Published online Dec 14, 2008. doi: 10.3748/wjg.14.7117
Revised: November 10, 2008
Accepted: November 17, 2008
Published online: December 14, 2008
AIM: To construct a noninvasive assessment model consisting of routine laboratory data to predict significant fibrosis and cirrhosis in patients with chronic hepatitis B (CHB).
METHODS: A total of 137 consecutive patients with CHB who underwent percutaneous liver biopsy were retrospectively analyzed. These patients were divided into two groups according to their aminotransferase (ALT) level. The sensitivity, specificity, positive predictive value (PPV), negative predictive value (NPV), the likelihood ratio (LR) of aminotransferase/platelet ratio index (APRI) ≥ 1.5 or < 1.5 in combination with different hyaluronic acid (HA) cut-off points were calculated for the presence of moderate to severe fibrosis/cirrhosis (fibrosis stages 2 and 4) and no to mild fibrosis/cirrhosis (fibrosis stages 0 and 1).
RESULTS: The APRI correlated with fibrosis stage in CHB patients. The APRI ≥ 1.5 in combination with a cut-off HA cut-off point > 300 ng/mL could detect moderate to severe fibrosis (stages 2-4) in CHB patients. The PPV was 93.7%, the specificity was 98.9%. The APRI < 1.5 in combination with different HA cut-off points could not detect no to mild fibrosis in CHB patients.
CONCLUSION: The APRI ≥ 1.5 in combination with a HA cut-off point > 300 ng/mL can detect moderate to severe fibrosis (stages 2-4) in CHB patients.
- Citation: Zhang YX, Wu WJ, Zhang YZ, Feng YL, Zhou XX, Pan Q. Noninvasive assessment of liver fibrosis with combined serum aminotransferase/platelet ratio index and hyaluronic acid in patients with chronic hepatitis B. World J Gastroenterol 2008; 14(46): 7117-7121
- URL: https://www.wjgnet.com/1007-9327/full/v14/i46/7117.htm
- DOI: https://dx.doi.org/10.3748/wjg.14.7117
| ALT≥2ULN | ALT < 2ULN | |
| Number of patients | 78 | 59 |
| Age (SD) | 35.2 (7) | 38.7 (7.4) |
| Gender | ||
| Male | 70.5% | 69.5% |
| Female | 29.5% | 30.5% |
| Stage of fibrosis (%) | ||
| F0 + F1 | 33 (42.3) | 24 (40.7) |
| F2 | 26 (33.3) | 20 (33.9) |
| F3 | 13 (16.7) | 9 (15.2) |
| F4 | 6 (7.7) | 6 (10.1) |
| Significant fibrosis (≥ F2) | 45 (57.3) | 35 (59.3) |
| APRI1(SD) | ||
| F0 + F1 | 0.55 (0.82) | 0.48 (0.33) |
| F2 | 1.44 (1.79) | 1.21 (1.57) |
| F3 | 1.98 (2.34) | 1.69 (1.62) |
| F4 | 2.11 (1.81) | 1.97 (1.73) |
| Significant fibrosis (≥ F2) | 1.84 (1.38) | 1.62 (1.45) |
| HA (SD) | ||
| F0 + F1 | 131.3 (82.7) | 129.9 (79.8) |
| F2 | 199.3 (158.2) | 190.5 (149.9) |
| F3 | 285.3 (188.6) | 285.7 (187.3) |
| F4 | 324.9 (212.6) | 333.3 (224.1) |
| Significant fibrosis (≥ F2) | 269.8 (214.1) | 268.9 (187.1) |
| Sensitivity (%) | Specificity (%) | PPV (%) | NPV (%) | +LR | -LR | |
| APRI ≥ 1.5 | 44.7 | 84.3 | 41.3 | 84.7 | 2.80 | 0.66 |
| +HA ≥ 150 | 46.6 | 95.6 | 88.6 | 89.5 | 10.6 | 0.56 |
| +HA ≥ 200 | 46.8 | 97.8 | 90.2 | 89.9 | 21.3 | 0.54 |
| +HA ≥ 250 | 47.3 | 98.7 | 93.2 | 90.2 | 36.4 | 0.53 |
| +HA ≥ 300 | 45.3 | 98.9 | 93.7 | 91.3 | 41.2 | 0.55 |
| Sensitivity (%) | Specificity (%) | PPV (%) | NPV (%) | +LR | -LR | |
| APRI < 1. 5 | 35.3 | 81.6 | 41.3 | 82.2 | 1.9 | 0.79 |
| +HA < 300 | 45.7 | 86.2 | 44.6 | 83.1 | 3.3 | 0.63 |
| +HA < 250 | 42.8 | 83.2 | 42.8 | 81.3 | 2.5 | 0.69 |
| +HA < 200 | 38.5 | 80.8 | 40.8 | 80.3 | 2.0 | 0.76 |
| +HA < 150 | 31.7 | 78.8 | 37.1 | 77.7 | 1.5 | 0.87 |
