Liposomal Nanovaccine Containing α-Galactosylceramide and Ganglioside GM3 Stimulates Robust CD8+ T Cell Responses via CD169+ Macrophages and cDC1
Abstract
:1. Introduction
2. Materials and Methods
2.1. Animals
2.2. Liposome Preparation
2.3. CD169-Fc ELISA
2.4. In Vivo Immunization, Spleen Digestion and Re-Stimulation
2.5. Flow Cytometry
2.6. Detection of Anti-OVA Ig in the Serum
2.7. Detection of Cytokines in the Serum
2.8. Statistical Analysis
3. Results
3.1. Incorporation of GM3 in Liposomes Results in Increased Uptake by Splenic CD169+ Macrophages
3.2. Combining αGC and GM3 in OVA-Containing Liposomes Results in Potent NKT and NK Activation and Generates Robust Antigen-Specific CD8+ T Cell Responses
3.3. CD169+ Macrophages are Necessary for Induction of CD8+ T Cells Responses, but not for B Cell, NKT and NK Cell Activation Generated by GM3-αGC-OVA Liposomes
3.4. cDC1 Play an Essential Role in GM3-αGC-OVA Liposomes-Mediated Activation of CD8+ T Cells
3.5. Immunization with GM3-αGC-OVA Liposomes Provides a Maturation Signal for DCs and Induces IL-12 Secretion
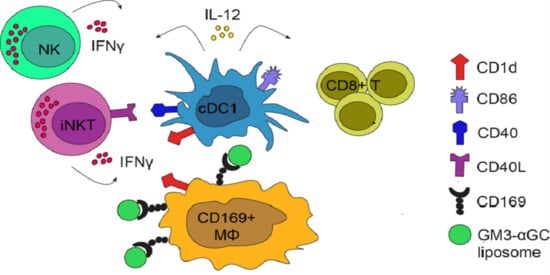
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Topalian, S.L.; Hodi, F.S.; Brahmer, J.R.; Gettinger, S.N.; Smith, D.C.; McDermott, D.F.; Powderly, J.D.; Carvajal, R.D.; Sosman, J.A.; Atkins, M.B.; et al. Safety, activity, and immune correlates of anti-PD-1 antibody in cancer. N. Engl. J. Med. 2012, 366, 2443–2454. [Google Scholar] [CrossRef] [PubMed]
- Larkin, J.; Chiarion-Sileni, V.; Gonzalez, R.; Grob, J.-J.; Rutkowski, P.; Lao, C.D.; Cowey, C.L.; Schadendorf, D.; Wagstaff, J.; Dummer, R.; et al. Five-Year Survival with Combined Nivolumab and Ipilimumab in Advanced Melanoma. N. Engl. J. Med. 2019, 381, 1535–1546. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Massarelli, E.; William, W.; Johnson, F.; Kies, M.; Ferrarotto, R.; Guo, M.; Feng, L.; Lee, J.J.; Tran, H.; Kim, Y.U.; et al. Combining Immune Checkpoint Blockade and Tumor-Specific Vaccine for Patients With Incurable Human Papillomavirus 16–Related Cancer: A Phase 2 Clinical Trial. JAMA Oncol. 2019, 5, 67–73. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ali, O.A.; Lewin, S.A.; Dranoff, G.; Mooney, D.J. Vaccines Combined with Immune Checkpoint Antibodies Promote Cytotoxic T-cell Activity and Tumor Eradication. Cancer Immunol. Res. 2016, 4, 95–100. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sánchez-Paulete, A.R.; Cueto, F.J.; Martínez-López, M.; Labiano, S.; Morales-Kastresana, A.; Rodríguez-Ruiz, M.E.; Jure-Kunkel, M.; Azpilikueta, A.; Aznar, M.A.; Quetglas, J.I.; et al. Cancer Immunotherapy with Immunomodulatory Anti-CD137 and Anti–PD-1 Monoclonal Antibodies Requires BATF3-Dependent Dendritic Cells. Cancer Discov. 2016, 6, 71. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Dudziak, D.; Kamphorst, A.O.; Heidkamp, G.F.; Buchholz, V.R.; Trumpfheller, C.; Yamazaki, S.; Cheong, C.; Liu, K.; Lee, H.-W.; Park, C.G.; et al. Differential Antigen Processing by Dendritic Cell Subsets in Vivo. Science 2007, 315, 107. [Google Scholar] [CrossRef]
