Published online Apr 21, 2008. doi: 10.3748/wjg.14.2358
Revised: March 5, 2008
Published online: April 21, 2008
AIM: To discuss the expression of α-adrenoreceptors in pancreatic cancer cell lines PC-2 and PC-3 and the effects of α1- and α2-adrenoreceptor antagonists, yohimbine and urapidil hydrochloride, on the cell lines in vitro.
METHODS: We cultured the human ductal pancreatic adenocarcinoma cell lines PC-2 and PC-3 and analyzed the mRNA expression of α1- and α2-adrenergic receptors by reverse transcription polymerase chain reaction (RT-PCR). The effects of yohimbine and urapidil hydrochloride on cell proliferation were assessed by 3-(4,5-dimethylthiazol-2-yl)-2,4,-diphenyltetrazolium bromide (MTT) assay. Apoptosis was detected using the terminal deoxyribonucleotidyl transferase (TdT)-mediated biotin-16-dUTP nick-end labeling (TUNEL) assay and flow cytometry (FCM).
RESULTS: PC-2 expressed mRNA in α1- and α2-adrenoreceptors. MTT assays showed that urapidil hydrochloride had no effect on PC-3 cell lines. However, exposure to urapidil hydrochloride increased DNA synthesis in PC-2 cell lines as compared to the control group. PC-2 cell lines were sensitive to both drugs. The proliferation of the 2 cell lines was inhibited by yohimbine. Cell proliferation was inhibited by yohimbine via apoptosis induction.
CONCLUSION: The expression of α1- and α2-adrenoreceptors is different in PC-2 and PC-3 cell lines, which might be indicative of their different functions. The α2-adrenoceptor antagonist, yohimbine, can inhibit the proliferation of both cell lines and induce their apoptosis, suggesting that yohimbine can be used as an anticancer drug for apoptosis of PC-2 and PC-3 cells.
-
Citation: Shen SG, Zhang D, Hu HT, Li JH, Wang Z, Ma QY. Effects of α-adrenoreceptor antagonists on apoptosis and proliferation of pancreatic cancer cells
in vitro . World J Gastroenterol 2008; 14(15): 2358-2363 - URL: https://www.wjgnet.com/1007-9327/full/v14/i15/2358.htm
- DOI: https://dx.doi.org/10.3748/wjg.14.2358
Pancreatic cancer is the fourth most common malignancy in the United States. The annual incidence rate is almost identical to the mortality rate, and the 5-year survival rate remains less than 5%[1]. Approximately 10%-20% of patients have a resectable pancreatic cancer at the time of its diagnosis, and the curative resection rate is only 14% with a median survival of 15-19 mo[2]. Because of the inability of early diagnosis, early vascular dissemination, and regional lymph node metastasis, pancreatic cancer has a poor prognosis[34].
Recent studies have demonstrated that age, cigarette smoking, alcohol, and chronic pancreatitis are the factors for pancreatic cancer[56]. However, the mechanism of pancreatic cancer is not entirely clear. It was reported that β-adrenoreceptor expressed in cancer of the pancreas, breast, colon, lung, and prostate cell lines is the key factor that induces tumor formation due to long-term smoking and chronic stress[7–11]. Chronic stress could elevate the level of norepinephrine and epinephrine, leading to β-adrenoreceptor activation. Adrenoceptors modulate diverse intracellular processes, such as DNA synthesis through activation of mitogen-activating protein kinases (MAPKs)[12].
The α-adrenoceptor subtypes have been classified as α1A-, α1B-, α1D-, α2A-, α2B-, and α2C-adrenoceptors and β-adrenoceptors as β1-, β2-, and β3-adrenoceptors[13–16]. Various cancer cell lines could express different adrenoceptor subtypes impacting tumor cell biological behavior and heteromorphism. It was recently reported that α2-adrenoceptor subtype is expressed in breast cancer cell lines at RNA and protein level. Moreover, these functional receptors are associated with an increase in cell proliferation[1718].
In this paper, we discuss the expression of α-adrenore-ceptors in pancreatic cancer cell lines PC-2 and PC-3, and the effects of the α1- and α2-adrenoreceptor antagonists, yohimbine and urapidil hydrochloride in vitro. We hypothesize that the α-adrenoreceptor antagonists could suppress the proliferation of pancreatic cancer cells via induction of apoptosis. The aim of this study was to describe and characterize the presence of α1- and α2-adrenoceptors in pancreatic cancer cell lines PC-2 and PC-3 at RNA level using reverse transcription polymerase chain reaction (RT-PCR). Moreover, apoptosis was detected by terminal deoxyribonucleotidyl transferase (TdT)-mediated biotin-16-dUTP nick-end labeling (TUNEL) assay and flow cytometry (FCM), when pancreatic cancer cells were stimulated with α-adrenoreceptor antagonists.
