Vector-Focused Approaches to Curb Malaria Transmission in the Brazilian Amazon: An Overview of Current and Future Challenges and Strategies
Abstract
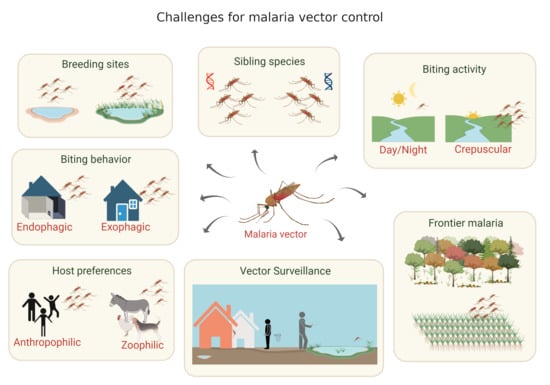
:1. Introduction
2. Malaria in Brazil, a Brief History and Current Status
3. Malaria Vectors in the Brazilian Amazon and the Importance of Vector Population Surveillance
4. Conventional Measures for Vector Control and Their Limitations
4.1. Long-Lasting Insecticidal Nets (LLINs)
4.2. Indoor Residual Spraying (IRS)
4.3. Larvae Control
4.4. Personal Protection
5. Promising Novel Vector Control Approaches
5.1. Genetic Control of Malaria Vectors
5.1.1. Population Suppression or Replacement
5.1.2. Self-Limiting or Sustaining Strategies
5.1.3. Challenges, Alternatives and Transfer to Neotropical Species (e.g., An. darlingi)
5.2. Microbial-Based Approaches to Control Malaria Transmission and Malaria Vector Populations
5.2.1. Entomopathogenic Organisms
5.2.2. Naturally Occurring Symbiotic Microorganisms with Anti-Pathogen Activity
5.2.3. Paratransgenesis
5.2.4. Wolbachia
6. Final Remarks and Perspectives
Author Contributions
Funding
Conflicts of Interest
References
- WHO. From Malaria Control to Malaria Elimination: A Manual for Elimination Scenario Planning. Available online: https://www.who.int/malaria/publications/atoz/9789241507028/en/ (accessed on 20 August 2020).
- Antiporta, D.A.; Rosas-Aguirre, A.; Chang, J.; Llanos-Cuentas, A.; Lescano, A.G. Malaria eradication. Lancet 2020, 395, e67. [Google Scholar] [CrossRef]
- WHO. Global Technical Strategy for Malaria 2016–2030. Available online: https://www.who.int/malaria/publications/atoz/9789241564991/en/ (accessed on 20 April 2020).
- WHO; UNICEF. Global Vector Control Response 2017–2030. Available online: https://www.who.int/vector-control/publications/global-control-response/en/ (accessed on 18 June 2020).
- PAHO. Plan of Action for Malaria Elimination 2016–2020. Available online: https://iris.paho.org/bitstream/handle/10665.2/31440/CD55-13-e.pdf?sequence=1&isAllowed (accessed on 22 April 2020).
- Brasil, M.d.S.d. Plano de Eliminação de Malária no Brasil. Available online: https://www.saude.gov.br/images/pdf/2017/janeiro/04/Plano-eliminacao-malaria-pub.pdf (accessed on 18 June 2020).
- Melo, J.; Padilha, M.; Barbosa, R.; Alonso, W.; Vittor, A.; Laporta, G. Evaluation of the malaria elimination policy in Brazil: A systematic review and epidemiological analysis study. Trop. Biomed. 2020, 37, 513–535. [Google Scholar]
- WHO. World MALARIA Report. Available online: https://www.who.int/publications/i/item/9789241565721 (accessed on 18 June 2020).
- Wilson, A.L.; Courtenay, O.; Kelly-Hope, L.A.; Scott, T.W.; Takken, W.; Torr, S.J.; Lindsay, S.W. The importance of vector control for the control and elimination of vector-borne diseases. PLoS Negl. Trop. Dis. 2020, 14, e0007831. [Google Scholar] [CrossRef] [Green Version]
- Coura, J.R.; Suárez-Mutis, M.; Ladeia-Andrade, S. A new challenge for malaria control in Brazil: Asymptomatic Plasmodium infection-a review. Mem. Inst. Oswaldo Cruz 2006, 101, 229–237. [Google Scholar] [CrossRef] [Green Version]
- Deane, L.M. Malaria vectors in Brazil. Mem. Inst. Oswaldo Cruz 1986, 81, 5–14. [Google Scholar] [CrossRef] [Green Version]
- Multini, L.C.; Marrelli, M.T.; Beier, J.C.; Wilke, A.B. Increasing Complexity Threatens the Elimination of Extra-Amazonian Malaria in Brazil. Trends Parasitol. 2019, 35, 383–387. [Google Scholar] [CrossRef]
- Pina-Costa, A.d.; Brasil, P.; Santi, S.M.D.; Araujo, M.P.D.; Suárez-Mutis, M.C.; Oliveira-Ferreira, J.; Lourenço-de-Oliveira, R.; Daniel-Ribeiro, C.T. Malaria in Brazil: What happens outside the Amazonian endemic region. Mem. Inst. Oswaldo Cruz 2014, 109, 618–633. [Google Scholar] [CrossRef]
- Brasil, M.d.S.d. Malária: O que é, Causas, Sintomas, Tratamento, Diagnóstico e Prevenção-Situação Epidemiológica da Malária. Available online: http://saude.gov.br/saude-de-a-z/malaria (accessed on 24 June 2020).
- Sampaio, V.S.; Siqueira, A.M.; Alecrim, M.d.G.C.; Mourão, M.P.G.; Marchesini, P.B.; Albuquerque, B.C.; Nascimento, J.; Figueira, É.A.G.; Alecrim, W.D.; Monteiro, W.M. Malaria in the State of Amazonas: A typical Brazilian tropical disease influenced by waves of economic development. Rev. Soc. Bras. Med. Trop. 2015, 48, 4–11. [Google Scholar] [CrossRef] [Green Version]
- Cruz, O.G. Madeira-Mamoré Railway Company: Considerações gerais sobre as condições sanitárias do rio Madeira. 1910. In Cruz, Oswaldo. Opera ominia; Instituto Oswaldo Cruz: Rio de Janeiro, Brazil, 1972; pp. 564–624. [Google Scholar]
- Lopes, G. Anopheles gambiae in Brazil: The background to a “silent spread”, 1930–1932. Historia Cienc. Saude–Manguinhos 2019, 26, 823–839. [Google Scholar] [CrossRef] [Green Version]
- Parmakelis, A.; Russello, M.A.; Caccone, A.; Marcondes, C.B.; Costa, J.; Forattini, O.P.; Sallum, M.A.M.; Wilkerson, R.C.; Powell, J.R. Historical analysis of a near disaster: Anopheles gambiae in Brazil. Am. J. Trop. Med. 2008, 78, 176–178. [Google Scholar] [CrossRef]
- Ferreira, M.U.; Castro, M.C. Challenges for malaria elimination in Brazil. Malar. J. 2016, 15, 1–18. [Google Scholar] [CrossRef] [PubMed]
- Oliveira-Ferreira, J.; Lacerda, M.V.; Brasil, P.; Ladislau, J.L.; Tauil, P.L.; Daniel-Ribeiro, C.T. Malaria in Brazil: An overview. Malar. J. 2010, 9, 115. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Killeen, G.F. Following in Soper’s footsteps: Northeast Brazil 63 years after eradication of Anopheles gambiae. Lancet Infect. Dis. 2003, 3, 663–666. [Google Scholar] [CrossRef]
- Silva, R.d.; Paiva, C.H.A. The Juscelino Kubitschek government and the Brazilian Malaria Control and Eradication Working Group: Collaboration and conflicts in Brazilian and international health agenda, 1958–1961. História, Ciências, Saúde-Manguinhos 2015, 22, 95–114. [Google Scholar] [CrossRef] [Green Version]
- Da Silva, R.; Hochman, G. Um método chamado Pinotti: Sal medicamentoso, malária e saúde internacional (1952–1960). História, Ciências, Saúde-Manguinhos 2011, 18, 519–543. [Google Scholar] [CrossRef]
- Marques, A.C. Migrations and the dissemination of malaria in Brazil. Mem. Inst. Oswaldo Cruz 1986, 81, 17–30. [Google Scholar] [CrossRef] [Green Version]
- Marques, A.C. Human migration and the spread of malaria in Brazil. Parasitol. Today 1987, 3, 166–170. [Google Scholar] [CrossRef]
- Camargo, E.P. Malária, maleita, paludismo. Cienc. Cult. 2003, 55, 26–29. [Google Scholar]
- Fearnside, P.M. Deforestation in Brazilian Amazonia: History, rates, and consequences. Conserv. Biol. 2005, 19, 680–688. [Google Scholar] [CrossRef]
- Carlos, B.C.; Rona, L.D.; Christophides, G.K.; Souza-Neto, J.A. A comprehensive analysis of malaria transmission in Brazil. Pathog. Glob. Health 2019, 113, 1–13. [Google Scholar] [CrossRef] [Green Version]
- Eichemberg Silva, L.C. O Que Mostram os Indicadores Sobre a Pobreza na Década Perdida; (Texto Para Discussão, 274); IPEA: Rio de Janeiro, Brazil, 1992. [Google Scholar]
- Conn, J.E.; Grillet, M.E.; Correa, M.; Sallum, M.A.M. Malaria transmission in South America—present status and prospects for elimination. In Towards Malaria Elimination—A Leap Forward; InTech: London, UK, 2018; pp. 281–313. [Google Scholar]
- Arruda-Barbosa, L.d.; Sales, A.F.G.; Souza, I.L.L.d. Reflexes of Venezuelan immigration on health care at the largest hospital in Roraima, Brazil: Qualitative analysis. Saude Soc. 2020, 29, e190730. [Google Scholar] [CrossRef]
- Grillet, M.E.; Moreno, J.E.; Hernandez, J.V.; Vincenti-Gonzalez, M.F.; Noya, O.; Tami, A.; Paniz-Mondolfi, A.; Llewellyn, M.; Lowe, R.; Escalante, A.A. Malaria in Southern Venezuela: The Hottest Hotspot in Latin America. bioRxiv 2020. [Google Scholar]
- Mosnier, E.; Roux, E.; Cropet, C.; Lazrek, Y.; Moriceau, O.; Gaillet, M.; Mathieu, L.; Nacher, M.; Demar, M.; Odonne, G. Prevalence of Plasmodium spp. in the Amazonian Border Context (French Guiana–Brazil): Associated Factors and Spatial Distribution. Am. J. Trop. Med. 2020, 102, 130–141. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tauil, P.L. Perspectives of vector borne diseases control in Brazil. Rev. Soc. Bras. Med. Trop. 2006, 39. [Google Scholar]