About 350 million individuals are chronically infected with hepatitis B virus (HBV) worldwide[1]. There are 30 million patients with chronic hepatitis B (CHB) in China, which will progress to cirrhosis or hepatocellular carcinoma (HCC) in 10%-30% of CHB patients. Although antiviral treatment with interferon or nucleoside analogues has been widely adopted, it has significant side effects. Liver biopsy can help decide the treatment modality for patients infected with HBV, especially for those whose alanine aminotransferase (ALT) is under 2 of the upper limit of normal (ULN) or normal, but its value is questioned because of its potential risk and the concern of sampling errors[2]. Therefore, there is a growing tendency to use noninvasive measures instead of histopathological analysis of liver tissue for the evaluation of disease progression in patients with chronic liver diseases. Up to date, several laboratory tests, scores, and indices have been proposed for noninvasive prediction of hepatic fibrosis in CHB patients[3,4]. However, the results of such tests are different in different study populations[5]. Aminotransferase/platelet ratio index (APRI) is easy to calculate, but it can only predict the severe hepatic fibrosis (F3, F4). Kuroiwa et al[6] reported that HA can predict all fibrosis stages, but its sensitivity and specificity are not very high. We hypothesized that APRI in combination with different hyaluronic acid (HA) cut-off points would be a better predictor of fibrosis than individual parameters.
A total of 137 consecutive patients with CHB who underwent percutaneous liver biopsy at Shanghai Public Health Clinical Center (China) from 2005 to April 2008 were included in this study. Real-time PCR showed that all patients were positive for HBV DNA and had no chronic liver disease confirmed by standard clinical, serological, biochemical, and radiological criteria. Additional exclusion criteria were antiviral treatment before liver biopsy, alcohol consumption in excess of 40 g/d. Liver biopsies were obtained by either blind or ultrasound-guided techniques using a 16-gauge Klatskin needle. The length of biopsy samples was longer than 1.5 cm. All biopsies were read by a pathologist who had no clinical information on the CHB patients. Formalin-fixed and formalin-embedded liver tissues were cut into 4-μm thick sections with a microtome. One section was stained with hematoxylin and eosin for assessment of hepatic inflammatory activity and the other sections were stained with Gomori stain for evaluation of hepatic fibrosis. Biopsy specimens with at least 4 portal fields were considered representative and scored by a pathologist unaware of the laboratory results. Fibrosis was staged as no (0), mild (1), moderate (2), severe (3), and cirrhosis (4), using the METAVIR score[7]. Hepatic inflammatory activity was also scored.
Serum aspartate aminotransferase (AST), ALT, HA and platelet count in all patients within 2 wk after liver biopsy were routinely determined. The ULN for ALT was 50 U/L, and transformed into ULN for further analysis. The reference range for platelet count was 100 × 109-300 × 109/L. HA was measured using the RIA and the reference range was 9-119 ng/mL. According to the ULN of ALT, we divided the patients into two groups with their ALT ≥ 2ULN and < 2ULN, respectively. APRI was calculated as previously described[8,9].
Baseline demographic data were evaluated for comparability of the two groups using Fisher’s exact test for categorical variables and Student’s t test for continuous variables. Student’s t test or analysis of variance was used to compare the means of different stage groups when appropriate. Correlation was evaluated by the Spearman correlation coefficient. The sensitivity, specificity, positive predictive value (PPV), negative predictive value (NPV) and likelihood ratio (LR) of APRI ≥ 1.5 and APRI < 1.5 in combination with different HA cut-off points were detected for the presence of moderate to severe fibrosis/cirrhosis (fibrosis stages 2 and 4) and no to mild fibrosis/cirrhosis (fibrosis stages 0 and 1). P < 0.05 was considered statistically significant.