Dulbecco’s modified Eagle’s medium (DMEM) and fetal bovine serum (FBS) were purchased from Gibco (Grand Island, NY, USA). Trizol was purchased from Invitrogen (Carlsbad, CA, USA). Urapidil hydrochloride, yohimbine, streptomycin, penicillin, and 3,3-dimethyl-2-thionotetrahydro-1,3,5-thiadiazine (MTT) were purchased from Sigma Chemicals (St. Louis, MO, USA).
PC-2 and PC-3 were obtained from the Pathology Department of China Beijing Union Medical University (PC-3 cell line established from human primary exocrine pancreatic head-cancer, PC-2 cell line from human exocrine pancreatic cancer metastasis). Cells were cultured in a high glucose-DMEM medium supplemented with 10% heat-inactivated fetal calf serum, penicillin (100 U/mL), and streptomycin (100 mg/mL) at 37°C in an atmosphere containing 50 mL/L CO2 and 950 mL/L with a relative humidity.
RNA was isolated from PC-2 and PC-3 cells lysed with Trizol. Dense RNA was quantified by UV spectrophotometry (at 260/280 nm) and stored at -70°C. For reverse transcriptase reaction, 0.5 &mgr;g of 18 oligo (dT) primers in diethyl pyrocarbonate (DEPC) water was heated to 70°C for 5 min to allow annealing of the primer to RNA followed by chilling on ice. A total of 10 mmol/L dNTPs, 20 units of RiboLock ribonuclease inhibitor, 5 × reaction buffer [250 mmol/L Tris-HCl (pH 8.3), 250 mmol/L KCl, 20 mmol/L MgCl2, and 50 mmol/L dithiothreitol (DTT)] were added and the reaction mixture was incubated at 37°C for 5 min. Then, 200 units of M-MuLV reverse transcriptase was added and the mixture was incubated at 42°C for 1 h, followed by heat inactivation for 10 min at 70°C.
PCR was performed with 5 &mgr;L RT reaction mixture containing 1 &mgr;L of 10 mmol/L dNTPs, 5 &mgr;L of 10× PCR buffer [100 mmol/L Tris-HCl (pH 8.3), 500 mmol/L KCl, and 15 mmol/L MgCl2, 1.5 &mgr;L of Taq polymerase (Perkin Elmer, Branchburg, NJ, USA), 1 &mgr;L primers for human α1A-, α1B-, α1D-, α2A-, α2C-, α2B-, β1-, and β2-adrenoceptors, and DEPC water in a final volume of 50 &mgr;L. Adrenoceptor primers were designed according to Genbank, NCBI. The α1A adrenoceptor primers (sense: 5’-CTCTGCGTCTGGGCACTCT-3’ and antisense: 5’-GAAGCAGCCGACCACGAT-3’) were used to amplify a 402-bp fragment. The α1B primers (sense: 5’-AAGAACTTTCACGAGGACACCC-3’ and antisense: 5’-CAGAACACCACCTTGAACACG-3’) were used to amplify a 233-bp fragment. The α1D primers (sense: 5’-TCACTCAAGTACCCAGCCATCA-3’ and antisense: 5’-GGAACCAGCAGAGCACGAAG-3’) were used to amplify a 487-bp fragment. The α2A primers (sense: 5’-ATCGGAGTGTTCGTGG-3’ and antisense: 5’-AGGAAGCAATAGTGATTAGGG-3’) were used to amplify a 489-bp fragment. The α2C primers (sense: 5’-TGTTCGTGCTCTGCTGGTTC-3’ and antisense: 5’-GGGGAAGGCAAAGGGGTC-3’) were used to amplify a 427-bp fragment. The α2B primers (sense: 5’-AAATCGGGGCGACAATAG-3’ and antisense: 5’-GAGACCCAAGCCACTAAAA-3’) were used to amplify a 367-bp fragment. The β1 primers (sense: 5’-GGGAGAAGCATTAGGG-3’ and antisense: 5’-CAAGGAAAGCAAGGTGGG-3’) were used to amplify a 270-bp fragment. The β2 primers (sense: 5’-CAGCAAAGGGACGAGGTG-3’ and antisense: 5’-AAGTAATGGCAAAGTAGCG-3’) were used to amplify a 334 bp fragment. The PCR conditions for the receptors of the above-mentioned primers were as follows: 1 cycle at 94°C for 2 min; 35 cycles at 94°C for 30 s, at 62°C for 60 s, and at 70°C for 2 min; and a final extension at 70°C for 7 min. Reactions were run on a MJ Research PTC-200 thermal cycler. The PCR products and a 100-bp DNA ladder were run on a 1.5% agarose gel for 2 h at 90 V. The gel was imaged using ethidium bromide staining with a UVP GDS 7500 or an Ultra Lum TUI-5000 gel documentation system.