- Mendes, A.M.; Lima, M.d.S.; Maciel, A.G.P.; Menezes, R.A.d.O.; Eugênio, N.C.C. Malaria among indigenous peoples on the Brazil-French Guiana border, 2007–2016: A descriptive study. Epidemiol. Serv. Saúde 2020, 29, e2019056. [Google Scholar] [PubMed]
- Robortella, D.R.; Calvet, A.A.; Amaral, L.C.; Fantin, R.F.; Guimarães, L.F.F.; França Dias, M.H.; Brito, C.F.A.d.; Sousa, T.N.d.; Herzog, M.M.; Oliveira-Ferreira, J. Prospective assessment of malaria infection in a semi-isolated Amazonian indigenous Yanomami community: Transmission heterogeneity and predominance of submicroscopic infection. PLoS ONE 2020, 15, e0230643. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Douine, M.; Lambert, Y.; Musset, L.; Hiwat, H.; Blume, L.R.; Marchesini, P.; Moresco, G.G.; Cox, H.; Sanchez, J.F.; Villegas, L. Malaria in Gold Miners in the Guianas and the Amazon: Current Knowledge and Challenges. Curr. Trop. Med. Rep. 2020, 1–11. [Google Scholar] [CrossRef] [Green Version]
- Mosnier, E.; Dusfour, I.; Lacour, G.; Saldanha, R.; Guidez, A.; Gomes, M.S.; Sanna, A.; Epelboin, Y.; Restrepo, J.; Davy, D. Resurgence risk for malaria, and the characterization of a recent outbreak in an Amazonian border area between French Guiana and Brazil. BMC Infect. Dis. 2020, 20, 1–14. [Google Scholar] [CrossRef]
- Consoli, R.A.; Oliveira, R.L.d. Principais Mosquitos de Importância Sanitária no Brasil; Editora Fiocruz: Rio de Janeiro, Brasil, 1994. [Google Scholar]
- Tadei, W.P.; Dutary Thatcher, B. Malaria vectors in the Brazilian Amazon: Anopheles of the subgenus Nyssorhynchus. Rev. Inst. Med. Trop. São Paulo 2000, 42, 87–94. [Google Scholar] [CrossRef]
- Marrelli, M.T.; Sallum, M.A.M.; Marinotti, O. The second internal transcribed spacer of nuclear ribosomal DNA as a tool for Latin American anopheline taxonomy: A critical review. Mem. Inst. Oswaldo Cruz 2006, 101, 817–832. [Google Scholar] [CrossRef] [Green Version]
- Galardo, A.K.R.; Arruda, M.; COUTO, A.A.D.A.; Wirtz, R.; Lounibos, L.P.; Zimmerman, R.H. Malaria vector incrimination in three rural riverine villages in the Brazilian Amazon. Am. J. Trop. Med. 2007, 76, 461–469. [Google Scholar] [CrossRef]
- Klein, T.A.; Lima, J. Seasonal distribution and biting patterns of Anopheles mosquitoes in Costa Marques, Rondonia, Brazil. J. Am. Mosq. Control Assoc. 1990, 6, 700–707. [Google Scholar]
- Arcos, A.N.; da Silva Ferreira, F.A.; da Cunha, H.B.; Tadei, W.P. Characterization of artificial larval habitats of Anopheles darlingi (Diptera: Culicidae) in the Brazilian Central Amazon. Rev. Bras. Entomol. 2018, 62, 267–274. [Google Scholar] [CrossRef]
- Tadei, W.P.; Rodrigues, I.B.; Rafael, M.S.; Sampaio, R.T.d.M.; Mesquita, H.; Pinheiro, V.C.S.; Zequi, J.A.C.; Roque, R.A.; Dos Santos, J.M.M. Adaptative processes, control measures, genetic background, and resilience of malaria vectors and environmental changes in the Amazon region. Hydrobiologia 2017, 789, 179–196. [Google Scholar] [CrossRef]
- Alves, M.R.; Codeço, C.T.; Peiter, P.C.; Souza-Santos, R. Malaria and fish farming in the Brazilian Amazon Region: A strengths, weaknesses, opportunities, and threats analysis. Rev. Soc. Bras. Med. Trop. 2019, 52. [Google Scholar] [CrossRef]
- Lourenço-de-Oliveira, R.; Luz, S.L. Simian malaria at two sites in the Brazilian Amazon-II: Vertical distribution and frequency of anopheline species inside and outside the forest. Mem. Inst. Oswaldo Cruz 1996, 91, 687–694. [Google Scholar] [CrossRef] [Green Version]
- Forattini, O.P. Culicidologia Médica: Identificaçäo, Biologia e Epidemiologia: V 2; Editora da Universidade de São Paulo: Sao Paulo, Brazil, 2002; p. 860. [Google Scholar]
- Tadei, W.P.; Santos, J.M.M.d.; Rodrigues, I.B.; Rafael, M.S. Malária e Dengue na Amazônia: Vetores e estratégias de controle. In Pesquisa e Ações em Saúde nos Institutos de Pesquisa do Ministério da Ciência e Tecnologia; Ministério da Ciência e Tecnologia: São Paulo, Brazil, 2010; pp. 113–125. [Google Scholar]
- Rafael, M.S.; Rohde, C.; Bridi, L.C.; Tadei, W.P. Salivary polytene chromosome map of Anopheles darlingi, the main vector of neotropical malaria. Am. J. Trop. Med. 2010, 83, 241–249. [Google Scholar] [CrossRef] [Green Version]
- Wolfarth-Couto, B.; Filizola, N.; Durieux, L. Seasonal pattern of malaria cases and the relationship with hydrologic variability in the Amazonas State, Brazil. Rev. Bras. Entomol. 2020, 23, e200018. [Google Scholar]
- Olson, S.H.; Gangnon, R.; Elguero, E.; Durieux, L.; Guégan, J.-F.; Foley, J.A.; Patz, J.A. Links between climate, malaria, and wetlands in the Amazon Basin. Emerg. Infect. Dis 2009, 15, 659. [Google Scholar] [CrossRef]
- Wolfarth, B.R.; Filizola, N.; Tadei, W.P.; Durieux, L. Epidemiological analysis of malaria and its relationships with hydrological variables in four municipalities of the State of Amazonas, Brazil. Hydrol. Sci. J. 2013, 58, 1495–1504. [Google Scholar] [CrossRef]
- Souza, P.F.; Xavier, D.R.; Suarez Mutis, M.C.; da Mota, J.C.; Peiter, P.C.; de Matos, V.P.; Magalhães, M.d.A.F.M.; Barcellos, C. Spatial spread of malaria and economic frontier expansion in the Brazilian Amazon. PLoS ONE 2019, 14, e0217615. [Google Scholar] [CrossRef] [PubMed]
- Chaves, L.S.M.; Fry, J.; Malik, A.; Geschke, A.; Sallum, M.A.M.; Lenzen, M. Global consumption and international trade in deforestation-associated commodities could influence malaria risk. Nat. Commun. 2020, 11, 1258. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- MacDonald, A.J.; Mordecai, E.A. Amazon deforestation drives malaria transmission, and malaria burden reduces forest clearing. Proc. Natl. Acad. Sci. USA 2019, 116, 22212–22218. [Google Scholar] [CrossRef] [PubMed]
- Conn, J.E.; Wilkerson, R.C.; Segura, M.N.O.; de Souza, R.T.; Schlichting, C.D.; Wirtz, R.A.; Póvoa, M.M. Emergence of a new Neotropical malaria vector facilitated by human migration and changes in land use. Am. J. Trop. Med. 2002, 66, 18–22. [Google Scholar] [CrossRef] [PubMed]
- Charlwood, J. Biological variation in Anopheles darlingi Root. Mem. Inst. Oswaldo Cruz 1996, 91, 391–398. [Google Scholar] [CrossRef] [Green Version]
- Montoya-Lerma, J.; Solarte, Y.A.; Giraldo-Calderón, G.I.; Quiñones, M.L.; Ruiz-López, F.; Wilkerson, R.C.; González, R. Malaria vector species in Colombia: A review. Mem. Inst. Oswaldo Cruz 2011, 106, 223–238. [Google Scholar] [CrossRef] [PubMed]
- Elliott, R. The influence of vector behavior on malaria transmission. Am. J. Trop. Med. 1972, 21, 755–763. [Google Scholar] [CrossRef]
- Hudson, J. Anopheles darlingi Root (Diptera: Culicidae) in the Suriname rain forest. Bull. Entomol. Res. 1984, 74, 129–142. [Google Scholar] [CrossRef]
- Magris, M.; Rubio-Palis, Y.; Menares, C.; Villegas, L. Vector bionomics and malaria transmission in the Upper Orinoco River, Southern Venezuela. Mem. Inst. Oswaldo Cruz 2007, 102, 303–312. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Quiñones, M.; Suarez, M. Indoor resting heights of some anophelines in Colombia. Proc. Natl. Acad. Sci. USA 1990, 6, 602–604. [Google Scholar]
- Rozendaal, J. Biting and resting behavior of Anopheles darlingi in the Suriname rainforest. J. Am. Mosq. Control. Assoc. 1989, 5, 351–358. [Google Scholar] [PubMed]
- Vittor, A.Y.; Gilman, R.H.; Tielsch, J.; Glass, G.; Shields, T.; Lozano, W.S.; Pinedo-Cancino, V.; Patz, J.A. The effect of deforestation on the human-biting rate of Anopheles darlingi, the primary vector of falciparum malaria in the Peruvian Amazon. Am. J. Trop. Med. 2006, 74, 3–11. [Google Scholar] [CrossRef] [Green Version]
- Charlwood, J.; Wilkes, T. Studies on the age-composition of samples of Anopheles darlingi Root (Diptera: Culicidae) in Brazil. Bull. Entomol. Res. 1979, 69, 337–342. [Google Scholar] [CrossRef]
- Forattini, O.P. Comportamento exófilo de Anopheles darlingi Root, em região meridional do Brasil. Rev. Saúde Públ. 1987, 21, 291–304. [Google Scholar] [CrossRef] [PubMed]
