Published online Mar 21, 2009. doi: 10.3748/wjg.15.1331
Revised: February 12, 2009
Accepted: February 19, 2009
Published online: March 21, 2009
AIM: To assess: (1) frequency and clinical relevance of gluten sensitive enteropathy (GSE) detected by serology in a mass screening program; (2) sensitivity of antitransglutaminase (tTGA) and antiendomysium antibodies (EmA); and (3) adherence to gluten-free diet (GFD) and follow-up.
METHODS: One thousand, eight hundred and sixty-eight subjects recruited from an occupational health department underwent analysis for tTGA and EmA and, if positive, duodenal biopsy, DQ2/DQ8 genotyping, clinical feature recording, blood tests, and densitometry were performed. Since > 98% of individuals had tTGA < 2 U/mL, this value was established as the cut-off limit of normality and was considered positive when confirmed twice in the same sample. Adherence to a GFD and follow up were registered.
RESULTS: Twenty-six (1.39%) subjects had positive tTGA and/or EmA, and 21 underwent biopsy: six Marsh III (one IIIa, four IIIb, one IIIc), nine Marsh I and six Marsh 0 (frequency of GSE 1:125). The sensitivity of EmA for GSE was 46.6% (11.1% for Marsh I, 100% for Marsh III), while for tTGA, it was 93.3% (88.8% for Marsh I, 100% for Marsh III). All 15 patients with abnormal histology had clinical features related to GSE. Marsh I and III subjects had more abdominal pain than Marsh 0 (P = 0.029), and a similar trend was observed for distension and diarrhea. No differences in the percentage of osteopenia were found between Marsh I and III (P = 0.608). Adherence to follow-up was 69.2%. Of 15 GSE patients, 66.7% followed a GFD with 80% responding to it.
CONCLUSION: GSE in the general population is frequent and clinically relevant, irrespective of histological severity. tTGA is the marker of choice. Mass screening programs are useful in identifying patients who can benefit from GFD and follow-up.
- Citation: Mariné M, Fernández-Bañares F, Alsina M, Farré C, Cortijo M, Santaolalla R, Salas A, Tomàs M, Abugattas E, Loras C, Ordás I, Viver JM, Esteve M. Impact of mass screening for gluten-sensitive enteropathy in working population. World J Gastroenterol 2009; 15(11): 1331-1338
- URL: https://www.wjgnet.com/1007-9327/full/v15/i11/1331.htm
- DOI: https://dx.doi.org/10.3748/wjg.15.1331
Screening for celiac disease (CD) in the general population is still a controversial issue. Recent papers have reviewed the benefits and drawbacks of CD diagnosis in this situation[1]. CD is a highly prevalent disease (1:100 to 1:300) which fulfils some of the criteria favoring mass screening. That is, the availability of accurate and non-invasive diagnostic methods and an effective treatment (gluten-free diet: GFD) which restores health and prevents disease-associated complications[2]. The major concerns regarding mass screening are the reported lack of GFD adherence in asymptomatic patients detected in screening programs[34] and the limited available data on the cost-effectiveness of such an approach[5]. In addition, the benefits of early diagnosis in patients with mild disease, in terms of preventing late complications, are poorly understood[6]. In this sense, knowledge of the natural history of mild CD has been hampered by its imprecise definition in the medical literature, with frequent overlapping of the terms “mild enteropathy” (i.e. lymphocytic enteritis and partial atrophy) and “silent CD”. In fact, it was recently demonstrated that these terms may not be considered synonymous since gluten sensitive enteropathy (GSE) with preserved villous architecture may be symptomatically similar to patients with atrophy[7] showing a good response to a GFD[89], and conversely, silent patients with atrophy do exist, and are at risk of subsequent severe complications[10].
Occupational health departments provide care for the working population on environmental issues associated with work processes, but also help detect very frequent preventable diseases. In this setting, the aims of the present study were: (1) to assess the frequency and clinical relevance of GSE (both Marsh I and Marsh III) detected by serology in a mass screening program; (2) to compare the sensitivity of antitransglutaminase antibodies (tTGA) and antiendomysium antibodies (EmA) in detecting the whole spectrum of GSE (Marsh I to Marsh III); and (3) to assess the degree of adherence to a mass-screening program and the effectiveness of GFD.
One thousand, eight hundred and sixty-eight individuals (1308 males, 560 females, 36.6 ± 0.5 years) were recruited from the Occupational Work Surveillance Department in Catalonia, Northeastern Spain, between January 2004 and December 2005. None of the 1868 subjects included had previously been diagnosed with CD. After obtaining written informed consent, blood sampling for serum EmA and tTGA assay was carried out and clinical features for gastrointestinal and systemic symptoms, and associated diseases (yes/no questions) were obtained. HLA DQ2/DQ8 genetic study and duodenal biopsy were offered to those subjects positive for either EmA or tTGA. Furthermore, as previously described, a self-administered questionnaire of symptom severity using a visual analogue scale (VAS) ranging from 0 to 100 was recorded in these subjects[711]. The symptoms evaluated were diarrhea, abdominal pain, abdominal distension, flatulence and asthenia. Anemia and hypertransaminasemia, as well as abnormalities in bone mineral density (BMD), were also recorded. Subjects were considered to be symptomatic when they complained of at least one of the above-mentioned symptoms and/or had impaired blood test and/or densitometry. A symptom was considered to be present when it scored more than 20 points and was considered severe when it scored more than 50 points on the VAS.
