [CrossRef] [Green Version]
- Bottino, R.; Fernandez, L.A.; Ricordi, C.; Lehmann, R.; Tsan, M.F.; Oliver, R.; Inverardi, L. Transplantation of allogeneic islets of Langerhans in the rat liver: Effects of macrophage depletion on graft survival and microenvironment activation. Diabetes 1998, 47, 316–323. [Google Scholar] [CrossRef]
- Bennet, W.; Groth, C.G.; Larsson, R.; Nilsson, B.; Korsgren, O. Isolated human islets trigger an instant blood mediated inflammatory reaction: Implications for intraportal islet transplantation as a treatment for patients with type 1 diabetes. Ups. J. Med. Sci. 2000, 105, 125–133. [Google Scholar] [CrossRef] [PubMed]
- Sakata, N.; Obenaus, A.; Chan, N.; Mace, J.; Chinnock, R.; Hathout, E. Factors affecting islet graft embolization in the liver of diabetic mice. Islets 2009, 1, 26–33. [Google Scholar] [CrossRef] [PubMed]
- Mellgren, A.; Schnell Landstrom, A.H.; Petersson, B.; Andersson, A. The renal subcapsular site offers better growth conditions for transplanted mouse pancreatic islet cells than the liver or spleen. Diabetologia 1986, 29, 670–672. [Google Scholar] [CrossRef] [PubMed]
- Fujita, M.; McGrath, K.M.; Bottino, R.; Dons, E.M.; Long, C.; Kumar, G.; Ekser, B.; Echeverri, G.J.; Hata, J.; Haruma, K.; et al. Technique of endoscopic biopsy of islet allografts transplanted into the gastric submucosal space in pigs. Cell Transplant. 2013, 22, 2335–2344. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cantarelli, E.; Citro, A.; Pellegrini, S.; Mercalli, A.; Melzi, R.; Dugnani, E.; Jofra, T.; Fousteri, G.; Mondino, A.; Piemonti, L. Transplant Site Influences the Immune Response After Islet Transplantation: Bone Marrow Versus Liver. Transplantation 2017, 101, 1046–1055. [Google Scholar] [CrossRef] [PubMed]
- Itoh, T.; Nishinakamura, H.; Kumano, K.; Takahashi, H.; Kodama, S. The Spleen Is an Ideal Site for Inducing Transplanted Islet Graft Expansion in Mice. PLoS ONE 2017, 12, e0170899. [Google Scholar] [CrossRef]
- Sakata, N.; Aoki, T.; Yoshimatsu, G.; Tsuchiya, H.; Hata, T.; Katayose, Y.; Egawa, S.; Unno, M. Strategy for clinical setting in intramuscular and subcutaneous islet transplantation. Diabetes Metab. Res. Rev. 2014, 30, 1–10. [Google Scholar] [CrossRef]
- Merani, S.; Toso, C.; Emamaullee, J.; Shapiro, A.M. Optimal implantation site for pancreatic islet transplantation. Br. J. Surg. 2008, 95, 1449–1461. [Google Scholar] [CrossRef]
- Outzen, H.C.; Leiter, E.H. Transplantation of pancreatic islets into cleared mammary fat pads. Transplantation 1981, 32, 101–105. [Google Scholar] [CrossRef]
- Cuthbertson, R.A.; Mandel, T.E. A comparison of portal versus systemic venous drainage in murine foetal pancreatic islet transplantation. Aust. J. Exp. Biol. Med. Sci. 1986, 64 Pt 2, 175–184. [Google Scholar] [CrossRef]
- Yasunami, Y.; Lacy, P.E.; Finke, E.H. A new site for islet transplantation—A peritoneal-omental pouch. Transplantation 1983, 36, 181–182. [Google Scholar] [CrossRef] [PubMed]
- Ao, Z.; Matayoshi, K.; Lakey, J.R.; Rajotte, R.V.; Warnock, G.L. Survival and function of purified islets in the omental pouch site of outbred dogs. Transplantation 1993, 56, 524–529. [Google Scholar] [CrossRef] [PubMed]
- Kin, T.; Korbutt, G.S.; Rajotte, R.V. Survival and metabolic function of syngeneic rat islet grafts transplanted in the omental pouch. Am. J. Transplant. 2003, 3, 281–285. [Google Scholar] [CrossRef]
- Lu, Y.; Zou, S.; Bertera, S.; Bottino, R.; Cooper, D.K.C.; Liu, Z.; Huang, Y.; Wang, C.; Hong, C.; He, T.; et al. A Method for Islet Transplantation to the Omentum in Mouse. J. Vis. Exp. 2019. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ibarra, V.; Appel, A.A.; Anastasio, M.A.; Opara, E.C.; Brey, E.M. This paper is a winner in the Undergraduate category for the SFB awards: Evaluation of the tissue response to alginate encapsulated islets in an omentum pouch model. J. Biomed. Mater. Res. A 2016, 104, 1581–1590. [Google Scholar] [CrossRef] [Green Version]
- Montazeri, L.; Hojjati-Emami, S.; Bonakdar, S.; Tahamtani, Y.; Hajizadeh-Saffar, E.; Noori-Keshtkar, M.; Najar-Asl, M.; Ashtiani, M.K.; Baharvand, H. Improvement of islet engrafts by enhanced angiogenesis and microparticle-mediated oxygenation. Biomaterials 2016, 89, 157–165. [Google Scholar] [CrossRef] [Green Version]
- Hajizadeh-Saffar, E.; Tahamtani, Y.; Aghdami, N.; Azadmanesh, K.; Habibi-Anbouhi, M.; Heremans, Y.; De Leu, N.; Heimberg, H.; Ravassard, P.; Shokrgozar, M.A.; et al. Inducible VEGF expression by human embryonic stem cell-derived mesenchymal stromal cells reduces the minimal islet mass required to reverse diabetes. Sci. Rep. 2015, 5, 9322. [Google Scholar] [CrossRef]