- Lourenço-de-Oliveira, R.; Guimarães, A.E.d.G.; Arlé, M.; Silva, T.F.d.; Castro, M.G.; Motta, M.A.; Deane, L.M. Anopheline species, some of their habits and relation to malaria in endemic areas of Rondonia State, Amazon region of Brazil. Mem. Inst. Oswaldo Cruz 1989, 84, 501–514. [Google Scholar] [CrossRef] [Green Version]
- Roberts, D.; Alecrim, W.; Tavares, A.; Radke, M. The house-frequenting, host-seeking and resting behavior of Anopheles darlingi in southeastern Amazonas, Brazil. J. Am. Mosq. Control Assoc. 1987, 3, 433. [Google Scholar]
- Tadei, W.P.; Thatcher, B.D.; Santos, J.; Scarpassa, V.M.; Rodrigues, I.B.; Rafael, M.S. Ecologic observations on anopheline vectors of malaria in the Brazilian Amazon. Am. J. Trop. Med. 1998, 59, 325–335. [Google Scholar] [CrossRef]
- Freitas-Sibajev, M.; Conn, J.; Mitchell, S.; Cockburn, A.; Seawright, J.; Momen, H. Mitochondrial DNA and morphological analyses of Anopheles darlingi populations from Brazil (Diptera: Culicidae). Mosq. Syst. 1995, 27, 78–99. [Google Scholar]
- Voorham, J. Intra-population plasticity of Anopheles darlingi’s (Diptera, Culicidae) biting activity patterns in the state of Amapá, Brazil. Rev. Saúde Públ. 2002, 36, 75–80. [Google Scholar] [CrossRef] [Green Version]
- De Oliveira, C.D.; Tadei, W.P.; Abdalla, F.C.; Paolucci Pimenta, P.F.; Marinotti, O. Multiple blood meals in Anopheles darlingi (Diptera: Culicidae). J. Vector Ecol. 2012, 37, 351–358. [Google Scholar] [CrossRef]
- Barrón, M.G.; Paupy, C.; Rahola, N.; Akone-Ella, O.; Ngangue, M.F.; Wilson-Bahun, T.A.; Pombi, M.; Kengne, P.; Costantini, C.; Simard, F.; et al. A new species in the major malaria vector complex sheds light on reticulated species evolution. Sci. Rep. 2019, 9, 14753. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sinka, M.E.; Bangs, M.J.; Manguin, S.; Chareonviriyaphap, T.; Patil, A.P.; Temperley, W.H.; Gething, P.W.; Elyazar, I.R.F.; Kabaria, C.W.; Harbach, R.E.; et al. The dominant Anopheles vectors of human malaria in the Asia-Pacific region: Occurrence data, distribution maps and bionomic précis. Parasit Vectors 2011, 4, 89. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tripathy, A.; Samanta, L.; Das, S.; Parida, S.K.; Marai, N.; Hazra, R.K.; Kar, S.K.; Mahapatra, N. Distribution of sibling species of Anopheles culicifacies s.l. and Anopheles fluviatilis s.l. and their vectorial capacity in eight different malaria endemic districts of Orissa, India. Mem. Inst. Oswaldo Cruz 2010, 105, 981–987. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rosa-Freitas, M.G.; Lourenço-de-Oliveira, R.; Carvalho-Pinto, C.J.d.; Flores-Mendoza, C.; Silva-do-Nascimento, T.F. Anopheline Species Complexes in Brazil. Current Knowledge of Those Related to Malaria Transmission. Mem. Inst. Oswaldo Cruz 1998, 93, 651–655. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Saraiva, J.F.; Souto, R.N.P.; Scarpassa, V.M. Molecular taxonomy and evolutionary relationships in the Oswaldoi-Konderi complex (Anophelinae: Anopheles: Nyssorhynchus) from the Brazilian Amazon region. PLoS ONE 2018, 13, e0193591. [Google Scholar] [CrossRef]
- Klein, T.A.; Lima, J.B.; Tada, M.S. Comparative susceptibility of anopheline mosquitoes to Plasmodium falciparum in Rondonia, Brazil. Am. J. Trop. Med. Hyg. 1991, 44, 598–603. [Google Scholar] [CrossRef]
- Marrelli, M.T.; Honório, N.A.; Flores-Mendoza, C.; Lourenco-de-Oliveira, R.; Marinotti, O.; Kloetzel, J.K. Comparative susceptibility of two members of the Anopheles oswaldoi complex, An. oswaldoi and An. konderi, to infection by Plasmodium vivax. Trans. R Soc. Trop. Med. Hyg. 1999, 93, 381–384. [Google Scholar] [CrossRef]
- Bourke, B.P.; Conn, J.E.; de Oliveira, T.M.P.; Chaves, L.S.M.; Bergo, E.S.; Laporta, G.Z.; Sallum, M.A.M. Exploring malaria vector diversity on the Amazon Frontier. Malar. J. 2018, 17, 342. [Google Scholar] [CrossRef]
- Swain, S.N.; Makunin, A.; Dora, A.S.; Barik, T.K. SNP barcoding based on decision tree algorithm: A new tool for identification of mosquito species with special reference to Anopheles. Acta Trop. 2019, 199, 105152. [Google Scholar] [CrossRef]
- WHO. Guidelines for Malaria VECTOR Control 2019. Available online: https://apps.who.int/iris/bitstream/handle/10665/310862/9789241550499-eng.pdf?ua=1 (accessed on 18 June 2020).
- WHO. Malaria Vector Control, Policy Guidance, Recommendations. Available online: https://www.who.int/malaria/policy-guidance/vector-control#tab=tab_2 (accessed on 5 May 2020).
- WHO. Malaria Vector Control, Policy Guidance, Operational Manuals. Available online: https://www.who.int/malaria/policy-guidance/vector-control#tab=tab_3 (accessed on 5 May 2020).
- Brasil, M.d.S.d.; Saúde, S.d.V.e.; Transmissíveis, D.d.I.e.D. Guia de Tratamento da Malária no Brasil. Available online: http://bvsms.saude.gov.br/bvs/publicacoes/guia_tratamento_malaria_brasil.pdf (accessed on 5 May 2020).
- WHO. Global Malaria Programme: Insecticide-Treated Mosquito Nets: A WHO Position Statement. Available online: https://www.who.int/mediacentre/news/releases/2007/pr43/en/ (accessed on 5 May 2020).
- Baia-da-Silva, D.C.; Brito-Sousa, J.D.; Rodovalho, S.R.; Peterka, C.; Moresco, G.; Lapouble, O.M.M.; Melo, G.C.d.; Sampaio, V.d.S.; Alecrim, M.d.G.C.; Pimenta, P. Current vector control challenges in the fight against malaria in Brazil. Rev. Soc. Bras. Med. Trop. 2019, 52. [Google Scholar] [CrossRef]
- Lorenz, L.M.; Bradley, J.; Yukich, J.; Massue, D.J.; Mageni Mboma, Z.; Pigeon, O.; Moore, J.; Kilian, A.; Lines, J.; Kisinza, W.; et al. Comparative functional survival and equivalent annual cost of 3 long-lasting insecticidal net (LLIN) products in Tanzania: A randomised trial with 3-year follow up. PLoS Med. 2020, 17, e1003248. [Google Scholar] [CrossRef] [PubMed]
- Briet, O.; Koenker, H.; Norris, L.; Wiegand, R.; Vanden Eng, J.; Thackeray, A.; Williamson, J.; Gimnig, J.E.; Fortes, F.; Akogbeto, M.; et al. Attrition, physical integrity and insecticidal activity of long-lasting insecticidal nets in sub-Saharan Africa and modelling of their impact on vectorial capacity. Malar. J. 2020, 19, 310. [Google Scholar] [CrossRef]
- Ngufor, C.; Agbevo, A.; Fagbohoun, J.; Fongnikin, A.; Rowland, M. Efficacy of Royal Guard, a new alpha-cypermethrin and pyriproxyfen treated mosquito net, against pyrethroid-resistant malaria vectors. Sci. Rep. 2020, 10, 12227. [Google Scholar] [CrossRef] [PubMed]
- Bayili, K.; N’do, S.; Namountougou, M.; Sanou, R.; Ouattara, A.; Dabiré, R.K.; Ouédraogo, A.G.; Malone, D.; Diabaté, A. Evaluation of efficacy of Interceptor® G2, a long-lasting insecticide net coated with a mixture of chlorfenapyr and alpha-cypermethrin, against pyrethroid resistant Anopheles gambiae s.l. in Burkina Faso. Malar. J. 2017, 16, 190. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Paton, D.G.; Childs, L.M.; Itoe, M.A.; Holmdahl, I.E.; Buckee, C.O.; Catteruccia, F. Exposing Anopheles mosquitoes to antimalarials blocks Plasmodium parasite transmission. Nature 2019, 567, 239–243. [Google Scholar] [CrossRef] [PubMed]
- De Oliveira Sousa, J.; de Albuquerque, B.C.; Coura, J.R.; Suárez-Mutis, M.C. Use and retention of long-lasting insecticidal nets (LLINs) in a malaria risk area in the Brazilian Amazon: A 5-year follow-up intervention. Malar. J. 2019, 18, 100. [Google Scholar] [CrossRef] [PubMed]
- Vieira, G.d.D.; Basano, S.d.A.; Katsuragawa, T.H.; Camargo, L.M.A. Insecticide-treated bed nets in Rondônia, Brazil: Evaluation of their impact on malaria control. Rev. Inst. Med. Trop. São Paulo 2014, 56, 493–497. [Google Scholar] [CrossRef] [Green Version]
- Murta, F.L.G.; Mendes, M.O.; Sampaio, V.S.; Junior, A.S.B.; Díaz-Bermúdez, X.P.; Monteiro, W.M.; Lacerda, M.V.G. Misperceptions of patients and health workers regarding malaria elimination in the Brazilian Amazon: A qualitative study. Malar. J. 2019, 18, 223. [Google Scholar] [CrossRef] [Green Version]
- WHO. Indoor Residual Spraying: An Operational Manual for Indoor Residual Spraying (IRS) for Malaria Transmission Control and Elimination. Available online: https://www.who.int/malaria/publications/atoz/9789241508940/en/ (accessed on 5 May 2020).