Serum IgA-EmA was determined by indirect immunofluorescence (IFI) assay in serum samples at 1/5 dilution, as previously described[12]. Commercial sections of monkey distal oesophagus (BioMedical Diagnostics, France) were used as the IFI substrate.
IgA-class tTGA was analyzed in serum using a quantitative automated ELISA method by means of a commercially available detection kit (Varelisa CelikeyTM, Phadia AB, Freiburg, Germany) using recombinant human tTG as antigen[13]. Since > 98% of individuals had tTGA < 2 U/mL, this value was established as the cut-off limit of normality and was considered positive when confirmed twice in the same sample (recommended cut-off by the manufacturer > 8 U/mL). Total serum IgA was measured using rate nephelometry (BN II, Siemens Healthcare Diagnostics SL, Marburg, Germany). In cases of IgA deficiency, IgG-class EmA was measured.
When one or both serological markers were positive, an upper endoscopy with duodenal biopsy was proposed.
Standard techniques for DNA extraction, PCR amplification and product detection were used. To purify genomic DNA from whole blood, a commercial reagent Generation® Capture Column Kit (Gentra Systems, Minnesota, USA) was used. HLA-DQ2 (DQA1*0501 and DQB1*0201 alleles) and HLA-DQ8 (DQA1*0301 and DQB1*0302 alleles) genotyping was performed by PCR amplification using sequence specific primers (PCR-SSP)[14] on a GeneAmp PCR 2400 System (Applied Biosystems, Foster City, CA, USA). PCR products were detected by electrophoresis on 2% agarose gel and were visualized under UV light. Analysis of HLA-DQ8 haplotype was performed only on those patients with negative DQ2.
Four endoscopic biopsies from the 2nd-3rd portions of the duodenum were processed by using hematoxylin/eosin staining and CD3 immunophenotyping, and were blindly evaluated by an expert gastrointestinal pathologist (A.S.). Histopathological findings were staged according to the Marsh criteria[15], as revised by Rostami et al[16]: ‘Infiltrative’ lesions with intraepithelial lymphocytosis are defined as Marsh type I, ‘infiltrative/hyperplastic’ lesions are defined as Marsh II, and ‘partial (A), subtotal (B) and total (C) villous atrophy’ as Marsh III. A cut-off of 25 intraepithelial lymphocytes (IELs)/100 epithelial cells was established to diagnose lymphocytic enteritis (LE)[17]. Other frequent causes of LE, such as parasites, NSAID ingestion, Crohn’s disease and autoimmune diseases were ruled out[18]. Helicobacter pylori (H pylori) infection was investigated by means of the urease test and histopathological assessment using hematoxylin/eosin staining of the gastric mucosa in all the cases.
The diagnosis of GSE was considered when some degree of histological abnormality was found and a good response to a GFD was demonstrated (see below) at least after one year of follow-up, according to AGA criteria[19].
BMD was assessed in all patients showing some degree of histological abnormality (Marsh I to III), both at baseline and after GFD. T and Z-scores were measured in the lumbar spine and left femoral neck using a dual-energy X-ray absorptiometry (DXA) (Lunar DPX-aph, Madison, WI, USA). According to World Health Organization criteria, osteopenia is defined as a value of BMD between 1 SD and 2.5 SD below the average for young adults (T score -1 to -2.5), and osteoporosis is defined as a value of BMD more than 2.5 SD below the average value for young adults (T score < -2.5)[20].
GFD was recommended to all patients with villous atrophy and to all symptomatic LE patients, and adherence was recorded. To ensure the correct intake of a strict GFD, patients were referred to the Catalan Celiac Society (‘Celiacs de Catalunya’, Barcelona). Iron and/or calcium plus vitamin D supplements were prescribed when deficiencies or bone density impairment were detected. Clinical, histological, analytical and serological assessments were carried out in all patients who adhered to a GFD at least 1 year after starting the diet. In addition, a second densitometry assessment was carried out when the basal assessment was impaired. For the remaining individuals with positive tTGA at baseline, who were on a gluten-containing diet, clinical, analytical and serological assessments were requested, as a minimum. A patient was considered to have achieved a complete clinical response when all symptoms disappeared (VAS < 20 points) and when normalization of analytical and bone densitometry abnormalities occurred. A partial clinical response was defined as more than a 30-point reduction in the VAS score and/or a significant improvement but not normalization of analytical and bone densitometry abnormalities. A complete histological response was defined as a decrease from Marsh III to Marsh I or Marsh 0, and in Marsh I cases, a normalization in the IEL count or a reduction of at least 50% from the basal biopsy. An improvement in the degree of atrophy (i.e. from Marsh IIIc to Marsh IIIa) or a reduction in the IEL count from 25% to 50% of the basal biopsy in Marsh I cases, was considered a partial histological response.
In patients who did not accept a biopsy after GFD, negative serology was considered a criterion of at least partial response. Patients with LE and those with the lowest tTGA positive values were particularly encouraged to undergo histological retesting during follow-up.
