Published online Apr 7, 2009. doi: 10.3748/wjg.15.1554
Revised: February 27, 2009
Accepted: March 6, 2009
Published online: April 7, 2009
A new, hypervirulent strain of Clostridium difficile, called NAP1/BI/027, has been implicated in C. difficile outbreaks associated with increased morbidity and mortality since the early 2000s. The epidemic strain is resistant to fluoroquinolones in vitro, which was infrequent prior to 2001. The name of this strain reflects its characteristics, demonstrated by different typing methods: pulsed-field gel electrophoresis (NAP1), restriction endonuclease analysis (BI) and polymerase chain reaction (027). In 2004 and 2005, the US Centers for Disease Control and Prevention (CDC) emphasized that the risk of C. difficile-associated diarrhea (CDAD) is increased, not only by the usual factors, including antibiotic exposure, but also gastrointestinal surgery/manipulation, prolonged length of stay in a healthcare setting, serious underlying illness, immune-compromising conditions, and aging. Patients on proton pump inhibitors (PPIs) have an elevated risk, as do peripartum women and heart transplant recipients. Before 2002, toxic megacolon in C. difficile-associated colitis (CDAC), was rare, but its incidence has increased dramatically. Up to two-thirds of hospitalized patients may be infected with C. difficile. Asymptomatic carriers admitted to healthcare facilities can transmit the organism to other susceptible patients, thereby becoming vectors. Fulminant colitis is reported more frequently during outbreaks of C. difficile infection in patients with inflammatory bowel disease (IBD). C. difficile infection with IBD carries a higher mortality than without underlying IBD. This article reviews the latest information on C. difficile infection, including presentation, vulnerable hosts and choice of antibiotics, alternative therapies, and probiotics and immunotherapy. We review contact precautions for patients with known or suspected C. difficile-associated disease. Healthcare institutions require accurate and rapid diagnosis for early detection of possible outbreaks, to initiate specific therapy and implement effective control measures. A comprehensive C. difficile infection control management rapid response team (RRT) is recommended for each health care facility. A communication network between RRTs is recommended, in coordination with each country’s department of health. Our aim is to convey a comprehensive source of information and to guide healthcare professionals in the difficult decisions that they face when caring for these oftentimes very ill patients.
-
Citation: Hookman P, Barkin JS.
Clostridium difficile associated infection, diarrhea and colitis. World J Gastroenterol 2009; 15(13): 1554-1580 - URL: https://www.wjgnet.com/1007-9327/full/v15/i13/1554.htm
- DOI: https://dx.doi.org/10.3748/wjg.15.1554
In our two previous reviews[12], we joined those who have written about the new more virulent strain of Clostridium difficile that was described in December 2005 in the National Institutes of Health (NIH)/Center for Disease Control and Prevention (CDC) Morbidity and Mortality Weekly Report. This CDC report emphasized that, in the past, C. difficile-associated diarrhea (CDAD) usually affected hospital inpatients, but now was appearing in relatively healthy adults, including some who had not even been exposed to a hospital setting.
Loo et al[3] and McDonald et al[4] have indicated that, not only is the rate of disease associated with
C. difficile increasing, but a previously uncommon strain of C. difficile has been identified. This strain of C. difficile, which has variations in its toxin genes, is more resistant to fluoroquinolones than prior strains. This newer and more virulent organism has emerged as a cause of geographically dispersed outbreaks of antibiotic-associated diarrhea (AAD), specifically C. difficile diseases, CDAD and C. difficile-associated colitis (CDAC).
CDAD has also become a more severe disease, and more often has progressed to toxic megacolon (TM). More severe CDAC and CDAD have started to increase in incidence and severity. C. difficile also accounts for an increasing percentage of community-acquired diarrhea cases. Fluoroquinolones, especially C-8-methoxy fluoroquinolones, such as moxifloxacin and gatifloxacin, have been incriminated in CDAD epidemics in different health care facilities. This current review attempts to provide an update on this new virulent organism that causes very severe CDAD and CDAC, and emphasizes the importance of early recognition of its complications and its treatment.
Typing of bacterial outbreaks characterize C. difficile as a Gram-positive, anaerobic, spore-forming bacillus that is spread indirectly via the fecal-oral route through spores left on surfaces. It produces two cytotoxins, which bind to receptors on intestinal epithelial cells, leading to inflammation and diarrhea. The toxins loosen the junctions of the epithelial cells that line the colon, allowing for penetration between epithelial cells[5]. This begins a cascade of tissue-damaging inflammatory processes that involve the release of destructive leukotrienes and cytokines.
Colonization of C. difficile is facilitated by the disruption of normal intestinal flora as a result of antimicrobial therapy. The antibiotics most frequently implicated in CDAD are clindamycin, penicillins, cephalosporins and fluoroquinolones[6].
There has been a dramatic increase in the frequency, severity and refractoriness of C. difficile as seen in multiple outbreaks, not only in North America, but around the world. These factors are attributed to this hypervirulent strain, NAP1/BI/027.
Bartlett documented that, over the first 5 years in which CDAD was acknowledged to exist, 1978 to 1983, the most common cause of CDAD was previous use of clindamycin[7]. The standard diagnostic test was a cytotoxin assay. Standard management was to withdraw the implicated antibiotic and treat with oral vancomycin. Most patients responded well, but 25% relapsed when vancomycin was withdrawn.
Over the next 20 years (1983-2003), the most commonly implicated antibiotics were the cephalosporins, which reflected their increased rates of use. Fluoroquinolones now are the major inducing agents, along with cephalosporins, a phenomenon which presumably reflects newly-acquired in vitro resistance and the escalating rates of use[8].
Between 2003 and 2006, C. difficile has become more frequent, more severe, more refractory to standard therapy, and more likely to relapse than in previous years. This pattern has been seen throughout the United States, Canada and Europe, and is now attributed to a new strain of C. difficile, alternatively designated as BI, NAP1, or ribotype 027 toxinotype III (all synonymous terms). Although this strain had been isolated as far back as 1984, it has recently emerged as a public concern with the development of fluouroquinolone resistance in our current era of widespread fluouroquinolone use.
The emergence of this hypervirulent C. difficile strain has vastly altered the face of the disease, with increased nosocomial outbreaks and concomitant morbidity. In 2007, Blossom and McDonald[9] reported on the increasing incidence and severity of C. difficile-associated disease attributable to this hypervirulent strain. This strain produces increased levels of toxins A and B, as well as an extra toxin, known as ‘binary toxin’, which accounts for its increased toxicity. This previously uncommon strain now has become epidemic, and has been reported in populations that previously had been thought to be at low risk, including peripartum women and healthy persons living in the community. Individuals with low or undetectable levels of antibody against C. difficile toxins are more likely to develop diarrhea than those with detectable antibody against the toxin. Careful adherence to infection control policies is critical to the control of C. difficile, especially at nursing facilities, long-term care and rehabilitation facilities and hospitals, as well as in the community. CDAD primarily occurs in hospitals, where exposure to antimicrobial drugs (the major risk factor for CDAD) and environmental contamination by C. difficile spores are more common[10].
Outbreaks of CDAD due to the new, highly-virulent strain of C. difficile have been recognized throughout European health care facilities, including 75 hospitals in England, 16 hospitals in the Netherlands, 13 healthcare facilities in Belgium, and nine healthcare facilities in France. In Germany, the first cases of the highly-virulent C. difficile strain, reported in 2007 and characterized as PCR ribotype 027, were associated with high mortality[11]. Larger outbreaks of C. difficile have been reported in northern France in particular[12]. These outbreaks are very difficult to control, and preliminary results from case-control studies indicate a correlation with the administration of fluoroquinolones and cephalosporins.
Seroprevalence increased in Denmark with increasing age in both 1990 and 1998. Unfortunately, the increase was about four times higher in 1998 than in 1990, which suggests a higher rate of exposure to C. difficile in the general Danish adult population[13].
In Dublin, Ireland, C. difficile is a major cause of infectious diarrhea in hospitalized patients[14]. Between August 2003 and January 2004, there was an appreciable increase in the incidence of C. difficile-associated disease, peaking at 21 cases per 1000 patient admissions. Of the C. difficile isolates recovered, 85 (95%) were identical toxin A-negative and toxin B-positive strains, corresponding to toxinotype VIII and PCR ribotype 017. All clonal isolates were resistant to multiple antibiotics, including ofloxacin, ciprofloxacin, levofloxacin, moxifloxacin and gatifloxacin [minimum inhibitory concentrations (MICs) > 32 &mgr;g/mL] and erythromycin, clarithromycin and clindamycin (MICs > 256 &mgr;g/mL). Recurrent C. difficile-associated disease occurred in 26 (36%) of the patients. At least 10 of these 26 patients (14%) developed C. difficile colitis. The authors found that careful attention to improving infection control interventions was the most important means of controlling this nosocomial pathogen.
Reported mortality rates from C. difficile-associated disease in the United States increased from 5.7 per million population in 1999 to 23.7 per million in 2004. These increased rates also may be caused by the emergence of a highly virulent strain of C. difficile. C. difficile infection, according to Schroeder[15], is now responsible for approximately 3 million cases of diarrhea and colitis annually in the United States, and has a mortality rate of 1%-2.5%. Zilberberg et al[16] have reviewed a sample of more than 36 million annual discharges from non-governmental US hospitals, and have concluded that 2.3% of the cases of C. difficile-related disease were fatal, amounting for roughly 5500 deaths. That was nearly double the percentage that resulted in death in 2000.
In Canada, Pépin et al[1718] have documented that, since 2002, an epidemic of CDAD caused by the same hypervirulent strain previously found in the United States, the United Kingdom and the Netherlands, has spread to as many as 30 hospitals in Quebec. More than half (55%) of the patients with CDAD at the investigators’ own hospital had received fluoroquinolones within the preceding 2 mo. Moreover, the excessive use of proton pump inhibitors might have facilitated this epidemic. This CDAD was associated with a very high case-fatality rate and with a 30-d mortality rate of 23.0% (37/161) compared with 7.0% (46/656) of matched control subjects (P < 0.001). Twelve months after diagnosis, mortality was 37.3% (60/161) among patients with CDAD vs 20.6% (135/656) among controls (P < 0.001), for a cumulative absolute attributable mortality of 16.7% [95% confidence interval (CI) 8.6%-25.2%]. Each case of nosocomial CDAD led, on average, to 10.7 additional hospital days. These investigators documented especially high attributable mortality among elderly patients with CDAD, mostly caused by this hypervirulent strain, which represents a dramatic change in the severity of this infection. Kuijper et al[19] have estimated that the financial impact of CDAD on the healthcare system is 5-15 000 Euros/case in England and $1.1 billion/year total expenditures in the USA. Assuming a European Union (EU) population of 457 million, the potential cost of CDAD in the EU can be estimated to be 3 billion Euros/year, and this is expected to almost double over the next four decades.