The effects of yohimbine and urapidil hydrochloride on cell proliferation were detected by colorimetric MTT assay based on the NADH-dependent enzymatic reduction of the tetrazolium salt MTT in metabolically active cells but not in dead cells. Cells were seeded in a basal medium at the concentration of 1 × 104 cells/well in 96-well plates, and allowed to grow and adhere in complete media at 37°C with 50 mL/L CO2 for 20-24 h. The cells were then transferred to basal media with 0.05% FBS and incubated for 24 h with control (no treatment), yohimbine (25-200 &mgr;mol/L), and urapidil hydrochloride (25-200 &mgr;mol/L). Following incubation, 20 &mgr;L MTT (5 mg/mL) was added to each well, and the cells grew in complete media at 37°C with 50 mL/L CO2 for 4 h. The supernatant was removed, then 150 &mgr;L DMSO was added to each well of the 96-well plate and swung for 10 min. The optical density at 490 nm was determined with an enzyme linked immunosorbent assay (ELISA) reader.
TUNEL assay was performed with the in situ cell apoptosis detection kit following its manufacturer’s instructions. Briefly, the cells were replaced on cover slips after exposure to urapidil and yohimbine for 24 h and fixed with 4% paraformaldehyde for 30 min and adhered to slides with balsam. Nonspecific chromogen reaction, induced by endogenous peroxidase, was inhibited with 3% H2O2 for 10 min at room temperature. Terminal deoxynucleotidyl transferase (TdT) and biotin-11-dUTP reactions were performed for 1 h at 37°C in a humidified box, and blocking reagent was applied for 30 min at room temperature, followed by Avidin-HRP for 1 h at 37°C in a humidified box. For biochemical controls, positive control slides were treated with DNase and negative control slides were treated with PBS instead of TdT. DNA fragments were stained using DAB as a substrate for peroxidase, and hematoxylin was used as a counter stain. Apoptotic index was calculated as a ratio of the number of apoptotic cells to the total number of tumor cells in each slide.
The apoptosis rate was measured by FCM according to the instructions supplied with the annexin V-FITC kit. In brief, after treatment with urapidil and yohimbine, PC-2 and PC-3 cells were harvested by centrifugation, washed once with ice-cold PBS and resuspended in binding buffer at a concentration of 1 × 106 cells/mL, in which 100 mL of cell suspension was added in a 5 mL FCM tube. A total of 5 mL of annexin V-FITC and 10 mL of 20 mg/mL PI were added and incubated for 15 min in the dark prior to a further addition of 400 mL PBS. Quantitative analysis of apoptotic level was performed using a flow cytometer (BD Biosystems, CA, USA). The apoptotic percentage of 10 000 cells was determined and all the experiments reported in this study were performed in triplicate.
In vitro data were analyzed by one-way ANOVA and nonparametric Kruskal-Wallis test by using SPSS version 13.0. Values were expressed as mean ± SD, and P < 0.05 was considered statistically significant.