Categorical parameters were expressed as proportions, whereas continous variables were expressed as both mean and standard error of the mean (SEM). Since intestinal biopsy was performed when EmA and/or tTGA were positive, the ratio of sensitivities of the two serological tests was calculated by an estimation of test sensitivity when disease confirmation was limited to positive results. Differences in sensitivities were assessed using a modified McNemar test as previously described[21]. Statistical comparisons for qualitative variables were made by an extension of Fisher’s exact test for 2 × 3 contingency tables (Freeman-Halton test)[22]. One-way ANOVA and paired Student t test were used to compare quantitative variables. P < 0.05 was considered statistically significant. All statistical analyses were performed using the SPSS for Windows Statistical package (SPSS Inc., Chicago, IL, USA).
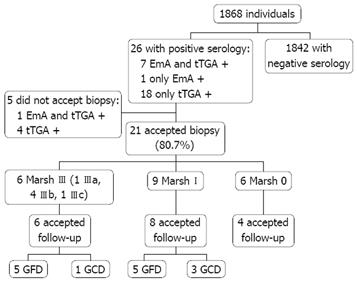
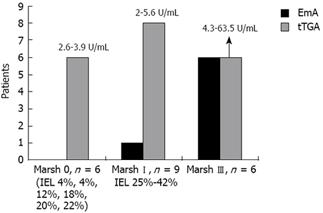
Figure 1 represents a flow diagram of the evaluated patients. Twenty-six of the 1868 individuals (1.39%) had positive markers for CD (18 males, eight females, mean age 37.7 ± 11.0 years). Of the 26 patients with positive markers, seven were positive for both EmA and tTGA, one was positive only for EmA, and the remaining 18 were positive only for tTGA. Twenty-one of these 26 individuals (80.7%) underwent an intestinal biopsy, which disclosed the following histological findings: six Marsh III (one IIIa, four IIIb, one IIIc), nine Marsh I and six Marsh 0. Three Marsh I cases had H pylori infection but the IEL count remained unchanged after 6 mo of eradication therapy. Thus, 0.80% of subjects initially tested had a biopsy proven lesion of the GSE spectrum (1:125) and 0.32% had villous atrophy (1:312). Values of tTGA related to the degree of mucosal damage are shown in Figure 2. All Marsh III patients were positive for both EmA and tTGA, and all Marsh I and 2 Marsh III patients had tTGA values higher than 2 U/mL but lower than the cut-off recommended by the manufacturer (8 U/mL).
The sensitivity of EmA for GSE diagnosis was 46.6% (11.1% for Marsh I and 100% for Marsh III), whereas the sensitivity of tTGA was 93.3% (88.8% for Marsh I and 100% for Marsh III) (P = 0.04). The sensitivity ratio demonstrated a two-fold sensitivity for tTGA compared with EmA to diagnose the whole spectrum of GSE (from Marsh I to III).
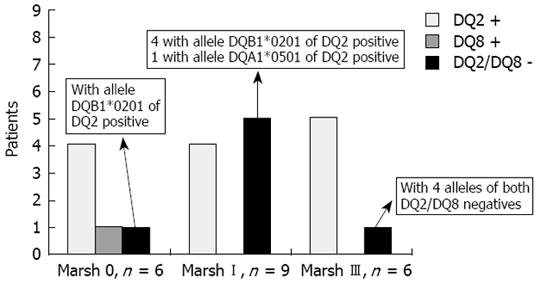
Thirteen of the 21 biopsied subjects (62%) were DQ2 +, 1 (4.7%) was DQ8 +, six had one allele of the DQ2 + (28.6%) (DQB1*0201 in five subjects and DQA1*0501 in one) and only one Marsh III (4.7%) was negative for both alleles of DQ2 and DQ8. A detailed description of the genetic markers related to the degree of histological damage is shown in Figure 3.
In five subjects with positive markers a biopsy was not performed, four because they did not accept the procedure and one patient could not be located [one was positive for both EmA (1/20) and tTGA (3.94 U/mL) and four were positive only for tTGA (2.21 to 5.10 U/mL)].
No significant differences were found in the percentage of autoimmune diseases (P = 0.415), thyroid diseases (P = 0.632), type 1 diabetes mellitus (P = 1), previous anemia (P = 0.765), diarrhea (P = 0.764), abdominal pain (P = 1), flatulence (P = 0.965) or abdominal distension (P = 0.621) between individuals, in the general working population, or in those with positive and negative serological markers.