- Pareta, R.; McQuilling, J.P.; Sittadjody, S.; Jenkins, R.; Bowden, S.; Orlando, G.; Farney, A.C.; Brey, E.M.; Opara, E.C. Long-term function of islets encapsulated in a redesigned alginate microcapsule construct in omentum pouches of immune-competent diabetic rats. Pancreas 2014, 43, 605–613. [Google Scholar] [CrossRef] [Green Version]
- Pedraza, E.; Brady, A.C.; Fraker, C.A.; Molano, R.D.; Sukert, S.; Berman, D.M.; Kenyon, N.S.; Pileggi, A.; Ricordi, C.; Stabler, C.L. Macroporous three-dimensional PDMS scaffolds for extrahepatic islet transplantation. Cell Transplant. 2013, 22, 1123–1135. [Google Scholar] [CrossRef] [Green Version]
- Kriz, J.; Vilk, G.; Mazzuca, D.M.; Toleikis, P.M.; Foster, P.J.; White, D.J. A novel technique for the transplantation of pancreatic islets within a vascularized device into the greater omentum to achieve insulin independence. Am. J. Surg. 2012, 203, 793–797. [Google Scholar] [CrossRef]
- Gupta, R.; Sefton, M.V. Application of an endothelialized modular construct for islet transplantation in syngeneic and allogeneic immunosuppressed rat models. Tissue Eng. Part A 2011, 17, 2005–2015. [Google Scholar] [CrossRef] [PubMed]
- Kim, H.I.; Yu, J.E.; Park, C.G.; Kim, S.J. Comparison of four pancreatic islet implantation sites. J. Korean Med. Sci. 2010, 25, 203–210. [Google Scholar] [CrossRef] [PubMed]
- Solari, M.G.; Srinivasan, S.; Boumaza, I.; Unadkat, J.; Harb, G.; Garcia-Ocana, A.; Feili-Hariri, M. Marginal mass islet transplantation with autologous mesenchymal stem cells promotes long-term islet allograft survival and sustained normoglycemia. J. Autoimmun. 2009, 32, 116–124. [Google Scholar] [CrossRef] [PubMed]
- Berman, D.M.; O’Neil, J.J.; Coffey, L.C.; Chaffanjon, P.C.; Kenyon, N.M.; Ruiz, P., Jr.; Pileggi, A.; Ricordi, C.; Kenyon, N.S. Long-term survival of nonhuman primate islets implanted in an omental pouch on a biodegradable scaffold. Am. J. Transplant. 2009, 9, 91–104. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kin, T.; O’Neil, J.J.; Pawlick, R.; Korbutt, G.S.; Shapiro, A.M.; Lakey, J.R. The use of an approved biodegradable polymer scaffold as a solid support system for improvement of islet engraftment. Artif. Organs 2008, 32, 990–993. [Google Scholar] [CrossRef]
- Kobayashi, T.; Aomatsu, Y.; Iwata, H.; Kin, T.; Kanehiro, H.; Hisanga, M.; Ko, S.; Nagao, M.; Harb, G.; Nakajima, Y. Survival of microencapsulated islets at 400 days posttransplantation in the omental pouch of NOD mice. Cell Transplant. 2006, 15, 359–365. [Google Scholar] [CrossRef] [Green Version]
- Guan, J.; Behme, M.T.; Zucker, P.; Atkison, P.; Hramiak, I.; Zhong, R.; Dupre, J. Glucose turnover and insulin sensitivity in rats with pancreatic islet transplants. Diabetes 1998, 47, 1020–1026. [Google Scholar] [CrossRef]
- al-Abdullah, I.H.; Anil Kumar, M.S.; Kelly-Sullivan, D.; Abouna, G.M. Site for unpurified islet transplantation is an important parameter for determination of the outcome of graft survival and function. Cell Transplant. 1995, 4, 297–305. [Google Scholar] [CrossRef]
- Kasoju, N.; Patikova, A.; Wawrzynska, E.; Vojtiskova, A.; Sedlacik, T.; Kumorek, M.; Pop-Georgievski, O.; Sticova, E.; Kri, Z.J.; Kubies, D. Bioengineering a pre-vascularized pouch for subsequent islet transplantation using VEGF-loaded polylactide capsules. Biomater. Sci. 2020, 8, 631–647. [Google Scholar] [CrossRef] [Green Version]
- Tuch, B.E.; Sheil, A.G.; Ng, A.B.; Turtle, J.R. Long-term survival of human fetal pancreatic tissue transplanted into an insulin-dependent diabetic patient. Diabet Med. 1986, 3, 24–28. [Google Scholar] [CrossRef]
- Chusyd, D.E.; Wang, D.; Huffman, D.M.; Nagy, T.R. Relationships between Rodent White Adipose Fat Pads and Human White Adipose Fat Depots. Front. Nutr. 2016, 3, 10. [Google Scholar] [CrossRef] [Green Version]
- Weaver, J.D.; Headen, D.M.; Aquart, J.; Johnson, C.T.; Shea, L.D.; Shirwan, H.; Garcia, A.J. Vasculogenic hydrogel enhances islet survival, engraftment, and function in leading extrahepatic sites. Sci. Adv. 2017, 3, e1700184. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rajotte, R.V.; Tighe, V.M.; Warnock, G.L.; Kneteman, N.M.; Finegood, D.T. New site for islet transplantation in rats—Liver-mesentery or liver-omental pouch. Transplant. Proc. 1994, 26, 675. [Google Scholar] [PubMed]
- Michalska, W.; Garnuszek, P.; Licinska, I.; Wilgus, J.; Szymanska, K.; Rowinski, W.; Mazurek, A.P.; Fiedor, P. Monitoring of pancreatic islets transplanted to colon mesentery. Transplant. Proc. 2002, 34, 653–654. [Google Scholar] [CrossRef]
