Published online Sep 14, 2008. doi: 10.3748/wjg.14.5344
Revised: August 8, 2008
Accepted: August 15, 2008
Published online: September 14, 2008
AIM: To investigate the effects of exogenously mutated p27kip1 (p27) on proliferation and apoptosis of human cholangiocarcinoma cell line, QBC939in vivo.
METHODS: Adenoviral vectors were used to transfect mutated p27 cDNA into human QBC939 cell line. Expression of p27 was detected by RT-PCR. Western blot. Cell growth, morphological change, cell cycle, apoptosis and cloning formation were determined by MTT assay and flow cytometry.
RESULTS: The expression of p27 protein and mRNA was increased significantly in QBC939 cell line transfected with Ad-p27mt. The transfer of Ad-p27mt could significantly inhibit the growth of QBC939 cells, decrease the cloning formation rate and induce apoptosis. p27 over expression caused cell cycle arrest at G0/G1 phase 72 h after infection with Ad-p27mt.
CONCLUSION: p27 may cause cell cycle arrest at G0/G1 phase and subsequently lead to apoptosis. Recombinant adenovirus expressing mutant p27 may be potentially useful in gene therapy for cholangiocarcinoma.
- Citation: Luo J, Chen YJ, Wang WY, Zou SQ. Effect of mutant p27kip1 gene on human cholangiocarcinoma cell line, QBC939. World J Gastroenterol 2008; 14(34): 5344-5348
- URL: https://www.wjgnet.com/1007-9327/full/v14/i34/5344.htm
- DOI: https://dx.doi.org/10.3748/wjg.14.5344
| Group | Cell clone count (ratio, cell clone amounts/500) | |||
| 3 d after transfect | 6 d after transfect | 9 d after transfect | 12 d after transfect | |
| Ad-p27mt group | 9 (1.8) | 14 (2.8) | 15 (3.0) | 21 (4.2) |
| Ad-LacZ group (n = 3) | 16 (3.2) | 27 (5.4) | 49 (9.8) | 56 (11.2) |
| QBC939 group (n = 3) | 18 (3.6) | 31 (6.2) | 46 (9.2) | 62 (12.4) |
| Group | G0/G1 (%)/apoptosis (%) | ||
| 24 h after transfect | 48 h after transfect | 72 h after transfect | |
| Ad-p27mt group (n = 3) | 61.02 ± 1.03/ 0.81 ± 0.052 | 73.32 ± 2.99/ 5.27 ± 0.030 | 83.63 ± 2.10/ 11.61 ± 1.23 |
| Ad-LacZgroup (n = 3) | 54.91 ± 2.32/ 0.76 ± 0.031 | 62.56 ± 2.71/ 1.28 ± 0.043 | 63.57 ± 2.32/ 4.16 ± 0.230 |
| QBC939 group (n = 3) | 49.76 ± 1.97/ 0.78 ± 0.041 | 56.95 ± 1.06/ 1.10 ± 0.071 | 62.56 ± 2.88/ 4.13 ± 0.454 |
It is well known that cell cycle progression is governed by cyclin-dependent kinases (CDKs). P27kip1 (p27), a key inhibitor of CDKs, can directly inhibit the entry of cell cycle from G1 phase into S phase. A major mechanism underlying the regulation of p27 is proteolysis by the ubiquitin–proteasome pathway. Phosphorylation of p27 on threonine 187 (T187) by Cdk2 creates a binding site for a Skp2-containing E3 ubiquitin-protein ligase, SCF. Ubiquitylation of p27 by SCF results in degradation of p27 by the proteasome.
In this study, a replication-deficient adenovirus vector encoding a mutated p27 at the Thr-187/pyrophosphorylation site was constructed and transfected into the cultured human cholangiocarcinoma cell line QBC939, in order to investigate the effects of adenovirus-mediated p27 on proliferation and apoptosis of cholangiocarcinoma cells.