All 15 subjects with abnormal histological findings had clinical features related to the disease. In one Marsh III and two Marsh I patients, the only clinical feature was osteopenia (20%). The frequencies of clinical manifestations related to the degree of histological severity are described in Table 1. Subjects with Marsh I and Marsh III lesions had significantly more abdominal pain (66.7%) than those with normal mucosa (0%; P = 0.029). A similar but non-significant trend was observed for distension and diarrhea, whereas asthenia was more often found in Marsh III patients. No differences were found for hypertransaminasemia and only one Marsh III patient had anemia. A progressive increase in the severity of most symptoms from Marsh 0 to Marsh III was observed when symptoms were assessed by means of a VAS (Table 2), reaching statistically significant differences for distension (P = 0.035) and asthenia (P = 0.031).
| Normal mucosa (Marsh 0) n = 6 (%) | LE (Marsh I) n = 9 (%) | Atrophy (Marsh III) n = 6 (%) | Pvalue | |
| Flatulence | 4 (66.7) | 6 (66.7) | 4 (66.7) | 1 |
| Distension | 2 (33.4) | 7 (77.8) | 4 (66.7) | 0.282 |
| Abdominal pain | 0 (0.0) | 6 (66.7) | 4 (66.7) | 0.029 |
| Diarrhea | 1 (16.7) | 4 (44.5) | 4 (66.7) | 0.300 |
| Asthenia | 2 (33.4) | 2 (22.3) | 4 (66.7) | 0.213 |
| Anemia | 0 (0) | 0 (0) | 1 (16.7) | 0.571 |
| Hypertransaminasemia | 1 (16.7) | 1 (11.9) | 2 (33.3) | 0.783 |
| Normal mucosa (Marsh 0) n = 6 | LE (Marsh I) n = 9 | Atrophy (Marsh III) n = 6 | Pvalue | |
| Flatulence | 36.6 ± 12.0 (0-60) | 28.8 ± 8.2 (0-60) | 36.6 ± 14.7 (0-80) | 0.839 |
| Distension | 6.67 ± 4.2 (0-20) | 28.8 ± 6.7 (0-60) | 50.0 ± 16.9 (0-100) | 0.035 |
| Abdominal pain | 0.0 ± 0.0 (0-20) | 31.1 ± 10.0 (0-80) | 26.6 ± 9.8 (0-60) | 0.06 |
| Diarrhea | 8.33 ± 8.3 (0-50) | 17.7 ± 9.1 (0-80) | 30.0 ± 12.3 (0-80) | 0.384 |
| Asthenia | 10.0 ± 6.8 (0-40) | 11.1 ± 7.5 (0-60) | 50.0 ± 16.9 (0-100) | 0.031 |
Moreover, severe abdominal pain (VAS > 50) was more frequent in Marsh I (33.4%) than in Marsh 0 (0%) and in Marsh III (16.7%) (P = 0.006), while distension and asthenia were more frequent in Marsh III (66.7%) than in Marsh 0 (0%) and Marsh I (11.2%) (P = 0.001 for both symptoms).
BMD was only assessed in those patients with abnormal biopsy. No significant differences in the percentage of osteopenia were found between Marsh I (55.6%) and III (33.4%) (P = 0.608). There were no patients with osteoporosis.
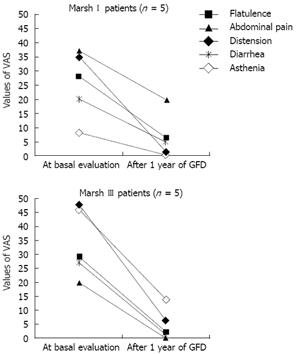
Eighteen of the 26 subjects with positive serology at baseline accepted follow-up, disclosing a 69.2% adherence to the mass-screening program, with a mean follow-up of 28 months (range, 20 to 33). Of the 15 patients with histopathological lesions compatible with GSE, 10 followed a GFD (66.7%, five Marsh I and five Marsh III). Table 3 shows a detailed description of the patients who adhered to a GFD. Overall, nine of 10 patients (90%) (five Marsh III and four Marsh I) had a complete histological and/or serological response to a GFD. A dramatic clinical improvement was observed in both Marsh I and Marsh III patients; response was complete for two of the 10 patients (one Marsh III and one Marsh I) and partial for five (three Marsh III and two Marsh I). The main reason for Marsh I patients’ adherence to GFD was the presence of osteopenia (four of five Marsh I patients). In contrast, osteopenia was only diagnosed in one Marsh I patient of the four who did not follow a GFD. No differences were found for either the number or the severity of symptoms between patients who followed a GFD and those who did not. At the end of follow-up, those patients who followed a GFD showed an improvement in the mean value of the VAS for all symptoms, and this was statistically significant for distension (P = 0.014), flatulence (P = 0.028) and abdominal pain (P = 0.007) (Table 4). In Figure 4, evolution of the mean values of the VAS are shown separately for Marsh I and Marsh III.