- Hendrawan, S.; Yusuf, I.; Hatta, M.; Aman, M.; Patellongi, I.; Serra, A.L.; Lawrence, G.; Weber, U.; Sutedja, B.; Baer, H.U. Allogeneic islet cells implant on poly-l-lactide matrix to reduce hyperglycaemia in streptozotocin-induced diabetic rat. Pancreatology 2017, 17, 411–418. [Google Scholar] [CrossRef]
- Phelps, E.A.; Templeman, K.L.; Thule, P.M.; Garcia, A.J. Engineered VEGF-releasing PEG-MAL hydrogel for pancreatic islet vascularization. Drug Deliv. Transl. Res. 2015, 5, 125–136. [Google Scholar] [CrossRef]
- Phelps, E.A.; Headen, D.M.; Taylor, W.R.; Thule, P.M.; Garcia, A.J. Vasculogenic bio-synthetic hydrogel for enhancement of pancreatic islet engraftment and function in type 1 diabetes. Biomaterials 2013, 34, 4602–4611. [Google Scholar] [CrossRef] [Green Version]
- Vernon, R.B.; Preisinger, A.; Gooden, M.D.; D’Amico, L.A.; Yue, B.B.; Bollyky, P.L.; Kuhr, C.S.; Hefty, T.R.; Nepom, G.T.; Gebe, J.A. Reversal of diabetes in mice with a bioengineered islet implant incorporating a type I collagen hydrogel and sustained release of vascular endothelial growth factor. Cell Transplant. 2012, 21, 2099–2110. [Google Scholar] [CrossRef] [Green Version]
- Minardi, S.; Guo, M.; Zhang, X.; Luo, X. An elastin-based vasculogenic scaffold promotes marginal islet mass engraftment and function at an extrahepatic site. J. Immunol. Regen. Med. 2019, 3, 1–12. [Google Scholar] [CrossRef]
- Najjar, M.; Manzoli, V.; Abreu, M.; Villa, C.; Martino, M.M.; Molano, R.D.; Torrente, Y.; Pileggi, A.; Inverardi, L.; Ricordi, C.; et al. Fibrin gels engineered with pro-angiogenic growth factors promote engraftment of pancreatic islets in extrahepatic sites in mice. Biotechnol. Bioeng. 2015, 112, 1916–1926. [Google Scholar] [CrossRef]
- Liu, J.M.H.; Zhang, X.; Joe, S.; Luo, X.; Shea, L.D. Evaluation of biomaterial scaffold delivery of IL-33 as a localized immunomodulatory agent to support cell transplantation in adipose tissue. J. Immunol. Regen. Med. 2018, 1, 1–12. [Google Scholar] [CrossRef] [PubMed]
- Liu, J.M.H.; Zhang, J.; Zhang, X.; Hlavaty, K.A.; Ricci, C.F.; Leonard, J.N.; Shea, L.D.; Gower, R.M. Transforming growth factor-beta 1 delivery from microporous scaffolds decreases inflammation post-implant and enhances function of transplanted islets. Biomaterials 2016, 80, 11–19. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gibly, R.F.; Zhang, X.; Graham, M.L.; Hering, B.J.; Kaufman, D.B.; Lowe, W.L., Jr.; Shea, L.D. Extrahepatic islet transplantation with microporous polymer scaffolds in syngeneic mouse and allogeneic porcine models. Biomaterials 2011, 32, 9677–9684. [Google Scholar] [CrossRef] [Green Version]
- Kheradmand, T.; Wang, S.; Gibly, R.F.; Zhang, X.; Holland, S.; Tasch, J.; Graham, J.G.; Kaufman, D.B.; Miller, S.D.; Shea, L.D.; et al. Permanent protection of PLG scaffold transplanted allogeneic islet grafts in diabetic mice treated with ECDI-fixed donor splenocyte infusions. Biomaterials 2011, 32, 4517–4524. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rios, P.D.; Zhang, X.; Luo, X.; Shea, L.D. Mold-casted non-degradable, islet macro-encapsulating hydrogel devices for restoration of normoglycemia in diabetic mice. Biotechnol. Bioeng. 2016, 113, 2485–2495. [Google Scholar] [CrossRef] [PubMed]
- Brubaker, C.E.; Kissler, H.; Wang, L.J.; Kaufman, D.B.; Messersmith, P.B. Biological performance of mussel-inspired adhesive in extrahepatic islet transplantation. Biomaterials 2010, 31, 420–427. [Google Scholar] [CrossRef] [Green Version]
- Salvay, D.M.; Rives, C.B.; Zhang, X.; Chen, F.; Kaufman, D.B.; Lowe, W.L., Jr.; Shea, L.D. Extracellular matrix protein-coated scaffolds promote the reversal of diabetes after extrahepatic islet transplantation. Transplantation 2008, 85, 1456–1464. [Google Scholar] [CrossRef]
- Manzoli, V.; Villa, C.; Bayer, A.L.; Morales, L.C.; Molano, R.D.; Torrente, Y.; Ricordi, C.; Hubbell, J.A.; Tomei, A.A. Immunoisolation of murine islet allografts in vascularized sites through conformal coating with polyethylene glycol. Am. J. Transplant. 2018, 18, 590–603. [Google Scholar] [CrossRef]
- Villa, C.; Manzoli, V.; Abreu, M.M.; Verheyen, C.A.; Seskin, M.; Najjar, M.; Molano, R.D.; Torrente, Y.; Ricordi, C.; Tomei, A.A. Effects of Composition of Alginate-Polyethylene Glycol Microcapsules and Transplant Site on Encapsulated Islet Graft Outcomes in Mice. Transplantation 2017, 101, 1025–1035. [Google Scholar] [CrossRef] [Green Version]
- Liu, X.Y.; Nothias, J.M.; Scavone, A.; Garfinkel, M.; Millis, J.M. Biocompatibility investigation of polyethylene glycol and alginate-poly-L-lysine for islet encapsulation. ASAIO J. 2010, 56, 241–245. [Google Scholar] [CrossRef]