- Corrêa, A.P.S.; Galardo, A.K.; Lima, L.A.; Câmara, D.C.; Müller, J.N.; Barroso, J.F.S.; Lapouble, O.M.; Rodovalho, C.M.; Ribeiro, K.A.N.; Lima, J.B.P. Efficacy of insecticides used in indoor residual spraying for malaria control: An experimental trial on various surfaces in a “test house”. Malar. J. 2019, 18, 345. [Google Scholar] [CrossRef]
- Dengela, D.; Seyoum, A.; Lucas, B.; Johns, B.; George, K.; Belemvire, A.; Caranci, A.; Norris, L.C.; Fornadel, C.M. Multi-country assessment of residual bio-efficacy of insecticides used for indoor residual spraying in malaria control on different surface types: Results from program monitoring in 17 PMI/USAID-supported IRS countries. Parasit Vectors 2018, 11, 1–14. [Google Scholar] [CrossRef]
- Rohani, A.; Fakhriy, H.A.; Suzilah, I.; Zurainee, M.N.; Najdah, W.M.A.W.; Ariffin, M.M.; Shakirudin, N.M.; Afiq, M.S.M.; Jenarun, J.; Tanrang, Y.; et al. Indoor and outdoor residual spraying of a novel formulation of deltamethrin K-Othrine® (Polyzone) for the control of simian malaria in Sabah, Malaysia. PLoS ONE 2020, 15, e0230860. [Google Scholar] [CrossRef]
- IVCC. NgenIRS is a Four Year Partnership (2016–2019), Led by IVCC and Funded by Unitaid, That Includes the US President’s Malaria Initiative, the Global Fund, Abt Associates and PATH. Available online: https://www.ivcc.com/market-access/ngenirs/ (accessed on 3 September 2020).
- Antonio-Nkondjio, C.; Sandjo, N.N.; Awono-Ambene, P.; Wondji, C.S. Implementing a larviciding efficacy or effectiveness control intervention against malaria vectors: Key parameters for success. Parasit Vectors 2018, 11, 57. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Fontoura, P.S.; da Costa, A.S.; Ribeiro, F.S.; Ferreira, M.S.; Castro, M.C.; Ferreira, M.U. Field Efficacy of VectoMax FG and VectoLex CG Biological Larvicides for Malaria Vector Control in Northwestern Brazil. J. Med. Entomol. 2020, 57, 942–946. [Google Scholar] [CrossRef]
- WHO. Larval Source Management: A Supplementary Malaria Vector Control Measure: An Operational Manual. Available online: https://apps.who.int/iris/bitstream/handle/10665/85379/9789241505604_eng.pdf (accessed on 5 May 2020).
- Soper, F.L.; Wilson, D.B. Anopheles Gambiae in Brazil 1930 to 1940; The Rockefeller Foundation: New York, NY, USA, 1943. [Google Scholar]
- Derua, Y.A.; Kweka, E.J.; Kisinza, W.N.; Githeko, A.K.; Mosha, F.W. Bacterial larvicides used for malaria vector control in sub-Saharan Africa: Review of their effectiveness and operational feasibility. Parasit Vectors 2019, 12, 426. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Becker, N. Microbial control of mosquitoes: Management of the Upper Rhine mosquito population as a model programme. Parasitol. Today 1997, 13, 485–487. [Google Scholar] [CrossRef]
- Kumar, A.; Sharma, V.; Sumodan, P.; Thavaselvam, D. Field trials of biolarvicide Bacillus thuringiensis var. israelensis strain 164 and the larvivorous fish Aplocheilus blocki against Anopheles stephensi for malaria control in Goa, India. J. Am. Mosq. Control Assoc. 1998, 14, 457–462. [Google Scholar]
- Kroeger, A.; Horstick, O.; Riedl, C.; Kaiser, A.; Becker, N. The potential for malaria control with the biological larvicide Bacillus thuringiensis israelensis (Bti) in Peru and Ecuador. Acta Tropica 1995, 60, 47–57. [Google Scholar] [CrossRef]
- Dambach, P. The use of Aquatic Predators for Larval Control of Mosquito Disease Vectors: Opportunities and Limitations. Biol. Control 2020, 104357. [Google Scholar] [CrossRef]
- Cuthbert, R.N.; Dalu, T.; Wasserman, R.J.; Weyl, O.L.; Froneman, P.W.; Callaghan, A.; Coughlan, N.E.; Dick, J.T. Alternative prey impedes the efficacy of a natural enemy of mosquitoes. Biol. Control 2020, 141, 104146. [Google Scholar] [CrossRef]
- Cavados, C.F.G.; Tadei, W.P.; Roque, R.A.; Regis, L.N.; de Oliveira, C.M.F.; Gil, H.B. Bacillus Entomopathogenic Based Biopesticides in Vector Control Programs in Brazil. In Bacillus Thuringiensis and Lysinibacillus Sphaericus; Springer: Berlin, Germany, 2017; pp. 223–237. [Google Scholar]
- Carrasco-Escobar, G.; Manrique, E.; Ruiz-Cabrejos, J.; Saavedra, M.; Alava, F.; Bickersmith, S.; Prussing, C.; Vinetz, J.M.; Conn, J.E.; Moreno, M. High-accuracy detection of malaria vector larval habitats using drone-based multispectral imagery. PLoS Negl. Trop. Dis. 2019, 13, e0007105. [Google Scholar] [CrossRef] [Green Version]
- Solano-Villarreal, E.; Valdivia, W.; Pearcy, M.; Linard, C.; Pasapera-Gonzales, J.; Moreno-Gutierrez, D.; Lejeune, P.; Llanos-Cuentas, A.; Speybroeck, N.; Hayette, M.-P.; et al. Malaria risk assessment and mapping using satellite imagery and boosted regression trees in the Peruvian Amazon. Sci. Rep. 2019, 9, 15173. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Schoepke, A.; Steffen, R.; Gratz, N. Effectiveness of personal protection measures against mosquito bites for malaria prophylaxis in travelers. J. Travel Med. 1998, 5, 188–192. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Loutan, L. Malaria: Still a threat to travellers. Int. J. Antimicrob. Agents 2003, 21, 158–163. [Google Scholar] [CrossRef]
- Richards, S.L.; Agada, N.; Balanay, J.A.G.; White, A.V. Permethrin treated clothing to protect outdoor workers: Evaluation of different methods for mosquito exposure against populations with differing resistance status. Pathog. Glob. Health 2018, 112, 13–21. [Google Scholar] [CrossRef] [PubMed]
- Tan, K.; Faierstein, G.B.; Xu, P.; Barbosa, R.M.; Buss, G.K.; Leal, W.S. A popular Indian clove-based mosquito repellent is less effective against Culex quinquefasciatus and Aedes aegypti than DEET. PLoS ONE 2019, 14, e0224810. [Google Scholar] [CrossRef] [Green Version]
- Roberts, D.R.; Tren, R. International advocacy against DDT and other public health insecticides for malaria control. Res. Rep. Trop. Med. 2011, 2, 23. [Google Scholar]
- Mwanga, E.P.; Mmbando, A.S.; Mrosso, P.C.; Stica, C.; Mapua, S.A.; Finda, M.F.; Kifungo, K.; Kafwenji, A.; Monroe, A.C.; Ogoma, S.B.; et al. Eave ribbons treated with transfluthrin can protect both users and non-users against malaria vectors. Malar. J. 2019, 18, 1–14. [Google Scholar] [CrossRef]
- Masalu, J.P.; Finda, M.; Killeen, G.F.; Ngowo, H.S.; Pinda, P.G.; Okumu, F.O. Creating mosquito-free outdoor spaces using transfluthrin-treated chairs and ribbons. Malar. J. 2020, 19, 109. [Google Scholar] [CrossRef]
- Sangoro, O.P.; Gavana, T.; Finda, M.; Mponzi, W.; Hape, E.; Limwagu, A.; Govella, N.J.; Chaki, P.; Okumu, F.O. Evaluation of personal protection afforded by repellent-treated sandals against mosquito bites in south-eastern Tanzania. Malar. J. 2020, 19, 148. [Google Scholar] [CrossRef] [Green Version]
- Burns, M.; Rowland, M.; N’Guessan, R.; Carneiro, I.; Beeche, A.; Ruiz, S.S.; Kamara, S.; Takken, W.; Carnevale, P.; Allan, R. Insecticide-treated plastic sheeting for emergency malaria prevention and shelter among displaced populations: An observational cohort study in a refugee setting in Sierra Leone. Am. J. Trop. Med. Hyg. 2012, 87, 242–250. [Google Scholar] [CrossRef] [Green Version]
- Kitau, J.; Oxborough, R.; Kaye, A.; Chen-Hussey, V.; Isaacs, E.; Matowo, J.; Kaur, H.; Magesa, S.M.; Mosha, F.; Rowland, M.; et al. Laboratory and experimental hut evaluation of a long-lasting insecticide treated blanket for protection against mosquitoes. Parasit Vectors 2014, 7, 129. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rowland, M.; Durrani, N.; Hewitt, S.; Mohammed, N.; Bouma, M.; Carneiro, I.; Rozendaal, J.; Schapira, A. Permethrin-treated chaddars and top-sheets: Appropriate technology for protection against malaria in Afghanistan and other complex emergencies. Trans. R Soc. Trop. Med. Hyg. 1999, 93, 465–472. [Google Scholar] [CrossRef]
- Hoermann, A.; Tapanelli, S.; Capriotti, P.; Masters, E.K.; Habtewold, T.; Christophides, G.K.; Windbichler, N. Converting endogenous genes of the malaria mosquito into simple non-autonomous gene drives for population replacement. bioRxiv 2020. [Google Scholar] [CrossRef]
- Raban, R.R.; Marshall, J.M.; Akbari, O.S. Progress towards engineering gene drives for population control. J. Exp. Biol. 2020, 223. [Google Scholar] [CrossRef] [Green Version]
- Terenius, O.; Marinotti, O.; Sieglaff, D.; James, A.A. Molecular genetic manipulation of vector mosquitoes. Cell Host Microbe. 2008, 4, 417–423. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Serebrovsky, A. On the possibility of a new method for the control of insect pests. Sterile-male technique for eradication or control of harmful insects. In Proceedings of the Panel on Application of the Sterile-Male Technique for the Eradication or Control of Harmful Species of Insects, Organised by the Joint FAO/IAEA Division of Atomic Energy in Food and Agriculture, Vienna, Austria, 27–31 May 1968; Volume 1969, pp. 123–237. [Google Scholar]