| At basal evaluation | After GFD | |||||||||
| Clinical features | EmA | tTGA (U/mL) | Biopsy | IELs | Clinical features | EmA | tTGA (U/mL) | Biopsy | IELs | |
| Case 1 female 47 years | Flatulence, distension, abdominal pain, diarrhea, asthenia, anemia, osteopenia | > 1/160 | 74 | Marsh IIIb | --- | Asthenia | Neg. | 3.3 | Marsh I | 35% |
| Case 2 female 24 years | Flatulence | > 1/160 | 159 | Marsh IIIb | --- | No symptoms | Neg. | 1.9 | --- | --- |
| Case 3 male 41 years | Flatulence, distension, abdominal pain, diarrhea, asthenia, hypertransaminasemia | > 1/160 | 63.4 | Marsh IIIc | --- | Asthenia, hypertransaminasemia | Neg. | 2.0 | Marsh I | 28% |
| Case 4 female 32 years | Osteopenia | 1/80 | 8.5 | Marsh IIIa | --- | Osteopenia | Neg. | 0 | --- | --- |
| Case 5 female 22 years | Distension, abdominal pain, diarrhea, asthenia, hypertransaminasemia, osteopenia | 1/10 | 4.34 | Marsh IIIb | --- | Distension, osteopenia | Neg. | 1.0 | Marsh I | 42% |
| Case 6 female 42 years | Flatulence, distension, osteopenia | Neg. | 2.0 | Marsh I | 40% | Osteopenia | Neg. | 2.4 | Marsh 0 | 22% |
| Case 7 male 41 years | Osteopenia | Neg. | 3.34 | Marsh I | 26% | No symptoms | Neg. | 1.24 | Marsh I | 26% |
| Case 8 female 38 years | Flatulence, distension, abdominal pain | Neg. | 4.04 | Marsh I | 42% | Flatulence | Neg. | 2.0 | Marsh 0 | 17% |
| Case 9 male 37 years | Distension, abdominal pain, diarrhea, osteopenia | Neg. | 3.20 | Marsh I | 26% | Abdominal pain, diarrhea, osteopenia | Neg. | 2.4 | Marsh I | 28% |
| Case 10 male 32 years | Flatulence, distension, abdominal pain, diarrhea, asthenia, osteopenia | Neg. | 2.34 | Marsh I | 35% | Flatulence, distension, abdominal pain, diarrhea, asthenia, osteopenia | Neg. | 1 | Marsh 0 | 14% |
| Basal (mean values of VAS) | After 1 year of GFD (mean values of VAS) | Pvalue | |
| Flatulence | 28 ± 8.9 | 5 ± 3.4 | 0.028 |
| Distension | 42 ± 10.9 | 7 ± 4.7 | 0.014 |
| Abdominal pain | 28 ± 9.0 | 7 ± 5.1 | 0.007 |
| Diarrhea | 24 ± 10.2 | 4 ± 2.6 | 0.063 |
| Asthenia | 28 ± 12.3 | 12 ± 6.6 | 0.133 |
Anemia and hypertransaminasemia reverted after GFD in those patients who had these conditions at basal evaluation. BMD normalized in two of the six patients with abnormal values at baseline. In the remaining cases, a trend for T score improvement in both the femoral neck (initial -0.74 ± 0.22, final -0.69 ± 0.18; P = 0.144) and lumbar spine (initial -1.27 ± 0.30, final -1.09 ± 0.30; P = 0.068) was observed.
Eight additional patients with initial positive serology, who decided to follow a gluten-containing diet (one Marsh III, three Marsh I and four Marsh 0), accepted a clinical, serological and/or histological follow-up (Table 5). A progression from Marsh 0 to Marsh I was observed in one case and from Marsh I to Marsh III in another. This progression was accompanied by worsening of symptoms and an increase in tTGA values in the Marsh III patient. In the remaining cases with the exception of one, tTGA values diminished below 2 U/mL in the follow-up, although clinical symptoms remained unchanged.
| At basal evaluation | Follow-up | |||||||||
| Clinical features | EmA | tTGA (U/mL) | Biopsy | IELs | Clinical features | EmA | tTGA (U/mL) | Biopsy | IELs | |
| Case 1 male 23 years | Flatulence, distension, abdominal pain, diarrhea, asthenia | 1/40 | 6.76 | Marsh IIIb | ---- | Flatulence, distension, abdominal pain, diarrhea, asthenia | Neg. | 1.70 | Not accepted | --- |
| Case 2 male 63 years | Osteopenia | Neg. | 4.07 | Marsh I | 29% | Osteopenia | Neg. | 2.2 | Marsh I | 25% |
| Case 3 male 24 years | Flatulence, distension, abdominal pain, diarrhea, asthenia | Neg. | 3.47 | Marsh I | 25% | Flatulence, distension, diarrhea, asthenia | Neg. | 1.0 | Not accepted | --- |
| Case 4 female 66 years | Flatulence, distension, abdominal pain | Neg. | 5.60 | Marsh I | 35% | Diarrhea, abdominal pain, distension, flatulence | Neg. | 15 | Marsh IIIa | --- |
| Case 5 male 45 years | Asthenia | Neg. | 2.68 | Marsh 0 | 5% | Asthenia | Neg. | 2.0 | Marsh I | 28% |
| Case 6 male 47 years | Flatulence | Neg. | 3.95 | Marsh 0 | 22% | Flatulence | Neg. | 1.0 | Marsh 0 | 5% |
| Case 7 male 36 years | Distension | Neg. | 2.78 | Marsh 0 | 20% | Distension, hypertransaminasemia | Neg. | 1.2 | Not accepted | --- |
| Case 8 male 36 years | Flatulence | Neg. | 2.80 | Marsh 0 | 18% | Flatulence | Neg. | 1.0 | Not accepted | --- |
The frequency of biopsy proven CD with atrophy found in this study (1:312) is within the range previously described in our geographical area[23]. All Marsh III cases in our study had positive tTGA and EmA, confirming that both serological tests have a similar high sensitivity for diagnosing CD with villous atrophy[24]. However, it is well known that the sensitivity of serology sharply decreases in mild forms of GSE. Although not universally accepted, the recognition of Marsh I patients is important since a significant proportion of these patients have severe symptoms[7] and could benefit from a GFD. The observation that less than 2% of individuals in the general population have tTGA values higher than 2 U/mL prompted us to establish this value as the normal cut-off limit, instead of the 8 U/mL recommended by the manufacturer. This fact allowed us to identify two additional Marsh III and eight Marsh I patients by using tTGA alone, and it increased the sensitivity of this serological marker with respect to EmA at the expense of a greater number of diagnosed patients with mild enteropathy (88.8% versus 11.1% sensitivity in Marsh I for tTGA and EmA, respectively). With the combined use of EmA and tTGA the prevalence of biopsy proven GSE increased to 1:125.
