JOURNAL OF CLINICAL AND MEDICAL RESEARCH
COVID-19 Vaccination Efficacy and Safety Literature Review
| ReceivedJan 11, 2021 | RevisedFeb 1, 2020 | AcceptedFeb 11, 2021 | PublishedMar 8, 2021 |
Michael Halim1*, Alice Halim2and Yovita Tjhin
1University of Salford, MSc Biomedical Science, Greater Manchester, United Kingdom
2Zhongshan Hospital, Shanghai Medical College of Fudan University, Shanghai, China
3National Heart and Lung Institute, Imperial College London, MSc Cardiovascular and Respiratory Healthcare, London, United Kingdom
*Corresponding Author: Michael Halim, University of Salford, MSc Biomedical Science, Greater Manchester, United Kingdom.
Accepted Date: 02-11-2021; Published Date: 03-08-2021
Copyright© 2021 by Halim M, et al. All rights reserved. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
Abstract
Since the emergency of the novel coronavirus disease (COVID-19) that is caused by SARS-Cov-2 in 2019, researchers have been on the move to find solutions to mitigate the spread of the virus. Various control measures have been put in place by governments under guidelines and recommendations of key global agencies with the world health organization (WHO) leading in providing information to help fight the pandemic. Multi-agency research efforts have been geared towards developing vaccines for active immunization to prevent COVID-19 infection. This paper is geared towards providing a detailed review and analysis of developments of the current vaccines in terms of safety and efficacy. Approaches that have been taken by different researchers and their findings are the subject of this work. Based on the mechanism by which a vaccine protects an individual against COVID-19 infection, it has been found that the already rolled out vaccines are mRNA (Pfizer and Moderna) and vector (Astrazeneca) vaccine structured. There is also China's Sinovac vaccine which has been in place for the past few years. The four vaccines reviewed here are administered in two doses some days apart. Currently, no vaccine has a safety threat and the efficacies are 95% for COVID-19 mRNA vaccine BNT162b2 (Pfizer), 94.1% for mRNA-1273 vaccine (Moderna), 70.4%forChAdOx1 nCoV-19 vaccine / AZD1222 (AstraZeneca) vaccine and 78% for sinovac respectively. Findings of this paper show that other vaccines are awaiting clinical roll out for trials. Even though these efficacies imply that the vaccines offer significant protection against the infection, further research and evaluation should go on to achieve higher efficacies while addressing any safety concerns that may go beyond local and systemic reactions that occur on patients after vaccination. This study concludes that even with the protection of the present vaccines, individuals must continue wearing personal protective equipment (PPEs) such as masks.
Keywords
Coronavirus disease, COVID-19, SARS-Cov-2, Pandemic, Vaccine, Safety, Efficacy, Clinical trial, mRNA, Vector vaccine, Protein subunit, mRNA vaccine BNT162b2 (Pfizer), mRNA-1273 vaccine (Moderna), ChAdOx1 nCoV-19 vaccine / AZD1222 (AstraZeneca) and Sinovac vaccine.
Background
The first COVID-19 infection case was recorded in Wuhan back in December 2019. The rate of spread of this SARS classified virus was not predetermined until it was established that it was highly contagious a month later. According to WHO [1], COVID-19 can be transmitted mainly through respiratory droplets when individuals are within a range of one metre. Thus, one can easily get infected if they use objects or surfaces that have been in contact with an infected person or directly being in contact with such a person. For these reasons, WHO recommends the appropriate use of personal protective equipment such as face masks to control the spread of the infection when respirators are inadequate. In addition, based centres for disease control (CDC) further recommend social distancing to minimize the chances of getting exposed to the virus [2]. Despite mitigation measures having been adopted globally, the virus exhibited a very high transmission rate. At the time when this work was done, there were an estimated 81, 947,503 COVID-19 infections and 1,808,041 mortalities according to the WHO COVID -19 online dashboard [3].