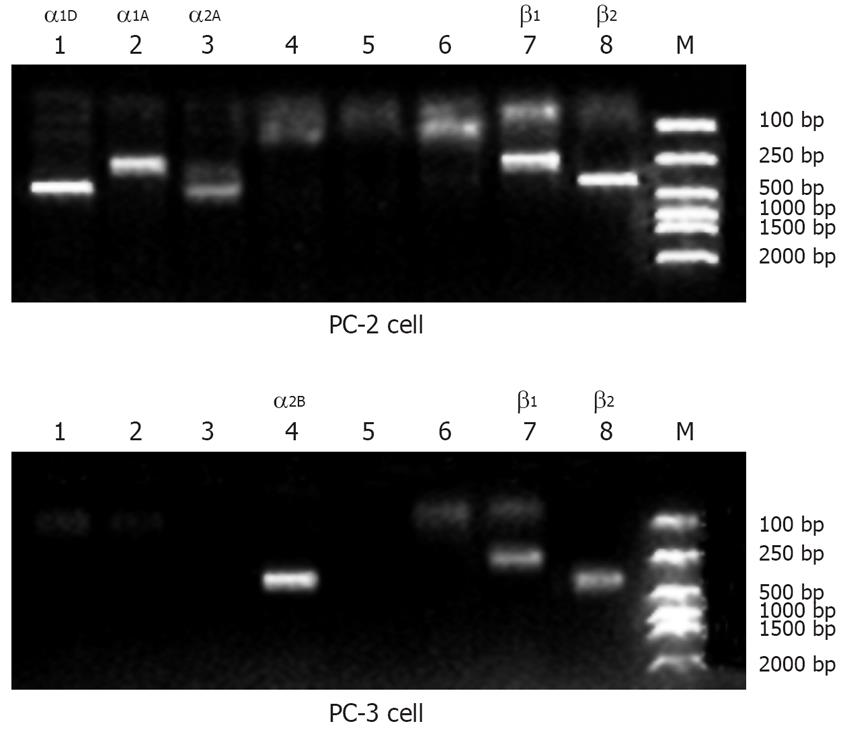
RT-PCR results demonstrated that PC-2 expressed mRNA in α1D, α1A, α2A, β1 and β2-adrenoceptors and PC-3 expressed mRNA in α2B, β1, and β2-adrenoceptors (Figure 1).
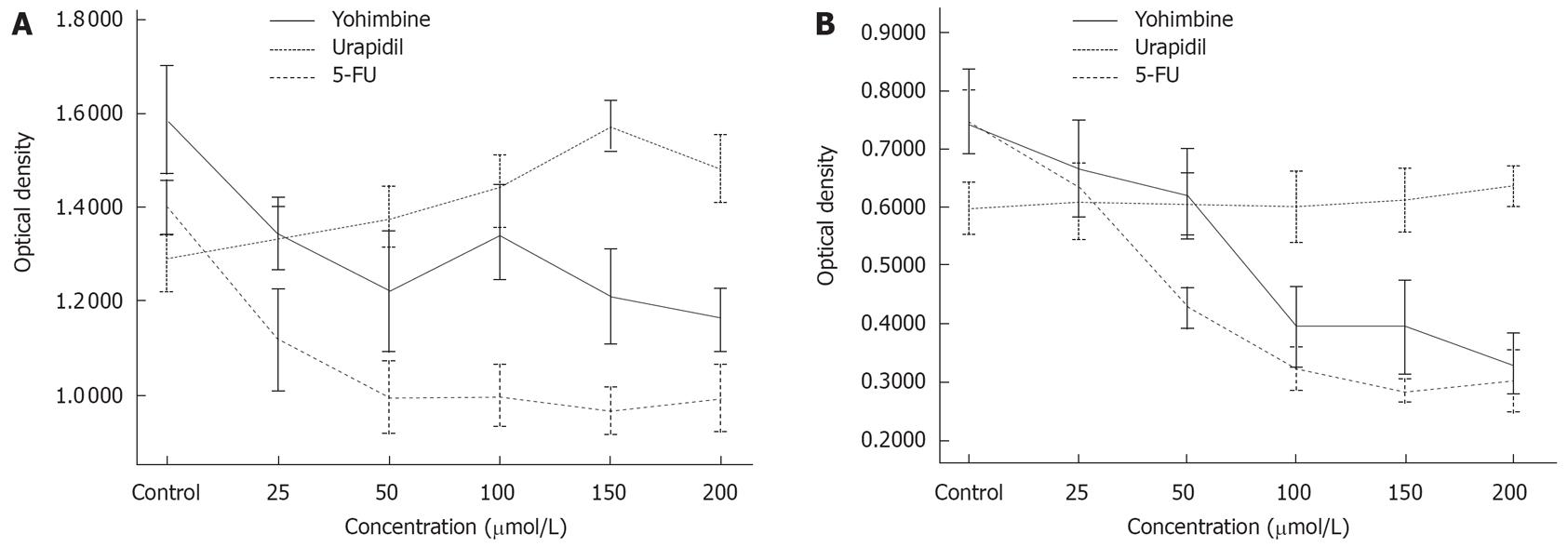
MTT assays showed that urapidil hydrochloride had no effect on PC-3 cell lines, whereas exposure to urapidil hydrochloride increased DNA synthesis in the PC-2 cell line as compared to the control group. PC-2 was sensitive to both drugs. The proliferation of 2 cell lines was inhibited by yohimbine. However, the rate of suppression by yohimbine was weaker than that by 5-FU used as a positive control (Figure 2).