- Salmon, H.; Idoyaga, J.; Rahman, A.; Leboeuf, M.; Remark, R.; Jordan, S.; Casanova-Acebes, M.; Khudoynazarova, M.; Agudo, J.; Tung, N.; et al. Expansion and Activation of CD103+ Dendritic Cell Progenitors at the Tumor Site Enhances Tumor Responses to Therapeutic PD-L1 and BRAF Inhibition. Immunity 2016, 44, 924–938. [Google Scholar] [CrossRef] [Green Version]
- Hildner, K.; Edelson, B.T.; Purtha, W.E.; Diamond, M.; Matsushita, H.; Kohyama, M.; Calderon, B.; Schraml, B.U.; Unanue, E.R.; Diamond, M.S.; et al. Batf3 deficiency reveals a critical role for CD8alpha+ dendritic cells in cytotoxic T cell immunity. Science 2008, 322, 1097–1100. [Google Scholar] [CrossRef] [Green Version]
- van Dinther, D.; Veninga, H.; Iborra, S.; Borg, E.G.F.; Hoogterp, L.; Olesek, K.; Beijer, M.R.; Schetters, S.T.T.; Kalay, H.; Garcia-Vallejo, J.J.; et al. Functional CD169 on Macrophages Mediates Interaction with Dendritic Cells for CD8+ T Cell Cross-Priming. Cell Rep. 2018, 22, 1484–1495. [Google Scholar] [CrossRef] [Green Version]
- Asano, K.; Nabeyama, A.; Miyake, Y.; Qiu, C.-H.; Kurita, A.; Tomura, M.; Kanagawa, O.; Fujii, S.-i.; Tanaka, M. CD169-Positive Macrophages Dominate Antitumor Immunity by Crosspresenting Dead Cell-Associated Antigens. Immunity 2011, 34, 85–95. [Google Scholar] [CrossRef] [Green Version]
- Backer, R.; Schwandt, T.; Greuter, M.; Oosting, M.; Jüngerkes, F.; Tüting, T.; Boon, L.; O’Toole, T.; Kraal, G.; Limmer, A.; et al. Effective collaboration between marginal metallophilic macrophages and CD8+ dendritic cells in the generation of cytotoxic T cells. Proc. Natl. Acad. Sci. USA 2010, 107, 216–221. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Grabowska, J.; Lopez-Venegas, M.A.; Affandi, A.J.; den Haan, J.M.M. CD169+ Macrophages Capture and Dendritic Cells Instruct: The Interplay of the Gatekeeper and the General of the Immune System. Front. Immunol. 2018, 9. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Komohara, Y.; Ohnishi, K.; Takeya, M. Possible functions of CD169-positive sinus macrophages in lymph nodes in anti-tumor immune responses. Cancer Sci. 2017, 108, 290–295. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- van Dinther, D.; Veninga, H.; Revet, M.; Hoogterp, L.; Olesek, K.; Grabowska, J.; Borg, E.G.F.; Kalay, H.; van Kooyk, Y.; den Haan, J.M.M. Comparison of Protein and Peptide Targeting for the Development of a CD169-Based Vaccination Strategy Against Melanoma. Front. Immunol. 2018, 9, 1997. [Google Scholar] [CrossRef] [PubMed]
- Chen, W.C.; Kawasaki, N.; Nycholat, C.M.; Han, S.; Pilotte, J.; Crocker, P.R.; Paulson, J.C. Antigen Delivery to Macrophages Using Liposomal Nanoparticles Targeting Sialoadhesin/CD169. PLoS ONE 2012, 7, e39039. [Google Scholar] [CrossRef]