These alarming statistics have kept researchers in different fields to find a solution to address this global health challenge. The outbreak of SARS-CoV-2 among humans made it the third zoonotic coronavirus to move from animals to humans after SARS-CoV [4] and MERS-CoV [5]. After establishing that the virus causing the acute respiratory disease was zoonotic [6], further epidemiology and pathology studies have been going on to understand the virus structure and possible development of vaccines to counter the infection and spread. Viral genome sequencing showed that this virus has 75 to 80% similarity to SARS-CoV, 50% to MERS-CoV and more similarity can be observed in other coronaviruses like those of bats [7]. Further, it can be cultured in conditions similar to those of Middle East respiratory syndrome coronavirus (MERS-CoV) and SARS-CoV. Due to this similarity i.e., causing acute respiratory distress (ARD) and its structure [8], this virus was named SARS CoV-2, and the disease it causes is called COVID -19, where 19 is associated with the year (2019) when this disease was first reported. Moreover, the binding affinity of SARS-CoV-2 and SARS-CoV spikes (S) is the same as they use the Angiotensin-converting enzyme 2 (ACE2) receptor to infect a cell [9].
With such an understanding of SARS-CoV-2's binding mechanism and its glycoproteins, researchers have developed vaccines that are at different stages of clinical trials and approved for use at a wider scope.For any vaccine to get general approval, it has to show promising results during the stages of its clinical trials. The trails always work towards establishing short-term safety, efficacy and the ability to generate enough immune response. According to the CDC, vaccine efficacy is defined as the differences between people who become sick following vaccination and those who become sick without receiving the vaccination. It is a measure identified during the third phase of a clinical trial in which the researcher vaccinate some people and give a placebo to others. The test subjects are then monitored for some months to witness whether or not the people who get vaccinated get infected at a lower rate compared to those who received placebo. For instance, suppose a COVID-19 vaccine is said to be having a vaccine efficacy of 80%, then it means that if one hundred people had been vaccinated, then on average, 80 people out of the 100 will not acquire COVID-19. On the same note, vaccine safety is defined as its ability not to cause any health complication, either at the present or in future, on the people who have been vaccinated.
According to [8], the four COVID -19 vaccines under development are based mainly on mRNA and DNA technologies.mRNA induces cells to produce spike proteins which trigger antibody production while Sinovac uses dead viral particle to induce antibody production. According to CDC, four vaccines have already been rolled out COVID-19 mRNA vaccine BNT162b2 (Pfizer), mRNA-1273 vaccine (Moderna), ChAdOx1 nCoV-19 vaccine / AZD1222 (AstraZeneca), and lastly, the China’s Sinovac vaccine.
Pfizer’s vaccine, commonly known with the formulaeCOVID-19 mRNA vaccine BNT162b2 has the active ingredient modRNA that encode the viral spike glycoprotein (S) of SARS-CoV-2, the lipid(4-hydroxybutyl), (hexane-6,1-diyl), (ALC-3015), 2-[(polyethylene glycol)-2000],salts such as potassium chloride and monobasic potassium phosphate, and lastly sucrose [15].The COVID-19 virus is studded with proteins that it uses while entering the human cells. The so identified spike proteins make an attempting target for potential vaccines as well as treatments. The illustration is shown in the figure below:
Figure 1: Illustrating the mechanical function of Pfizer’s vaccine [15].
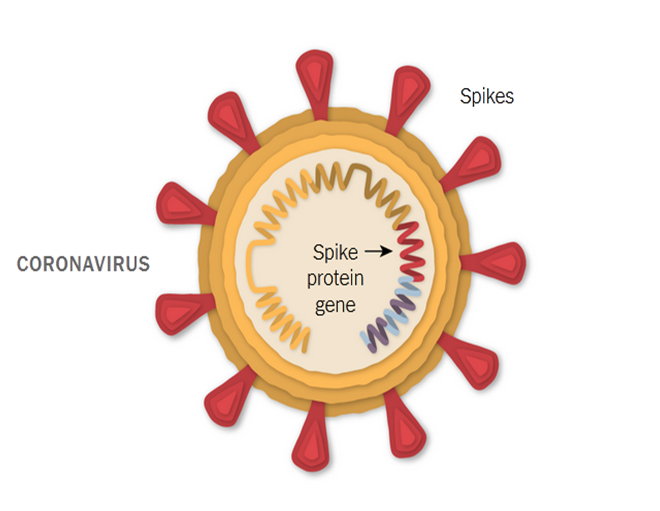
As demonstrated in the figure above, the Pfizer-BioNTech vaccine is specifically based on the genetic instructions of the virus to build the spike protein [16]. The vaccine was invented by BioNTech, specifically by wife and husband team of researchers OzlemTureci, 53, and UgurSahin, 55, both based in Mainz city of German. The vaccine can be stored at a refrigerated temperature of 2-8°C (36 and 46 degrees Fahrenheit). It is reported that Pfizer vaccine will be charged $20 per dose [17]. Presently there is no clear pre-cautionary guideline about the vaccine though women with severe allergic reaction are advised to get the vaccine but with great caution.