In Zimbabwe, C. difficile was isolated from 29.0% of 100 chicken feces samples and from 22.0% of 100 soil samples. Some of the C. difficile isolates from chickens (89.7%) and soil (95.5%) were toxigenic. All of the isolates were resistant to cefotaxime, gentamicin, ciprofloxacin, norfloxacin and nalidixic acid. The results of this study suggest that broiler chickens sold at marketplaces can be an important source of C. difficile, and may infect humans through consumption[20].
The incidence of CDAD in Singapore has remained relatively low, with isolates remaining susceptible to metronidazole and vancomycin[21].
The new, hypervirulent strain, NAP1/BI/027, has been implicated as the responsible pathogen in selected C. difficile outbreaks since the early 2000s. The epidemic strain is resistant to fluoroquinolones in vitro, a characteristic which was an infrequent observation in C. difficile strains prior to 2001. Five main characteristics of this strain contribute to the clinical and epidemiological observations. (1) The epidemic strain produces a binary toxin, an additional toxin that is not present in other C. difficile strains[2223]. (2) Binary toxin is related to the iota-toxin found in Clostridium perfringens, and its role in C. difficile pathogenesis is not fully understood[2425]. (3) The epidemic strain produces substantially larger quantities of toxins A and B in vitro than other C. difficile strains[26]. (4) Toxin production by an emerging strain of C. difficile has been associated with outbreaks of severe disease in North America and Europe[27]. The epidemic strain is toxinotype III; most other C. difficile strains are toxinotype 0[28]. Toxinotyping is based on analysis of the pathogenic locus (PaLoc) of the C. difficile genome, the region that includes the genes for toxin A (tcdA), toxin B (tcdB), and neighboring regulatory genes. (5) The epidemic strain has a partial deletion of tcdC, a gene in PaLoc that is responsible for down-regulation of toxin production[29].
C. difficile produces at least two distinct toxins[30]. These have been labeled toxin A and toxin B. Although initially thought to have distinctive actions, both now appear to be cytotoxic and enteropathic. Previous animal experiments have suggested that only toxin A mediates diarrhea and enterocolitis, even though C. difficile releases two structurally similar exotoxins. But when toxin A-negative/toxin B-positive strains of C. difficile are isolated from patients with AAD and colitis, this indicated that toxin B also may also be pathogenic in humans. C. difficile toxin B, like toxin A, has been found to be a potent inflammatory enterotoxin in the human intestine[31].
Both toxins disrupt the actin cytoskeleton of intestinal epithelial cells by uridine diphosphate-glucose dependent glycosylation of Rho and Ras proteins[32]. Stabler et al[33] have reported that toxin B from 027 strains may have a different binding capacity than their less-virulent counterparts and may, in addition to the mutated tcdC regulator, be responsible for the increased virulence of the 027 strains.
The most widely used laboratory assays for C. difficile infection involve toxin A and/or toxin B detection, and both are usually detected if diarrhea is present. Atypical toxin variant strains that may cause symptoms have also been described in Asia[34].
Kuijper et al[19] have claimed that C. difficile has more than 150 PCR ribotypes and 24 toxinotypes, and has a PaLoc with genes that encode for enterotoxin A (tcdA) and cytotoxin B (tcdB). Genes for the binary toxin are located outside the PaLoc. The recently completed genome sequence of C. difficile 630 has revealed a large proportion (11%) of mobile genetic elements, mainly in the form of conjugative transposons.
Drudy et al[35] have reported on several C. difficile outbreaks due to PCR ribotype 027 (PCR-027) associated with a mutation in gyrA that is associated with high-level resistance to fluoroquinolones. This strain type, which contains genes for the binary toxin, has an 18-bp deletion and a frameshift mutation in tcdC, which results in deregulated expression of toxins A and B. These strains can produce up to 16 times more toxin A and 23 times more toxin B in vitro than toxinotype 0 strains. The strain demonstrates universally high-level resistance to fluoroquinolones, in contrast to PCR 027 isolates that were collected before 2001. Mutations at the active site or the quinolone resistance determining region of DNA gyrase and topoisomerase IV have been associated with increased resistance to fluoroquinolones in several bacteria. In Escherichia coli, amino acid substitutions that occur at Ser-83 in gyrA have also been associated with fluoroquinolone resistance. Thus, the emergence of the hypervirulent NAP1/O27 C. difficile strain, also known as BI NAP1, has vastly altered the face of the disease, with increased nosocomial outbreaks and concomitant morbidity in countries worldwide.
In an epidemic of C. difficile-associated disease in the Canadian province of Quebec, Warny et al[26] documented that the dominant strain produced higher amounts of toxins A and B than those produced by non-epidemic strains. The epidemic strain was characterized as toxinotype III, North American PFGE type 1, and PCR-ribotype 027 (NAP1/027). This strain carried the binary toxin gene cdtB and an 18-bp deletion in tcdC. The authors isolated this strain from 72 patients with C. difficile-associated disease. Peak median (IQR) toxin A and toxin B concentrations produced in vitro by NAP1/027 were 16 and 23 times higher, respectively, than those measured in isolates representing 12 different PFGE types, known as toxinotype 0 [toxin A, median 848 &mgr;g/L (IQR 504-1022) vs 54 &mgr;g/L (23-203); toxin B, 180 &mgr;g/L (137-210) vs 8 &mgr;g/L (5-25); P < 0.0001 for both toxins]. Thus, the severity of C. difficile-associated disease caused by NAP1/027 appears to be the result of hyper-production of toxins A and B. The dissemination of this strain across North America and Europe has led to dangerous changes in the epidemiology of C. difficile-associated disease.
A nationwide epidemiological study conducted in Korea has revealed that tcdA(-)tcdB(+) C. difficile strains already have spread extensively throughout the country, The use of enzyme immunoassays capable of detecting TcdA and TcdB is strongly recommended for the diagnosis of CDAD in microbiology laboratories, in order to control the spread of the tcdA(-)tcdB(+) strains of C. difficile[36]. Sixty to 80% of C. difficile isolates in Korea have been reported to be toxigenic. Endoscopy, performed on 55/106 patients, revealed 29 with pseudomembranous colitis (PMC), five with colitis, 14 with other colon diseases, and seven normal colons. Among the 29 PMC cases, 21 (72.4%) were associated with tcdA-tcdB + strains (P = 0.0016). These results reveal the emergence of tcdA-tcdB+ C. difficile strains in Korea, and these variant strains could evoke a higher rate of PMC than tcdA + tcdB + strains[37].
C. difficile toxin A elicits intestinal fluid secretion and neutrophil infiltration by both mast cell-dependent and -independent pathways, and substance P participates in both pathways[38].
Extensive mitochondrial damage occurs within 15 min in cells exposed to toxin A. Diminished ATP concentrations and increased oxygen radicals contribute to cytotoxicity from this bacterial toxin[39].
The toxins damage the tight junctions of the intestinal epithelium. Tight junctions are crucial determinants of barrier function in intestinal epithelia, and are regulated by Rho guanosine triphosphatase. Rho kinase (ROCK) is a downstream effector of Rho. ROCK inhibition in calcium switch assays has shown that ROCK is necessary for the assembly of tight and adherens junctions. ROCK also is critical for assembly of apical junctional proteins and F-actin cytoskeleton organization during junctional formation[40].
C. difficile toxins A and B are glucosyltrans-ferases, which catalyze the inactivation of Rho proteins. C. difficile toxins act via translocation into target cells, and do their damage through autocatalytic processes by inactivating low-molecular-mass GTP-binding proteins of the Rho GTPase family involved in cellular signaling. This leads to cytotoxicity, including depolymerization of the target cell’s actin cytoskeleton. Thus, these toxins glycosylate members of the Rho GTPase family, and this GTPase inactivation leads to depolymerization of the cell’s actin cytoskeleton and, ultimately, cell death[41]. In addition, the C. difficile toxins further damage the intestine’s target cells by initiating massive cellular immune responses; i.e. neutrophilic infiltration with up-regulation and release of cytokines, such as interleukin (IL)-8, IL-6, IL-1β, leukotrienes B4 and interferon-γ.
Part of the mammalian immune response falls to the innate immune system called defensins and, specifically, human α-defensins produced by leukocytes, mucosal epithelial cells, and skin. Defensins, one of evolution’s major groups of antibiotic peptides, have broad-spectrum antibiotic activity against Gram-positive and Gram-negative bacteria, fungi, and viruses[42–44]. Defensins are characterized by a conserved 6-cysteine array. Each cysteine has intra-molecular disulfide bonds that are essential to protection against proteolysis[45]. Defensins also are known to contribute to wound healing, chemotaxis, and cytokine function[4647]. Defensins are part of two major groups of antimicrobial peptides: defensins and cathelicidins. These groups of human defensins consist in part of alpha, beta and omega defensins, human neutrophil protein (HNP)-1, HNP-3, cathelicidin LL-37 and enteric human defensin (HD)-5. These peptides play a role in the innate immune response, by deactivating various microbial pathogens, as well as specific bacterial exotoxins.
The antibiotic activity of both HNPs and HD5 is well documented in host defenses against enteric pathogens[4849]. HD5 and HD6 are produced and stored in Paneth cell secretory granules[50], along with a variety of additional Paneth cell products demonstrated to have antimicrobial and immune activity[51–55]. The impact of defensins on C. difficile disease has been described by Giesemann et al[56] and others[57–60].