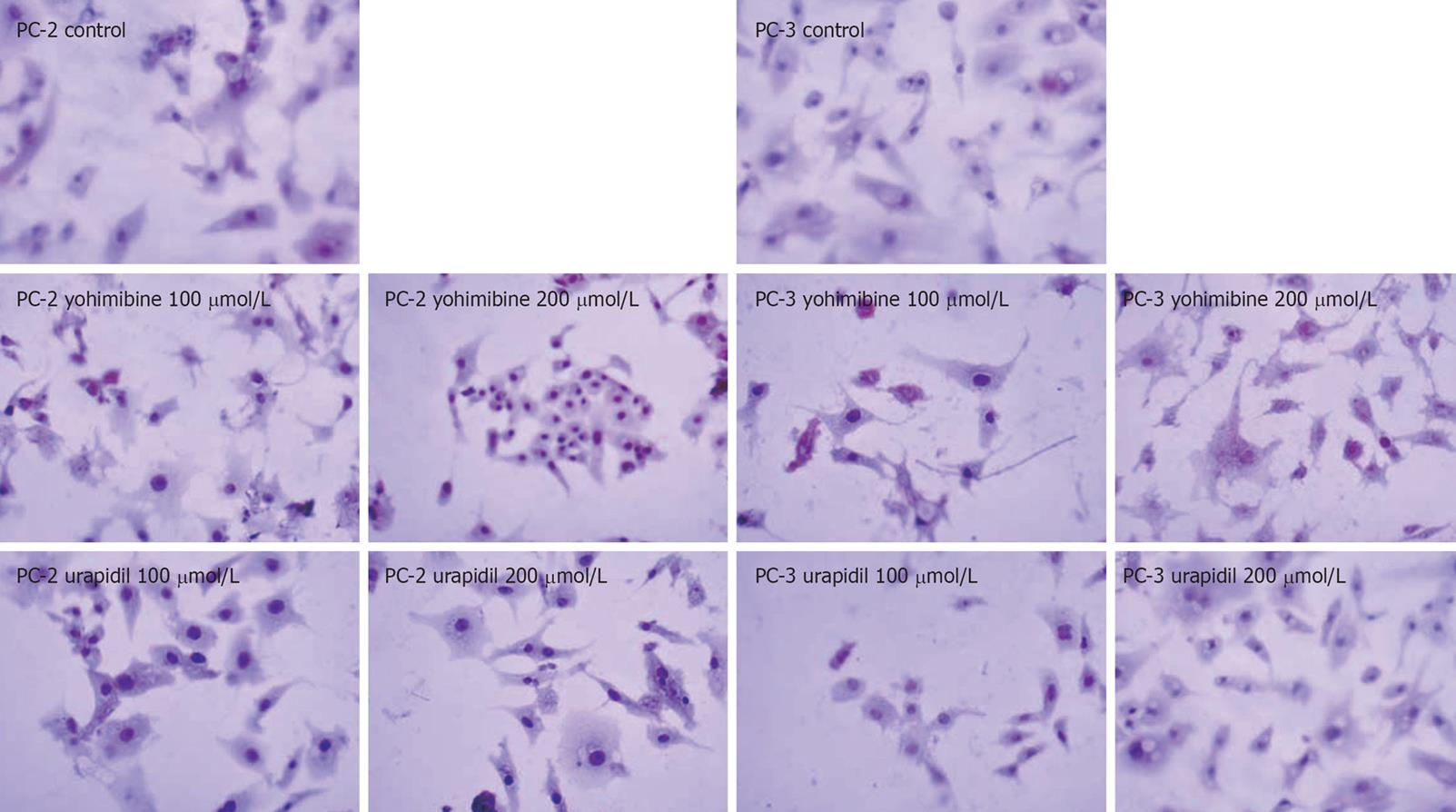
Nuclei of apoptotic cells were condense and stained brown whereas the negative cells were stained blue in the TUNEL assay. The results were in accordance with the MTT assay and revealed that yohimbine could induce apoptosis of both cell lines whereas urapidil hydrochloride had no effect on the 2 cell lines (Figure 3 and Table 1).