mRNA-1273 vaccine (Moderna) work nearly in the same manner as Pfizer-BioNTech vaccine. The team which invented Moderna vaccine was led by Tal Zaks, a chief medical officer of Moderna who had worked previously as the president of global oncology at Sanofi [15].The vaccine is stored at -25°C and -15°C (-13°F and 5°F) temperature. Each dose ofModerna will cost between $32 and $37 [16].
ChAdOx1 nCoV-19 vaccine / AZD1222 (AstraZeneca) vaccine on the other hand uses replication-deficient chimpanzee adenovirus to provide a SARS-CoV-2 protein that subsequently induces immune response to protect the body [18]. The SARS-CoV genome is a kind of single strand RNA molecule that encodes four different structural proteins: nucleocapsid (N), envelope (E) protein, membrane (M) protein and lastly spike (S) glycoprotein. By being vaccinated by ChAdOx1 nCoV-19 vaccine / AZD1222 (AstraZeneca) vaccine, it is expected that the body will be in a position to recognise and subsequently develop a form of the protected response to Spike protein that will assist halt the SARS-CoV-2 virus from getting into human cells [19]. ChAdOx1 nCoV-19 vaccine / AZD1222 (AstraZeneca) was invented by AstraZeneca and Oxford University.One can store the vaccine at 2-8°C (36 and 46 degrees Fahrenheit) [19]. AstraZeneca will be charged $3 per single dose [19].
Lastly is China's Sinovac vaccine which uses inactivated form of Covid-19 virus, though somehow different from the Pfizer jab. CoronaVac specifically uses a slightly destroyed coronavirus particles which are inactivated [20]. CoronaVac was invented by Chinese biopharmaceutical company Sinovac. The vaccine is kept at 2–8 °C (36–46 °F). A CoronaVac dose will cost US$10.30 for a single dosage [21].
Once the immune system gets into contact with such particles, it begins to generate the appropriate antibodies to help protect against possible future infection [21]. The next section explores the methods used to search and select the most relevant publications reviewed in this study.
Methods
To begin with, keywords were combined during the search of articles focusing on COVID -19 vaccines. For instance, the google scholar database was used to find publications by typing coronavirus and vaccines. Using the ‘and’ word between words increased the scope of the articles retrieved from the databases. Articles relevant to this study were screened using their titles are abstracts. Publications with articles that lacked a combination of keywords such as COVID -19 vaccine, efficacy, and safety were excluded from the pool of found articles. Further in the abstract, if it revealed that the paper involved the development of a vaccine and its subsequent clinical trial, such kind of an article was included for review. Google Scholar was used as the main article database for this work. After an article’s title and abstract showed relevance to the study, the full file was retrieved from the journal for further screening. The methods section of the selected articles was explored to determine what the research entailed. Articles showing an approach of vaccine development of review of a vaccine's performance in terms of efficacy and safety were given a high priority for this review. Further, the results section of the article was also very useful in screening the article further to evaluate if the findings were in line with the topic. Articles that did not meet these criteria were excluded at this stage while those that showed positive results were retained. PubMed indexed journal articles were included during the search. A systematic review followed by metanalyses of the clinical trials done for the vaccines based on previous studies was carried out for this paper.
Results
Based on research that has been conducted, only four vaccines have been published officially and authorized for use against COVID-19 in various parts of the world. Therefore, this section contains four peers reviewed results of authorized vaccines. Other vaccines are still in clinical trials and their efficacy and safety data are not readily available. Of the four, two are mRNA(COVID-19 mRNA vaccine BNT162b2 (Pfizer), mRNA-1273 vaccine (Moderna) while the other two (ChAdOx1 nCoV-19 vaccine / AZD1222 (AstraZeneca) and China’s Sinovac ) are vector vaccines.