Giesemann et al[56] have studied the effects of α-defensin HNP-1, HNP-3, and enteric HD-5 on the activity of C. difficile toxins A and B. They found that the treatment of cells with human α-defensins caused a loss of cytotoxicity of toxin B, but not of toxin A. In this study, only α-defensins, but not β-defensin-1 or cathelicidin LL-37, inhibited toxin B-catalyzed in vitro glucosylation of Rho GTPases in a competitive manner. This indicates that human α-defensins interact with high affinity for C. difficile toxin B. Defensins thereby provide a defense mechanism against clostridial glucosylating cytotoxins. At high concentrations, defensins (HNP-1 ≥ 2 &mgr;mol/L) also cause high-molecular-mass aggregates of C. difficile toxins, thus further decreasing their toxic effects on target cells.
C. difficile has been found in approximately 3% of normal adults and up to 40% of hospitalized patients[7]. However, as Salzman emphasizes: “only about one third of patients harboring C. difficile develop colitis, whereas the rest remain asymptomatic[61]”. Giesemann et al[56] have shown that α-defensins inhibit C. difficile toxin B, which offers insight into the possibility of different inflammatory responses in patients who develop CDAC versus others who do not. Salzman feels that “α-defensins show an additional antitoxin activity, in which HD5 is more effective; i.e. the stimulation of toxin aggregation.” Giesemann has shown that HD5, used at concentrations that normally can be found in the small intestine, is effective at causing aggregation of toxin B, thus effectively preventing the toxin’s ability to enter cells and interact with its target. These findings suggest an additional mechanism of antitoxin activity by α-defensin HD5.
This ability of HD5 to cause toxin B aggregation may provide an explanation for both the asymptomatic carriage of this pathogen and the frequency of patient relapse following antibiotic treatment, especially if the small intestine is a reservoir for C. difficile carriage in the gut. Salzman postulated that C. difficile is able to maintain colonization of the small intestine, but unable to cause colitis, because the high concentration of HD5 at this site neutralizes the secreted exotoxin.
In summary, Salzman feels that, in the small intestine, high concentrations of HD5 result in toxin B aggregation and therefore, the prevention of intoxication. While, in the large intestine, inadequate amounts of α-defensin are present to aggregate or inhibit toxin B, resulting in epithelial intoxication, inflammation, and neutrophilic infiltration.
Usually, C. difficile that transits through the large bowel will be prevented from finding a niche by the normal colonic microbiota. Yet, if the microbial ecology of the colon is disrupted, perhaps through antibiotic treatment, C. difficile can colonize the large intestine. Salzman postulates that, under these conditions, HD5 concentration is reduced by diffusion and dilution; thus, C. difficile exotoxins become free to interact with colonic enterocytes, resulting in intoxication, inflammatory responses, and infectious colitis.
Many patients are colonized with C. difficile, but have no symptoms. Perhaps C. difficile is harbored in the small intestine, where its toxic effects are well neutralized. Lawrence has claimed that about 20% of hospitalized adults are C. difficile carriers; and, in LTCFs, the carriage rate may approach 50%[62]. Although asymptomatic, these individuals shed pathogenic organisms and serve as a reservoir for environmental contamination. About 3% of healthy adults and 20%-40% of hospitalized patients are colonized with C. difficile, which in healthy persons is metabolically inactive in the spore form. Many patients have C. difficile as an asymptomatic organism in their intestine on hospital admission, and it only becomes a problem after they are treated with antibiotics, if, in fact, it ever induces symptoms. Exposure to antibiotics that disrupt the colonic microbial flora appears to be the most important risk factor for CDAD.
Asymptomatic colonized patients can act as a reservoir for the transmission of CDAD. Data, however, are limited regarding whether the treatment of these asymptomatic carriers leads to a decrease in the nosocomial transmission of C. difficile. Thirty asymptomatic C. difficile carriers were randomly assigned to one of three treatment groups: oral vancomycin 125 mg four times daily; metronidazole 500 mg orally twice daily; or placebo. Johnson et al[63] have found that nine of 10 patients receiving vancomycin became culture-negative during and immediately after treatment, compared to three of 10 receiving metronidazole and two of 10 receiving placebo. However, this decolonization was transient, as most patients became re-colonized within weeks. Thus, metronidazole does not appear to be effective for the treatment of asymptomatic carriers. In the setting of a hospital outbreak in which temporary elimination of the organism is felt necessary to reduce horizontal transmission, vancomycin may be a useful tool[63].
Riggs et al[64] have reported on molecular typing of C. difficile performed on asymptomatic carriers using pulsed-field gel electrophoresis. They found that 35 (51%) of 68 asymptomatic patients were carriers of toxigenic C. difficile, and 13 (37%) of these patients carried epidemic strains. They have also reported that 87% of isolates found in skin samples and 58% of isolates found in environmental samples were identical to concurrent isolates found in stool samples. Spores on the skin of asymptomatic patients were transferred easily to the investigators’ hands, again accounting for spread to persons in contact. This might be an explanation for the McFarland et al[65] observation that nosocomial CDAD frequently is transmitted between hospitalized patients, and that the organism often is present on the hands of hospital personnel caring for such patients. Kyne et al[66] have studied prospectively C. difficile infections in hospitalized patients who were receiving antibiotics, and identified no evidence of immune protection against repeat colonization by C. difficile. However, after colonization, there is an association between a systemic anamnestic response to toxin A, as demonstrated by increased serum levels of IgG antibody against toxin A, and asymptomatic carriage of C. difficile.
C. difficile infection causes diarrhea, often watery, rather than bloody, and it generally develops within 48-72 h of infection. In some, the symptoms may be delayed for 2-3 mo, usually after an antimicrobial agent has been administered. In some, only a single antibiotic tablet may lead to severe disease. Over time, the clinical spectrum has become better appreciated, with illness severity noted to be broad-ranging, from an asymptomatic carrier state (without detectable toxin) to severe and life-threatening pseudomembranous colitis with toxic megacolon[67].
The clinician must be ever on the alert to make an early diagnosis of C. difficile-related disease in the setting of new-onset loose stools or symptoms of abdominal distension and/or or leukocytosis, since unexplained leukocytosis in hospitalized patients, even in the absence of diarrhea, may reflect underlying C. difficile infection[68]. In a prospective study, Bulusu et al[69] found that, of 60 patients with unexplained leukocytosis (with a white blood cell count > 15 000/&mgr;L), a positive stool C. difficile toxin was observed more frequently in cases than in controls (58% versus 12%, respectively). Age over 75 years and immunosuppression were associated with a poor outcome. Earlier surgical consultation is warranted in severe cases to consider potentially life-saving colectomy, as well as alterations in the hospital-based standard of care for prevention.
Usually, the disease affects the colon and, in many cases, is made evident by the presence of colonic pseudomembranes. However, in patients with underlying Crohn’s or ulcerative colitis, pseudomembranous changes may not occur; therefore, typicial endoscopic findings of C. difficile may not be present, and the colonic mucosa will reflect only the underlying inflammatory bowel disease.
C. difficile infection may present with an acute abdomen but either absent or mild diarrhea, as described by Triadafilopoulos and Hallstone[70] in 1991. Plain abdominal radiographs revealed megacolon in these patients. This was combined with small and large bowel dilation in one who exhibited a volvulus-like pattern, and isolated small-bowel ileus in another. Diagnosis was revealed by emergency colonoscopy. All patients had positive results for C. difficile, and two tested positive for cytotoxicity. All were treated with IV metronidazole, resulting in the resolution of all symptoms and abdominal findings.
An unusual manifestation of CDAC was described in 1981 by Dansinger et al[71]. They reported that up to half of patients with indolent C. difficile infection develop manifestations of protein-losing enteropathy, including ascites, peripheral edema, and hypoalbuminemia. Inflammation of the bowel may allow leakage of albumin into the lumen, causing colonic loss of albumin with inadequate compensatory hepatic synthesis. As a result, serum albumin levels may drop below 20 g/L (20 g/L)[7172]. Older patients may present with pedal edema, and be mistakenly diagnosed with CHF.
Rubin et al[73] studied patients who had developed a more aggressive form of CDAD versus those who developed milder disease. They found that 21 of 710 patients (3%) either required intensive care unit (ICU) admission or died as a result of their infection. The factors predisposing to the development of severe C. difficile colitis included concurrent malignancy, chronic obstructive pulmonary disease, immunosuppressive or anti-peristaltic medications, renal failure, and the administration of clindamycin (P < 0.05 for all). Patients with severe C. difficile colitis were more likely to have abdominal pain, tenderness and distention, peritonitis, hemoconcentration (> 5 points), hypoalbuminemia (< 30 mg/L), and an elevated (> 25 000) or suppressed (< 1500) white blood cell count (P < 0.05 for all). Therefore, we must initiate aggressive diagnostic and therapeutic modalities in this patient group.
Extra-colonic features may occur in CDAD patients[74]. These include small bowel involvement in those patients with previous small bowel surgery, and visceral abscesses, primarily in the spleen, and less commonly in the pancreas. Other features include a reactive polyarticular arthritis, cellulitis, necrotizing fasciitis, osteomyelitis, and prosthetic device infections. Arthritis after C. difficile was further characterized by Birnbaum as being an asymmetric oligoarthritis[74]. C. difficile colitis has also been reported associated with intra abdominal hypertension and abdominal compartment syndrome[75].
In 2004 and 2005, the CDC emphasized that the risk of CDAD is increased in certain susceptible populations (Table 1).
| Patients who take the following drugs |
| Antibiotics |
| Proton pump inhibitors |
| Valacyclovir |
| Patient characteristics |
| IBD |
| Serous underlying illness-comorbidities |
| Gastrointestinal surgery/manipulations |
| Advanced age |
| Immune-compromising conditions (post transplantation) |
| Peri-partum |
| Environment |
| Prolonged stay in health-care settings |
| Laboratory factors |
| Hypoalbuminemia |
| Low levels of anti-toxin and B antibodies |
Although the antibiotics most frequently implicated in predisposition to C. difficile infection are fluoroquinolones, clindamycin, cephalosporins and penicillins, virtually all antibiotics, including metronidazole and vancomycin, can predispose to C. difficile. De Andrés et al[76] reported a case of C. difficile colitis associated with valacyclovir treatment.