A total of 137 patients with CHB who underwent percutaneous liver biopsy were included in this study. In order to adequately estimate the predictive model, the patients were divided into 2 groups with their ALT ≥ 2ULN and ALT < 2ULN, respectively. There was no significant correlation between age, gender, HA, APRI and disease stage. The characteristics of patients in the two groups are shown in Table 1.
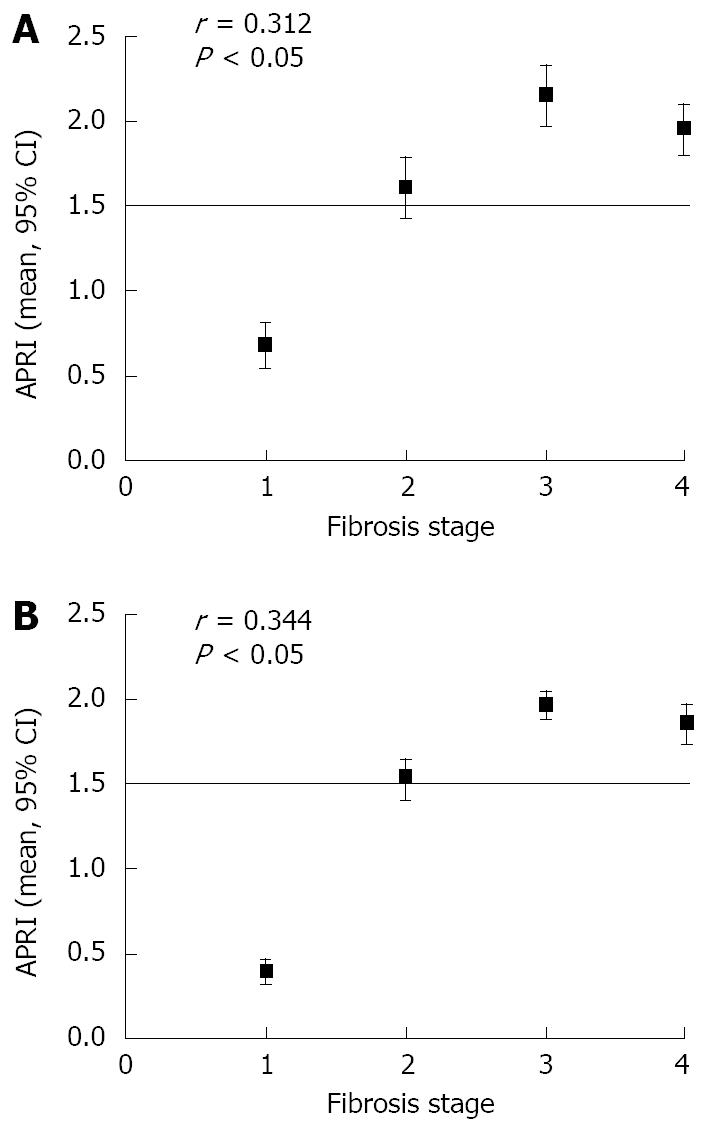
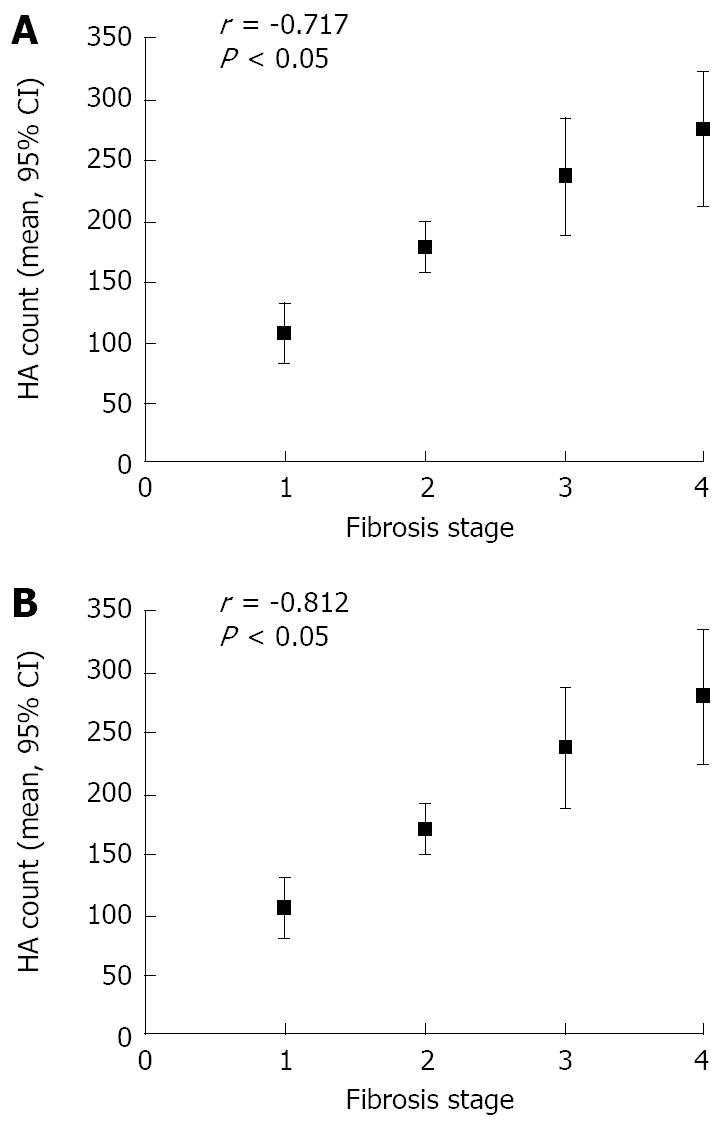
The distribution of fibrosis stages in the two groups is shown in Table 1. Of the 137 patients, 57 (41.6%) had no or only mild fibrosis (stages 0 and 1), 46 (33.6%) had moderate fibrosis (stage 2), and 34 (24.8%) had severe fibrosis or cirrhosis (stages 3 and 4). As expected, the APRI and HA cut-off points increased with the stage of fibrosis, but there was no significant difference between the two groups. The mean APRI was ≥ 1.5 in patients with moderate to severe fibrosis or cirrhosis (stages 2-4) and < 1.5 in patients with no or mild fibrosis (stages 0 and 1).
Among the patients with their APRI ≥ 1.5, the Spearman correlation coefficient was r = 0.312 and 0.344 between APRI and fibrosis stage (P < 0.05, Figure 1), and r = -0.717 and -0.812 between HA and fibrosis stage (P < 0.05, Figure 2). However, there was no significant difference in patients with their APRI < 1.5 between APRI or HA count and fibrosis stage.
APRI ≥ 1.5 in combination with different HA cut-off points could detect moderate to severe fibrosis (stages 2-4) in CHB patients (Table 2). The sensitivity, specificity, PPV, NPV, +LR and -LR were determined. APRI alone had a PPV of 41.3% and a specificity of 84.7%. When HA was added, the PPV and specificity increased significantly, indicating that APRI ≥ 1.5 in combination