Safety and Efficacy of COVID-19 mRNA vaccine BNT162b2 (Pfizer) Vaccine
From the trial involving a sample size of 21720 [10], the vaccine candidates, ≥16 years received 30μg of this mRNA vaccine administered in 2 doses 21 days apart. Among the 21720 candidates who received the vaccine, 8 of them exhibited COVID-19 signs at least one week after the second dose of the vaccine. BNT162b2 showed a protection percentage of 95% (95% CI, 90.3 -97.6) with safety issues indicated by temporary pain at the point of injection, fatigue, and headache which were rated as normal local reactions. Less than 1% experienced severe pain at the injection spot. The vaccine is considered safe for the prevention of COVID-19 infection and the antibodies last for 2 months. The vaccine was approved by U.S. Food and Drug Administration (FDA) [12]. The reported side effects were mainly tiredness and headache (59% and 52%, respectively [10].
Safety and Efficacy of mRNA-1273 vaccine (Moderna)
Clinical trials done by researchers and involving a sample size of 30,000 [11] reveal that the mRNA-1273 vaccine was administered in 100 μg doses 28 days apart. Just like, BNT162b2, this lipid nanoparticle encapsulated vaccine was tested in the placebo and the mRNA vaccine at a ratio of 1:1. COVID-19 symptoms were observed in 11 patients out of the 15,210 individuals who received the mRNA-1273 vaccine. The efficacy was found to be 94.1% i.e. (95% CI, 89.5 – 96.8%; p<0.001). According to the researchers, there are no safety issues with the vaccines since it is only the expected local and systemic reactions that were observed. The antibody last for 4 months upon vaccination. The vaccine was approved by U.S. Food and Drug Administration (FDA) [11]. The reported side effects comprised of painat the point of injection (91.6%), fatigue (68.5%), headache (63.0%), muscle pain (59.6%), and joint pain (44.8%) [16].
Safety and Efficacy of ChAdOx1 nCoV-19 vaccine / AZD1222 (AstraZeneca)
This vaccine was tried in South Africa, United Kingdom, and recently in Brazil with participants receiving 5×〖10〗^10 molecules of the vaccine based on research done by [13]. The Clinical trial phase 3 involved a sample size of 23 848 participants [13]. The overall vaccine efficacy was computed as 70.4% (95% CI, 54.8-80.6, 30[0.5%] of 5807 patients). This viral vector vaccine was shown to be efficacious and safe for combating COVID-19 since only 79 patients out of 5807 who received ChAdOx1 vaccine showed COVID-19 symptoms. The antibody last for 6 months upon vaccination. The vaccine was approved by Institutional Biosafety Committee (IBC) [13]. The major side effects included fatigue and headache [19].
Safety and Efficacy of China’s Sinovac vaccine
China's CoronaVac COVID-19 vaccine that was developed by Sinovac has been proven to be harmless and protective after its third phase trials in various countries across the world, a factor that has boosted the public confidence regarding its rollout in different parts of the world [21]. According to scholarly results, Sinovac's vaccine is 100 per cent efficient and effective in preventing moderate infections, 77.9% effective in preventing possible mild cases, and poses an overall efficacy of at least 50.4 per cent in Brazil latest final trials [20]. Vaccine experts have indicated that the trial results are good enough for the vaccine to be enrolled for use among the general population. It is estimated that the antibody last for 6 months upon vaccination. The vaccine was approved by Chinese government following phase 3 clinical trials done in Brazil and other countries that involved 50,000 participants [20]. Allergies [21] were reported as a major side effect among the perticipants.
Summary of Results
Table 1: Vaccine types and Efficacies.