- Crocker, P.R.; Kelm, S.; Dubois, C.; Martin, B.; McWilliam, A.S.; Shotton, D.M.; Paulson, J.C.; Gordon, S. Purification and properties of sialoadhesin, a sialic acid-binding receptor of murine tissue macrophages. EMBO J. 1991, 10, 1661–1669. [Google Scholar] [CrossRef]
- Yu, X.; Feizpour, A.; Ramirez, N.-G.P.; Wu, L.; Akiyama, H.; Xu, F.; Gummuluru, S.; Reinhard, B.M. Glycosphingolipid-functionalized nanoparticles recapitulate CD169-dependent HIV-1 uptake and trafficking in dendritic cells. Nat. Commun. 2014, 5, 4136. [Google Scholar] [CrossRef] [Green Version]
- Affandi, A.J.; Grabowska, J.; Olesek, K.; Lopez Venegas, M.; Barbaria, A.; Rodríguez, E.; Mulder, P.P.G.; Pijffers, H.J.; Ambrosini, M.; Kalay, H.; et al. Selective tumor antigen vaccine delivery to human CD169(+) antigen-presenting cells using ganglioside-liposomes. Proc. Natl. Acad. Sci. USA 2020, 117, 27528–27539. [Google Scholar] [CrossRef]
- Nijen Twilhaar, M.K.; Czentner, L.; Grabowska, J.; Affandi, A.J.; Lau, C.Y.J.; Olesek, K.; Kalay, H.; van Nostrum, C.F.; van Kooyk, Y.; Storm, G.; et al. Optimization of Liposomes for Antigen Targeting to Splenic CD169+ Macrophages. Pharmaceutics 2020, 12, 1138. [Google Scholar] [CrossRef]
- Kawasaki, N.; Vela, J.L.; Nycholat, C.M.; Rademacher, C.; Khurana, A.; van Rooijen, N.; Crocker, P.R.; Kronenberg, M.; Paulson, J.C. Targeted delivery of lipid antigen to macrophages via the CD169/sialoadhesin endocytic pathway induces robust invariant natural killer T cell activation. Proc. Natl. Acad. Sci. USA 2013, 110, 7826–7831. [Google Scholar] [CrossRef] [Green Version]
- Barral, P.; Polzella, P.; Bruckbauer, A.; van Rooijen, N.; Besra, G.S.; Cerundolo, V.; Batista, F.D. CD169+ macrophages present lipid antigens to mediate early activation of iNKT cells in lymph nodes. Nat. Immunol. 2010, 11, 303–312. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Barral, P.; Sánchez-Niño, M.D.; van Rooijen, N.; Cerundolo, V.; Batista, F.D. The location of splenic NKT cells favours their rapid activation by blood-borne antigen. EMBO J. 2012, 31, 2378–2390. [Google Scholar] [CrossRef] [PubMed]
- Matsuda, J.L.; Mallevaey, T.; Scott-Browne, J.; Gapin, L. CD1d-restricted iNKT cells, the ‘Swiss-Army knife’ of the immune system. Curr. Opin. Immunol. 2008, 20, 358–368. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Eberl, G.; MacDonald, H.R. Selective induction of NK cell proliferation and cytotoxicity by activated NKT cells. Eur. J. Immunol. 2000, 30, 985–992. [Google Scholar] [CrossRef]
- Stolk, D.; van der Vliet, H.J.; de Gruijl, T.D.; van Kooyk, Y.; Exley, M.A. Positive & Negative Roles of Innate Effector Cells in Controlling Cancer Progression. Front. Immunol. 2018, 9. [Google Scholar] [CrossRef] [Green Version]