with a HA cut-off point > 300 ng/mL can detect moderate to severe fibrosis or cirrhosis (stages 2-4) in CHB patients. In the present study, the PPV was 93.7% and the specificity was 98.9%. On the other hand, not all patients with moderate to severe fibrosis or cirrhosis could be correctly identified. The identification methods for patients with no or mild fibrosis (stages 0 and 1) using APRI < 1.5 in combination with different HA cut-off points are shown in Table 3. Since the sensitivity, specificity, PPV and NPV were low, mild fibrosis in CHB patients could not be detected using these laboratory parameters.
It is well known that the exact staging of liver fibrosis is crucial for the therapeutic decision and assessing the prognosis of CHB patients. The gold standard for fibrosis staging is liver biopsy. However, a simple noninvasive method for detection of fibrosis would be beneficial. Some studies have shown that APRI is only sensitive to F3-F4 stages of fibrosis[10,11].
HA, mainly metabolized in liver, is one of the important components of extracellular matrix, and can reflect the level of hepatic fibrosis in some degree. Guéchot et al[12] showed that the sensitivity and specificity of serum HA at a cut-off point of 110 μg/L for the diagnosis of hepatic fibrosis are 79% and 89%, respectively. Patel et al[13] reported that as a noninvasive valuable marker, serum HA concentration is correlated with hepatic fibrosis. Kuroiwa et al[6] showed that the AUC value of HA for any fibrosis and cirrhosis is higher than 0.5. Montazeri et al[14] demonstrated that serum HA is a preferred marker of severe fibrogenesis and inflammation in CHB patients. HA at a cutoff point of 126.4 μg/L can detect severe fibrosis with a sensitivity of 90.9% and a specificity of 98.1%.