The risk of CDAD in hospitalized patients receiving antibiotics may be compounded by co-existing disorders that require treatment with PPI therapy, which inhibits one’s defenses against ingested bacteria by virtually eliminating gastric acid[77]. Dial et al[78] estimated an adjusted risk ratio for C. difficile-associated disease with the current use of PPIs as 2.9 (95% CI: 2.4-3.4); and with H2-receptor antagonists, the rate ratio was 2.0 (95% CI: 1.6-2.7). The authors also uncovered an elevated rate of CDAD in patients on non-steroidal anti-inflammatory drugs (rate ratio, 1.3; 95% CI: 1.2-1.5). Thus, the consumption of drugs other than antibiotics may put one at increased risk for community-acquired C. difficile.
PPI therapy is also associated with an increased risk of recurrent C. difficile colitis. Patients receiving PPIs have been found to be 4.17 times as likely to have recurrence as their counterparts not receiving them[79]. This relationship between PPI therapy and C. difficile was elucidated by Jump who found that the survival of vegetative C. difficile in gastric contents obtained from patients receiving PPIs was also increased at a pH of > 5[80].
The incidence of severe CDAD is increasing in peripartum women. A PubMed search identified 24 recorded cases of peripartum C. difficile infection. Most patients (91%) had received prophylactic antibiotics during delivery or for treatment of bacterial infections. Two cases without known risk factors were found, by polymerase chain reaction analysis, to be infected with an epidemic and hypervirulent C. difficile strain. These cases demonstrate the need for clinicians to consider C. difficile infection in pregnant and peripartum patients with diarrhea, even if they do not have the traditional risk factors for C. difficile infection, such as antibiotic use or concurrent hospitalizations[81].
The Agency for Healthcare Research and Quality (AHRQ) is the lead US Federal agency charged with improving the quality, safety, efficiency, and effectiveness of health care. AHRQ data make clear that one of the challenges in accurately diagnosing CDAD is that it is not unusual for patients who acquire C. difficile to have multiple co-morbidities. Thus, multiple co-morbidities put patients at risk for C. difficile infection. AHRQ found that hospitalized patients with CDAD had over 10 diagnoses, versus six diagnoses among patients without CDAD[82]. According to recent AHRQ data, four out of the top 20 most common principle diagnoses observed with CDAD are infections (sepsis, pneumonia, urinary tract infection, and skin infection), where antibiotic use would be difficult to avoid[82].
Sixteen patients, representing an incidence rate of 0.16%, developed a C. difficile infection after total joint arthroplasty (TJA) at one institution. Those at risk for developing CDAD after TJA were patients with deteriorated physical status and those who had received more than one antibiotic postoperatively[83].
In addition, C. difficile is now considered to be a significant cause of diarrhea in heart transplant recipients, and the post-transplantation period is now considered one of greater risk[84]. With C. difficile infection, CDAC prior to 2000 was a rare complication in this patient group; but 38 of the 43 reported cases of CDAC in these patients occurred after 2000. Therefore, C. difficile is now also one of the most common causes of diarrhea in patients who have undergone solid organ transplantation[85]. Another group of patients at increased risk are post orthotopic liver transplant patients. Testing for C. difficile toxins among orthotopic liver transplant patients with nosocomial diarrhea revealed that 63% of samples are toxin-positive[86].
The development of life-threatening toxic megacolon secondary to CDAC now must be considered in solid organ recipients. Toxic megacolon was reported in five patients by Stelzmueller et al[85].
The risk of C. difficile infection was 14.9 cases per 1000 surgical procedures among patients who received preoperative prophylaxis (PAP) during the period 2003-2005, which is a significant increase compared with 0.7 cases per 1000 surgical procedures during the period 1999-2002 (P < 0.001). Independent risk factors associated with C. difficile infection in patients given PAP alone, were older age, the administration of cefoxitin (rather than cefazolin) alone or in combination with another drug, and the year of surgery. Thus, in the context of a large epidemic of C. difficile infection associated with the emergence of a novel strain of organism, 1.5% of patients who had received PAP as their sole antibiotic treatment developed C. difficile infection. In situations in which the only purpose of PAP is to prevent infrequent and relatively benign infections, the risks of PAP may outweigh its benefits, especially in elderly patients[87].
Unfortunately, the incidence of C. difficile infection is increasing in US surgical patients even without PAP, and infection with C. difficile is most prevalent after emergency operations and among patients who have undergone intestinal tract resections[88].
IBD patients are at greater risk than the general population for acquiring C. difficile infection[89]. Issa et al[90] performed a retrospective, observational study in IBD patients to evaluate the impact of C. difficile. They found that the rate of C. difficile infection had increased from 1.8% of IBD patients in 2004 to 4.6% in 2005 (P < 0.01). The proportion of IBD patients within the total number of C. difficile infections at their institution increased from 7% in 2004 to 16% in 2005 (P < 0.01). In 2005, IBD colonic involvement was found in the vast majority (91%) of C. difficile-infected patients, a clear majority (76%) had contracted infection as an outpatient, and antibiotic exposure was identified in 61% of IBD patients with C. difficile infection. Over the period 2004-2005, more than half of the infected IBD patients required hospitalization, and 20% required colectomy. Univariate and multivariate analyses identified maintenance immunomodulator use and colonic involvement as independent risk factors for C. difficile infection in IBD. The authors also reported a nationwide doubling in the rate of C. difficile infection among hospitalized UC patients between 1998 and 2004. The pathologic/endoscopic features of pseudomembranous colitis CDAC varies as a spectrum, with some patients exhibiting only mild inflammatory changes confined to the superficial epithelium, and typical pseudomembranes and crypt abscesses may not be present. The more severe cases demonstrate marked mucin secretion, and more intense inflammation. Intense necrosis of the full thickness of the mucosa, with a confluent pseudo-membrane, can become more prominent as disease severity increases.
The association between IBD and C. difficile may be due to a variety of factors, including antibiotic use for treatment of other gastrointestinal pathogens and frequent hospitalizations for the management of IBD flares. Many of these patients are taking immunosuppressive medications that may confer additional risk of C. difficile infection. C. difficile, and specifically its toxins, have been implicated as a risk factor for the exacerbation of the inflammatory process in up to 5% of patients with ulcerative colitis or Crohn’s disease. A severe clinical course may result from C. difficile infection superimposed on IBD, including the precipitation of toxic colitis and toxic megacolon.
CDAC in patients with IBD carries a higher mortality than in patients with C. difficile without underlying IBD. On multivariate analysis, patients in the C. difficile-IBD group had a four times greater mortality than patients admitted to hospital for IBD alone (AOR = 4.7, 95% CI: 2.9 to 7.9) or C. difficile alone (AOR = 2.2, 95% CI: 1.4 to 3.4), and stayed in the hospital for 3 d longer (95% CI: 2.3 to 3.7 d). Significantly higher mortality, endoscopy and surgery rates were found in patients with ulcerative colitis compared with Crohn’s disease (P < 0.05) who had associated C. difficile[91]. The median times from admission to a positive C. difficile test result for non-IBD was much longer than in Crohn’s disease and ulcerative colitis patients (4.0, 0.8, and 0.5 d, respectively). C. difficile infections in IBD are confirmed predominantly within 48 h of admission, suggesting most were acquired before hospitalization. CDAD rates approximately doubled in Crohn’s disease (9.5 to 22.3/1000 admissions) and tripled in ulcerative colitis (18.4 to 57.6/1000). Length of stay was similar among the groups. For all years combined, the adjusted odds ratios for CDAD in all IBD, Crohn’s disease, and ulcerative colitis admissions were 2.9 (95% CI: 2.1-4.1), 2.1 (1.3-3.4), and 4.0 (2.4-6.6), respectively[92].
Patients with severe C. difficile infection, especially IBD patients, require prompt diagnosis and management, since failure to diagnose the infection can lead to inappropriate treatment with glucocorticoids or immunosuppressive therapy. Furthermore, C. difficile may be difficult to distinguish from an IBD relapse, given the similar symptoms of diarrhea, abdominal pain, and low-grade fever. Thus, a high index of suspicion is required when evaluating IBD patients with apparent flares, especially those who recently have received antibiotics and/or have been hospitalized.
Thus, speedy diagnosis largely requires the use of laboratory tools, since endoscopy may not be helpful early, because IBD patients may not develop pseudomembranes. Given the underlying colonic pathology, patients with IBD who develop C. difficile colitis frequently require colectomy (20 percent in one series)[90].
Delays in both diagnosing and treating both initial and recurrent CDAD[93] are due to the fact that CDAD can mimic the more common ‘benign’ antibiotic-associated diarrhea that is not caused by C. difficile[94]. Thus, the diarrhea from C. difficile will be ascribed to other causes; e.g. food poisoning, viral infection, or other causes. Klebsiella pneumoniae, Candida species and Staphylococcus aureus have been identified as potential causative organisms in C. difficile negative AAD patients[95].
Patients can be infected with this microorganism and may have no symptoms of colitis. They, therefore, may not be tested for C. difficile infection (see section on presentation). These asymptomatic carriers, who are admitted to healthcare facilities and hospitals, become vectors during outbreaks and can transmit the organism to other susceptible patients. Most cases of CDAD occur at 4-9 d after discontinuation of antibiotic therapy, according to Schroeder[15]; however, CDAD can occur up to 8 wk after the discontinuation of antibiotics.
Lower endoscopy is a useful tool for the diagnosis of C. difficile. This is especially when: (1) there is a high level of clinical suspicion for C. difficile, despite a negative laboratory assay; (2) prompt C. difficile diagnosis is needed before laboratory results can be obtained; (3) C. difficile infection fails to respond to antibiotic therapy; or (4) when there is an atypical disease presentation, and C. difficile is suspected, as with ileus, acute abdomen, leukocytosis or diarrhea.
Endoscopy is not indicated in patients with classic clinical findings and a positive stool toxin assay. Conversely, endoscopy may be contra-indicated, especially in the setting of fulminant colitis, due to the risk of perforation.