|
Vaccine |
mRNA
vaccine BNT162b2 (Pfizer) |
mRNA-1273
vaccine (Moderna) |
ChAdOx1
nCoV-19 vaccine / AZD1222 (AstraZeneca) |
Sinovac
Vaccine |
|
Inventors |
OzlemTureci
and UgurSahin [17] |
Tal
Zaks [15] |
AstraZeneca
and Oxford University [19] |
Chinese
biopharmaceutical company Sinovac [20] |
|
Country and manufacturer |
Country:
USA Manufacturer:BioNTech
[17] |
Country:
US Manufacturer:
Moderna [16]. |
Country:
UK Manufacturer:
Oxford [19] |
Country:
China Manufacturer:
Sinovac [21] |
|
Approving institutions |
FDA
[12] |
FDA
[15]. |
Institutional
Biosafety Committee (IBC) [19] |
Chinese
government [20] |
|
Clinical trial and sample size |
Clinical
trial: phase 3 Sample
size: 21720 [10] |
Clinical
trial: phase 3 Sample
size:30,000 [15]. |
Clinical
trial: phase 3 Sample
size:23 848 [19] |
Clinical
trial: phase 3 Sample
size:around 50,000 [21] |
|
Doses and days between doses |
30
µg administered
in 2 doses 21 days apart [10] |
2
injections of mRNA-1273 given 28 days apart [15]. |
Intramuscular
injection given
28 days apart |
Injection |
|
2
doses 14 days apart [20] |
||||
|
Type and Mechanism of Action |
Type:
mRNA Mechanism
of actions: mRNA induces cells to produce spike proteins which trigger
antibody production [8] |
Type:
mRNA Mechanism
of actions: mRNA vaccines have strands of genetic material known as mRNA
inside a coating that help protects the mRNA from enzymes found in the body
[8]. |
Type:
vector vaccine Mechanism
of actions: Uses modified adenovirus particle to induce antibody production
[13]. |
Type:
Inactivated virus |
|
Mechanism
of action: Sinovac uses inactivated viral particle to induce antibody
production [21]. |
||||
|
Storage temperature |
2-8°C
(36 and 46 degrees Fahrenheit) [17]. |
-25°C
and -15°C (-13°F and 5°F) [16]. |
2-8°C
(36 and 46 degrees Fahrenheit)[19] |
2–8
°C (36–46 °F) [20] |
|
Cost |
$20
per dose [17] |
$32
and $37 [16] |
$3
per single dose [19] |
US$10.30
for a single dosage [21] |
|
Efficacy (%) |
95%[10] |
94.1%[16] |
70.4%
[19] |
78%
[20] |
|
Antibodies duration post-vaccination |
2
months [10] |
4
Months [16] |
6
Months [19] |
6
Months [20] |
|
Safety |
No
single adverse effects in as far as safety and health complications are
concerned [10] |
Safety
data indicate that the vaccine is safe [16] |
No
serious adverse events related [19]. |
No
major complications recorded [21]. |
|
Adverse Effects |
fatigue
and headache (59% and 52%, respectively [10] |
injection
site pain (91.6%), fatigue (68.5%), headache (63.0%), muscle pain (59.6%),
joint pain (44.8%) [16]. |
Fatigue
and headache [19] |
Allergies
[21] |
|
Future development and trajectories |
Public
sensitization on vaccine efficacy and safety [10] |
More
studies to establish the side effects of the vaccine [16]. |
Further
trials and monitoring assure safety and effectiveness [19] |
As
the trials have revealed mixed results, more studies need to be done to
establish the efficacy and safety of the vaccine [20] |
Discussion
The study report shows COVID-19 vaccination efficacy and safety on the selected clinical trials. Based on the mechanism by which an individual is protected against covid-19 infection, China's Sinovac, mRNA (Pfizer and Moderna), and AstraZeneca vaccines are rolled out. The four vaccines are administered in two doses and some days apart based on the theory of multiple doses, for instance, immunization routine. In some cases, multi-doses are essential for
an individual to receive the highest level of immunity. In contrast, in others, the
second dose is necessary since not all individuals respond proportionally and sufficiently to a single dose.
Following vaccination series, a particular vaccine requires boosters for the entire population or a specific group of people to improve weakening immunity after a specific period. Therefore, two doses are recommended in COVID-19 vaccines to ensure individuals achieve the highest level of immunity. According to [8], the four COVID-19 vaccines are based on mRNA and DNA technologies. The diagnosis of COVID-19 infection is achieved through viral RNA detection from saliva or nasal pharyngeal swab [22]. According to [22], protection from viral infections is achieved using the viral neutralizing antibodies principle for immune vaccination. With the understanding of SARS-CoV-2's binding mechanism and its glycoproteins, the vaccines induce mRNA cells that generate spike proteins triggering antibody production [22].