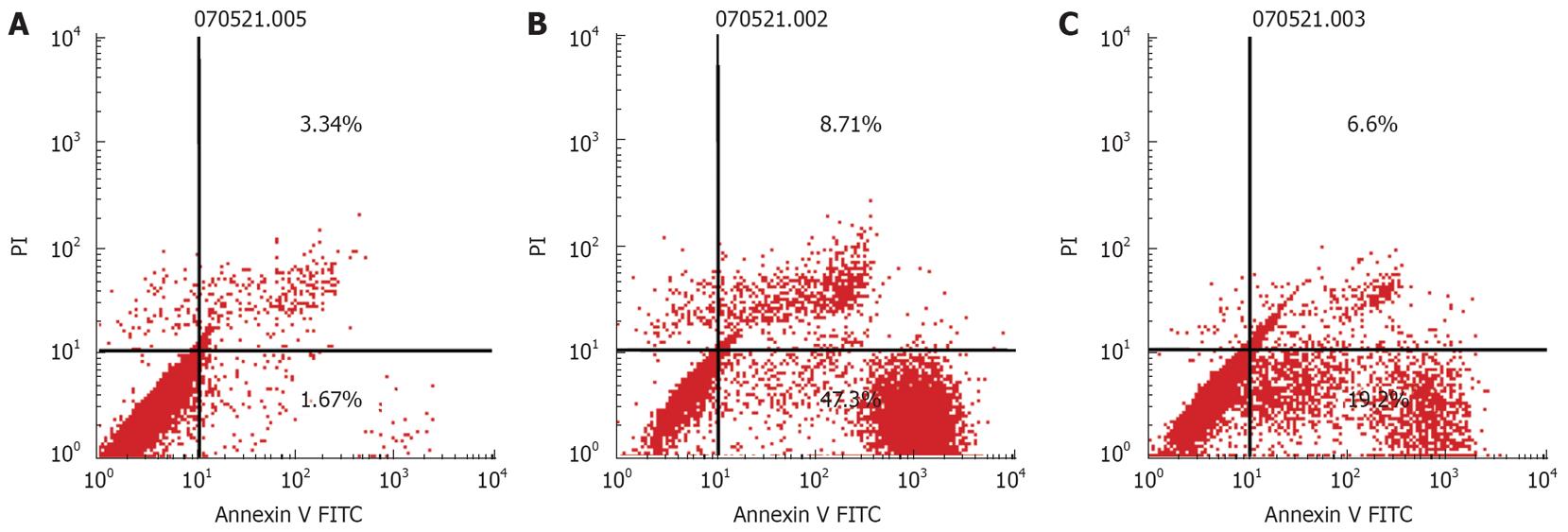
PC-2 and PC-3 cells were treated with the α-adrenoreceptor antagonists, urapidil hydrochloride (200 &mgr;mol/L) and yohimbine (200 &mgr;mol/L) for 24 h. The cells treated with urapidil hydrochloride and yohimbine were double stained with annexin V and PI and analyzed by FCM (Figure 4).
In this study, α1-adrenoceptors were only expressed in PC-2 cells, while α2-adrenoceptors were expressed in both PC-2 and PC-3 cells. MTT assay demonstrated that urapidil hydrochloride had no effect on PC-3 cell line, but increased DNA synthesis in PC-2 cell line as compared to the control group. The proliferation of the 2 cell lines was inhibited by yohimbine. The rate of suppression by yohimbine was weaker than by 5-FU used as a positive control.
α-adrenoceptor is a member of the superfamily of G protein-coupled adrenoceptors, which mediate reactions of endogenous catecholamines in a variety of target cells[12]. Currently, the following 6 native α-adrenoceptor subtypes have been identified: α1A, α1B, α1D, α2A, α2C, and α2B, which have been studied more extensively in diseases of the cardiovascular system, such as vascular constriction and hypertension mechanism[172021]. However, there are few reports on the relationship between α-adrenoceptor subtypes and malignant tumors. Our study identified the mRNA expression of α-adrenoceptor subtypes in PC-2 and PC-3 cell lines, and showed that the PC-2 cell line expressed α1D-, α1A-, and α2A-adrenoceptor subtype mRNA, and the PC-3 cell line expressed the α2B-adrenoceptor subtype mRNA.
In the present study, prostatic cells expressed α1-adrenoceptors. Catecholamine could promote prostatic cell proliferation[22]. Growth of human prostate cancer cells could be suppressed by α1-adrenoceptor antagonists, doxazosin and terazosin, by inducing apoptosis[2324]. On the other hand, the α1-adrenoceptor antagonist, urapidil hydrochloride, in our study, had effects on the proliferation of PC-2 cells but not on that of PC-3 cells, which might be related to the α-adrenoceptor subtype, but the mechanism of action is not clear.