- Carnaud, C.; Lee, D.; Donnars, O.; Park, S.H.; Beavis, A.; Koezuka, Y.; Bendelac, A. Cutting edge: Cross-talk between cells of the innate immune system: NKT cells rapidly activate NK cells. J. Immunol. 1999, 163, 4647–4650. [Google Scholar]
- Fujii, S.-I.; Shimizu, K.; Smith, C.; Bonifaz, L.; Steinman, R.M. Activation of natural killer T cells by alpha-galactosylceramide rapidly induces the full maturation of dendritic cells in vivo and thereby acts as an adjuvant for combined CD4 and CD8 T cell immunity to a coadministered protein. J. Exp. Med. 2003, 198, 267–279. [Google Scholar] [CrossRef] [PubMed]
- Nagato, K.; Motohashi, S.; Ishibashi, F.; Okita, K.; Yamasaki, K.; Moriya, Y.; Hoshino, H.; Yoshida, S.; Hanaoka, H.; Fujii, S.; et al. Accumulation of activated invariant natural killer T cells in the tumor microenvironment after α-galactosylceramide-pulsed antigen presenting cells. J. Clin. Immunol. 2012, 32, 1071–1081. [Google Scholar] [CrossRef]
- Kunii, N.; Horiguchi, S.; Motohashi, S.; Yamamoto, H.; Ueno, N.; Yamamoto, S.; Sakurai, D.; Taniguchi, M.; Nakayama, T.; Okamoto, Y. Combination therapy of in vitro-expanded natural killer T cells and alpha-galactosylceramide-pulsed antigen-presenting cells in patients with recurrent head and neck carcinoma. Cancer Sci. 2009, 100, 1092–1098. [Google Scholar] [CrossRef]
- Nakagawa, R.; Serizawa, I.; Motoki, K.; Sato, M.; Ueno, H.; Iijima, R.; Nakamura, H.; Shimosaka, A.; Koezuka, Y. Antitumor activity of alpha-galactosylceramide, KRN7000, in mice with the melanoma B16 hepatic metastasis and immunohistological study of tumor infiltrating cells. Oncol. Res. 2000, 12, 51–58. [Google Scholar] [CrossRef]
- Shibolet, O.; Alper, R.; Zlotogarov, L.; Thalenfeld, B.; Engelhardt, D.; Rabbani, E.; Ilan, Y. NKT and CD8 lymphocytes mediate suppression of hepatocellular carcinoma growth via tumor antigen-pulsed dendritic cells. Int. J. Cancer 2003, 106, 236–243. [Google Scholar] [CrossRef] [PubMed]
- Chung, Y.; Qin, H.; Kang, C.Y.; Kim, S.; Kwak, L.W.; Dong, C. An NKT-mediated autologous vaccine generates CD4 T-cell dependent potent antilymphoma immunity. Blood 2007, 110, 2013–2019. [Google Scholar] [CrossRef] [PubMed]
- Macho-Fernandez, E.; Cruz, L.J.; Ghinnagow, R.; Fontaine, J.; Bialecki, E.; Frisch, B.; Trottein, F.; Faveeuw, C. Targeted delivery of α-galactosylceramide to CD8α+ dendritic cells optimizes type I NKT cell-based antitumor responses. J. Immunol. 2014, 193, 961–969. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Dölen, Y.; Kreutz, M.; Gileadi, U.; Tel, J.; Vasaturo, A.; van Dinther, E.A.; van Hout-Kuijer, M.A.; Cerundolo, V.; Figdor, C.G. Co-delivery of PLGA encapsulated invariant NKT cell agonist with antigenic protein induce strong T cell-mediated antitumor immune responses. Oncoimmunology 2016, 5, e1068493. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ghinnagow, R.; De Meester, J.; Cruz, L.J.; Aspord, C.; Corgnac, S.; Macho-Fernandez, E.; Soulard, D.; Fontaine, J.; Chaperot, L.; Charles, J.; et al. Co-delivery of the NKT agonist α-galactosylceramide and tumor antigens to cross-priming dendritic cells breaks tolerance to self-antigens and promotes antitumor responses. Oncoimmunology 2017, 6, e1339855. [Google Scholar] [CrossRef] [PubMed]