- Benedict, M.Q.; Robinson, A.S. The first releases of transgenic mosquitoes: An argument for the sterile insect technique. Trends Parasitol. 2003, 19, 349–355. [Google Scholar] [CrossRef]
- Thomas, D.D.; Donnelly, C.A.; Wood, R.J.; Alphey, L.S. Insect population control using a dominant, repressible, lethal genetic system. Science 2000, 287, 2474–2476. [Google Scholar] [CrossRef]
- Knipling, E.F. Sterile-Male Method of Population Control: Successful with some insects, the method may also be effective when applied to other noxious animals. Science 1959, 130, 902–904. [Google Scholar] [CrossRef]
- Mastrangelo, T.; Welch, J.B. An overview of the components of AW-IPM campaigns against the New World screwworm. Insects 2012, 3, 930–955. [Google Scholar] [CrossRef] [Green Version]
- Orozco-Dávila, D.; Quintero, L.; Hernández, E.; Solís, E.; Artiaga, T.; Hernández, R.; Ortega, C.; Montoya, P. Mass rearing and sterile insect releases for the control of A nastrepha spp. pests in Mexico–A review. Entomol. Exp. Appl. 2017, 164, 176–187. [Google Scholar] [CrossRef] [Green Version]
- Lindquist, D.; Abusowa, M.; Hall, M. The New World screwworm fly in Libya: A review of its introduction and eradication. Med. Vet. Entomol. 1992, 6, 2–8. [Google Scholar] [CrossRef] [PubMed]
- Vargas-Terán, M.; Hursey, B.; Cunningham, E. Eradication of the screwworm from Libya using the sterile insect technique. Parasitol. Today 1994, 10, 119–122. [Google Scholar] [CrossRef]
- Lowe, R.E.; Bailey, D.L.; Dame, D.A.; Savage, K.E.; Kaiser, P.E. Efficiency of techniques for the mass release of sterile male Anopheles albimanus Wiedemann in El Salvador. Am. J. Trop. Med. 1980, 29, 695–703. [Google Scholar] [CrossRef] [PubMed]
- Carvalho, D.O.; McKemey, A.R.; Garziera, L.; Lacroix, R.; Donnelly, C.A.; Alphey, L.; Malavasi, A.; Capurro, M.L. Suppression of a field population of Aedes aegypti in Brazil by sustained release of transgenic male mosquitoes. PLoS Negl. Trop. Dis. 2015, 9, e0003864. [Google Scholar] [CrossRef] [Green Version]
- Vreysen, M.J.; Saleh, K.; Mramba, F.; Parker, A.; Feldmann, U.; Dyck, V.A.; Msangi, A.; Bouyer, J. Sterile insects to enhance agricultural development: The case of sustainable tsetse eradication on Unguja Island, Zanzibar, using an area-wide integrated pest management approach. PLoS Negl. Trop. Dis. 2014, 8, e2857. [Google Scholar] [CrossRef] [Green Version]
- Hammond, A.M.; Galizi, R. Gene drives to fight malaria: Current state and future directions. Pathog. Glob. Health 2017, 111, 412–423. [Google Scholar] [CrossRef]
- Curtis, C. Possible use of translocations to fix desirable genes in insect pest populations. Nature 1968, 218, 368–369. [Google Scholar] [CrossRef]
- Leftwich, P.T.; Edgington, M.P.; Harvey-Samuel, T.; Carabajal Paladino, L.Z.; Norman, V.C.; Alphey, L. Recent advances in threshold-dependent gene drives for mosquitoes. Biochem. Soc. Trans. 2018, 46, 1203–1212. [Google Scholar] [CrossRef]
- Flores, H.A.; O’Neill, S.L. Controlling vector-borne diseases by releasing modified mosquitoes. Nat. Rev. Microbiol. 2018, 16, 508–518. [Google Scholar] [CrossRef]
- Quinn, C.M.; Nolan, T. Nuclease-based gene drives, an innovative tool for insect vector control: Advantages and challenges of the technology. Curr. Opin. Insect Sci. 2020, 39, 77–83. [Google Scholar] [CrossRef]
- Kyrou, K.; Hammond, A.M.; Galizi, R.; Kranjc, N.; Burt, A.; Beaghton, A.K.; Nolan, T.; Crisanti, A. A CRISPR–Cas9 gene drive targeting doublesex causes complete population suppression in caged Anopheles gambiae mosquitoes. Nat. Biotechnol. 2018, 36, 1062–1066. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Simoni, A.; Hammond, A.M.; Beaghton, A.K.; Galizi, R.; Taxiarchi, C.; Kyrou, K.; Meacci, D.; Gribble, M.; Morselli, G.; Burt, A. A male-biased sex-distorter gene drive for the human malaria vector Anopheles gambiae. Nat. Biotechnol. 2020, 1–7. [Google Scholar]
- Hammond, A.; Galizi, R.; Kyrou, K.; Simoni, A.; Siniscalchi, C.; Katsanos, D.; Gribble, M.; Baker, D.; Marois, E.; Russell, S. A CRISPR-Cas9 gene drive system targeting female reproduction in the malaria mosquito vector Anopheles gambiae. Nat. Biotechnol. 2016, 34, 78–83. [Google Scholar] [CrossRef] [Green Version]
- Galizi, R.; Doyle, L.A.; Menichelli, M.; Bernardini, F.; Deredec, A.; Burt, A.; Stoddard, B.L.; Windbichler, N.; Crisanti, A. A synthetic sex ratio distortion system for the control of the human malaria mosquito. Nat. Commun. 2014, 5, 3977. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gantz, V.M.; Jasinskiene, N.; Tatarenkova, O.; Fazekas, A.; Macias, V.M.; Bier, E.; James, A.A. Highly efficient Cas9-mediated gene drive for population modification of the malaria vector mosquito Anopheles stephensi. Proc. Natl. Acad. Sci. USA 2015, 112, E6736–E6743. [Google Scholar] [CrossRef] [Green Version]
- Pham, T.B.; Phong, C.H.; Bennett, J.B.; Hwang, K.; Jasinskiene, N.; Parker, K.; Stillinger, D.; Marshall, J.M.; Carballar-Lejarazú, R.; James, A.A. Experimental population modification of the malaria vector mosquito, Anopheles stephensi. PLoS Genet. 2019, 15, e1008440. [Google Scholar] [CrossRef]
- Akbari, O.S.; Matzen, K.D.; Marshall, J.M.; Huang, H.; Ward, C.M.; Hay, B.A. A synthetic gene drive system for local, reversible modification and suppression of insect populations. Curr. Biol. 2013, 23, 671–677. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Buchman, A.; Marshall, J.M.; Ostrovski, D.; Yang, T.; Akbari, O.S. Synthetically engineered Medea gene drive system in the worldwide crop pest Drosophila suzukii. Proc. Natl. Acad. Sci. USA 2018, 115, 4725–4730. [Google Scholar] [CrossRef] [Green Version]
- Kandul, N.P.; Liu, J.; Buchman, A.; Gantz, V.M.; Bier, E.; Akbari, O.S. Assessment of a split homing based gene drive for efficient knockout of multiple genes. G3-Genes Genom. Genet 2020, 10, 827–837. [Google Scholar] [CrossRef] [Green Version]
- Nash, A.; Urdaneta, G.M.; Beaghton, A.K.; Hoermann, A.; Papathanos, P.A.; Christophides, G.K.; Windbichler, N. Integral gene drives for population replacement. Biol. Open 2019, 8. [Google Scholar] [CrossRef] [Green Version]
- Oberhofer, G.; Ivy, T.; Hay, B.A. Cleave and Rescue, a novel selfish genetic element and general strategy for gene drive. Proc. Natl. Acad. Sci. USA 2019, 116, 6250–6259. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Collins, C.; Bonds, J.; Quinlan, M.; Mumford, J. Effects of the removal or reduction in density of the malaria mosquito, Anopheles gambiae sl, on interacting predators and competitors in local ecosystems. Med. Vet. Entomol. 2019, 33, 1–15. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Baker, D.A.; Nolan, T.; Fischer, B.; Pinder, A.; Crisanti, A.; Russell, S. A comprehensive gene expression atlas of sex-and tissue-specificity in the malaria vector, Anopheles gambiae. BMC Genom. 2011, 12, 1–12. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cassone, B.J.; Kay, R.G.; Daugherty, M.P.; White, B.J. Comparative transcriptomics of malaria mosquito testes: Function, evolution, and linkage. G3-Genes Genom. Genet 2017, 7, 1127–1136. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Papa, F.; Windbichler, N.; Waterhouse, R.M.; Cagnetti, A.; D’Amato, R.; Persampieri, T.; Lawniczak, M.K.; Nolan, T.; Papathanos, P.A. Rapid evolution of female-biased genes among four species of Anopheles malaria mosquitoes. Genome Res. 2017, 27, 1536–1548. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rose, G.; Krzywinska, E.; Kim, J.; Revuelta, L.; Ferretti, L.; Krzywinski, J. Dosage compensation in the African malaria mosquito Anopheles gambiae. Genome Biol. Evol. 2016, 8, 411–425. [Google Scholar]
- Taxiarchi, C.; Kranjc, N.; Kriezis, A.; Kyrou, K.; Bernardini, F.; Russell, S.; Nolan, T.; Crisanti, A.; Galizi, R. High-resolution transcriptional profiling of Anopheles gambiae spermatogenesis reveals mechanisms of sex chromosome regulation. Sci. Rep. 2019, 9, 1–12. [Google Scholar] [CrossRef]
- Araujo, M.d.S.; Andrade, A.O.; Santos, N.A.C.d.; Pereira, D.B.; Costa, G.d.S.; Paulo, P.F.M.d.; Rios, C.T.; Moreno, M.; Pereira-da-Silva, L.H.; Medeiros, J.F.d. Brazil’s first free-mating laboratory colony of Nyssorhynchus darlingi. Rev. Soc. Bras. Med. Trop. 2019, 52. [Google Scholar] [CrossRef] [Green Version]
- Moreno, M.; Tong, C.; Guzmán, M.; Chuquiyauri, R.; Llanos-Cuentas, A.; Rodriguez, H.; Gamboa, D.; Meister, S.; Winzeler, E.A.; Maguina, P. Infection of laboratory-colonized Anopheles darlingi mosquitoes by Plasmodium vivax. Am. J. Trop. Med. 2014, 90, 612–616. [Google Scholar] [CrossRef] [Green Version]
- Villarreal-Treviño, C.; Vásquez, G.M.; López-Sifuentes, V.M.; Escobedo-Vargas, K.; Huayanay-Repetto, A.; Linton, Y.-M.; Flores-Mendoza, C.; Lescano, A.G.; Stell, F.M. Establishment of a free-mating, long-standing and highly productive laboratory colony of Anopheles darlingi from the Peruvian Amazon. Malar. J. 2015, 14, 227. [Google Scholar] [CrossRef] [Green Version]