α2-adrenoceptor is classically linked to the inhibition of adenylyl cyclase leading to a reduction in intracellular cAMP. Recent studies revealed that yohimbine inhibits both intracellular and extracellular cAMP levels in human mammary tumor MCF-7 cells and also suppresses the proliferation of MCF-7 cells, indicating that α-adrenergic receptors may mediate this kind of action[1619]. Our study demonstrated that the α2-adrenoceptor antagonist, yohimbine, could inhibit PC-2 and PC-3 cell proliferation by inducing apoptosis.
The molecular mechanism underlying apoptosis of PC-2 and PC-3 cells induced by yohimbine is unknown. It was reported that expression of a cloned α2-adrenergic receptor allows the coupling of this receptor to the p21ras- mitogen-activated protein (MAP) kinase cascade in hamster lung fibroblasts via a Gi-mediated pathway that is independent of adenylyl cyclase inhibition or phospholipase stimulation[2425]. MAPK pathway plays a pivotal role in pancreatic cancer progression by stimulating distinct mitotic, antiapoptotic, and angiogenic cascades, and by controlling different intracellular signaling elements activated by other growth factors in pancreatic cancer cells[26]. Based on our hypothesis that yohimbine induces apoptosis of PC-2 and PC-3 cells by inhibiting the MAPK or other pathways, our group will document the molecular mechanism underlying the apoptosis induced by yohimbine in future studies.
In conclusion, α2-adrenoceptor antagonist, yohimbine but not urapidil hydrochloride, exerts a negative effect on pancreatic cancer cell growth by inducing apoptosis. Although the potential underlying mechanism is presently unknown, yohimbine may suppress pancreatic cell growth by deregulating signal transduction pathways, potentially involving the MAPK or other pathways. The mechanistic aspects of the antigrowth effect of yohimbine against pancreatic tumors are under investigation.
It is supposed that neurobiology would play a key role in development of malignant tumors, by its direct effect on malignant tumors or on tumor micro-environment. Autonomic nerves are closely related with various activities of the body. A few reports are available on the beta-adrenergic receptors in cancer of colon, breast, and prostate, etc. However, little is known about alpha-adrenergic receptors in malignant tumor, we, therefore, studied the distribution and function of alpha-adrenergic receptors of malignant tumour cells.
Pancreas is a complex organ, including blood, nerve and lymph. Pancreatic cancer is generally characterized by less blood supply, innervation complex, and lymphatic irregularity, which have been brought difficulty to diagnosis and treatment of pancreatic cancer.
In recent years, basic researches of malignant tumor concentrated on their biological characteristics, such as invasion and metastasis. This study explored the development, metastasis and infiltration of pancreatic cancer by neurobiology.
The study is only a part of our research. The study would screen the drugs suppressing malignant tumor, from cardiovascular drugs.
The study investigated the effects of α-adrenoreceptor antagonists on proliferation and apoptosis of pancreatic cancer cells. The subject is important because very little is known about the role of adrenergic stimulation in the regulation of proliferation and death of pancreatic cancer cells. The study was well designed.
| 1. | Mancuso A, Calabro F, Sternberg CN. Current therapies and advances in the treatment of pancreatic cancer. Crit Rev Oncol Hematol. 2006;58:231-241. [Cited in This Article: ] |
| 2. | Michalski CW, Weitz J, Buchler MW. Surgery insight: surgical management of pancreatic cancer. Nat Clin Pract Oncol. 2007;4:526-535. [Cited in This Article: ] |
| 3. | Verslype C, Van Cutsem E, Dicato M, Cascinu S, Cunningham D, Diaz-Rubio E, Glimelius B, Haller D, Haustermans K, Heinemann V. The management of pancreatic cancer. Current expert opinion and recommendations derived from the 8th World Congress on Gastrointestinal Cancer, Barcelona, 2006. Ann Oncol. 2007;18 Suppl 7:vii1-vii10. [Cited in This Article: ] |