- Klaas, M.; Oetke, C.; Lewis, L.E.; Erwig, L.P.; Heikema, A.P.; Easton, A.; Willison, H.J.; Crocker, P.R. Sialoadhesin promotes rapid proinflammatory and type I IFN responses to a sialylated pathogen, Campylobacter jejuni. J. Immunol. 2012, 189, 2414–2422. [Google Scholar] [CrossRef] [Green Version]
- Miyake, Y.; Asano, K.; Kaise, H.; Uemura, M.; Nakayama, M.; Tanaka, M. Critical role of macrophages in the marginal zone in the suppression of immune responses to apoptotic cell-associated antigens. J. Clin. Investig. 2007, 117, 2268–2278. [Google Scholar] [CrossRef]
- Saito, M.; Iwawaki, T.; Taya, C.; Yonekawa, H.; Noda, M.; Inui, Y.; Mekada, E.; Kimata, Y.; Tsuru, A.; Kohno, K. Diphtheria toxin receptor-mediated conditional and targeted cell ablation in transgenic mice. Nat. Biotechnol. 2001, 19, 746–750. [Google Scholar] [CrossRef]
- Stolk, D.A.; de Haas, A.; Vree, J.; Duinkerken, S.; Lübbers, J.; van de Ven, R.; Ambrosini, M.; Kalay, H.; Bruijns, S.; van der Vliet, H.J.; et al. Lipo-Based Vaccines as an Approach to Target Dendritic Cells for Induction of T- and iNKT Cell Responses. Front. Immunol. 2020, 11. [Google Scholar] [CrossRef]
- Rouser, G.; Fkeischer, S.; Yamamoto, A. Two dimensional then layer chromatographic separation of polar lipids and determination of phospholipids by phosphorus analysis of spots. Lipids 1970, 5, 494–496. [Google Scholar] [CrossRef]
- Puryear, W.B.; Akiyama, H.; Geer, S.D.; Ramirez, N.P.; Yu, X.; Reinhard, B.M.; Gummuluru, S. Interferon-inducible mechanism of dendritic cell-mediated HIV-1 dissemination is dependent on Siglec-1/CD169. PLoS Pathog. 2013, 9, e1003291. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Den Haan, J.M.; Lehar, S.M.; Bevan, M.J. CD8(+) but not CD8(-) dendritic cells cross-prime cytotoxic T cells in vivo. J. Exp. Med. 2000, 192, 1685–1696. [Google Scholar] [CrossRef] [PubMed]
- Schnorrer, P.; Behrens, G.M.; Wilson, N.S.; Pooley, J.L.; Smith, C.M.; El-Sukkari, D.; Davey, G.; Kupresanin, F.; Li, M.; Maraskovsky, E.; et al. The dominant role of CD8+ dendritic cells in cross-presentation is not dictated by antigen capture. Proc. Natl. Acad. Sci. USA 2006, 103, 10729–10734. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Fujii, S.; Liu, K.; Smith, C.; Bonito, A.J.; Steinman, R.M. The linkage of innate to adaptive immunity via maturing dendritic cells in vivo requires CD40 ligation in addition to antigen presentation and CD80/86 costimulation. J. Exp. Med. 2004, 199, 1607–1618. [Google Scholar] [CrossRef]
- Smyth, M.J.; Thia, K.Y.T.; Street, S.E.A.; Cretney, E.; Trapani, J.A.; Taniguchi, M.; Kawano, T.; Pelikan, S.B.; Crowe, N.Y.; Godfrey, D.I. Differential Tumor Surveillance by Natural Killer (Nk) and Nkt Cells. J. Exp. Med. 2000, 191, 661–668. [Google Scholar] [CrossRef] [Green Version]