Human cholangiocarcinoma cell line QBC939 was kindly donated by Professor Wang Shu-Guang of the Hepatobiliary Department of Xinan Hospital, Third Military Medical University. Tetramethyl-azo-zole-cyan (MTT) and iodized-dine (PI) were purchased from Sigma Ltd. Human-source rat anti-p27 monoclonal antibody was purchased from Beijing Zhongshan Ltd. Sense and anti-sense primers of p27 were synthesized by Shanghai Sangon Bioengineering and Technology Service Co. Ltd. Recombinant adenovirus vehicle Ad-p27mt and adenovirus control vehicle Ad-LacZ were kindly donated by Professor Xu Shao-Yong at Digestive Medical Department and Doctor Wang Jia-Ning at Cardiovascular Department, Yunyang Medical College. CO2 gas incubator(Binder, Germany), inverted phase contrast microscope (Olympus, Japan), FACsort flow cytometry (USA BD Ltd.) were used in this study. Cells used in experiments were divided into control group (QBC939 group), Ad-LacZ group and Ad-p27mt group.
Human cholangiocarcinoma cell line QBC939 was incubated in 10% FCS-containing RPMI 1640 culture medium at 37°C at an atmosphere containing 50mL/L CO2, and infected with Ad-LacZ or with Ad-p27mtatant at multiplicity of infection (MOI) of 50 as the density reached to 40%-50%.
Ad-LacZ was used to infect QBC939 cholangiocarcinoma cells when the MOI was set at 25, 50, 100 and 200. X-gal staining was performed after 48 h culture. Blue-staining cells were counted and the percentage was calculated to confirm recombinant adenovirus infection efficacy. Results demonstrated that as MOI ≥ 50, recombinant adenovirus was able to implement an approximately 100% transduction efficacy rate on the two types of cells.
Cells (4000-6000 cells/well) were inoculated in 96-well plates. The culture fluid was discarded after 48h of grouping, and 150 μL /well (0.5 mg/mL) MTT solution was supplemented at 37°C for 4 h followed by 150 μL/well DMSO, and shaken for 10 min. Absorbance (A) value was detected with an autokinetic enzyme scaling meter at 492 nm wavelength. Cell growth suppressive rate = (1-A value of experimental group/A value of the same titre QBC939 group) × 100%.
The cells infected with Ad-LacZ or with Ad-p27mutant at a MOI of 50 were transferred into a 12-well plate (500 cells/well) in triplicate and cultured for 3, 6, 9, and 12 d, respectively, then fixed with methanol and stained with 0.4% crystal violet. Clones containing at least 50 cells were counted under inverse microscope. Clone formation ratio (%) = cell clone amounts/500 × 100%.
Total cellular RNA was extracted from QBC939 cells transfected with Ad-p27mt and Ad-LacZ for 48 h using the Trizol method. PCR was performed after reverse transcription. The sequences of P27mt gene are upstream primer: 5'-CCTAGAGGGCAAGTACGAGTG-3', downstream primer: 5'-GAAGAATCGTCGGTTGCAGGTCGCT-3'. Reaction parameters were pre-degenerated at 95°C for 5 min, degenerated at 94°C for 30 s, 39 cycles of annealing at 56.3°C for 35 s, extension at 72°C for 35 s, a final extension at 72°C for 10 min. Electrophoresis was performed for the PCR products on a 2% agarose gel.
Cellular protein disposed for 72 h was extracted with the same method as described above. The proteins electro-transferred onto nitrocellulose membranes and blocked by confining liquid were bound to p27 monoclonal antibody and secondary antibody, and colored by enhanced chemiluminescence (ECL).
Cells of each group (above 106 cells in each group) were harvested at different time points. RNA enzyme was added at 37°C and reacted for 1 h after cells were fixed in 70% alcohol at 4°C for 24 h (final concentration 50 μg/mL). After 20-30 min of PI solution (concentration 100 μg/mL) staining, cells were counted by monochromatic fluorescence flow cytometry to observe the apoptosis rate.