Endoscopic findings: Pseudomembranes are pathognomonic for CDAC, but are not found in all areas of the colon, even in severe cases; thus, findings may be patchy. Pseudomembranes may be absent in the rectosigmoid area, but may be visualized more proximally with colonoscopy. This is true in patients with co-existing IBD. Pseudomembranes are raised yellow or off-white plaques, up to 2 cm in diameter, which are randomly scattered over the colorectal mucosa with normal intervening mucosa, and that cannot be removed by lavage. The pseudomembranes form when C. difficile toxin-induced cytoskeleton disruption causes shallow ulcerations on the intestinal mucosal surface. It is postulated that ulcer formation allows for the release of serum proteins, mucus, and inflammatory cells, which appear grossly on the colorectal mucosal surface as pseudomembranes. Light and scanning electron microscopy after exposure to either of the C. difficile toxins reveal patchy damage and exfoliation of superficial epithelial cells, while crypt epithelium remains intact. Fluorescent microscopy of phalloidin-stained sections shows that both toxins cause the disruption and condensation of cellular F-actin[96].
Other colonic mucosal findings include bowel-wall edema, erythema, friability, and inflammation, with or without pseudomembranes. This manifests on the abdominal CT scan as thickening of the colonic wall.
Colonoscopic findings among 16 patients with histologically-proven antibiotic-associated PMC or CDAC were described by Seppälä et al[97]. Pseudomembranes were found in only five of 16 (31%) patients by sigmoidoscopy, but were found in 11 of 13 patients (85%) in whom colonoscopy also was performed. These findings suggest the importance of colonoscopy in the early diagnosis of CDAC, because the typical endoscopic changes of pseudomembranes are limited to the colon above the rectosigmoid area in most patients. Consequently, colonoscopy should be performed, instead of sigmoidoscopy, at least in clinically suspected CDAC cases[98].
C. difficile colitis is usually associated with a mild/moderate course, but may progress to fulminant colitis. Fulminant colitis develops in 3%-8% of patients. The manifestations of fulminant colitis typically include severe lower quadrant or diffuse abdominal pain, diarrhea, abdominal distention, fever, hypovolemia, lactic acidosis, and marked leukocytosis (up to 40 000 white blood cells/microL or higher). Diarrhea may be less prominent in patients with prolonged ileus, due to pooling of secretions in the dilated, atonic colon. Other potential complications of fulminant colitis include toxic megacolon and bowel perforation[73].
Toxic megacolon is a clinical diagnosis based upon the finding of colonic dilatation (> 7 cm in its greatest diameter) accompanied by severe systemic toxicity. Abdominal plain films also may demonstrate small-bowel dilatation, air-fluid levels (mimicking an intestinal obstruction or ischemia), and ‘thumb printing’ (scalloping of the bowel wall) due to submucosal edema. Toxic megacolon may be complicated by bowel perforation.
This latter complication presents with abdominal rigidity, involuntary guarding, diminished bowel sounds, rebound tenderness, and severe localized tenderness in the left or right lower quadrants. Abdominal radiographs may demonstrate free intra-abdominal air. Thus, patients with toxic megacolon must be followed with daily upright abdominal X-rays to ascertain if perforation has occurred. Patients with toxic megacolon should be evaluated for surgical resection. Once fulminant colitis is diagnosed, subtotal colectomy with ileostomy usually is required. In these patients who develop a marked leukocytosis or bandemia, surgery is advisable, because the leukocytosis frequently precedes hypotension. The requirement for vasopressor therapy carries a poor prognosis, according to Shen et al[99].
Lamontagne et al[100] has documented that emergency colectomy reduces mortality in patients with fulminant CDAD. The independent predictors of 30-d mortality in their study were leukocytosis ≥ 50 × 109/L (AOR, 18.6; 95% CI: 3.7-94.7); serum lactate ≥ 5 mmol/L (AOR, 12.4; 95% CI: 2.4-63.7); age ≥ 75 years (AOR, 6.5; 95% CI: 1.7-24.3); immunosuppression (AOR, 7.9; 95% CI: 2.3-27.2); and shock requiring vasopressor therapy (AOR, 3.4; 95% CI: 1.3-8.7). After adjusting for these confounders, patients who had an emergency colectomy were less likely to die than those treated medically. Colectomy also seemed more beneficial in patients 65 years or older; in those who were immune-competent; and those with leukocytosis ≥ 20 × 109/L or a serum lactate level between 2.2 and 4.9 mmol/L.
Small-bowel involvement with C. difficile enteritis is unusual[101]. Potential risk factors for small-bowel involvement with C. difficile enteritis include prior gastrointestinal surgery (including colonic resection) and advanced age[102]. Such patients may present with ileitis and high ileostomy output and may be at increased risk for fulminant disease.
Small-bowel involvement with C. difficile infection enteritis has been described increasingly since 2000. Usually, this occurs in patients with a history of a prior colectomy or total procto-colectomy for severe and extensive IBD. The ileal mucosa appears to be at increased risk for inflammatory disease in the specific subset of patients who have undergone a prior colectomy[67]. Serious post-colectomy concerns, like severe ileostomy dysfunction with high ileostomy volumes and marked diarrhea, have been known to occur after pan-procto-colectomy and restorative ileo-anal pouch formation. They are almost always due to a non-C. difficile enteritis. This non-CDAD post colectomy enteritis can be life threatening; fortunately it is steroid/ immunosuppressive responsive, according to Gooding et al[103]. This picture can be mimicked by C. difficile infection.
Lundeen et al[104] reported that high ileostomy volumes may result from C. difficile enteritis in patients who have undergone colectomy for ulcerative colitis. All of the ileostomy output was positive for C. difficile toxins. These patients responded to metronidazole and/or vancomycin, in contrast to subjects with the former, non-CDAD entity.
Refractory or treatment-resistant pouchitis also may occur with C. difficile infection[105]. C. difficile infection involving ileal pouch-anal anastomosis is common, and occurs with or without the previous receipt of antibiotics[99]. Diagnosing recurrent C. difficile infection can be difficult in this group of patients, especially in the 20% without diarrhea.
All health care facilities must develop rapid communica-tion between the laboratory and the treating physician. At the Mayo Medical School, the time between electronic medical record reporting of a positive result for a test for C. difficile toxin in stool and the ordering of antimicrobial therapy was compared during consecutive periods when results were not telephoned (n = 274) and when results were telephoned (n = 90) to the clinical service[106]. The mean times to the ordering of antimicrobial therapy were 11.9 and 3.6 h, respectively (P < 0.001). The clinical implications of this 8-h delay may be important, especially in patients with severe disease. Early recognition of CDAD caused by NAP1/027, followed by the initiation of rapid treatment, can help to prevent complications and further spread of the bacterium[107].
Current laboratory testing lacks a single assay that is sensitive, specific, and rapid. Peterson et al[108] used clinical criteria that required at least three loose stools in one day, as part of the reference standard for a positive test result supporting CDAD (Table 2). They found that real-time PCR and anaerobic culture assays were significantly more sensitive than the enzyme immunoassay (P < 0.01 to P < 0.05). Real-time PCR has an assay turnaround time of < 4 h, and is both more sensitive than, and as rapid as enzyme immunoassay. They feel that it is a feasible laboratory option to replace enzyme immunoassay for toxigenic C. difficile detection in clinical practice, as well as for use during the development of new therapeutic agents.
| Test | Sensitivity (%) | Specificity (%) | PPV | NPV |
| Enzyme immunoassay | 73 | 98 | 73 | 98 |
| Real-time PCR | 93 | 97 | 76 | 99 |
| Cell culture assay | 77 | 97 | 70 | 98 |
| Anaerobic culture for toxigenic C. difficile strains | 100 | 96 | 68 | 100 |
Tests for the presence of C. difficile and its toxins are imperfect, and false positives and false negatives are not uncommon. McFarland[30] found that false-negative results occur in 29%-56% of cases. False-negative results may occur when specimens are not promptly tested or not kept refrigerated until testing is performed. Also, there is a relatively high false-negative rate, due to the fact that 100-1000 pg of toxin must be present for an EIA test to be positive. Utilizing up to three serial EIA tests may increase the diagnostic yield by as much as 10 percent, if the initial test is negative. If CDAD is suspected, despite negative initial testing, submission of multiple specimens and verifying that the laboratory is testing for both the A and B toxins is mandatory (Table 3).
| Enzyme immunoassay for toxins A & B - 80% sensitive |
| Use 3 samples |
| Cytotoxicity assay-more sensitive and specific, but takes 24-48 h |
Enzyme immunoassays are labor-intensive tests, requiring several hours of technician time and an assay reader. The batching of specimens increases cost efficiency, but may delay the reporting of results, especially if tests are not done every day. Rapid enzyme immunoassay is more costly for each test performed but, for laboratories that process only occasional samples, it appears to provide prompt, reliable, and cost-effective results.
Enzyme immunoassay rapid cards have been evaluated, in terms of their ability to detect C. difficile toxins A and B. For one such card, the EIAPrem, the positive predictive value (PPV) was 75/85 samples (88.2%; CI: 79% to 94%) and the negative predictive value (NPV) was 360/361 samples (99.7%; CI: 98% to 99%). For a review of all card performances, see references[109–112].
Killgore et al[113] compared the results of analyses done with seven C. difficile typing techniques: multi-locus variable-number tandem-repeat analysis (MLVA); amplified fragment length polymorphism; surface layer protein A gene sequence typing; PCR-ribotyping; restriction endonuclease analysis (REA); multi-locus sequence typing; and pulsed-field gel electrophoresis (PFGE). All techniques appeared to be capable of detecting outbreak strains; but only REA and MLVA exhibited sufficient discrimination to distinguish strains from different outbreaks.
Comparison of four enzyme immunoassays (Bartels Prima System C. difficile Toxin A EIA, Cambridge Biotech Cytoclone A+B EIA, Meridian Diagnostics Premier C. difficile Toxin A EIA, and TechLab C. difficile Tox-A Test EIA) found that, although enzyme immunoassays were less sensitive than cytotoxin assay, they provide same-day results and may be useful in laboratories without tissue culture facilities[114].
ELISA Toxin A+B is a reliable method with 100% specificity and sensitivity in the rapid diagnosis of
C. difficile. Its results can be utilized until culture results are obtained. The specificity of the Toxin A latex test is 100%; however, its use alone as a primary rapid diagnostic test is not recommended, because of its low (30.7%) sensitivity. This was shown when all of the culture positive samples underwent testing by ELISA Toxin A+B method and were found to be 100% positive, but only four of these positive culture samples (30.7%) yielded positive results with the Toxin A latex test[115].