- Tugues, S.; Burkhard, S.H.; Ohs, I.; Vrohlings, M.; Nussbaum, K.; vom Berg, J.; Kulig, P.; Becher, B. New insights into IL-12-mediated tumor suppression. Cell Death Differ. 2015, 22, 237–246. [Google Scholar] [CrossRef] [Green Version]
- Wculek, S.K.; Cueto, F.J.; Mujal, A.M.; Melero, I.; Krummel, M.F.; Sancho, D. Dendritic cells in cancer immunology and immunotherapy. Nat. Rev. Immunol. 2020, 20, 7–24. [Google Scholar] [CrossRef]
- Waldman, A.D.; Fritz, J.M.; Lenardo, M.J. A guide to cancer immunotherapy: From T cell basic science to clinical practice. Nat. Rev. Immunol. 2020, 20, 651–668. [Google Scholar] [CrossRef]
- Hermans, I.F.; Silk, J.D.; Gileadi, U.; Salio, M.; Mathew, B.; Ritter, G.; Schmidt, R.; Harris, A.L.; Old, L.; Cerundolo, V. NKT cells enhance CD4+ and CD8+ T cell responses to soluble antigen in vivo through direct interaction with dendritic cells. J. Immunol. 2003, 171, 5140–5147. [Google Scholar] [CrossRef] [Green Version]
- Semmling, V.; Lukacs-Kornek, V.; Thaiss, C.A.; Quast, T.; Hochheiser, K.; Panzer, U.; Rossjohn, J.; Perlmutter, P.; Cao, J.; Godfrey, D.I.; et al. Alternative cross-priming through CCL17-CCR4-mediated attraction of CTLs toward NKT cell–licensed DCs. Nat. Immunol. 2010, 11, 313–320. [Google Scholar] [CrossRef]
- Gottschalk, C.; Mettke, E.; Kurts, C. The Role of Invariant Natural Killer T Cells in Dendritic Cell Licensing, Cross-Priming, and Memory CD8+ T Cell Generation. Front. Immunol. 2015, 6. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Starbeck-Miller, G.R.; Harty, J.T. The Role of Il-12 and Type I Interferon in Governing the Magnitude of CD8 T Cell Responses. Adv. Exp. Med. Biol. 2015, 850, 31–41. [Google Scholar] [CrossRef] [PubMed]
- Farrand, K.J.; Dickgreber, N.; Stoitzner, P.; Ronchese, F.; Petersen, T.R.; Hermans, I.F. Langerin+ CD8alpha+ dendritic cells are critical for cross-priming and IL-12 production in response to systemic antigens. J. Immunol. 2009, 183, 7732–7742. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Backer, R.A.; Diener, N.; Clausen, B.E. Langerin+CD8+ Dendritic Cells in the Splenic Marginal Zone: Not So Marginal After All. Front. Immunol. 2019, 10. [Google Scholar] [CrossRef]
- Ashour, D.; Arampatzi, P.; Pavlovic, V.; Förstner, K.U.; Kaisho, T.; Beilhack, A.; Erhard, F.; Lutz, M.B. IL-12 from endogenous cDC1, and not vaccine DC, is required for Th1 induction. JCI Insight 2020, 5, e135143. [Google Scholar] [CrossRef]
- Park, J.E.; Wu, D.Y.; Prendes, M.; Lu, S.X.; Ragupathi, G.; Schrantz, N.; Chapman, P.B. Fine specificity of natural killer T cells against GD3 ganglioside and identification of GM3 as an inhibitory natural killer T-cell ligand. Immunology 2008, 123, 145–155. [Google Scholar] [CrossRef]