All data were expressed as mean ± SD. The data were analyzed with SPSS 10.0 software. Variance analysis SNK method was employed in comparison of multi-groups. P < 0.05 was considered statistically significant.
Ultraviolet spectrophotometry showed that the titre of recombinant adenovirus after multiplication, amplification, and purification was up to 7.95 × 1012 CFU/mL.
Ad-LacZ was used to infect QBC939 cholangiocarcinoma cells. The multiplicity of infection (MOI) was 25, 50, 100 and 200. X-gal staining was performed after 48 h culture. Blue-staining cells were counted and the percentage was calculated to confirm the recombinant adenovirus infection efficacy. Results demonstrated that as MOI ≥ 50, recombinant adenovirus was able to implement an approximately transduction efficacy rate of 100% in the two types of cells.
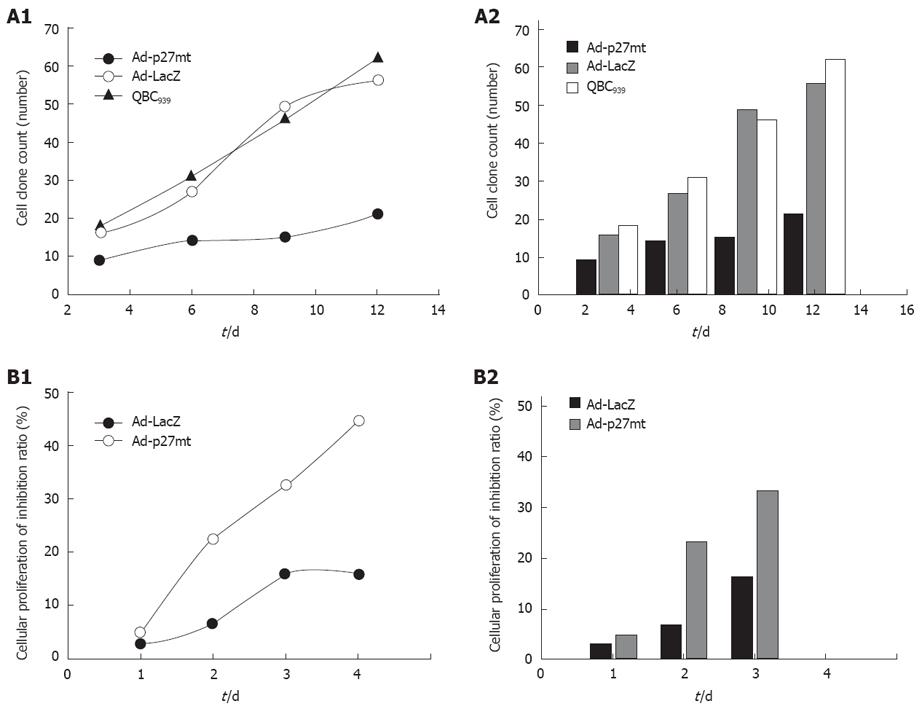
Clone formation test: The number and ratio of cellular clones in different groups are shown in Table 1 and Figure 1. The transfer of Ad-p27mt significantly inhibited the growth of QBC939 cells, decreased the clone formation, which was significantly different from the Ad-LacZ-infected and uninfected groups (F = 10.361, P = 0.011) with no statistical difference.
MTT assay for cell growth and viability: MTT assays also indicated that the proliferation of QBC939 cells was significantly inhibited after Adp27 infection, with its inhibitory effect peaked at 72 h. The transfer of Ad-p27mt could significantly inhibit the growth of QBC939 cells and decrease clone formation. After 24, 48 and 72 h of Adp27 infection, the average CD value was remarkably lower in Adp27-infected group than in Ad LacZ-infected and uninfected groups, revealing that introduction of exogenously mutated p27 gene via a recombinant adenovirus vector could significantly suppress the growth of QBC939 cells in a non time-dependent manner within 72 h.