In this study, the APRI was correlated with fibrosis stage in CHB patients. The sensitivity, specificity, PPV, NPV, +LR and -LR of APRI in detecting were 35.3%, 81.6%, 41.3%, 82.2%, 1.9 and 0.79, respectively, for the detection of mild fibrosis (stage 1) in CHB patients. However, its sensitivity, specificity, PPV, NPV, +LR and -LR of APRI were 44.7%, 84.3%, 41.3%. 84.7%, 2.8 and 0.66, respectively, for the detection of moderate to severe fibrosis (stages 2-4) in CHB patients. The sensitivity, specificity, PPV, and NPV of HA were very low for the detection of fibrosis stages in CHB patients. APRI does not involve a complicated formula, thus allowing it to be quickly calculated. In addition, it uses 2 laboratory tests and is not associated with the added expense of a reference laboratory, and does not contain subjective parameters such as ethanol intake.
We established a noninvasive assessment model of liver fibrosis consisting of APRI and HA. APRI ≥1.5 in combination with different HA cut-off points was used to predict moderate to severe fibrosis (stages 2-4) in CHB patients. APRI alone had a PPV of 41.3% and a specificity of 84.7%. When different HA cut-off points were added, the PPV and specificity increased significantly, especially when a HA cut-off point was greater than 300 ng/mL, indicating that APRI ≥ 1.5 in combination with a HA cut-off point > 300 ng/mL can predict moderate to severe fibrosis (stages 2-4) in CHB patients. The PPV, specificity and LR of this model were 93.7%, 98.9% and 41.2, respectively. On the other hand, not all patients with moderate to severe fibrosis could be correctly identified. We want to know if serum ALT level is correlated with HA and APRI values. However, the APRI and HA increased with the stage of fibrosis, but there was no significant difference in patients with their ALT ≥ 2ULN or < 2ULN, respectively, which may be due to the small number of patients.
Since the rate of APRI < 1.5 in combination with different HA cut-off points for the detection of mild fibrosis (stage 1) in CHB patients was low in this study, liver biopsy was needed for the detection of mild fibrosis in CHB patients.
Finally, the model was established based on liver biopsy as the gold standard[15]. Since sampling error and inter-observer variability are known limitations of a liver biopsy, we should interpret the results of noninvasive tests for hepatic fibrosis with caution within a broader clinical context.
In conclusion, a predictive model for assessing the probability of significant hepatic fibrosis can be established in CHB patients. APRI ≥ 1.5 in combination with a HA cut-off point > 300 ng/mL can detect moderate to severe fibrosis (stages 2-4) in CHB patients.
Liver biopsy is the gold standard for hepatic fibrosis in chronic hepatitis B (CHB) patients, especially in those whose alanine aminotransferase (ALT) is under 2ULN or normal, but its value is questioned because of its potential risk and sampling error. Noninvasive markers of liver fibrosis have been recently proposed as substitutes for liver biopsy, but their reported accuracy is around 80%. They have been mostly validated in hepatitis C but not in hepatitis B. They applied their method in a cohort of patients with chronic hepatitis B.
Since sampling error and inter-observer variability are known limitations of liver biopsy, some noninvasive methods for liver fibrosis have been proposed, but international guidelines still do not recommend a routine use of the markers due to lack of reproducibility and a misdiagnosis rate of 20%. Thus, a trusted method that avoids a number of liver biopsies by maintaining an excellent accuracy is urgently needed.
In this study, they established a noninvasive assessment model consisting of APRI and HA for the detection of liver fibrosis and cirrhosis in CHB patients. APRI ≥ 1.5 in combination with a HA cut-off point > 300 ng/mL could detect moderate to severe fibrosis (stages 2-4) in CHB patients. The PPV and specificity of this model were 93.7% and 98.9%, respectively, showing that it can be used as a non-invasive marker for the detection of liver fibrosis in CHB patients.
The predictive model can be used as a first line assessment of significant hepatic fibrosis in CHB patients, limiting liver biopsy to those who are unclassified or show a low predictive value. In the future, priority should be given to large scale validation studies and the most promising non-invasive markers in patients with all major etiologies of chronic liver disease and most frequent cofactors affecting the diagnostic performance of fibrosis markers.
APRI: a simple test combining aspartate aminotransferase (AST) and platelet count for non-invasive prediction of significant fibrosis and cirrhosis in hepatitis C patients. It is a very simple and economic tool, but it is somehow less accurate than fibrotest.
This is an interesting paper addressing a clinical problem in the management of chronic hepatitis B. The usefulness of this combination of non-invasive markers of fibrosis and its place in clinical practice needs to be further studied.