- Paget, C.; Deng, S.; Soulard, D.; Priestman, D.A.; Speca, S.; von Gerichten, J.; Speak, A.O.; Saroha, A.; Pewzner-Jung, Y.; Futerman, A.H.; et al. TLR9-mediated dendritic cell activation uncovers mammalian ganglioside species with specific ceramide backbones that activate invariant natural killer T cells. PLoS Biol. 2019, 17, e3000169. [Google Scholar] [CrossRef] [Green Version]
- Valente, M.; Dölen, Y.; van Dinther, E.; Vimeux, L.; Fallet, M.; Feuillet, V.; Figdor, C.G. Cross-talk between iNKT cells and CD8 T cells in the spleen requires the IL-4/CCL17 axis for the generation of short-lived effector cells. Proc. Natl. Acad. Sci. USA 2019, 116, 25816. [Google Scholar] [CrossRef]
- Allan, L.L.; Stax, A.M.; Zheng, D.J.; Chung, B.K.; Kozak, F.K.; Tan, R.; van den Elzen, P. CD1d and CD1c expression in human B cells is regulated by activation and retinoic acid receptor signaling. J. Immunol. 2011, 186, 5261–5272. [Google Scholar] [CrossRef] [Green Version]
- Bai, L.; Constantinides, M.G.; Thomas, S.Y.; Reboulet, R.; Meng, F.; Koentgen, F.; Teyton, L.; Savage, P.B.; Bendelac, A. Distinct APCs explain the cytokine bias of α-galactosylceramide variants in vivo. J. Immunol. 2012, 188, 3053–3061. [Google Scholar] [CrossRef]
- Doherty, D.G.; Melo, A.M.; Moreno-Olivera, A.; Solomos, A.C. Activation and Regulation of B Cell Responses by Invariant Natural Killer T Cells. Front. Immunol. 2018, 9. [Google Scholar] [CrossRef] [PubMed]
- Giaccone, G.; Punt, C.J.; Ando, Y.; Ruijter, R.; Nishi, N.; Peters, M.; von Blomberg, B.M.; Scheper, R.J.; van der Vliet, H.J.; van den Eertwegh, A.J.; et al. A phase I study of the natural killer T-cell ligand alpha-galactosylceramide (KRN7000) in patients with solid tumors. Clin. Cancer Res. Off. J. Am. Assoc. Cancer Res. 2002, 8, 3702–3709. [Google Scholar]
- Ishikawa, A.; Motohashi, S.; Ishikawa, E.; Fuchida, H.; Higashino, K.; Otsuji, M.; Iizasa, T.; Nakayama, T.; Taniguchi, M.; Fujisawa, T. A phase I study of alpha-galactosylceramide (KRN7000)-pulsed dendritic cells in patients with advanced and recurrent non-small cell lung cancer. Clin. Cancer Res. Off. J. Am. Assoc. Cancer Res. 2005, 11, 1910–1917. [Google Scholar] [CrossRef] [Green Version]
- Wolf, B.J.; Choi, J.E.; Exley, M.A. Novel Approaches to Exploiting Invariant NKT Cells in Cancer Immunotherapy. Front. Immunol. 2018, 9. [Google Scholar] [CrossRef] [PubMed]
- Bulbake, U.; Doppalapudi, S.; Kommineni, N.; Khan, W. Liposomal Formulations in Clinical Use: An Updated Review. Pharmaceutics 2017, 9, 12. [Google Scholar] [CrossRef]
- Grabbe, S.; Haas, H.; Diken, M.; Kranz, L.M.; Langguth, P.; Sahin, U. Translating nanoparticulate-personalized cancer vaccines into clinical applications: Case study with RNA-lipoplexes for the treatment of melanoma. Nanomedicine 2016, 11, 2723–2734. [Google Scholar] [CrossRef]