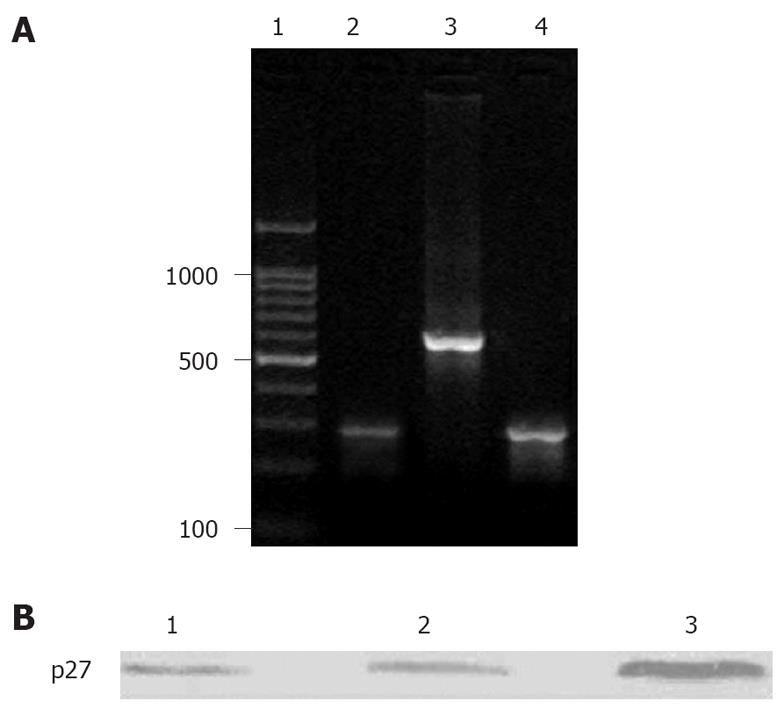
Gel electrophoresis for the RT- PCR products displayed that the expression of p27 was decreased in normal control group, but the expression of QBC939 cells was significantly elevated with a distinct 275bp objective gene strap (Figure 2A).
The expression of p27 was significantly increased in Ad-p27mt-tranfected QBC939 cells. However, the expression of p27 could be detected in a small number of Ad-LacZ-tranfected QBC939 cells (endogenous) and in the control group (Figure 2B).
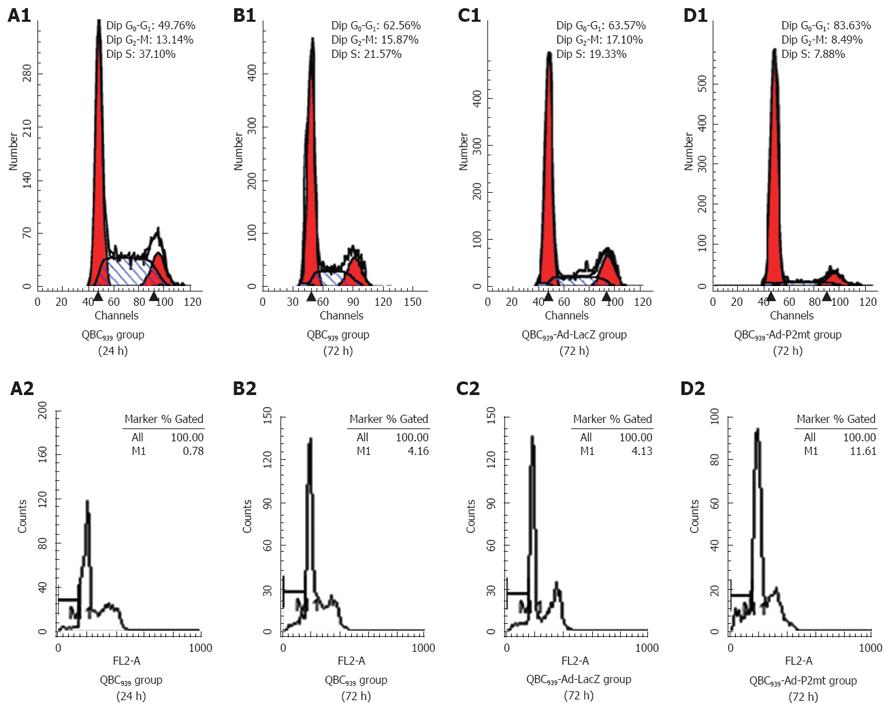
A high expression level of exogenous p27 protein in QBC939 cells evoked a strong cell cycle arrest at G0/G1 phase in a time-dependent manner within 72 h. The cell ratio was stabilized at about 83.63% ± 2.10% in a non time-dependent manner after 72 h, which was significantly different from that in the Ad-LacZ-infected and uninfected groups (F = 15.954, P = 0.012; Table 2 and Figure 3). The apoptosis rate was 11.61% ± 1.23% when the cells were infected with Ad-p27mt for 72 h. The sub-G1 apoptosis was more significant in Ad-p27mt group than in AdLacZ and control groups (Table 2 and Figure 3). All results were obtained from experiments performed in triplicate.