- Marinotti, O.; Cerqueira, G.C.; De Almeida, L.G.P.; Ferro, M.I.T.; Loreto, E.L.d.S.; Zaha, A.; Teixeira, S.M.; Wespiser, A.R.; Almeida e Silva, A.; Schlindwein, A.D. The genome of Anopheles darlingi, the main neotropical malaria vector. Nucleic Acids Res. 2013, 41, 7387–7400. [Google Scholar] [CrossRef] [PubMed]
- Compton, A.; Sharakhov, I.V.; Tu, Z. Recent Advances and Future Perspectives in Vector-omics. Curr. Opin. Insect Sci. 2020, 40, 94–103. [Google Scholar] [CrossRef] [PubMed]
- Da Silva, A.N.; Dos Santos, C.C.; Lacerda, R.N.; Santa Rosa, E.P.; De Souza, R.T.; Galiza, D.; Sucupira, I.; Conn, J.E.; Póvoa, M.M. Laboratory colonization of Anopheles aquasalis (Diptera: Culicidae) in Belém, Pará, Brazil. J. Med. Entomol. 2006, 43, 107–109. [Google Scholar] [CrossRef] [Green Version]
- Giglio, N.; Sousa-Lima, A.; Gallardo, A.; Lima, J. Laboratory Colonization of Anopheles (Nyssorhynchus) marajoara (Diptera: Culicidae) by Induced Copulation. J. Med. Entomol. 2015, 52, 3–8. [Google Scholar] [CrossRef] [PubMed]
- Horosko, S.; Lima, J.B.; Brandolini, M. Establishment of a free-mating colony of Anopheles albitarsis from Brazil. J. Am. Mosq. Control Assoc. 1997, 95–96. [Google Scholar]
- Dahmana, H.; Mediannikov, O. Mosquito-Borne Diseases Emergence/Resurgence and How to Effectively Control It Biologically. Pathogens 2020, 9, 310. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Poopathi, S.; Mani, C.; Thirugnanasambantham, K.; Praba, V.L.; Ahangar, N.A.; Balagangadharan, K. Identification and characterization of a novel marine Bacillus cereus for mosquito control. Parasitol. Res. 2014, 113, 323–332. [Google Scholar] [CrossRef]
- Sharma, L.; Bohra, N.; Singh, R.K.; Marques, G. Potential of Entomopathogenic Bacteria and Fungi. In Microbes for Sustainable Insect Pest Management; Springer: Cham, Switzerland, 2019; pp. 115–149. [Google Scholar]
- Soares-da-Silva, J.; Queirós, S.G.; de Aguiar, J.S.; Viana, J.L.; dos RAV Neta, M.; da Silva, M.C.; Pinheiro, V.C.; Polanczyk, R.A.; Carvalho-Zilse, G.A.; Tadei, W.P. Molecular characterization of the gene profile of Bacillus thuringiensis Berliner isolated from Brazilian ecosystems and showing pathogenic activity against mosquito larvae of medical importance. Acta Trop. 2017, 176, 197–205. [Google Scholar] [CrossRef]
- Arantes, O.; Vilas-Bôas, L.; Vilas-Bôas, G. Bacillus thuringiensis: Estratégias no controle biológico. In Biotecnologia: Avanços na Agricultura e na Agroindústria; Agropecuária: Rio de Janeiro, Brazil, 2002; pp. 269–293. [Google Scholar]
- Galardo, A.K.R.; Zimmerman, R.; Galardo, C.D. Larval control of Anopheles (Nyssorhinchus) darlingiusing granular formulation of Bacillus sphaericus in abandoned gold-miners excavation pools in the Brazilian Amazon Rainforest. Rev. Soc. Bras. Med. Trop. 2013, 46, 172–177. [Google Scholar] [CrossRef] [Green Version]
- Fillinger, U.; Knols, B.G.; Becker, N. Efficacy and efficiency of new Bacillus thuringiensis var. israelensis and Bacillus sphaericus formulations against Afrotropical anophelines in Western Kenya. Trop. Med. Int. Health 2003, 8, 37–47. [Google Scholar] [CrossRef]
- Majambere, S.; Pinder, M.; Fillinger, U.; Ameh, D.; Conway, D.J.; Green, C.; Jeffries, D.; Jawara, M.; Milligan, P.J.; Hutchinson, R. Is mosquito larval source management appropriate for reducing malaria in areas of extensive flooding in The Gambia? A cross-over intervention trial. Am. J. Trop. Med. 2010, 82, 176–184. [Google Scholar] [CrossRef] [PubMed]
- Afrane, Y.A.; Mweresa, N.G.; Wanjala, C.L.; Gilbreath III, T.M.; Zhou, G.; Lee, M.-C.; Githeko, A.K.; Yan, G. Evaluation of long-lasting microbial larvicide for malaria vector control in Kenya. Malar. J. 2016, 15, 1–9. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Derua, Y.A.; Kahindi, S.C.; Mosha, F.W.; Kweka, E.J.; Atieli, H.E.; Wang, X.; Zhou, G.; Lee, M.C.; Githeko, A.K.; Yan, G. Microbial larvicides for mosquito control: Impact of long lasting formulations of Bacillus thuringiensis var. israelensis and Bacillus sphaericus on non-target organisms in western Kenya highlands. Ecol. Evol. 2018, 8, 7563–7573. [Google Scholar] [CrossRef] [Green Version]
- Zhou, G.; Wiseman, V.; Atieli, H.E.; Lee, M.-C.; Githeko, A.K.; Yan, G. The impact of long-lasting microbial larvicides in reducing malaria transmission and clinical malaria incidence: Study protocol for a cluster randomized controlled trial. Trials 2016, 17, 423. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Johnson, B.J.; Manby, R.; Devine, G.J. Performance of an aerially applied liquid Bacillus thuringiensis var. israelensis formulation (strain AM65-52) against mosquitoes in mixed saltmarsh-mangrove systems and fine-scale mapping of mangrove canopy cover using affordable drone-based imagery. Pest Manag. Sci. 2020. [Google Scholar] [CrossRef]
- Ramirez, J.L.; Short, S.M.; Bahia, A.C.; Saraiva, R.G.; Dong, Y.; Kang, S.; Tripathi, A.; Mlambo, G.; Dimopoulos, G. Chromobacterium Csp_P reduces malaria and dengue infection in vector mosquitoes and has entomopathogenic and in vitro anti-pathogen activities. PLoS Pathog. 2014, 10, e1004398. [Google Scholar] [CrossRef]
- Short, S.M.; Van Tol, S.; MacLeod, H.J.; Dimopoulos, G. Hydrogen cyanide produced by the soil bacterium Chromobacterium sp. Panama contributes to mortality in Anopheles gambiae mosquito larvae. Sci. Rep. 2018, 8, 8358. [Google Scholar] [CrossRef] [Green Version]
- Valero-Jiménez, C.A.; van Kan, J.A.; Koenraadt, C.J.; Zwaan, B.J.; Schoustra, S.E. Experimental evolution to increase the efficacy of the entomopathogenic fungus Beauveria bassiana against malaria mosquitoes: Effects on mycelial growth and virulence. Evol. Appl. 2017, 10, 433–443. [Google Scholar] [CrossRef]
- Lovett, B.; Bilgo, E.; Diabate, A.; St. Leger, R. A review of progress toward field application of transgenic mosquitocidal entomopathogenic fungi. Pest Manag. Sci. 2019, 75, 2316–2324. [Google Scholar] [CrossRef]
- Azizoglu, U.; Jouzani, G.S.; Yilmaz, N.; Baz, E.; Ozkok, D. Genetically modified entomopathogenic bacteria, recent developments, benefits and impacts: A review. Sci. Total Environ. 2020, 734, 139169. [Google Scholar] [CrossRef]
- Federici, B.A. Recombinant bacterial larvicides for control of important mosquito vectors of disease. In Vector Biology, Ecology and Control; Springer: Dordrecht, The Netherlands, 2010; pp. 163–176. [Google Scholar]
- Federici, B.A.; Park, H.-W.; Bideshi, D.K.; Wirth, M.C.; Johnson, J.J.; Sakano, Y.; Tang, M. Developing recombinant bacteria for control of mosquito larvae. J. Am. Mosq. Control Assoc. 2007, 23, 164–175. [Google Scholar] [CrossRef]
- Borovsky, D.; Nauwelaers, S.; Shatters, R., Jr. Biochemical and Molecular Characterization of Pichia pastoris Cells Expressing Multiple TMOF Genes (tmfA) for Mosquito Larval Control. Front. Physiol. 2020, 11, 527. [Google Scholar] [CrossRef] [PubMed]
- Deng, S.-Q.; Zou, W.-H.; Li, D.-L.; Chen, J.-T.; Huang, Q.; Zhou, L.-J.; Tian, X.-X.; Chen, Y.-J.; Peng, H.-J. Expression of Bacillus thuringiensis toxin Cyt2Ba in the entomopathogenic fungus Beauveria bassiana increases its virulence towards Aedes mosquitoes. PLoS. Negl. Trop. Dis. 2019, 13, e0007590. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Karabörklü, S.; Azizoglu, U.; Azizoglu, Z.B. Recombinant entomopathogenic agents: A review of biotechnological approaches to pest insect control. World J. Microbiol. Biotechnol. 2018, 34, 14. [Google Scholar] [CrossRef]
- Cirimotich, C.M.; Dong, Y.; Clayton, A.M.; Sandiford, S.L.; Souza-Neto, J.A.; Mulenga, M.; Dimopoulos, G. Natural microbe-mediated refractoriness to Plasmodium infection in Anopheles gambiae. Science 2011, 332, 855–858. [Google Scholar] [CrossRef] [Green Version]
- Dong, Y.; Manfredini, F.; Dimopoulos, G. Implication of the mosquito midgut microbiota in the defense against malaria parasites. PLoS Pathog. 2009, 5, e1000423. [Google Scholar] [CrossRef] [Green Version]
- Ramirez, J.L.; Souza-Neto, J.; Cosme, R.T.; Rovira, J.; Ortiz, A.; Pascale, J.M.; Dimopoulos, G. Reciprocal tripartite interactions between the Aedes aegypti midgut microbiota, innate immune system and dengue virus influences vector competence. PLoS Negl. Trop. Dis. 2012, 6, e1561. [Google Scholar] [CrossRef]
- Van Tol, S.; Dimopoulos, G. Influences of the mosquito microbiota on vector competence. In Advances in Insect Physiology; Elsevier: Amsterdam, The Netherlands, 2016; Volume 51, pp. 243–291. [Google Scholar]
- Bai, L.; Wang, L.; Vega-Rodríguez, J.; Wang, G.; Wang, S. A Gut Symbiotic Bacterium Serratia marcescens Renders Mosquito Resistance to Plasmodium Infection Through Activation of Mosquito Immune Responses. Front. Microbiol. 2019, 10. [Google Scholar] [CrossRef] [Green Version]
- Gonzalez-Ceron, L.; Santillan, F.; Rodriguez, M.H.; Mendez, D.; Hernandez-Avila, J.E. Bacteria in midguts of field-collected Anopheles albimanus block Plasmodium vivax sporogonic development. J. Med. Entomol. 2003, 40, 371–374. [Google Scholar] [CrossRef] [Green Version]