A recently published study performed in Iran[25], evaluating both Marsh I and Marsh III patients, found a similar number of GSE patients to that found in our study, with most Marsh I patients detected only by positive tTGA and not with EmA. These results demonstrated, as did ours, the increased sensitivity of tTGA in detecting mild enteropathy. Unfortunately, the clinical characteristics of these patients and response to a GFD were not assessed in the Akbari et al study, raising some doubts about the reliability of the diagnosis of GSE in LE patients[26]. In fact, other causes of LE should be ruled out before considering the possibility of GSE diagnosis[18].
Interestingly, despite the limitations of small sample size, the clinical features of Marsh I patients identified in the general working population duplicate those previously published in a group of first degree relatives of CD patients, confirming that Marsh I patients may be symptomatic similar to patients with atrophy[7]. Again, distension, abdominal pain and asthenia were the symptoms most consistently associated with GSE irrespective of the severity of the intestinal lesions, whereas non-significant differences were found for diarrhea and flatulence. In addition, as previously described, similar percentages of osteopenia were found in Marsh I and Marsh III patients, suggesting the existence of a similar degree of calcium and/or vitamin D-impaired absorption. This study also demonstrated that the Marsh I patients detected in this mass-screening program, with low positive levels of tTGA, were true GSE patients with a gluten-dependent lesion and with a similar response to a GFD as those with Marsh III.
It is noticeable that tTGA values ranging from 2.6 to 3.9 U/mL were detected in six Marsh 0 individuals who could be considered false positives. However, a frequency higher than expected of DQ2/DQ8 positivity in these individuals, as well as the progression from Marsh 0 to Marsh I in one case, suggests that these individuals might have latent CD and therefore merit follow-up.
The genetic characteristics of the 21 individuals with available duodenal histology merit an additional comment. Sixty-two per cent and 4.7% were DQ2 and DQ8 positive, respectively, and 28.6% (five Marsh I and one Marsh 0) had only one allele of the DQ2 (DQB*0201 in five cases and DQA1*0501 in one more case). Thus, the percentage of DQ2 positivity in the present study was lower than that described for CD patients, among whom more than 90% express both DQ2 alleles[27]. However, it has been reported that in the majority of DQ2-negative CD patients (approximately 5%), one of the DQ2 alleles is present, generally DQB*0201 and rarely DQA1*0501[28]. Consistent with this, the DQ2 negative patients in the present study, most of them Marsh I, expressed only one allele of the DQ2, predominantly DQB*0201. It may be speculated that the presence of only the β chain or α chain of the DQ2 heterodimer, encoded by DQB*0201 or DQA1*0501, respectively, could impede the progression from mild enteropathy to atrophy in these subjects. Taking together all these data, individuals with tTGA values ≥ 2 U/mL detected in this study have, with a very high probability, some form of the GSE spectrum of conditions (from Marsh 0 to Marsh III).
The present study also shows that GSE patients in the general population may not be identified by clinical features, since a similar percentage of related CD symptoms was found in individuals with positive and negative markers. This fact explains why CD remains underdiagnosed in a high proportion of affected subjects and is an additional argument for mass-screening using other approaches, such as serology, irrespective of clinical symptoms. Unfortunately, serology had limitations due to its low sensitivity in detecting individuals with mild GSE. In addition, fluctuations from time to time in tTGA values may allow the identification of CD patients at some time points but not in others. In fact, most of the tTGA values in patients on a gluten-containing diet were negative in the follow-up.
Almost 70% of subjects with positive serology adhered to the follow-up program, which included GFD compliance or simple clinical, histological and serological surveillance. The reported degree of GFD adherence has been shown to vary greatly in different studies ranging from less than 10% (4) to 90%[29], and is probably highly dependent on the patient-doctor relationship and confidence. In addition, the GFD adherence in this and other studies of CD detected by screening is similar to or better than that reported for other diseases, such as hypercholesterolemia or coronary heart disease[30], in which specific diets or changes in lifestyle are required to prevent life-threatening complications.
It has been argued that the lack of adherence to a GFD in patients identified in screening programs is due to an absence of symptoms in these cases. We have demonstrated in the present study that, when systematically assessed, signs or symptoms related to GSE may be identified in all cases, taking into account that osteopenia was the only clinical feature detected in 20% of patients. Thus, the low GFD adherence due to an absence of potential benefit perceived by the patient should never be used as an argument against the performance of mass screening for CD in the general population.
In conclusion, GSE in the general population is frequent and is clinically relevant, irrespective of the severity of the histological lesion. Mass screening programs are useful for identifying these patients in order to initiate either a GFD or close follow-up monitoring.