- Nguyen, T.T.; Emami, F.; Yook, S.; Nguyen, H.T.; Pham, T.T.; Pathak, S.; Regmi, S.; Kim, J.O.; Yong, C.S.; Kim, J.R.; et al. Local release of NECA (5′-(N-ethylcarboxamido)adenosine) from implantable polymeric sheets for enhanced islet revascularization in extrahepatic transplantation site. J. Control Release 2020, 321, 509–518. [Google Scholar] [CrossRef] [PubMed]
- Wang, K.; Wang, X.; Han, C.S.; Chen, L.Y.; Luo, Y. Scaffold-supported Transplantation of Islets in the Epididymal Fat Pad of Diabetic Mice. J. Vis. Exp. 2017, 54995. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Wang, K.; Zhang, W.; Qiang, M.; Luo, Y. A bilaminated decellularized scaffold for islet transplantation: Structure, properties and functions in diabetic mice. Biomaterials 2017, 138, 80–90. [Google Scholar] [CrossRef] [PubMed]
- Mao, D.; Zhu, M.; Zhang, X.; Ma, R.; Yang, X.; Ke, T.; Wang, L.; Li, Z.; Kong, D.; Li, C. A macroporous heparin-releasing silk fibroin scaffold improves islet transplantation outcome by promoting islet revascularisation and survival. Acta Biomater. 2017, 59, 210–220. [Google Scholar] [CrossRef] [PubMed]
- Buitinga, M.; Assen, F.; Hanegraaf, M.; Wieringa, P.; Hilderink, J.; Moroni, L.; Truckenmuller, R.; van Blitterswijk, C.; Romer, G.W.; Carlotti, F.; et al. Micro-fabricated scaffolds lead to efficient remission of diabetes in mice. Biomaterials 2017, 135, 10–22. [Google Scholar] [CrossRef] [PubMed]
- Gibly, R.F.; Zhang, X.; Lowe, W.L., Jr.; Shea, L.D. Porous scaffolds support extrahepatic human islet transplantation, engraftment, and function in mice. Cell Transplant. 2013, 22, 811–819. [Google Scholar] [CrossRef] [Green Version]
- Brady, A.C.; Martino, M.M.; Pedraza, E.; Sukert, S.; Pileggi, A.; Ricordi, C.; Hubbell, J.A.; Stabler, C.L. Proangiogenic hydrogels within macroporous scaffolds enhance islet engraftment in an extrahepatic site. Tissue Eng. Part A 2013, 19, 2544–2552. [Google Scholar] [CrossRef] [Green Version]
- Stiegler, P.; Matzi, V.; Pierer, E.; Hauser, O.; Schaffellner, S.; Renner, H.; Greilberger, J.; Aigner, R.; Maier, A.; Lackner, C.; et al. Creation of a prevascularized site for cell transplantation in rats. Xenotransplantation 2010, 17, 379–390. [Google Scholar] [CrossRef]
- Forster, N.A.; Penington, A.J.; Hardikar, A.A.; Palmer, J.A.; Hussey, A.; Tai, J.; Morrison, W.A.; Feeney, S.J. A prevascularized tissue engineering chamber supports growth and function of islets and progenitor cells in diabetic mice. Islets 2011, 3, 271–283. [Google Scholar] [CrossRef] [Green Version]
- Yasunami, Y.; Nakafusa, Y.; Nitta, N.; Nakamura, M.; Goto, M.; Ono, J.; Taniguchi, M. A Novel Subcutaneous Site of Islet Transplantation Superior to the Liver. Transplantation 2018, 102, 945–952. [Google Scholar] [CrossRef]
- Gregoire, F.M.; Smas, C.M.; Sul, H.S. Understanding adipocyte differentiation. Physiol. Rev. 1998, 78, 783–809. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Divoux, A.; Clement, K. Architecture and the extracellular matrix: The still unappreciated components of the adipose tissue. Obes. Rev. 2011, 12, e494–e503. [Google Scholar] [CrossRef] [PubMed]
- Weiner, F.R.; Shah, A.; Smith, P.J.; Rubin, C.S.; Zern, M.A. Regulation of collagen gene expression in 3T3-L1 cells. Effects of adipocyte differentiation and tumor necrosis factor alpha. Biochemistry 1989, 28, 4094–4099. [Google Scholar] [CrossRef] [PubMed]
- Hynes, R.O.; Zhao, Q. The evolution of cell adhesion. J. Cell Biol. 2000, 150, F89–F96. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Popova, S.N.; Lundgren-Akerlund, E.; Wiig, H.; Gullberg, D. Physiology and pathology of collagen receptors. Acta Physiol. 2007, 190, 179–187. [Google Scholar] [CrossRef]
- Kantengwa, S.; Baetens, D.; Sadoul, K.; Buck, C.A.; Halban, P.A.; Rouiller, D.G. Identification and characterization of alpha 3 beta 1 integrin on primary and transformed rat islet cells. Exp. Cell Res. 1997, 237, 394–402. [Google Scholar] [CrossRef]
- Belkin, A.M.; Stepp, M.A. Integrins as receptors for laminins. Microsc. Res. Tech. 2000, 51, 280–301. [Google Scholar] [CrossRef]
- Johansson, S.; Svineng, G.; Wennerberg, K.; Armulik, A.; Lohikangas, L. Fibronectin-integrin interactions. Front. Biosci. 1997, 2, d126–d146. [Google Scholar] [CrossRef] [Green Version]
- Hamidi, H.; Ivaska, J. Vascular Morphogenesis: An Integrin and Fibronectin Highway. Curr. Biol. 2017, 27, R158–R161. [Google Scholar] [CrossRef]
- Olaniru, O.E.; Persaud, S.J. Identifying novel therapeutic targets for diabetes through improved understanding of islet adhesion receptors. Curr. Opin. Pharmacol. 2018, 43, 27–33. [Google Scholar] [CrossRef] [Green Version]