- Dennison, N.J.; Saraiva, R.G.; Cirimotich, C.M.; Mlambo, G.; Mongodin, E.F.; Dimopoulos, G. Functional genomic analyses of Enterobacter, Anopheles and Plasmodium reciprocal interactions that impact vector competence. Malar. J. 2016, 15, 1–15. [Google Scholar] [CrossRef]
- Cappelli, A.; Damiani, C.; Mancini, M.V.; Valzano, M.; Rossi, P.; Serrao, A.; Ricci, I.; Favia, G. Asaia activates immune genes in mosquito eliciting an anti-Plasmodium response: Implications in Malaria control. Front. Genet. 2019, 10, 836. [Google Scholar] [CrossRef] [Green Version]
- Cappelli, A.; Valzano, M.; Cecarini, V.; Bozic, J.; Rossi, P.; Mensah, P.; Amantini, C.; Favia, G.; Ricci, I. Killer yeasts exert anti-plasmodial activities against the malaria parasite Plasmodium berghei in the vector mosquito Anopheles stephensi and in mice. Parasit Vectors 2019, 12, 329. [Google Scholar] [CrossRef] [PubMed]
- Herren, J.K.; Mbaisi, L.; Mararo, E.; Makhulu, E.E.; Mobegi, V.A.; Butungi, H.; Mancini, M.V.; Oundo, J.W.; Teal, E.T.; Pinaud, S. A microsporidian impairs Plasmodium falciparum transmission in Anopheles arabiensis mosquitoes. Nat. Commun. 2020, 11, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Sougoufara, S.; Ottih, E.C.; Tripet, F. The need for new vector control approaches targeting outdoor biting Anopheline malaria vector communities. Parasit Vectors 2020, 13, 1–15. [Google Scholar] [CrossRef] [PubMed]
- Huang, W.; Wang, S.; Jacobs-Lorena, M. Use of Microbiota to Fight Mosquito-Borne Disease. Front. Genet. 2020, 11. [Google Scholar] [CrossRef]
- Ren, X.; Hoiczyk, E.; Rasgon, J.L. Viral paratransgenesis in the malaria vector Anopheles gambiae. PLoS Pathog. 2008, 4, e1000135. [Google Scholar] [CrossRef]
- Fang, W.; Vega-Rodríguez, J.; Ghosh, A.K.; Jacobs-Lorena, M.; Kang, A.; Leger, R.J.S. Development of transgenic fungi that kill human malaria parasites in mosquitoes. Science 2011, 331, 1074–1077. [Google Scholar] [CrossRef] [Green Version]
- Mancini, M.V.; Spaccapelo, R.; Damiani, C.; Accoti, A.; Tallarita, M.; Petraglia, E.; Rossi, P.; Cappelli, A.; Capone, A.; Peruzzi, G. Paratransgenesis to control malaria vectors: A semi-field pilot study. Parasit Vectors 2016, 9, 1–9. [Google Scholar] [CrossRef] [Green Version]
- Raharimalala, F.N.; Boukraa, S.; Bawin, T.; Boyer, S.; Francis, F. Molecular detection of six (endo-) symbiotic bacteria in Belgian mosquitoes: First step towards the selection of appropriate paratransgenesis candidates. Parasitol. Res. 2016, 115, 1391–1399. [Google Scholar] [CrossRef]
- Villegas, L.M.; Pimenta, P.F.P. Metagenomics, paratransgenesis and the Anopheles microbiome: A portrait of the geographical distribution of the anopheline microbiota based on a meta-analysis of reported taxa. Mem. Inst. Oswaldo Cruz 2014, 109, 672–684. [Google Scholar] [CrossRef] [Green Version]
- Wang, S.; Dos-Santos, A.L.; Huang, W.; Liu, K.C.; Oshaghi, M.A.; Wei, G.; Agre, P.; Jacobs-Lorena, M. Driving mosquito refractoriness to Plasmodium falciparum with engineered symbiotic bacteria. Science 2017, 357, 1399–1402. [Google Scholar] [CrossRef] [Green Version]
- Wang, S.; Ghosh, A.K.; Bongio, N.; Stebbings, K.A.; Lampe, D.J.; Jacobs-Lorena, M. Fighting malaria with engineered symbiotic bacteria from vector mosquitoes. Proc. Natl. Acad. Sci. USA 2012, 109, 12734–12739. [Google Scholar] [CrossRef] [Green Version]
- Yoshida, S.; Ioka, D.; Matsuoka, H.; Endo, H.; Ishii, A. Bacteria expressing single-chain immunotoxin inhibit malaria parasite development in mosquitoes. Mol. Biochem. Parasitol. 2001, 113, 89–96. [Google Scholar] [CrossRef]
- Arruda, A.; Ferreira, G.S.; da Silva Lima, N.C.; dos Santos Júnior, A.; Custódio, M.G.F.; Benevides-Matos, N.; Ozaki, L.S.; Stabeli, R.G.; Silva, A.A. A simple methodology to collect culturable bacteria from feces of Anopheles darlingi (Diptera: Culicidae). J. Microbiol. Methods 2017, 141, 115–117. [Google Scholar] [CrossRef] [PubMed]
- Bascuñán, P.; Niño-Garcia, J.P.; Galeano-Castañeda, Y.; Serre, D.; Correa, M.M. Factors shaping the gut bacterial community assembly in two main Colombian malaria vectors. Microbiome 2018, 6, 1–12. [Google Scholar] [CrossRef] [PubMed]
- Galeano-Castañeda, Y.; Bascuñán, P.; Serre, D.; Correa, M.M. Trans-stadial fate of the gut bacterial microbiota in Anopheles albimanus. Acta Tropica 2020, 201, 105204. [Google Scholar] [CrossRef] [PubMed]
- Galeano-Castañeda, Y.; Urrea-Aguirre, P.; Piedrahita, S.; Bascuñán, P.; Correa, M.M. Composition and structure of the culturable gut bacterial communities in Anopheles albimanus from Colombia. PLoS ONE 2019, 14, e0225833. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kämpfer, P.; Glaeser, S.P.; Marinotti, O.; Guy, L.; Håkansson, S.; Tadei, W.P.; Busse, H.-J.; Terenius, O. Coetzeea brasiliensis gen. nov., sp. nov. isolated from larvae of Anopheles darlingi. Int. J. Syst. Evol. Microbiol. 2016, 66, 5211–5217. [Google Scholar] [CrossRef]
- Nilsson, L.K.; de Oliveira, M.R.; Marinotti, O.; Rocha, E.M.; Håkansson, S.; Tadei, W.P.; de Souza, A.Q.L.; Terenius, O. Characterization of Bacterial Communities in Breeding Waters of Anopheles darlingi in Manaus in the Amazon Basin Malaria-Endemic Area. Microb. Ecol. 2019, 78, 781–791. [Google Scholar] [CrossRef] [Green Version]
- Oliveira, T.M.; Sanabani, S.S.; Sallum, M.A.M. Asaia (Rhodospirillales: Acetobacteraceae) and Serratia (Enterobacterales: Yersiniaceae) associated with Nyssorhynchus braziliensis and Nyssorhynchus darlingi (Diptera: Culicidae). Rev. Bras. Entomol. 2020, 64. [Google Scholar] [CrossRef]
- Oliveira, T.M.P.; Sanabani, S.S.; Sallum, M.A.M. Bacterial diversity associated with the abdomens of naturally Plasmodium-infected and non-infected Nyssorhynchus darlingi. BMC Microbiol. 2020, 20, 1–8. [Google Scholar] [CrossRef]
- Prussing, C.; Saavedra, M.P.; Bickersmith, S.A.; Alava, F.; Guzmán, M.; Manrique, E.; Carrasco-Escobar, G.; Moreno, M.; Gamboa, D.; Vinetz, J.M. Malaria vector species in Amazonian Peru co-occur in larval habitats but have distinct larval microbial communities. PLoS Negl. Trop. Dis. 2019, 13, e0007412. [Google Scholar] [CrossRef] [Green Version]
- Terenius, O.; De Oliveira, C.D.; Pinheiro, W.D.; Tadei, W.P.; James, A.A.; Marinotti, O. 16S rRNA gene sequences from bacteria associated with adult Anopheles darlingi (Diptera: Culicidae) mosquitoes. J. Med. Entomol. 2008, 45, 172–175. [Google Scholar] [CrossRef] [Green Version]
- Hilgenboecker, K.; Hammerstein, P.; Schlattmann, P.; Telschow, A.; Werren, J.H. How many species are infected with Wolbachia?–a statistical analysis of current data. FEMS Microbiol. Lett. 2008, 281, 215–220. [Google Scholar] [CrossRef] [Green Version]
- Sinkins, S.P. Wolbachia and cytoplasmic incompatibility in mosquitoes. Insect Biochem. Mol. Biol. 2004, 34, 723–729. [Google Scholar] [CrossRef]
- Werren, J.H.; Baldo, L.; Clark, M.E. Wolbachia: Master manipulators of invertebrate biology. Nat. Rev. Microbiol. 2008, 6, 741–751. [Google Scholar] [CrossRef]
- Rousset, F.; Bouchon, D.; Pintureau, B.; Juchault, P.; Solignac, M. Wolbachia endosymbionts responsible for various alterations of sexuality in arthropods. Proc. R. Soc. B 1992, 250, 91–98. [Google Scholar]
- Stouthamer, R.; Breeuwer, J.A.; Hurst, G.D. Wolbachia pipientis: Microbial manipulator of arthropod reproduction. Annu. Rev. Microbiol. 1999, 53, 71–102. [Google Scholar] [CrossRef]
- Yen, J.H.; Barr, A.R. New hypothesis of the cause of cytoplasmic incompatibility in Culex pipiens L. Nature 1971, 232, 657–658. [Google Scholar] [CrossRef]
- Caragata, E.P.; Dutra, H.L.; Moreira, L.A. Exploiting intimate relationships: Controlling mosquito-transmitted disease with Wolbachia. Trends Parasitol. 2016, 32, 207–218. [Google Scholar] [CrossRef] [Green Version]
- Hughes, G.L.; Koga, R.; Xue, P.; Fukatsu, T.; Rasgon, J.L. Wolbachia infections are virulent and inhibit the human malaria parasite Plasmodium falciparum in Anopheles gambiae. PLoS Pathog. 2011, 7, e1002043. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ferreira, A.G.; Fairlie, S.; Moreira, L.A. Insect vectors endosymbionts as solutions against diseases. Curr. Opin. Insect Sci. 2020, 40, 56–61. [Google Scholar] [CrossRef]
- Zhang, D.; Xi, Z.; Li, Y.; Wang, X.; Yamada, H.; Qiu, J.; Liang, Y.; Zhang, M.; Wu, Y.; Zheng, X. Toward implementation of combined incompatible and sterile insect techniques for mosquito control: Optimized chilling conditions for handling Aedes albopictus male adults prior to release. PLoS Negl. Trop. Dis. 2020, 14, e0008561. [Google Scholar] [CrossRef] [PubMed]
- Baldini, F.; Segata, N.; Pompon, J.; Marcenac, P.; Shaw, W.R.; Dabiré, R.K.; Diabaté, A.; Levashina, E.A.; Catteruccia, F. Evidence of natural Wolbachia infections in field populations of Anopheles gambiae. Nat. Commun. 2014, 5, 3985. [Google Scholar] [CrossRef] [Green Version]