| 4. | Ghaneh P, Slavin J, Sutton R, Hartley M, Neoptolemos JP. Adjuvant therapy in pancreatic cancer. World J Gastroenterol. 2001;7:482-489. [Cited in This Article: ] |
| 5. | Lowenfels AB, Maisonneuve P. Epidemiology and risk factors for pancreatic cancer. Best Pract Res Clin Gastroenterol. 2006;20:197-209. [Cited in This Article: ] |
| 6. | Del Chiaro M, Zerbi A, Falconi M, Bertacca L, Polese M, Sartori N, Boggi U, Casari G, Longoni BM, Salvia R. Cancer risk among the relatives of patients with pancreatic ductal adenocarcinoma. Pancreatology. 2007;7:459-469. [Cited in This Article: ] |
| 7. | Weddle DL, Tithoff P, Williams M, Schuller HM. Beta-adrenergic growth regulation of human cancer cell lines derived from pancreatic ductal carcinomas. Carcinogenesis. 2001;22:473-479. [Cited in This Article: ] |
| 8. | Schuller HM, Tithof PK, Williams M, Plummer H 3rd. The tobacco-specific carcinogen 4-(methylnitrosamino)-1-(3-pyridyl)-1-butanone is a beta-adrenergic agonist and stimulates DNA synthesis in lung adenocarcinoma via beta-adrenergic receptor-mediated release of arachidonic acid. Cancer Res. 1999;59:4510-4515. [Cited in This Article: ] |
| 9. | Badino GR, Novelli A, Girardi C, Di Carlo F. Evidence for functional beta-adrenoceptor subtypes in CG-5 breast cancer cell. Pharmacol Res. 1996;33:255-260. [Cited in This Article: ] |
| 10. | Odore R, Badino P, Stammati AL, De Angelis I, Zucco F, Belloli C, Re G. Identification of functional beta-adrenoceptors in Caco-2 cell membranes. Vet Res Commun. 2003;27 Suppl 1:415-418. [Cited in This Article: ] |
| 11. | Nagmani R, Pasco DS, Salas RD, Feller DR. Evaluation of beta-adrenergic receptor subtypes in the human prostate cancer cell line-LNCaP. Biochem Pharmacol. 2003;65:1489-1494. [Cited in This Article: ] |
| 12. | Schuller HM. Mechanisms of smoking-related lung and pancreatic adenocarcinoma development. Nat Rev Cancer. 2002;2:455-463. [Cited in This Article: ] |
| 13. | Lutz H, Brian K, Kobilka . Adrenergic Receptors From Molecular Structure to in vivo Function. Trends Cardiovasc Med. 1997;7:137-145. [Cited in This Article: ] |
| 14. | Civantos Calzada B, Aleixandre de Artinano A. Alpha-adrenoceptor subtypes. Pharmacol Res. 2001;44:195-208. [Cited in This Article: ] |
| 16. | Summers RJ, Kompa A, Roberts SJ. Beta-adrenoceptor subtypes and their desensitization mechanisms. J Auton Pharmacol. 1997;17:331-343. [Cited in This Article: ] |
| 17. | Heusch G, Baumgart D, Camici P, Chilian W, Gregorini L, Hess O, Indolfi C, Rimoldi O. alpha-adrenergic coronary vaso-constriction and myocardial ischemia in humans. Circulation. 2000;101:689-694. [Cited in This Article: ] |
| 18. | Vazquez SM, Mladovan AG, Perez C, Bruzzone A, Baldi A, Luthy IA. Human breast cell lines exhibit functional alpha2-adrenoceptors. Cancer Chemother Pharmacol. 2006;58:50-61. [Cited in This Article: ] |
| 19. | Vazquez SM, Pignataro O, Luthy IA. Alpha2-adrenergic effect on human breast cancer MCF-7 cells. Breast Cancer Res Treat. 1999;55:41-49. [Cited in This Article: ] |
| 20. | Docherty JR. Subtypes of functional alpha1- and alpha2-adrenoceptors. Eur J Pharmacol. 1998;361:1-15. [Cited in This Article: ] |
| 21. | Davey M. Mechanism of alpha blockade for blood pressure control. Am J Cardiol. 1987;59:18G-28G. [Cited in This Article: ] |
| 22. | McGrath JC, Naghadeh MA, Pediani JD, Mackenzie JF, Daly CJ. Importance of agonists in alpha-adrenoceptor classification and localisation of alpha1-adrenoceptors in human prostate. Eur Urol. 1999;36 Suppl 1:80-88. [Cited in This Article: ] |
| 23. | Kyprianou N. Doxazosin and terazosin suppress prostate growth by inducing apoptosis: clinical significance. J Urol. 2003;169:1520-1525. [Cited in This Article: ] |
| 24. | Kyprianou N, Benning CM. Suppression of human prostate cancer cell growth by alpha1-adrenoceptor antagonists doxazosin and terazosin via induction of apoptosis. Cancer Res. 2000;60:4550-4555. [Cited in This Article: ] |
| 25. | Alblas J, van Corven EJ, Hordijk PL, Milligan G, Moolenaar WH. Gi-mediated activation of the p21ras-mitogen-activated protein kinase pathway by alpha 2-adrenergic receptors expressed in fibroblasts. J Biol Chem. 1993;268:22235-22238. [Cited in This Article: ] |