Screening for celiac disease (CD) in the general population is still a controversial issue. CD is a highly prevalent disease (1:100 to 1:300) that fulfils most of the criteria favoring mass screening. The benefits of early diagnosis in patients with mild disease, in terms of preventing late complications, are poorly understood.
In this study, the authors demonstrate that mass screening programs allow detection of CD cases with the whole gluten sensitive enteropathy (GSE) spectrum, which otherwise would have not been diagnosed, and that the adherence and response to a gluten-free diet (GFD) in these subjects was much better than that previously reported for other chronic diseases.
This study shows unpublished information on GSE detected in the general population. It was demonstrated that Marsh I subjects detected by antitransglutaminase (tTGA) in this setting are as symptomatic as Marsh III and that they also respond equally to a GFD, reinforcing the final diagnosis of GSE in mild enteropathy.
Understanding the evolution of GSE with mild enteropathy can help important decision-making, such as initiating a GFD in particular cases. Starting a GFD in symptomatic patients may prevent potential complications such as anemia and osteoporosis. The risk of lymphoma in mild GSE is at present unknown. A close follow-up of patients on a gluten-containing diet may help clarify this point.
GSE is characterized by a permanent intolerance to ingested gluten in susceptible individuals and leads to immunologically mediated inflammation of the small intestine mucosa. Common histopathology findings range from mild enteropathy, which consists of an increase in intraepithelial lymphocytes (> 25 IEL/100 IELs) (Marsh I lesion), to ‘classic’ celiac disease with crypt hyperplasia and partial (Marsh IIIa), subtotal (Marsh IIIb) or total (Marsh IIIc) atrophy.
In this interesting retrospective study the power of screening tests for gluten sensitive enteropathy are critically evaluated in a cohort of 1868 patients. The authors conclude from their data that evaluation of tTGA is more essential in diagnosis of gluten sensitive enteropathy than EmA and histology. In order to select patients for gluten free diet and follow-up mass screening programs are proposed as useful. Statistical analyses are well. The manuscript is well-written and illustrated. The topic is of high interest for a readership interested in gastrointestinal diseases.
| 1. | Mearin ML, Ivarsson A, Dickey W. Coeliac disease: is it time for mass screening? Best Pract Res Clin Gastroenterol. 2005;19:441-452. [Cited in This Article: ] |
| 2. | Wilson JM, Jugner G. Principles and practice of screening for disease. Geneva: World Health Organisation 1968; . [Cited in This Article: ] |
| 3. | Fabiani E, Taccari LM, Ratsch IM, Di Giuseppe S, Coppa GV, Catassi C. Compliance with gluten-free diet in adolescents with screening-detected celiac disease: a 5-year follow-up study. J Pediatr. 2000;136:841-843. [Cited in This Article: ] |
| 4. | Shamir R, Yehezkely-Schildkraut V, Hartman C, Eliakim R. Population screening for celiac disease: follow up of patients identified by positive serology. J Gastroenterol Hepatol. 2007;22:532-535. [Cited in This Article: ] |
| 5. | Shamir R, Hernell O, Leshno M. Cost-effectiveness analysis of screening for celiac disease in the adult population. Med Decis Making. 2006;26:282-293. [Cited in This Article: ] |
| 6. | Cranney A, Rostom A, Sy R, Dubé C, Saloogee N, Garritty C, Moher D, Sampson M, Zhang L, Yazdi F. Consequences of testing for celiac disease. Gastroenterology. 2005;128:S109-S120. [Cited in This Article: ] |
| 7. | Esteve M, Rosinach M, Fernández-Bañares F, Farré C, Salas A, Alsina M, Vilar P, Abad-Lacruz A, Forné M, Mariné M. Spectrum of gluten-sensitive enteropathy in first-degree relatives of patients with coeliac disease: clinical relevance of lymphocytic enteritis. Gut. 2006;55:1739-1745. [Cited in This Article: ] |
| 8. | Tursi A, Brandimarte G. The symptomatic and histologic response to a gluten-free diet in patients with borderline enteropathy. J Clin Gastroenterol. 2003;36:13-17. [Cited in This Article: ] |
| 9. | Rosinach M, Fernández-Bañares F, Esteve M, Alsina M, Farré C, Casalots J, Santaolalla R, Forné M, Espinós J, Salas A. Lymphocytic enteritis (lesion type Marsh I): Response to gluten free diet. Gastroenterol. 2006;130:A662. [Cited in This Article: ] |
| 10. | Kaukinen K, Peräaho M, Lindfors K, Partanen J, Woolley N, Pikkarainen P, Karvonen AL, Laasanen T, Sievänen H, Mäki M. Persistent small bowel mucosal villous atrophy without symptoms in coeliac disease. Aliment Pharmacol Ther. 2007;25:1237-1245. [Cited in This Article: ] |