Peer reviewer: Miguel C De Moura, Professor, Department of Gastroenterology, Medical School of Lisbon, Av Prof Egas Moniz, 1649-028 Lisboa, Portugal
S- Editor Tian L L- Editor Wang XL E- Editor Yin DH
| 1. | Lavanchy D. Hepatitis B virus epidemiology, disease burden, treatment, and current and emerging prevention and control measures. J Viral Hepat. 2004;11:97-107. [Cited in This Article: ] |
| 2. | Bedossa P, Dargere D, Paradis V. Sampling variability of liver fibrosis in chronic hepatitis C. Hepatology. 2003;38:1449-1457. [Cited in This Article: ] |
| 3. | Rosenberg WM, Voelker M, Thiel R, Becka M, Burt A, Schuppan D, Hubscher S, Roskams T, Pinzani M, Arthur MJ. Serum markers detect the presence of liver fibrosis: a cohort study. Gastroenterology. 2004;127:1704-1713. [Cited in This Article: ] |
| 4. | Forns X, Ampurdanes S, Llovet JM, Aponte J, Quinto L, Martinez-Bauer E, Bruguera M, Sanchez-Tapias JM, Rodes J. Identification of chronic hepatitis C patients without hepatic fibrosis by a simple predictive model. Hepatology. 2002;36:986-992. [Cited in This Article: ] |
| 5. | Giannini E, Testa R. Noninvasive diagnosis of fibrosis: the truth is rarely pure and never simple. Hepatology. 2003;38:1312-1313; author reply 1313. [Cited in This Article: ] |
| 6. | Kuroiwa Y, Suzuki N, Yamamoto M, Hatakeyama N, Hori T, Mizue N. [Prognostic value of serum markers for liver fibrosis in transient abnormal myelopoiesis (TAM)]. Rinsho Ketsueki. 2005;46:1179-1186. [Cited in This Article: ] |
| 7. | Bedossa P, Poynard T. An algorithm for the grading of activity in chronic hepatitis C. The METAVIR Cooperative Study Group. Hepatology. 1996;24:289-293. [Cited in This Article: ] |
| 8. | Pohl A, Behling C, Oliver D, Kilani M, Monson P, Hassanein T. Serum aminotransferase levels and platelet counts as predictors of degree of fibrosis in chronic hepatitis C virus infection. Am J Gastroenterol. 2001;96:3142-3146. [Cited in This Article: ] |
| 9. | Wai CT, Greenson JK, Fontana RJ, Kalbfleisch JD, Marrero JA, Conjeevaram HS, Lok AS. A simple noninvasive index can predict both significant fibrosis and cirrhosis in patients with chronic hepatitis C. Hepatology. 2003;38:518-526. [Cited in This Article: ] |
| 10. | Williams AL, Hoofnagle JH. Ratio of serum aspartate to alanine aminotransferase in chronic hepatitis. Relationship to cirrhosis. Gastroenterology. 1988;95:734-739. [Cited in This Article: ] |
| 11. | Sebastiani G, Vario A, Guido M, Alberti A. Sequential algorithms combining non-invasive markers and biopsy for the assessment of liver fibrosis in chronic hepatitis B. World J Gastroenterol. 2007;13:525-531. [Cited in This Article: ] |
| 12. | Guéchot J, Laudat A, Loria A, Serfaty L, Poupon R, Giboudeau J. Diagnostic accuracy of hyaluronan and type III procollagen amino-terminal peptide serum assays as markers of liver fibrosis in chronic viral hepatitis C evaluated by ROC curve analysis. Clin Chem. 1996;42:558-563. [Cited in This Article: ] |
| 13. | Patel K, Lajoie A, Heaton S, Pianko S, Behling CA, Bylund D, Pockros PJ, Blatt LM, Conrad A, McHutchison JG. Clinical use of hyaluronic acid as a predictor of fibrosis change in hepatitis C. J Gastroenterol Hepatol. 2003;18:253-257. [Cited in This Article: ] |
| 14. | Montazeri G, Estakhri A, Mohamadnejad M, Nouri N, Montazeri F, Mohammadkani A, Derakhshan MH, Zamani F, Samiee S, Malekzadeh R. Serum hyaluronate as a non-invasive marker of hepatic fibrosis and inflammation in HBeAg-negative chronic hepatitis B. BMC Gastroenterol. 2005;5:32. [Cited in This Article: ] |