Based on the conducted research, the four vaccines have different findings on safety and efficacy on COVID-19. Pfizer and Moderna mRNA vaccines recorded a percentage efficacy of 95% and 94.1%. On the other hand, AstraZeneca and Sinovac vaccine recorded a percentage efficacy of 70.4% and 78%, respectively.Although the COVID-19 vaccine is deployed based on immunogenicity data and safety measures, vaccine development aims to obtain evidence on vaccine efficacy in protecting individuals against SARS-CoV-2 infection [23]. Assessment for vaccine efficacy for COVID-19 is complex where a fundamental understanding of pathogen evolving is crucial [23]. According to World Health Organization, any vaccine on COVID-19 with an efficacy above 50 % would be worth approving [23]. Therefore, the research's four vaccines are worth being approved because each vaccine's percentage efficacy is above 70 %. For instance, the efficacy of 70.4 % in the AstraZeneca vaccine does not mean the remaining 30 % of the vaccinated people can contract Coronavirus. The 70.4% efficacy illustrates that 30 out of 5807 individuals who received the vaccine developed Coronavirus compared to 101 out of 5807 individuals who received placebo, thereby resulting in a 70% decrease in the incidence of COVID-19 [27].
A vaccine with lower vaccine efficacy is essential in vaccinating a specific group to protect against the local outbreak [25]. As the study shows, even if a vaccine does not fight an epidemic, it can be useful in saving many human lives, costs, and hospitalization [25]. Therefore, the AstraZeneca vaccine is still effective besides its lowest efficacy. Additionally, based on the research, the Pfizer vaccine has the highest percentage efficacy of 95 %, showing that the vaccine is effective [26]. Pfizer antibodies take the shortest time compared to other vaccines, approximately 20 months. On the COVID-19 vaccine's safety, all the participants who participated in the clinical trial came out safe as far as health complications are concerned [24].
Nevertheless, the report contains some limitations. Owning to the high efficacy obtained in Pfizer's clinical trial, the initial plan of following the placebo participants for two years will not be followed considering ethical concerns. As a result, the clinical trial does not provide a placebo group essential in assessing long-term efficacy and safety. Furthermore, the trial did not include children, elders, and pregnant women, and therefore, it is challenging to identify the safety of the vaccine when administered to the group.
Conclusion and Recommendation
In this work, three vaccines have been reviewed. Based on the clinical trials that were done by the researchers using randomized testing, a significant level of efficacy has been achieved so far with the highest mRNA vaccine having a 95% percentage. The mRNA vaccines have higher efficacy compared to the vector vaccine reviewed in this study. Further research shows that there are more vaccines under development, more investigation on the general safety and effectiveness of COVID-19 vaccines should go on. People should also continue wearing PPEs and keeping social distance in addition to the protection offered by the vaccines.
Future Perspectives
Since the development of the first COVID-19 vaccine, members of the public have been reluctant to take up the vaccines as they fear for their safety and efficacy. Therefore, there is need to sensitize members of the public regarding COVID-19 vaccine efficacy and safety so as to improve the rate of acceptance of these vaccines. Moreover, more studies need to be conducted to establish the side effects that are linked to the four vaccines.
Reference
1. Modes of transmission of the virus causing COVID-19: implications for IPC precaution recommendations. WHO. 2020.
2. COVID-19 and Your Health. Centres for Disease Control and Prevention. 2020.
3. WHO Coronavirus disease (COVID-19) dashboard. Covid19.who.int. 2021.
4. Drosten C, Günther S, Preiser W, Van Der Werf S, Brodt HR, Becker S, et al. Identification of a novel coronavirus in patients with severe acute respiratory syndrome. N Engl J Med. 2003;348(20):1967-76.
5. Zaki AM, Van Boheemen S, Bestebroer TM, Osterhaus AD, Fouchier RA. Isolation of a novel coronavirus from a man with pneumonia in Saudi Arabia. N Engl J Med. 2012;367(19):1814-20.
6. Rothan HA, Byrareddy SN. The epidemiology and pathogenesis of coronavirus disease (COVID-19) outbreak. J Autoimmun. 2020;109:102433.
7. Zhu N, Zhang D, Wang W, Li X, Yang B, Song J, et al. A novel coronavirus from patients with pneumonia in China, 2019. N Engl J Med. 2020.