- Kaido, T.; Yebra, M.; Cirulli, V.; Montgomery, A.M. Regulation of human beta-cell adhesion, motility, and insulin secretion by collagen IV and its receptor alpha1beta1. J. Biol. Chem. 2004, 279, 53762–53769. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bosco, D.; Meda, P.; Halban, P.A.; Rouiller, D.G. Importance of cell-matrix interactions in rat islet beta-cell secretion in vitro: Role of alpha6beta1 integrin. Diabetes 2000, 49, 233–243. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wang, R.; Li, J.; Lyte, K.; Yashpal, N.K.; Fellows, F.; Goodyer, C.G. Role for beta1 integrin and its associated alpha3, alpha5, and alpha6 subunits in development of the human fetal pancreas. Diabetes 2005, 54, 2080–2089. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yashpal, N.K.; Li, J.; Wheeler, M.B.; Wang, R. Expression of {beta}1 integrin receptors during rat pancreas development—Sites and dynamics. Endocrinology 2005, 146, 1798–1807. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Saleem, S.; Li, J.; Yee, S.P.; Fellows, G.F.; Goodyer, C.G.; Wang, R. beta1 integrin/FAK/ERK signalling pathway is essential for human fetal islet cell differentiation and survival. J. Pathol. 2009, 219, 182–192. [Google Scholar] [CrossRef]
- Krishnamurthy, M.; Li, J.; Fellows, G.F.; Rosenberg, L.; Goodyer, C.G.; Wang, R. Integrin {alpha}3, but not {beta}1, regulates islet cell survival and function via PI3K/Akt signaling pathways. Endocrinology 2011, 152, 424–435. [Google Scholar] [CrossRef] [Green Version]
- Baer, P.C.; Geiger, H. Adipose-derived mesenchymal stromal/stem cells: Tissue localization, characterization, and heterogeneity. Stem Cells Int. 2012, 2012, 812693. [Google Scholar] [CrossRef] [Green Version]
- Zuk, P.A.; Zhu, M.; Mizuno, H.; Huang, J.; Futrell, J.W.; Katz, A.J.; Benhaim, P.; Lorenz, H.P.; Hedrick, M.H. Multilineage cells from human adipose tissue: Implications for cell-based therapies. Tissue Eng. 2001, 7, 211–228. [Google Scholar] [CrossRef] [Green Version]
- Cavallari, G.; Olivi, E.; Bianchi, F.; Neri, F.; Foroni, L.; Valente, S.; La Manna, G.; Nardo, B.; Stefoni, S.; Ventura, C. Mesenchymal stem cells and islet cotransplantation in diabetic rats: Improved islet graft revascularization and function by human adipose tissue-derived stem cells preconditioned with natural molecules. Cell Transplant. 2012, 21, 2771–2781. [Google Scholar] [CrossRef] [Green Version]
- Bhang, S.H.; Jung, M.J.; Shin, J.Y.; La, W.G.; Hwang, Y.H.; Kim, M.J.; Kim, B.S.; Lee, D.Y. Mutual effect of subcutaneously transplanted human adipose-derived stem cells and pancreatic islets within fibrin gel. Biomaterials 2013, 34, 7247–7256. [Google Scholar] [CrossRef]
- Arzouni, A.A.; Vargas-Seymour, A.; Rackham, C.L.; Dhadda, P.; Huang, G.C.; Choudhary, P.; Nardi, N.; King, A.J.F.; Jones, P.M. Mesenchymal stromal cells improve human islet function through released products and extracellular matrix. Clin. Sci. 2017, 131, 2835–2845. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ren, G.; Rezaee, M.; Razavi, M.; Taysir, A.; Wang, J.; Thakor, A.S. Adipose tissue-derived mesenchymal stem cells rescue the function of islets transplanted in sub-therapeutic numbers via their angiogenic properties. Cell Tissue Res. 2019, 376, 353–364. [Google Scholar] [CrossRef] [PubMed]
- Navaei-Nigjeh, M.; Moloudizargari, M.; Baeeri, M.; Gholami, M.; Lotfibakhshaiesh, N.; Soleimani, M.; Vasheghani-Farahani, E.; Ai, J.; Abdollahi, M. Reduction of marginal mass required for successful islet transplantation in a diabetic rat model using adipose tissue-derived mesenchymal stromal cells. Cytotherapy 2018, 20, 1124–1142. [Google Scholar] [CrossRef] [PubMed]
- Song, L.; Sun, Z.; Kim, D.S.; Gou, W.; Strange, C.; Dong, H.; Cui, W.; Gilkeson, G.; Morgan, K.A.; Adams, D.B.; et al. Adipose stem cells from chronic pancreatitis patients improve mouse and human islet survival and function. Stem Cell Res. Ther. 2017, 8, 192. [Google Scholar] [CrossRef]
- Ohmura, Y.; Tanemura, M.; Kawaguchi, N.; Machida, T.; Tanida, T.; Deguchi, T.; Wada, H.; Kobayashi, S.; Marubashi, S.; Eguchi, H.; et al. Combined transplantation of pancreatic islets and adipose tissue-derived stem cells enhances the survival and insulin function of islet grafts in diabetic mice. Transplantation 2010, 90, 1366–1373. [Google Scholar] [CrossRef]
- Mohammadi Ayenehdeh, J.; Niknam, B.; Rasouli, S.; Hashemi, S.M.; Rahavi, H.; Rezaei, N.; Soleimani, M.; Liaeiha, A.; Niknam, M.H.; Tajik, N. Immunomodulatory and protective effects of adipose tissue-derived mesenchymal stem cells in an allograft islet composite transplantation for experimental autoimmune type 1 diabetes. Immunol. Lett. 2017, 188, 21–31. [Google Scholar] [CrossRef]