| 11. | Jaeschke R, Singer J, Guyatt GH. A comparison of seven-point and visual analogue scales. Data from a randomized trial. Control Clin Trials. 1990;11:43-51. [Cited in This Article: ] |
| 12. | Chorzelski TP, Beutner EH, Sulej J, Tchorzewska H, Jablonska S, Kumar V, Kapuscinska A. IgA anti-endomysium antibody. A new immunological marker of dermatitis herpetiformis and coeliac disease. Br J Dermatol. 1984;111:395-402. [Cited in This Article: ] |
| 13. | Wong RC, Wilson RJ, Steele RH, Radford-Smith G, Adelstein S. A comparison of 13 guinea pig and human anti-tissue transglutaminase antibody ELISA kits. J Clin Pathol. 2002;55:488-494. [Cited in This Article: ] |
| 14. | Olerup O, Aldener A, Fogdell A. HLA-DQB1 and -DQA1 typing by PCR amplification with sequence-specific primers (PCR-SSP) in 2 hours. Tissue Antigens. 1993;41:119-134. [Cited in This Article: ] |
| 15. | Marsh MN. Gluten, major histocompatibility complex, and the small intestine. A molecular and immunobiologic approach to the spectrum of gluten sensitivity ('celiac sprue'). Gastroenterology. 1992;102:330-354. [Cited in This Article: ] |
| 16. | Rostami K, Kerckhaert JP6, Tiemessen R, Meijer JW, Mulder CJ. The relationship between anti-endomysium antibodies and villous atrophy in coeliac disease using both monkey and human substrate. Eur J Gastroenterol Hepatol. 1999;11:439-442. [Cited in This Article: ] |
| 17. | Hayat M, Cairns A, Dixon MF, O'Mahony S. Quantitation of intraepithelial lymphocytes in human duodenum: what is normal? J Clin Pathol. 2002;55:393-394. [Cited in This Article: ] |
| 18. | Brown I, Mino-Kenudson M, Deshpande V, Lauwers GY. Intraepithelial lymphocytosis in architecturally preserved proximal small intestinal mucosa: an increasing diagnostic problem with a wide differential diagnosis. Arch Pathol Lab Med. 2006;130:1020-1025. [Cited in This Article: ] |
| 19. | AGA Institute Medical Position Statement on the Diagnosis and Management of Celiac Disease. Gastroenterology. 2006;131:1977-1980. [Cited in This Article: ] |
| 20. | Assessment of fracture risk and its application to screening for postmenopausal osteoporosis. Report of a WHO Study Group. World Health Organ Tech Rep Ser. 1994;843:1-129. [Cited in This Article: ] |
| 21. | Schatzkin A, Connor RJ, Taylor PR, Bunnag B. Comparing new and old screening tests when a reference procedure cannot be performed on all screenees. Example of automated cytometry for early detection of cervical cancer. Am J Epidemiol. 1987;125:672-678. [Cited in This Article: ] |
| 22. | Freeman GH, Halton JH. Note on an exact treatment of contingency, goodness of fit and other problems of significance. Biometrika. 1951;38:141-149. [Cited in This Article: ] |
| 23. | Riestra S, Fernández E, Rodrigo L, Garcia S, Ocio G. Prevalence of Coeliac disease in the general population of northern Spain. Strategies of serologic screening. Scand J Gastroenterol. 2000;35:398-402. [Cited in This Article: ] |
| 24. | Lewis NR, Scott BB. Systematic review: the use of serology to exclude or diagnose coeliac disease (a comparison of the endomysial and tissue transglutaminase antibody tests). Aliment Pharmacol Ther. 2006;24:47-54. [Cited in This Article: ] |
| 25. | Akbari MR, Mohammadkhani A, Fakheri H, Javad Zahedi M, Shahbazkhani B, Nouraie M, Sotoudeh M, Shakeri R, Malekzadeh R. Screening of the adult population in Iran for coeliac disease: comparison of the tissue-transglutaminase antibody and anti-endomysial antibody tests. Eur J Gastroenterol Hepatol. 2006;18:1181-1186. [Cited in This Article: ] |
| 26. | Feighery C, Conlon N, Jackson J. Adult population screening for coeliac disease: comparison of tissue-transglutaminase antibody and anti-endomysial antibody tests. Eur J Gastroenterol Hepatol. 2006;18:1173-1175. [Cited in This Article: ] |
| 27. | Kagnoff MF. Celiac disease: pathogenesis of a model immunogenetic disease. J Clin Invest. 2007;117:41-49. [Cited in This Article: ] |
| 28. | Karell K, Louka AS, Moodie SJ, Ascher H, Clot F, Greco L, Ciclitira PJ, Sollid LM, Partanen J. HLA types in celiac disease patients not carrying the DQA1*05-DQB1*02 (DQ2) heterodimer: results from the European Genetics Cluster on Celiac Disease. Hum Immunol. 2003;64:469-477. [Cited in This Article: ] |
| 29. | Tommasini A, Not T, Kiren V, Baldas V, Santon D, Trevisiol C, Berti I, Neri E, Gerarduzzi T, Bruno I. Mass screening for coeliac disease using antihuman transglutaminase antibody assay. Arch Dis Child. 2004;89:512-515. [Cited in This Article: ] |
| 30. | Chiuve SE, McCullough ML, Sacks FM, Rimm EB. Healthy lifestyle factors in the primary prevention of coronary heart disease among men: benefits among users and nonusers of lipid-lowering and antihypertensive medications. Circulation. 2006;114:160-167. [Cited in This Article: ] |