- Loquai, C.; Hassel, J.C.; Oehm, P.; Derhovanessian, E.; Jabulowsky, R.A.; Gold, M.; Schwarck-Kokarakis, D.; Attig, S.; Cuk, K.; Vogler, I.; et al. A shared tumor-antigen RNA-lipoplex vaccine with/without anti-PD1 in patients with checkpoint-inhibition experienced melanoma. J. Clin. Oncol. 2020, 38, 3136. [Google Scholar] [CrossRef]





| Antigen/reagent | Clone | Fluorochrome | Company |
|---|---|---|---|
| NK1.1 | PK136 | BV711 | Biolegend |
| CD25 | PC61 | BV650 | Biolegend |
| CD69 | H1.2F3 | AF700 | Biologend |
| CD3 | KT-3 | Alexa Fluor 488 | In-house made |
| CD4 | GK1.5 | BV510 | Biolegend |
| CD8 | 53-6.7 | PerCP-Cy5.5 | Biolegend |
| IL-4 | 11B11 | BV421 | Biolegend |
| CD169 | SER-4 | Alexa Fluor 488 | In-house made |
| B220 | 6B2 | Alexa Fluor 405 | In-house made |
| F4/80 | T45-2342 | PE-CF594 | BD Biosciences |
| CD8a | 53-6.7 | PE-Cy7 | BD Biosciences |
| CD11c | HL3 | BV650 | BD Biosciences |
| I-A/I-E | M5/114.15.2 | PE | eBioscience |
| I-A/I-E | M5/114.15.2 | Alexa Fluor 488 | In-house made |
| CD80 | 16-10A1 | PE | Immunotools |
| CD86 | GL-1 | PE-Cy7 | BD Biosciences |
| XCR1 | ZET | BV421 | Biolegend |
| CD40 | 1C10 | Biotin | In-house made |
| CD40L | MR1 | PE-Cy7 | Biolegend |
| CD70 | FR70 | biotin | BD Biosciences |
| CD8a | 53-6.7 | APC | BD Biosciences |
| CD44 | KM81 | FITC | Immunotools |
| H-Kb/SIINFEKL | N/A | PE tetramer | LUMC, Leiden |
| B220 | RA3-6B2 | BV510 | Biolegend |
| CD38 | 90/CD38 | PE | BD Biosciences |
| GL7 | GL-7 | PE-Cy7 | Biolegend |
| OVA | N/A | Alexa Fluor 488 | Invitrogen |
| CD11a | M17/4 | FITC | eBioscience |
| CD8a | 53-6.7 | PE-Cy7 | BD Biosciences |
| CD4 | GK1.5 | PE | eBioscience |
| CD1d PBS-57 | N/A | PE | NIH tetramer core facility |
| IFNγ | XMG1.2 | APC | eBioscience |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Grabowska, J.; Stolk, D.A.; Nijen Twilhaar, M.K.; Ambrosini, M.; Storm, G.; van der Vliet, H.J.; de Gruijl, T.D.; van Kooyk, Y.; den Haan, J.M.M. Liposomal Nanovaccine Containing α-Galactosylceramide and Ganglioside GM3 Stimulates Robust CD8+ T Cell Responses via CD169+ Macrophages and cDC1. Vaccines 2021, 9, 56. https://0-doi-org.brum.beds.ac.uk/10.3390/vaccines9010056
Grabowska J, Stolk DA, Nijen Twilhaar MK, Ambrosini M, Storm G, van der Vliet HJ, de Gruijl TD, van Kooyk Y, den Haan JMM. Liposomal Nanovaccine Containing α-Galactosylceramide and Ganglioside GM3 Stimulates Robust CD8+ T Cell Responses via CD169+ Macrophages and cDC1. Vaccines. 2021; 9(1):56. https://0-doi-org.brum.beds.ac.uk/10.3390/vaccines9010056
Chicago/Turabian StyleGrabowska, Joanna, Dorian A. Stolk, Maarten K. Nijen Twilhaar, Martino Ambrosini, Gert Storm, Hans J. van der Vliet, Tanja D. de Gruijl, Yvette van Kooyk, and Joke M.M. den Haan. 2021. "Liposomal Nanovaccine Containing α-Galactosylceramide and Ganglioside GM3 Stimulates Robust CD8+ T Cell Responses via CD169+ Macrophages and cDC1" Vaccines 9, no. 1: 56. https://0-doi-org.brum.beds.ac.uk/10.3390/vaccines9010056