Overall, the new-generation assays still are less sensitive than the cytotoxin assay; however, their advantages are that they provide same-day results; they can be used as a screening test; and they may be useful in laboratories without tissue-culture facilities. Results from a study by Vanpoucke et al[111] could not recommend one single assay over the other for the diagnosis of CDAD.
Therefore, the cytoxin assay test (CYTA) is highly sensitive and specific, but it is difficult to perform, and results are not available for 24-48 h[15]. What further complicates efforts to determine if toxin was present on admission is that C. difficile toxin is very unstable. The toxin degrades at room temperature and may be undetectable within 2 h after collection of a stool specimen. Given the cost and complexity of culture and cytotoxicity assays, most laboratories rely on tests for toxin A detection only. Moreover, enzyme immunoassays generally are available at lower cost and provide more rapid results, usually within 4 h. Their sensitivity generally ranges from 60% to 90%, and specificity from 75% to 100%. Testing of a single diarrheal stool generally is sufficient to make the diagnosis of CDAD; but unfortunately, doing so misses a substantial proportion of cases. Therefore, testing only should be performed on three loose stool specimens.
The cytotoxin assay test, though the ‘gold standard’ for assaying C. difficile toxins A and B, is labor-intensive, requires tissue-cultured cells and an inverted microscope, and needs overnight incubation before results can be read.
Recent experience has not altered the principles of management for the individual patient, but it does serve to emphasize the need for: (1) recognition of clinical characteristics that indicate severe CDAD (Table 4); (2) early recognition of C. difficile; (3) improved methods to manage severe relapsing disease; and (4) greater attention to infection control and antibiotic restraint. Previously published C. difficile infection management is available: [Fekety “Guidelines for the diagnosis and management of Clostridium difficile-associated diarrhea and colitis”Am J Gastroenterol, 1997; 92(5): 739-750] and the CDC’s own guidelines found at http://www.cdc.gov/ncidod/dhqp/id_CdiffFAQ_HCP.html and at http://www.cdc.gov/ncidod/dhqp/pdf/isolation2007.pdf.
| Patient characteristics |
| Older patients (> 65 yr) |
| Presence of comorbid conditions |
| Immune compromising conditions |
| Systemic immune response syndrome |
| Organ failure |
| Renal |
| Respiratory |
| Hypotension |
| Laboratory markers |
| Marked leukocytosis > 15 000 |
| Renal failure Cr > 2.3 mg/L |
| Hypoalbuminemia |
| Extent of disease |
| Pancolitis by imaging modalities |
| Complications |
| Ileus |
| Toxic megacolon |
| Intestinal perforation |
The efficacy of metronidazole or vancomycin prophylaxis to prevent C. difficile infection in patients who are receiving other antimicrobials is unproven, and treatment with these agents is ineffective against C. difficile in asymptomatic carriers[116].
The usual treatment for C. difficile-associated disease has been to stop antibiotics being given for other purposes and immediately start treatment with metronidazole or vancomycin. Patients who remain on antibiotics while undergoing treatment of CDAD have a high likelihood of treatment failure with metronidazole[117].
In 1983, before the virulent C. difficile epidemics, metronidazole and vancomycin were shown to have equivalent efficacy and relapse rates, and to be tolerated to a similar extent by patients with C. difficile-related diarrhea and colitis, but metronidazole was considerably more economical. Metronidazole was favored because the pharmacy cost for the dosage used was $387.48 to $520.00 for vancomycin and $11.84 for metronidazole[118].
Findings from another study suggest that metronidazole and vancomycin are equally effective for the treatment of mild CDAD, but that vancomycin is superior for treating patients with severe CDAD. Among the patients with mild CDAD, treatment with metronidazole or vancomycin resulted in clinical cure in 90% and 98% of the patients, respectively (P = 0.36). On the other hand, among the patients with severe CDAD, treatment with metronidazole or vancomycin resulted in clinical cure in 76% and 97% of the patients, respectively (P = 0.02). Clinical symptoms recurred in 15% of the patients treated with metronidazole and 14% of those treated with vancomycin[119].
In order to reduce vancomycin resistance, current guidelines still recommend the first-line use of metronidazole over vancomycin. However, the new strain of C. difficile may not respond as well to treatment with metronidazole, despite the absence of laboratory evidence of metronidazole resistance.
Comparison of the clinical and microbiological effects of vancomycin and metronidazole reveal that vancomycin-treated patients are more likely to develop undetectable levels of C. difficile (adjusted hazard ratio, 3.99; 95% CI: 1.41-11.3; P = 0.009) and to have resolution of diarrhea (adjusted hazard ratio, 4.17; 95% CI: 1.53-11.40; P = 0.005) during the first 5 d of therapy[120].
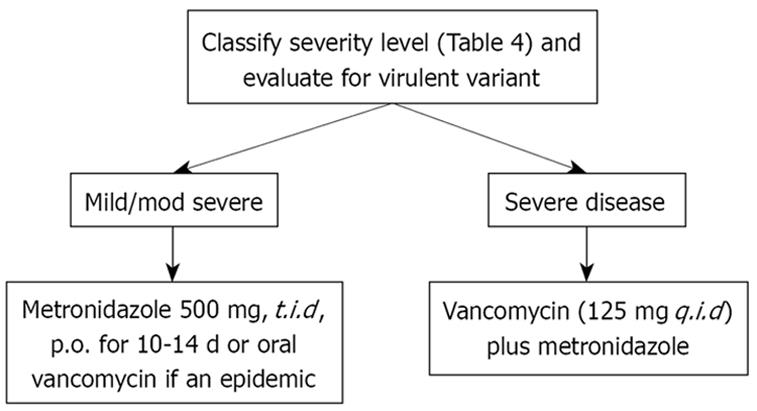
Recent studies demonstrate a high rate of failure of metronidazole, due either to infection with NAP-1 or to the presence, in hospitals, of older and sicker adults who previously have been treated with many broad-spectrum antibiotics. This raises the question as to what drug should be used as the initial therapy of C. difficile infection. The standard of care seems to be shifting towards using vancomycin first, if one is facing either a virulent organism or if risk factors for severe disease or several risk factors are present, like advanced age, immune deficiency, or pre-existing IBD (Table 4).
In addition, the cure rate seems to be significantly higher with vancomycin than metronidazole (97% versus 76%). In clinical practice, there is a shift toward using oral vancomycin as initial therapy for severe CDAD; and some clinicians are endorsing vancomycin as the preferred therapy for moderate to severe disease caused by this new epidemic strain. Currently, the treatment for hypervirulent C. difficile strains appears to be no different than for other C. difficile infections, and includes oral vancomycin[121].
Failure with metronidazole treatment may be attributable to a slower and less consistent microbiological response than that with oral the next sentence is deleted because it is repeated exactly from a previous paragraph. Vancomycin-treated patients are more likely to develop undetectable levels of C. difficile (adjusted hazard ratio, 3.99; 95% CI: 1.41-11.3; P = 0.009) and to have resolution of diarrhea (adjusted hazard ratio, 4.17; 95% CI: 1.53-11.40; P =0.005) during the first 5 d of therapy[120].
Freeman et al[122] found that duration of cytotoxin production by C. difficile ribotype 027 markedly exceeds that of ribotype 001. These findings may help to explain the increased severity of symptoms and higher case-fatality ratio associated with infections with C. difficile ribotype 027. The authors also found that sub-optimal gut concentrations of metronidazole, possibly due to inactivation by components of normal gut flora, are associated with continued toxin production. The persistence of C. difficile spores suggests that additional strategies to restore the normal colonic microflora also may be beneficial[123]. However we must take this paradigm change from metronidazole to vancomycin as initial therapy with caution. Pépin et al[1718] reported a large epidemic of CDAD in Quebec that included large numbers of patients with severe and complicated disease. They examined the relative efficacy of metronidazole and vancomycin in the wake of this hypervirulent strain. Pépin et al[1718] described a greater incidence of severe complications associated with CDAD (defined as 30-d mortality, sepsis, toxic megacolon, emergent colectomy, or intestinal perforation) with the coincident emergence of NAP1/027 in Quebec in 2003. They observed an overall 79% decrease in progression to severe complicated CDAD in patients initially treated with vancomycin, rather than metronidazole, between 1991 and 2003. They also noted that marked leukocytosis or renal failure predicted a significant risk of complications and mortality. In 2004, this led to a change in guidelines in Quebec, which recommended that oral vancomycin be used as initial treatment in patients with these markers of severity. In some cases, rectal vancomycin (0.5-1 g dissolved in 1-2 L of isotonic saline) can be given as a single 60-min retention enema every 4-12 h. Rifaximin administered as a ‘chaser’, after control of acute C. difficile infection with a standard 10-14-d course of vancomycin, appeared to prevent recurrence in seven of eight patients, even though they were rifaximin resistant[124].
An albumin level < 2.5 g/L and ICU stay are predictors of failure of metronidazole therapy for CDAD. These patients may benefit from oral vancomycin therapy at the outset[125].