- Kuppan, P.; Seeberger, K.; Kelly, S.; Rosko, M.; Adesida, A.; Pepper, A.R.; Korbutt, G.S. Co-transplantation of human adipose-derived mesenchymal stem cells with neonatal porcine islets within a prevascularized subcutaneous space augments the xenograft function. Xenotransplantation 2020, e12581. [Google Scholar] [CrossRef]
- Tanaka, T.; Kojima, D.; Mera, T.; Matsumoto, M.; Yasunami, Y.; Yanase, T. Expansion of transplanted islets in mice by co-transplantation with adipose tissue-derived mesenchymal stem cells. Heliyon 2018, 4, e00632. [Google Scholar] [CrossRef]
- Karaoz, E.; Okcu, A.; Unal, Z.S.; Subasi, C.; Saglam, O.; Duruksu, G. Adipose tissue-derived mesenchymal stromal cells efficiently differentiate into insulin-producing cells in pancreatic islet microenvironment both in vitro and in vivo. Cytotherapy 2013, 15, 557–570. [Google Scholar] [CrossRef]
- Anitha, R.; Vaikkath, D.; Shenoy, S.J.; Nair, P.D. Tissue-engineered islet-like cell clusters generated from adipose tissue-derived stem cells on three-dimensional electrospun scaffolds can reverse diabetes in an experimental rat model and the role of porosity of scaffolds on cluster differentiation. J. Biomed. Mater. Res. A 2020, 108, 749–759. [Google Scholar] [CrossRef]
- Badimon, L.; Cubedo, J. Adipose tissue depots and inflammation: Effects on plasticity and resident mesenchymal stem cell function. Cardiovasc. Res. 2017, 113, 1064–1073. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gupta, R.K. Adipocytes. Curr. Biol. 2014, 24, R988–R993. [Google Scholar] [CrossRef] [Green Version]
- Hotamisligil, G.S.; Shargill, N.S.; Spiegelman, B.M. Adipose expression of tumor necrosis factor-alpha: Direct role in obesity-linked insulin resistance. Science 1993, 259, 87–91. [Google Scholar] [CrossRef] [PubMed]
- Xu, H.; Uysal, K.T.; Becherer, J.D.; Arner, P.; Hotamisligil, G.S. Altered tumor necrosis factor-alpha (TNF-alpha) processing in adipocytes and increased expression of transmembrane TNF-alpha in obesity. Diabetes 2002, 51, 1876–1883. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kalupahana, N.S.; Claycombe, K.J.; Moustaid-Moussa, N. (n-3) Fatty acids alleviate adipose tissue inflammation and insulin resistance: Mechanistic insights. Adv. Nutr. 2011, 2, 304–316. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Takahashi, K.; Mizuarai, S.; Araki, H.; Mashiko, S.; Ishihara, A.; Kanatani, A.; Itadani, H.; Kotani, H. Adiposity elevates plasma MCP-1 levels leading to the increased CD11b-positive monocytes in mice. J. Biol. Chem. 2003, 278, 46654–46660. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kamei, N.; Tobe, K.; Suzuki, R.; Ohsugi, M.; Watanabe, T.; Kubota, N.; Ohtsuka-Kowatari, N.; Kumagai, K.; Sakamoto, K.; Kobayashi, M.; et al. Overexpression of monocyte chemoattractant protein-1 in adipose tissues causes macrophage recruitment and insulin resistance. J. Biol. Chem. 2006, 281, 26602–26614. [Google Scholar] [CrossRef] [Green Version]
- Kanda, H.; Tateya, S.; Tamori, Y.; Kotani, K.; Hiasa, K.; Kitazawa, R.; Kitazawa, S.; Miyachi, H.; Maeda, S.; Egashira, K.; et al. MCP-1 contributes to macrophage infiltration into adipose tissue, insulin resistance, and hepatic steatosis in obesity. J. Clin. Investig. 2006, 116, 1494–1505. [Google Scholar] [CrossRef]
- Melzi, R.; Mercalli, A.; Sordi, V.; Cantarelli, E.; Nano, R.; Maffi, P.; Sitia, G.; Guidotti, L.G.; Secchi, A.; Bonifacio, E.; et al. Role of CCL2/MCP-1 in islet transplantation. Cell Transplant. 2010, 19, 1031–1046. [Google Scholar] [CrossRef]
- Citro, A.; Pellegrini, S.; Dugnani, E.; Eulberg, D.; Klussmann, S.; Piemonti, L. CCL2/MCP-1 and CXCL12/SDF-1 blockade by L-aptamers improve pancreatic islet engraftment and survival in mouse. Am. J. Transplant. 2019, 19, 3131–3138. [Google Scholar] [CrossRef]
- Min, B.H.; Shin, J.S.; Kim, J.M.; Kang, S.J.; Kim, H.J.; Yoon, I.H.; Park, S.K.; Choi, J.W.; Lee, M.S.; Park, C.G. Delayed revascularization of islets after transplantation by IL-6 blockade in pig to non-human primate islet xenotransplantation model. Xenotransplantation 2018, 25. [Google Scholar] [CrossRef] [PubMed]
- Landecho, M.F.; Tuero, C.; Valenti, V.; Bilbao, I.; de la Higuera, M.; Fruhbeck, G. Relevance of Leptin and Other Adipokines in Obesity-Associated Cardiovascular Risk. Nutrients 2019, 11, 2664. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Denroche, H.C.; Quong, W.L.; Bruin, J.E.; Tuduri, E.; Asadi, A.; Glavas, M.M.; Fox, J.K.; Kieffer, T.J. Leptin administration enhances islet transplant performance in diabetic mice. Diabetes 2013, 62, 2738–2746. [Google Scholar] [CrossRef] [Green Version]