Cholangiocarcinoma remains one of the most difficult tumors to treat in clinical practice. Currently, there is no effective chemotherapy for this disease. Surgery offers the only opportunity to cure it. However, the majority of patients fail to qualify for such a treatment. Therefore, new therapeutic modalities are needed. Gene therapy is regard as one of the most important and potential new modalities for this disease.
The CDK inhibitor p27 plays a major role in controlling the cell cycle, which negatively regulates the transition from the G1 into the S phase. Moreover, p27kip1 is also a tumor suppressor. Loss of p27 function weakens the control of G1/S checkpoint, thus accelerating cell cycle progression and predisposing cells to malignant transformation[1,2]. Ganoth et al[3] and Troncone et al[4] reported that the degradation of p27 is mainly regulated by post-translational ubiquitin-proteasome-mediated proteolysis of phosphorylation in threonine (Thr) 187. In order to inhibit the degradation of p27 and restore the function of G1/S checkpoint, we transfected mutated p27 into cholangiocarcinoma QBC939 cell line, which has a mutation of Thr-187/Pro-188 (ACGCCC) to Met-187/Ile-188(ATGATC).
Western blot analysis showed that 72 h after infection with Ad-p27mt, p27 in the CBC939 cells expressed a strong band, whereas Ad-LacZ-infected QBC939 cells showed a faint p27 protein product in the uninfected groups, suggesting that tansgenes can be successfully induced and expressed. The elevated level of p27 expression demonstrated that mutated p27 was resistant to degradation and more stable than wild p27, indicating that phosphorylation of threonine (Thr) 187 can trigger degradation of wild p27. The transfer of Ad-p27mt significantly inhibited the proliferation of QBC939 cells, decreased clone formation, strongly induced cell cycle arrest and apoptosis at G1/S phase within 72 h after infection, which is consistent with the previous findings[5].
It has been well documented that over expression of wild p27 via adenoviral gene transfer on p27-deficient tumor cells could strongly inhibit cell cycle arrest and even lead to significantly apoptosis in disparate types of human cancers, such as spongiocytoma, lung cancer, leukaemia. It is a common phenomenon that recombinant adenovirus-mediated p27 can eliminate carcinoma cells through apoptosis. Although there was no significant difference in apoptosis between Adp27mt- and AdLacZ-infected cells, uninfected cells at any time point, our data show that 72 h after infection with Adp27mt, the typical sub-G1 apoptotic peak could be observed by flow cytometry, which was more apparent than in AdLacZ-infected and uninfected cells. Although the precise mechanism by which p27 induces apoptosis is unclear, transfer of p27 is associated with a moderate level of apoptosis as shown by FACS analysis. Since QBC939 cells have mutated p53, the mechanism underlying apoptosis induced by transfer of p27 must be p53-independent. Further investigation is needed on how p27 regulates and induces apoptosis.