8. Vaccines and Related Biological Products Advisory Committee Meeting. FDA; 2020.
9. Walls AC, Park YJ, Tortorici MA, Wall A, McGuire AT, Veesler D. Structure, function, and antigenicity of the SARS-CoV-2 spike glycoprotein. Cell. 2020;181(2):281-92.
10. Polack FP, Thomas SJ, Kitchin N, Absalon J, Gurtman A, Lockhart S, et al. Safety and efficacy of the BNT162b2 mRNA Covid-19 vaccine. N Engl J Med. 2020;383(27):2603-15.
11. Baden LR, El Sahly HM, Essink B, Kotloff K, Frey S, Novak R, et al. Efficacy and safety of the mRNA-1273 SARS-CoV-2 vaccine. N Engl J Med. 2021;384(5):403-16.
12. Mahase E. Covid-19: Pfizer vaccine efficacy was 52% after first dose and 95% after second dose, paper shows.
13. Voysey M, Clemens SA, Madhi SA, Weckx LY, Folegatti PM, Aley PK, et al. Safety and efficacy of the ChAdOx1 nCoV-19 vaccine (AZD1222) against SARS-CoV-2: an interim analysis of four randomised controlled trials in Brazil, South Africa, and the UK. Lancet. 2021;397(10269):99-111.
14. Coronavirus. Centre for disease control. 2020.
15. Wadman M. Public needs to prep for vaccine side effects.
16. Callaway E. Oxford covid vaccine results puzzle scientists. Nature. 2020;588.
17. Dooling K, McClung N, Chamberland M, Marin M, Wallace M, Bell BP, et al. The Advisory Committee on Immunization Practices’ Interim Recommendation for Allocating Initial Supplies of COVID-19 Vaccine—United States, 2020. Morb Mortal Wkly Rep. 2020;69(49):1857.
18. Voysey M, Clemens SA, Madhi SA, Weckx LY, Folegatti PM, Aley PK, et al. Safety and efficacy of the ChAdOx1 nCoV-19 vaccine (AZD1222) against SARS-CoV-2: an interim analysis of four randomised controlled trials in Brazil, South Africa, and the UK. Lancet. 2021;397(10269):99-111.
19. Knoll MD, Wonodi C. Oxford–AstraZeneca COVID-19 vaccine efficacy. Lancet. 2021;397(10269):72-4.
20. Palacios R, Patiño EG, de Oliveira Piorelli R, Conde MT, Batista AP, Zeng G, et al. Double-Blind, Randomized, Placebo-Controlled Phase III Clinical Trial to Evaluate the Efficacy and Safety of treating Healthcare Professionals with the Adsorbed COVID-19 (Inactivated) Vaccine Manufactured by Sinovac–PROFISCOV: A structured summary of a study protocol for a randomised controlled trial. Trials. 2020;21(1):1-3.
21. Cohen J. Vaccine designers take first shots at COVID-19.
22. Speiser DE, Bachmann MF. COVID-19: Mechanisms of vaccination and immunity. Vaccines. 2020;8(3):404.
23. Hodgson SH, Mansatta K, Mallett G, Harris V, Emary KR, Pollard AJ. What defines an efficacious COVID-19 vaccine? A review of the challenges assessing the clinical efficacy of vaccines against SARS-CoV-2. Lancet Infect Dis. 2020.
24. Baden LR, El Sahly HM, Essink B, Kotloff K, Frey S, Novak R, et al. Efficacy and safety of the mRNA-1273 SARS-CoV-2 vaccine. N Engl J Med. 2021;384(5):403-16.
25. Bartsch SM, O'Shea KJ, Ferguson MC, Bottazzi ME, Wedlock PT, Strych U, et al. Vaccine efficacy needed for a COVID-19 coronavirus vaccine to prevent or stop an epidemic as the sole intervention. Am J Prev Med. 2020;59(4):493-503.
26. Mahase E. Covid-19: Pfizer vaccine efficacy was 52% after first dose and 95% after second dose, paper shows.
27. World Health Organization. COVID-19: operational guidance for maintaining essential health services during an outbreak: interim guidance, 25 March 2020. World Health Organization. 2020.