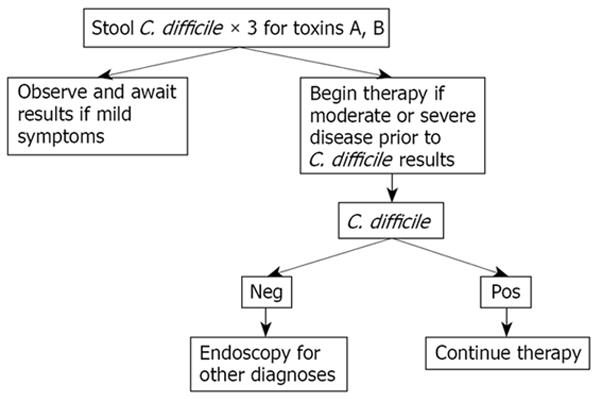
Regardless of what therapy is used, patients should be monitored carefully to ensure that they are responding to therapy, and not developing complications. If deterioration is suspected, or if the patient fits the criteria for very severe disease or is immunosuppressed or elderly, it may be wise to utilize vancomycin initially (Table 5). Our approach to patients with suspected or known C. difficile infection is based on the severity of their illness (Figures 1 and 2).
| Oral vancomycin, 500 mg q.i.d |
| Substitute intracolonic vancomycin infusion if ileus and add metronidazole 500 mg q.i.d., IV |
| Consider IV immunoglobulin therapy (400 mg/kg) |
| Surgical evaluation for acute abdomen |
Twenty percent of C. difficile infection patients relapse, despite adequate therapy. Risk factors for relapse are presented in Table 6. Diagnosing recurrent C. difficile infection can be difficult, especially in the 20% without diarrhea. The usual treatment for recurrent C. difficile infection is a repeat course of metronidazole, unless the patient has severe disease. Tapered and pulsed dosing schedules of vancomycin have been investigated for the treatment of C. difficile infection that recurs after an initial course of vancomycin (Table 7). An example of an oral vancomycin taper schedule is as follows: 125 mg qid× 10-14 d; 125 mg bid× 7 d; 125 mg daily × 7 d; 125 mg once every 2 d × 8 d; and 125 mg once every 3 d × 15 d[126]. The treatment of recurrent C. difficile infection with various vancomycin daily doses (2 g/d, 1 g/d, and 500 mg/d) and administration schedules (daily vancomycin followed by tapered or pulsed dose vancomycin therapy) was reported by McFarland et al[123]. They found that tapered and pulsed dosing schedules of vancomycin result in significantly better C. difficile infection cure rates than traditional vancomycin dosing.
| Prolonged antibiotic usage |
| Prolonged hospitalization |
| Age > 65 yr |
| Diverticulosis |
| Comorbid medical condition(s) |
| Second course of initial antibiotic, if the patient has mild/moderate disease; if severe disease, begin vancomycin |
| If recurrence after vancomycin, re-evaluate and treat with oral vancomycin and add tapering vancomycin regime and s. boulardii |
| If recurrence despite above, consider |
| Rifampicin |
| Cholestyramine |
| Fecal bacteriotherapy |
Wenisch et al[127] conducted a prospective, randomized study to compare the efficacy of the oral drugs fusidic acid, metronidazole, vancomycin, and teicoplanin in the treatment of CDAD. Treatment resulted in clinical cure greater than 90% with all the agents: 94% vancomycin, 96% teicoplanin, 93% fusidic acid, and 94% metronidazole. However, recurrent clinical symptoms occurred in 16% of patients treated with vancomycin or metronidazole, 7% of those treated with teicoplanin, and 28% of those treated with fusidic acid. There was asymptomatic carriage of C. difficile toxin in 13% of patients treated with vancomycin, 16% with metronidazole, 4% with teicoplanin and 24% with fusidic acid. No adverse effects related to therapy were observed with vancomycin or teicoplanin. Considering the costs of treatment, their findings suggest that metronidazole is the drug of choice for CDAD, and that glycopeptides should be reserved for patients who cannot tolerate metronidazole or who do not respond to treatment with this drug.
Studies on probiotics for C. difficile infection have been inconclusive and conflicting, with respect to treatment benefit. Nonetheless, the use of probiotics is becoming more widespread.
Pillai and Nelson conducted a meta-analysis to assess the potential therapeutic effects of probiotics for C. difficile infection[128]. Randomized, prospective studies (1966-2007) using probiotics alone or in conjunction with conventional antibiotics for the treatment of documented C. difficile colitis were eligible for inclusion. Ultimately, four studies met the inclusion criteria and were included in the review. The four studies examined the use of probiotics in conjunction with conventional antibiotics (vancomycin or metronidazole) for the treatment of recurrence or the initial episode of C. difficile colitis in adults. All of the studies were small and had methodological issues. A statistically-significant benefit of probiotics combined with antibiotics was detected in only one study. The authors concluded that, overall, there is insufficient evidence to recommend probiotic therapy as an adjunct to antibiotic therapy for C. difficile colitis. There also is no evidence to support the use of probiotics alone in the treatment of C. difficile colitis.
In 1994, McFarland et al[129] reported that patients receiving Saccharomyces boulardii were significantly less likely than patients receiving placebo to experience a recurrence of C. difficile diarrhea (RR 0.59; 95% CI: 0.35 to 0.98). Consequently, in a later meta-analysis, he compared the efficacy of probiotics for the prevention of AAD and the treatment of CDAD. Across 25 randomized controlled trials (RCTs), probiotics significantly reduced the relative risk of AAD (RR 0.43, 95% CI: 0.31 to 0.58, P < 0.001)[130]. Across six randomized trials, probiotics had significant efficacy for CDAD (RR 0.59, 95% CI: 0.41 to 0.85, P = 0.005).
This time, McFarland et al[129] concluded that a variety of different types of probiotic show promise as effective therapies for these two diseases. Again using meta-analysis, three types of probiotics (S. boulardii, Lactobacillus rhamnosus GG, and probiotic mixtures) were found to significantly reduce the development of AAD. Only S. boulardii was effective for CDAD.
Treatment of recurrent C. difficile infection with high-dose vancomycin plus S. boulardii is the only treatment combination that has been evaluated in a prospective, randomized, controlled trial and found to generate a significant trend toward reduced recurrent C. difficile infection[131]. Lactobacillus spp. have been evaluated for use in recurrent C. difficile infection, but data on regimens containing these organisms are poorly derived and conflicting. Fungemia with its administration has been reported in immunocompromised hosts. Therefore, its use is not appropriate in this group[132].
Relapse of C. difficile occurs in 10%-25% of patients treated with metronidazole or vancomycin. Furthermore, multiple relapses may occur in the same patient. An alternative approach to patients with recurrent CDAD involves the administration of the entire fecal flora from a healthy individual, which is referred to as fecal bacteriotherapy. Borody et al[133] reviewed 84 fecal transplantation therapies for severe cases of relapsing, or recurrent C. difficile infection (via various routes of administration). They found that 80% resulted in a good clinical response, resolution, or cure[133]. A review of eight reports on the infusion of feces or fecal bacteria revealed an optimistic cure rate, without recurrence in most patients[134]. In a study involving 18 patients treated with healthy donor stools via a nasogastric tube, 15 patients were recurrence-free at 90 d (two died of unrelated causes and one experienced recurrence)[135]. The patients described in these reports[133136–144] included those with symptomatic relapse after receiving multiple courses of antibiotics; e.g. vancomycin, and/or metronidazole, and/or rifampicin together with cholestyramine. Case series have suggested a clinical benefit of fecal bacteriotherapy in patients with severe or recurrent CDAD who have failed to respond to standard approaches. Although the data are limited to case series, fecal bacteriotherapy has been used successfully to treat relapsing C. difficile infection. The precise mechanisms for the benefits of fecal bacteriotherapy are unclear. The reappearance of Bacteroides species after treatment suggests that Bacteroides species may be involved in the restoration of the presumably antibiotic-damaged flora in the colon.
Successful treatment with two or more fecal enemas has been described in other reports, according to Borody, Leis, & Gerald Pang (http://www.up-to-date.com2008), involving a total of 23 patients with PMC who were refractory to antibiotic therapy, or who had experienced multiple relapses. In one study of 16 patients with severe, refractory disease treated over an 18-year period, 13 responded dramatically with decreases in diarrhea, temperature and leukocytosis. In a report describing nine patients, the single administration of a fecal enema (5-10 gm homogenized stool in pasteurized cow’s milk) was effective in seven. In another case report, according to Borody, Leis, & Gerald Pang (http://www.up-to-date.com), the one-time administration of bacteriotherapy was effective when 500 mL of fecal infusion in saline was delivered throughout the colon via a colonoscope. The authors hypothesized that the greater area of re-colonization by fecal bacteria created a greater capacity to inhibit spore formation proximal to the splenic flexure. The use of the colonoscope to deliver fecal bacteria has an added theoretical advantage of permitting delivery of the active flora components to the distal small bowel, where C. difficile can reside. In addition, the colonoscope may permit the proximal delivery of flora in patients with a dilated colon, although colonoscopy must be performed extremely cautiously in this setting, because of the risk of perforation. One of the current authors (JSB) has utilized this modality with similar results.
Aas et al[135] reported on 18 subjects who received donor stool by nasogastric tube for recurrent C. difficile infection over a 9-year period at a single institution. During the period between the initial diagnosis of C. difficile colitis and the stool treatments, the 18 subjects received a total of 64 courses of antimicrobials (range, 2-7 courses; median, three courses). During the first 90 d after receipt of treatment with stool, two patients died of unrelated illnesses. Only one of the 16 survivors experienced a single recurrence of C. difficile colitis over the 90-d follow-up. No adverse effects associated with stool treatment were observed. Patients with recurrent C. difficile colitis may benefit from the introduction of stool from healthy donors via a nasogastric tube.
Lund-Tønnesen et al[140] reported on 18 patients with CDAD who were given homologous feces from one healthy donor. In 17 patients, feces were instilled via a colonoscope, and in one patient via a gastric stoma. Fifteen patients were clinically cured, and no relapses were observed; however, it is important to note that three patients with severe colitis did not respond to the treatment.
In recalcitrant, recurrent C. difficile infection, one should attempt initially to use probiotics that have been shown to be effective in published studies. Subsequently, in patients who remain seriously ill from recurrent C. difficile infection, fecal bacteriotherapy may be used when other approaches have been unsuccessful[133145]. The above-mentioned study by Lund-Tønnesen et al[140], in which three patients with severe colitis did not respond to the treatment, while only the remaining less-severely ill patients were clinically cured, and no relapses were observed may indicate that this may serve as rescue therapy for patients with recurrent C. difficile. But its role in patients with severe C. difficile infection remains unproven.