- Gomes, F.M.; Hixson, B.L.; Tyner, M.D.; Ramirez, J.L.; Canepa, G.E.; e Silva, T.L.A.; Molina-Cruz, A.; Keita, M.; Kane, F.; Traoré, B. Effect of naturally occurring Wolbachia in Anopheles gambiae sl mosquitoes from Mali on Plasmodium falciparum malaria transmission. Proc. Natl. Acad. Sci. USA 2017, 114, 12566–12571. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jeffries, C.L.; Lawrence, G.G.; Golovko, G.; Kristan, M.; Orsborne, J.; Spence, K.; Hurn, E.; Bandibabone, J.; Tantely, L.M.; Raharimalala, F.N. Novel Wolbachia strains in Anopheles malaria vectors from sub-Saharan Africa. Wellcome Open Res. 2018, 3. [Google Scholar] [CrossRef] [PubMed]
- Shaw, W.R.; Marcenac, P.; Childs, L.M.; Buckee, C.O.; Baldini, F.; Sawadogo, S.P.; Dabiré, R.K.; Diabaté, A.; Catteruccia, F. Wolbachia infections in natural Anopheles populations affect egg laying and negatively correlate with Plasmodium development. Nat. Commun. 2016, 7, 1–7. [Google Scholar] [CrossRef] [PubMed]
- Chrostek, E.; Gerth, M. Is Anopheles gambiae a natural host of Wolbachia? MBio 2019, 10. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Joshi, D.; Pan, X.; McFadden, M.J.; Bevins, D.; Liang, X.; Lu, P.; Thiem, S.; Xi, Z. The maternally inheritable Wolbachia wAlbB induces refractoriness to Plasmodium berghei in Anopheles stephensi. Front. Microbiol. 2017, 8, 366. [Google Scholar] [CrossRef] [Green Version]
- Bian, G.; Joshi, D.; Dong, Y.; Lu, P.; Zhou, G.; Pan, X.; Xu, Y.; Dimopoulos, G.; Xi, Z. Wolbachia invades Anopheles stephensi populations and induces refractoriness to Plasmodium infection. Science 2013, 340, 748–751. [Google Scholar] [CrossRef]
- Epis, S.; Varotto-Boccazzi, I.; Crotti, E.; Damiani, C.; Giovati, L.; Mandrioli, M.; Biggiogera, M.; Gabrieli, P.; Genchi, M.; Polonelli, L. Chimeric symbionts expressing a Wolbachia protein stimulate mosquito immunity and inhibit filarial parasite development. Commun. Biol. 2020, 3, 1–10. [Google Scholar] [CrossRef] [PubMed]
- United Nations General Assembly. Transforming Our World: The 2030 Agenda for Sustainable Development; Division for Sustainable Development Goals: New York, NY, USA, 2015. [Google Scholar]
- Akhavan, D.; Musgrove, P.; Abrantes, A.; Gusmão, R.d.A. Cost-effective malaria control in Brazil: Cost-effectiveness of a malaria control program in the Amazon Basin of Brazil, 1988–1996. Soc. Sci. Med. 1999, 49, 1385–1399. [Google Scholar] [CrossRef]
- Bôtto-Menezes, C.; Bardají, A.; dos Santos Campos, G.; Fernandes, S.; Hanson, K.; Martínez-Espinosa, F.E.; Menéndez, C.; Sicuri, E. Costs associated with malaria in pregnancy in the Brazilian Amazon, a low endemic area where Plasmodium vivax predominates. PLoS Negl. Trop. Dis. 2016, 10, e0004494. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Dev, V.; Manguin, S. Fast Forward: From Malaria Elimination to Malaria Eradication. Available online: https://0-blogs-biomedcentral-com.brum.beds.ac.uk/bugbitten/2020/06/16/fast-forward-from-malaria-elimination-to-malaria-eradication/ (accessed on 17 June 2020).
- Pang, L.W.; Piovesan-Alves, F. Economic advantage of a community-based malaria management program in the Brazilian Amazon. Am. J. Trop. Med. 2001, 65, 883–886. [Google Scholar] [CrossRef] [Green Version]
- Shretta, R.; Avanceña, A.L.; Hatefi, A. The economics of malaria control and elimination: A systematic review. Malar. J. 2016, 15, 593. [Google Scholar] [CrossRef] [Green Version]
- Huang, C.-Y.; Zhang, S.-Y.; Chen, Z.-Y.; Xie, H.-G.; Yang, R.O. Cost-benefit analysis of malaria elimination phase surveillance measures in Fujian Province. Res. Sq. 2020. [Google Scholar] [CrossRef]



| World Health Organization | Brazilian Ministry of Health |
|---|---|
| GUIDELINES FOR MALARIA VECTOR CONTROL 2019 [83] Malaria vector control, Policy guidance, Recommendations, accessed on May 5, 2020 [84] Malaria vector control, Policy guidance, Operational manuals, accessed on May 5, 2020 [85] | Malária: o que é, causas, sintomas, tratamento, diagnóstico e prevenção, accessed on May 5, 2020 [14] Plano de Eliminação da Malária no Brasil 2016 [6] Guia de tratamento da malária no Brasil 2020 [86] |
| Core interventions | |
| INSECTICIDE-TREATED NETS: Pyrethroid-only Long-lasting insecticidal nets (LLINs) prequalified by WHO are recommended for deployment as a core intervention in all malaria-endemic settings. Pyrethroid-PBO nets prequalified by WHO are conditionally recommended for deployment instead of pyrethroid-only LLINs where the principal malaria vector(s) exhibit pyrethroid resistance. Strongly recommended as an intervention of public health value, high-certainty evidence. | INSECTICIDE-TREATED NETS: Use of long-lasting impregnated mosquito nets in priority locations for each municipality and increase coverage in locations where LLIN is already used, together with monitoring of LLIN replacement plan to ensure availability. |
| INDOOR RESIDUAL SPRAYING (IRS): IRS deploying a product prequalified by WHO is recommended as a core intervention in all malaria-endemic settings. DDT has not been prequalified; it may be used for IRS if no equally effective and efficient alternative is available, and if it is used in line with the Stockholm Convention on Persistent Organic Pollutants. Strongly recommended as an intervention of public health value, low-certainty evidence. | INDOOR RESIDUAL SPRAYING: IRS following technical recommendations of the Brazilian Health Surveillance Secretariat (SVS), in buildings located in areas responsible for 80% of malaria transmission by Infection, and in cycles that allow residual insecticide to be maintained throughout the year. |
| HOUSING AND WORKING PLACE IMPROVEMENT: doors and windows screen installation and maintenance. | |
| Supplementary interventions | |
| LARVICIDING: Regular application of biological or chemical insecticides to water bodies is recommended in areas where high coverage with a core intervention has been achieved, where aquatic habitats of the principal malaria vector(s) are few, fixed and findable, and where its application is both feasible and cost-effective. Conditionally recommended as an intervention of public health value, low-certainty evidence. | LARVICIDING: Carrying out management of water collections to eliminate breeding sites of anopheline mosquitoes in urban locations with malaria transmission. Drainage; minor sanitation work to eliminate vector breeding sites; landfill; cleaning the margins of breeding sites; modification of water flow; control of aquatic vegetation. |
| Personal protection measures | |
| TOPICAL REPELLENTS: Deployment of topical repellents is not recommended as a public health intervention; however, topical repellents may be beneficial as an intervention to provide personal protection. Conditionally recommended against deployment as an intervention with public health value, low-certainty evidence. | TOPICAL REPELLENTS: DEET (N-N-dietilmetatoluamida) |
| INSECTICIDE-TREATED CLOTHING: Use of insecticide-treated clothing is not recommended as an intervention with public health value; however, insecticide-treated clothing may be beneficial as an intervention to provide personal protection in specific population groups. Conditionally recommended against deployment as an intervention with public health value, low-certainty evidence. | CLOTHING: Clothing that protects legs (pants) and arms (long sleeve) |
| SPACE SPRAYING: Space spraying should not be undertaken for malaria control, and IRS or LLINs should be prioritized instead. Conditionally recommended against deployment, very low-certainty evidence. | SPACE SPRAYING: Performing chemical spatial control, when in outbreak situations. |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Rocha, E.M.; Katak, R.d.M.; Campos de Oliveira, J.; Araujo, M.d.S.; Carlos, B.C.; Galizi, R.; Tripet, F.; Marinotti, O.; Souza-Neto, J.A. Vector-Focused Approaches to Curb Malaria Transmission in the Brazilian Amazon: An Overview of Current and Future Challenges and Strategies. Trop. Med. Infect. Dis. 2020, 5, 161. https://0-doi-org.brum.beds.ac.uk/10.3390/tropicalmed5040161
Rocha EM, Katak RdM, Campos de Oliveira J, Araujo MdS, Carlos BC, Galizi R, Tripet F, Marinotti O, Souza-Neto JA. Vector-Focused Approaches to Curb Malaria Transmission in the Brazilian Amazon: An Overview of Current and Future Challenges and Strategies. Tropical Medicine and Infectious Disease. 2020; 5(4):161. https://0-doi-org.brum.beds.ac.uk/10.3390/tropicalmed5040161
Chicago/Turabian StyleRocha, Elerson Matos, Ricardo de Melo Katak, Juan Campos de Oliveira, Maisa da Silva Araujo, Bianca Cechetto Carlos, Roberto Galizi, Frederic Tripet, Osvaldo Marinotti, and Jayme A. Souza-Neto. 2020. "Vector-Focused Approaches to Curb Malaria Transmission in the Brazilian Amazon: An Overview of Current and Future Challenges and Strategies" Tropical Medicine and Infectious Disease 5, no. 4: 161. https://0-doi-org.brum.beds.ac.uk/10.3390/tropicalmed5040161