- Lee, Y.; Ravazzola, M.; Park, B.H.; Bashmakov, Y.K.; Orci, L.; Unger, R.H. Metabolic mechanisms of failure of intraportally transplanted pancreatic beta-cells in rats: Role of lipotoxicity and prevention by leptin. Diabetes 2007, 56, 2295–2301. [Google Scholar] [CrossRef] [Green Version]
- Achari, A.E.; Jain, S.K. Adiponectin, a Therapeutic Target for Obesity, Diabetes, and Endothelial Dysfunction. Int. J. Mol. Sci. 2017, 18, 1321. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ohashi, K.; Shibata, R.; Murohara, T.; Ouchi, N. Role of anti-inflammatory adipokines in obesity-related diseases. Trends Endocrinol. Metab. 2014, 25, 348–355. [Google Scholar] [CrossRef]
- Ohashi, K.; Ouchi, N.; Matsuzawa, Y. Anti-inflammatory and anti-atherogenic properties of adiponectin. Biochimie 2012, 94, 2137–2142. [Google Scholar] [CrossRef]
- Antoniades, C.; Antonopoulos, A.S.; Tousoulis, D.; Stefanadis, C. Adiponectin: From obesity to cardiovascular disease. Obes. Rev. 2009, 10, 269–279. [Google Scholar] [CrossRef]
- Du, X.; He, S.; Jiang, Y.; Wei, L.; Hu, W. Adiponectin prevents islet ischemia-reperfusion injury through the COX2-TNFalpha-NF-kappaB-dependent signal transduction pathway in mice. J. Endocrinol. 2013, 218, 75–84. [Google Scholar] [CrossRef] [Green Version]
- Delaune, V.; Berney, T.; Lacotte, S.; Toso, C. Intraportal islet transplantation: The impact of the liver microenvironment. Transpl. Int. 2017, 30, 227–238. [Google Scholar] [CrossRef] [Green Version]
- Weisberg, S.P.; McCann, D.; Desai, M.; Rosenbaum, M.; Leibel, R.L.; Ferrante, A.W., Jr. Obesity is associated with macrophage accumulation in adipose tissue. J. Clin. Investig. 2003, 112, 1796–1808. [Google Scholar] [CrossRef] [PubMed]
- Lumeng, C.N.; Bodzin, J.L.; Saltiel, A.R. Obesity induces a phenotypic switch in adipose tissue macrophage polarization. J. Clin. Investig. 2007, 117, 175–184. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Russo, L.; Lumeng, C.N. Properties and functions of adipose tissue macrophages in obesity. Immunology 2018, 155, 407–417. [Google Scholar] [CrossRef] [PubMed]
- Stice, M.J.; Dunn, T.B.; Bellin, M.D.; Skube, M.E.; Beilman, G.J. Omental Pouch Technique for Combined Site Islet Autotransplantation Following Total Pancreatectomy. Cell Transplant. 2018, 27, 1561–1568. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Baidal, D.A.; Ricordi, C.; Berman, D.M.; Alvarez, A.; Padilla, N.; Ciancio, G.; Linetsky, E.; Pileggi, A.; Alejandro, R. Bioengineering of an Intraabdominal Endocrine Pancreas. N. Engl. J. Med. 2017, 376, 1887–1889. [Google Scholar] [CrossRef] [Green Version]
- Sacks, H.; Symonds, M.E. Anatomical locations of human brown adipose tissue: Functional relevance and implications in obesity and type 2 diabetes. Diabetes 2013, 62, 1783–1790. [Google Scholar] [CrossRef] [Green Version]
- Xu, K.; Xie, R.; Lin, X.; Jia, J.; Zeng, N.; Li, W.; Xiao, D.; Du, T. Brown adipose tissue: A potential site for islet transplantation. Transplantation 2020. [Google Scholar] [CrossRef]

| Advantages | Disadvantages | |
|---|---|---|
| Liver |
|
|
| Kidney |
|
|
| Spleen |
|
|
| Muscle/ subcutaneous tissue |
|
|
| Omentum (white adipose tissue) |
|
|
| Mesentery (white adipose tissue) |
|
|
| Author (Year) | Transplant Model (Animal) | Number of Transplanted Islets | Additional Treatment | Outcome | Reference |
|---|---|---|---|---|---|
| Kasoju (2020) | Syngeneic (rat) | Not described | Using biomaterial spacer and growth factor | Islet engraftment | [39] |
| Lu (2019) | Not described | 450–500 islets | Using hydrogel | Normoglycemia achieved immediately | [24] |
| Ibarra (2016) | Allogeneic (rat) | 800–1000 beads (accurate number not described) | Encapsulation | Transplant efficacy was unclear | [25] |
| Montazeri (2016) | Xenogeneic (rat to nude mouse) | 250 islets (IEQs) | Using oxygenation technique with growth factor | Improved blood glucose level | [26] |
| Hajizadeh-Saffar (2015) | Allogeneic (mouse) | 200 islets (IEQs) 400 islets (IEQs) | Co-transplantation with growth factor-releasing cells (derived from mesenchymal stromal cells) | Normoglycemia rates were 80% in 200 IEQs with MSCs and 40% in 400 IEQs | [27] |
| Pareta (2014) | Syngeneic Allogeneic (rat) | 800 islets | Encapsulation | Failed to achieve normoglycemia | [28] |
| Pedraza (2013) | Syngeneic (rat) | 1800 islets | With or without bioscaffold | Achieved normoglycemia over 110 days | [29] |
| Kriz (2012) | Syngeneic (rat) | 10,000 islets (IEQs)/kg (2000–3000 IEQs) | Using biomaterial spacer | Normoglycemia rate was 70% at 100 days after transplantation | [30] |
| Gupta (2011) | Syngeneic Allogeneic (rat) | 2000 islets | Using endothelialized modules | Normoglycemia rate was 40% (syngeneic) | [31] |