In conclusion, Ad-p27mt at Thr-187 can be used as a novel, potent, tumor-suppressing gene therapy tool in the treatment of cholangiocarcinoma.
As a cyclin-dependent kinase inhibitor, p27Kip1 (p27) regulates cell cycle progression by transcriptional, translational and proteolytic mechanisms. G1/S cell cycle progression requires p27 proteolysis, which is triggered by its phosphorylation of threonine (Thr) 187. Increased p27 causes proliferating cells to exit from the cell cycle, while decreased p27 is required for quiescent cells to resume cell division. Low levels of p27 are associated with excessive cell proliferation in pathological conditions such as inflammation and cancers. High levels of p27 are observed in such conditions of diminished cell proliferation as in late stages of arterial wound repair in atherosclerosis. Interestingly, in many types of tumors such as gastric, prostate and breast carcinomas, the expression of p27 gene is down-regulated. Loss of p27 expression may result in tumor development and/or progression.
The research involved cell morphologyoncology, cell morphology, molecular biology and gene therapy for cholangiocarcinoma.
It is well know that the degradation of p27 is mainly regulated by post-translational ubiquitin-proteasome-mediated proteolysis during phosphorylation of threonine (Thr) 187. In order to inhibit the degradation of p27 and restore the function of G1/S checkpoint, we transfected mutated p27 into cholangiocarcinoma QBC939 cell line, which can mutate from Thr-187/Pro-188 (ACGCCC) to Met-187/Ile-188(ATGATC). The recombinant adenoviral vector cannot replicate in target cells because it lacks the E1 gene, thus only expressing the inserted gene. Because the foreign gene fragment is not incorporated into the genome of target cells, the danger of mutations affecting treatment is reduced. Meanwhile, adenoviral vectors are stable and easy to purify. This technique can effectively affect both proliferating and quiescent cells ex vivo. The potential for gene therapy by using the recombinant adenovirus is worthy of extensive attention. The results of our study suggest that adenovirus-mediated p27 gene transfection can be used as a novel gene therapy for cholangiocarcinoma.
The prognosis of cholangiocarcinoma is extremely poor although aggressive multidisciplinary cancer therapies have been used in clinical practice. Thus, it is imperative to develop new and effective treatment modalities for cholangiocarcinoma, such as gene therapy.
The authors showed that transfection of a human cholangiocarcinoma cell line (QBC939) could cause cell cycle arrest and apoptosis, which are of interest in developing new treatment modalities for cholangiocarcinoma. The methods used were well described. The results are of scientific interest.
Peer reviewer: Gustav Paumgartner, Professor, University of Munich, Klinikum Grosshadern, Marchioninistr. 15, Munich, D-81377, Germany
S- Editor Zhong XY L- Editor Wang XL E- Editor Lin YP
| 1. | Milde-Langosch K, Hagen M, Bamberger AM, Loning T. Expression and prognostic value of the cell-cycle regulatory proteins, Rb, p16MTS1, p21WAF1, p27KIP1, cyclin E, and cyclin D2, in ovarian cancer. Int J Gynecol Pathol. 2003;22:168-174. [Cited in This Article: ] |
| 2. | Fukunaga M. Immunohistochemical characterization of cyclin E and p27KIP1 expression in early hydatidiform moles. Int J Gynecol Pathol. 2004;23:259-264. [Cited in This Article: ] |
| 3. | Ganoth D, Bornstein G, Ko TK, Larsen B, Tyers M, Pagano M, Hershko A. The cell-cycle regulatory protein Cks1 is required for SCF(Skp2)-mediated ubiquitinylation of p27. Nat Cell Biol. 2001;3:321-324. [Cited in This Article: ] |
| 4. | Troncone G, Martinez JC, Iaccarino A, Zeppa P, Caleo A, Russo M, Migliaccio I, Motti ML, Califano D, Palmieri EA. p27Kip1 is expressed in proliferating cells in its form phosphorylated on threonine 187. BMC Clin Pathol. 2005;5:3. [Cited in This Article: ] |