| Berman (2009) | Autologous Allogeneic (monkey) | 5093 IEQ/kg (autologous) 4200–14,544 IEQ/kg (allogeneic) | Bioscaffold Immunosuppressants (allogeneic) | Achieved normoglycemia in autologous islet transplantation. Therapeutic effect was similar to that of intrahepatic islet transplantation | [34] |
| Kobayashi (2006) | Syngeneic (mouse) | 1500 islets | Encapsulation | The normoglycemia rate was 90% over 100 days after transplantation | [36] |
| Kin (2003) | Syngeneic (rat) | 2000 islets | No additional treatment | Achieving normoglycemia at 56 days after transplantation | [23] |
| Guan (1998) | Syngeneic (rat) | Approximately 3000 islets | No additional treatment | Achieving normoglycemia at 2 months after transplantation | [37] |
| Author (Year) | Transplant Model | Number of Transplanted Islets | Additional Treatment | Outcome | Reference |
|---|---|---|---|---|---|
| Nguyen (2020) | Xenogeneic (rat to nude mouse) | 500 islets (IEQs) | With or without bioscaffold | Achieved normoglycemia (88% with bioscaffold, 44% without bioscaffold) | [61] |
| Minardi (2019) | Syngeneic (mouse) | 70 islets | With bioscaffold | Achieved normoglycemia at 1 month after transplantation | [49] |
| Liu (2018) | Syngeneic Allogeneic (mouse) | 250 islets | Using immunomodulation technique and bioscaffolds | Achieved normoglycemia in all mice | [51] |
| Manzoli (2018) | Allogeneic (mouse) | 750–1000 islets (IEQs) | Encapsulation | Achieved normoglycemia at 10 days after transplantation | [58] |
| Weaver (2017) | Syngeneic (mice) | 600 islets (IEQs) | Using bioscaffold with growth factor | The normoglycemia rates were 75% and 60% using bioscaffold with and without growth factor, respectively | [42] |
| Wang (2017) | Syngeneic (mouse) | 150–500 islets | Using bioscaffold | Mice achieved normoglycemia: 10/12 (500 islets + scaffold), 10/15 (250 islets + scaffold), 9/19 (150 islets + scaffold), 3/10 (250 islets) | [62,63] |
| Mao (2017) | Syngeneic (mouse) | 300 islets | Using bioscaffold | No mice achieved normoglycemia in ITx only. All mice achieved normoglycemia with bioscaffold | [64] |
| Buitinga (2017) | Syngeneic (mouse) | 300 islets | Using bioscaffold | Achieved normoglycemia (75% with bioscaffold, 29% without bioscaffold) | [65] |
| Villa (2017) | Allogeneic (mouse) Xeneogeneic (baboon to NOD/scid mouse) | 750 islets (IEQs) | Encapsulation | Allogeneic: Normoglycemia achieved and maintained in all the mice (7/7) for 100 days after transplantation Xenogeneic: Normoglycemia achieved and maintained in all the mice (4/4) for 30 days after transplantation | [59] |
| Rios (2016) | Syngeneic (mouse) | 300 and 500 islets | Using bioscaffold | Achieved normoglycemia (100% in 500 islets, 25% in 300 islets) | [55] |
| Liu (2016) | Syngeneic (mouse) | 250 islets | Using bioscaffold | Normoglycemia achieved and maintained for 80 days after transplantation | [52] |
| Najjar (2015) | Syngeneic (mouse) | 250 islets (IEQs) | Using bioscaffold | Achieved normoglycemia (60% with bioscaffold, 10% without bioscaffold) | [50] |
| Gibly (2013) | Xenogeneic (Human to NOD/scid mice) | 2000 islets (IEQs) | Using bioscaffold | Normoglycemia achieved and maintained over 140 days after transplantation | [66] |
| Brady (2013) | Syngeneic (mouse) | 250 islets (IEQs) | Using bioscaffold | Achieved normoglycemia (100% with bioscaffold, 87.5% without bioscaffold) | [67] |
| Gibly (2011) | Syngeneic (mouse) | 75 islets | Using bioscaffold | Normoglycemia achieved and maintained for 42 days after transplantation | [53] |
| Kheradmand (2011) | Allogeneic (mouse) | 500 islets | Using bioscaffold with splenocytes | Achieved normoglycemia for 150 days (80% with splenocytes) | [54] |
| Brubaker (2010) | Syngeneic (mouse) | 150 islets | Using bioscaffold | Normoglycemia achieved and maintained for 110 days after transplantation (both with and without bioscaffold) | [56] |
| Salvay (2008) | Syngeneic (mouse) | 125 islets | Using bioscaffold | Normoglycemia achieved and maintained for 300 days after transplantation | [57] |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sakata, N.; Yoshimatsu, G.; Kodama, S. White Adipose Tissue as a Site for Islet Transplantation. Transplantology 2020, 1, 55-70. https://0-doi-org.brum.beds.ac.uk/10.3390/transplantology1020006
Sakata N, Yoshimatsu G, Kodama S. White Adipose Tissue as a Site for Islet Transplantation. Transplantology. 2020; 1(2):55-70. https://0-doi-org.brum.beds.ac.uk/10.3390/transplantology1020006
Chicago/Turabian StyleSakata, Naoaki, Gumpei Yoshimatsu, and Shohta Kodama. 2020. "White Adipose Tissue as a Site for Islet Transplantation" Transplantology 1, no. 2: 55-70. https://0-doi-org.brum.beds.ac.uk/10.3390/transplantology1020006