Suggested protocol for fecal bacteriotherapy: Barody’s protocol is as follows. (a) Donor stool and blood are screened for pathogens and viruses before infusion. CBC, serological testing for hepatitis A, B, and C; HIV-1 and HIV-2 and syphilis, stool culture for enteric bacterial pathogens, and light microscopy examination of stool sample for parasites and ova are performed. (b) The donor is clinically well, with the passage of normal, daily stools, and has had no intake of antibiotics for the last 6 mo. (c) The donor should not be a close relative, living in the same household such as a husband or child, theoretically to avoid use of flora from a silent carrier of the same pathogen. (d) The recipient is evaluated for HIV and hepatitis markers to avoid future questions about transmission. (e) Oral vancomycin (500 mg twice daily for 7 d) is administered, and then followed by a single oral lavage with 3-4 L of polyethylene glycol with electrolyte purgative (such as GoLYTELY). (f) Although the lavage is skipped in patients too ill to tolerate it, vancomycin pretreatment is used, whenever possible. (g) 200-300 gm of donor stool suspended in 200-300 mL of sterile normal saline (homogenized briefly in a kitchen blender to a liquid consistency) is administered via an enema within 10 min of preparation, and this is repeated daily for 5 d. (h) Initial infusion may be filtered and infused via colonoscopy, preferably into the terminal ileum to address known ileal presence of C. difficile. (i) At least five consecutive days of rectal enemas are administered, using donor stools. (j) The enema should be retained for at least 6 h (loperamide pretreatment may help), followed by a high-fiber meal and overall diet. (k) Although some patients are unable to retain the enema initially for prolonged periods, it appears that coating of the mucosa by the infusate is adequate. (l) Adverse effects have been transient and mild, and have consisted primarily of abdominal gurgling, gas and borborygymi-expected post-enema symptoms. Recurrence has not been observed with follow-up of 1-3 years in most patients, even though a number of patients subsequently have required antibiotics for unrelated infections.
In summary, patients who develop a second episode of C. difficile infection after successful treatment of the first episode may be at increased risk for developing complications. Although different drugs and regimens have been used, vancomycin may be the best option; and the combination of high-dose vancomycin plus S. boulardii is the only treatment combination that has been evaluated in a prospective, randomized, controlled trial to demonstrate a significant trend toward reduced recurrent C. difficile infection. Fecal bacteriotherapy seems promising and is undergoing further testing at this time
Other therapeutic options for CDAD are being developed, and drugs used for other infections are being studied as alternatives to metronidazole and vancomycin.
Nitazoxanide, a nitrothiazolide and metabolic precursor of tizoxanide, has broad-spectrum activity against helminths and protozoa, as well as bacterial enteric pathogens, including C. difficile. It is marketed in the US and has been widely used throughout the world to treat parasitic diseases of the gastrointestinal tract; several million children have been treated with this drug over the past decade. Nitazoxanide is a US FDA approved drug that is used as an anti-protozoal agent for oral administration in pediatric patients, aged 1-11 years, with diarrhea. The drug acts by interfering with anaerobic metabolic pathways, and it has been shown to have excellent in vitro activity against C. difficile. An ongoing double-blind study comparing metronidazole with nitazoxanide for C. difficile infection involved the treatment of 16 patients. The response rate for nitazoxanide was recently shown to be comparable to metronidazole for CDAD treatment in a prospective, randomized, double-blinded clinical trial[146–148]. It is associated with fewer side effects than metronidazole, which should improve compliance.
Tinidazole is a structural analogue of metronidazole, with similar bioavailability (100%) and fewer drug-related adverse effects, but similar in vitro activity against C. difficile[149150].
OPT-80, previously known as tiacumicin B, and with the proposed name difimicin, is a novel 18-membered macrocycle antibiotic. It has little or no systemic absorption after oral administration, and a narrow activity spectrum against Gram-positive aerobic and anaerobic bacteria, and has tested well in patients with C. difficile infection[151152].
Rifalazil and rifaximin are rifamycin derivatives. Rifalazil is an orally-absorbed systemic antibiotic with a broad spectrum of activity that has been shown to prevent and treat CDAD recurrence in a hamster model. Rifaximin, a non-systemic antibiotic approved by the US FDA for travelers’ diarrhea, currently is under evaluation for the treatment of CDAD[153154].
In unresponsive cases (e.g. those who have had no improvement after 3 d on metronidazole), one should add oral vancomycin, 500 g four times daily and intracolonic vancomycin (500 mg of IV vancomycin in 100 mL of normal saline per rectal Foley catheter, clamping for 60 min, repeating every 6 h). In addition, if there is an ileus, metronidazole can be given intravenously. While there is still no significant experience with nitazoxanide or rifaximin, these would be reasonable choices. As well, Pullman et al[155] report that ramoplanin, a poorly-absorbed glycolipodepsipeptide that has been evaluated for the prevention of vancomycin-resistant enterococci, has good in vitro activity against C. difficile.
Teicoplanin may be a good choice, because some empirical evidence suggests that it is better than vancomycin for bacteriologic cure. It has borderline superior effectiveness in terms of symptomatic cure, but it is not readily available in the United States.
Therefore, in addition to nitazoxanide, bacitracin, teicoplanin, and fusidic acid, agents that have published efficacy, are several drugs, like rifaximin and PAR-101, which currently are under investigation. Other therapies, including polymers that bind C. difficile toxin, monoclonal antibodies to toxins, and preventative measures like toxoid vaccines, also are under study.
Taylor et al[156] examined the safety and pharmacokinetics of a novel neutralizing human monoclonal antibody against C. difficile toxin A (CDA1) in 30 healthy adults whose median age was 27.5 years. While there were no serious adverse events related to its use, 21 of 30 reported non-serious adverse events were possibly related to CDA1. These included transient blood pressure changes requiring no treatment, nasal congestion, headache, abdominal cramps, nausea, and self-limited diarrhea. The authors concluded that, at least in healthy subjects, the administration of CDA1 as a single intravenous infusion is safe and well tolerated.
The importance of toxin production in the patho-physiology of C. difficile diarrhea has prompted consideration of anion-binding resins as a possible alternative to antimicrobial therapy. An advantage of resin therapy is that the bowel flora is not altered, as occurs with antibiotics (e.g. vancomycin or metronidazole), which may allow for more rapid reconstitution of normal colonic flora. Anion-exchange resins bind vancomycin as well as toxins; thus, the resin must be taken at least 2 h or 3 h apart from the vancomycin. Suggested regimens are colestipol (5 g every 12 h) or cholestyramine (4 g three or four times daily) for 1-2 wk, usually in conjunction with vancomycin.
Tolevamer, a novel toxin-binding polymer, has been developed to ameliorate C. difficile-associated disease without adversely affecting normal flora. Tolevamer has been tested for its ability to neutralize clostridial toxins produced by the epidemic BI/027 strains, thereby preventing toxin-mediated tissue culture cell rounding. The titers of toxin-containing C. difficile culture supernatants were determined using confluent cell monolayers, and then the supernatants were used in assays containing dilutions of tolevamer to determine the lowest concentration of drug that prevented ≥ 90% cytotoxicity. Tolevamer neutralized toxins in the supernatants of all C. difficile strains tested. Specific antibodies against the large clostridial toxins TcdA and TcdB also neutralized the cytopathic effect, suggesting that tolevamer specifically neutralizes these toxins, and that the binary toxin (whose genes are carried by the BI/027 strains) is not a significant source of cytopathology against tissue culture cells in vitro[157].
However, tolevamer is not FDA approved or commercially available, to date. Castanospermine has been identified as an inhibitor of the Rho/Ras-glucosylating Clostridium sordellii lethal toxin and C. difficile toxin B. Microinjection of castanospermine into embryonic bovine lung cells prevents the cytotoxic effects of toxins. The inhibitor binds in a conformation that brings its four hydroxyl groups and its N atom almost exactly into the positions of the four hydroxyls and the ring oxygen of the glucosyl moiety of UDP-glucose, respectively[158]. It is in its early stage of development.
Testing the feasibility of active vaccination against C. difficile and its toxins in high-risk individuals currently is ongoing[159]. C. difficile toxoid vaccine has induced immune responses to toxins A and B in patients with CDAD, and has been associated with resolution of recurrent diarrhea. This parenteral C. difficile vaccine, which contains toxoid A and toxoid B, has been reported to be safe and immunogenic in healthy volunteers. Three patients with multiple episodes of recurrent CDAD were vaccinated. Two of the three exhibited an increase in serum IgG antitoxin A antibodies (three- and four-fold increases), and in serum IgG antitoxin B antibodies (52 and 20-fold). Both individuals also developed cytotoxin-neutralizing activity against toxins A and B. Prior to vaccination, the subjects had required nearly continuous treatment with oral vancomycin for 7, 9, and 22 mo, respectively, to treat recurrent episodes of CDAD. After vaccination, all three subjects discontinued treatment with oral vancomycin without any further recurrence. Thus, C. difficile toxoid vaccine induced immune responses to toxins A and B in patients with CDAD, and was associated with resolution of recurrent diarrhea.
Vaccination with a partially-purified preparation of inactivated toxins A and B is also undergoing current study. Several studies have shown that the humoral immune response of the host to C. difficile toxins A and B influences the clinical course of CDAD, as well as the risk of relapse[160–162].
Another vaccine, containing toxoids A and B, has been shown to induce adequate antibody responses in healthy volunteers[163]. The efficacy of this vaccine subsequently was evaluated in an open-label study involving three patients with recurrent C. difficile colitis[159]. Following four intramuscular inoculations over an 8-wk period, all three patients discontinued antibiotic treatment without further recurrence over 6 mo of follow-up.
A retrospective review was performed on 264 C. difficile toxin-positive patients (November 2003-January 2005), which documented 14 patients with severe, refractory, recurrent C. difficile diarrhea who were treated with intravenous immunoglobulin (Flebogamma, 150-400 mg/kg)[164]. Patients received a median of three (range, 1-5 g/L) courses of vancomycin or metronidazole before receiving intravenous immunoglobulin. All had hypoalbuminemia (median, 22 g/L; range, 18-33 g/L) and raised C-reactive protein (median, 47 mg/L; range, 25-255 g/L) at the time of infusion. The median white cell count was 15.3 × 109/L (range, 4-24 g/L). Eight patients had evidence of pancolitis on abdominal imaging, suggesting severe C. difficile diarrhea. All patients tolerated intravenous immunoglobulin without side effects. Nine (64%) responded with bowel habits normalizing in a median of 10 (range, 2-26) d; one patient received two doses. One patient had a partial response from two doses, but died 2 mo later after a recurrence. Thus, intravenous immunoglobulin may be effective for severe, refractory, or recurrent C. difficile diarrhea after failed conventional treatment.
In patients with C. difficile colitis, a progressive, systemic inflammatory state may develop that is unresponsive to medical therapy; some cases ultimately will progress to colectomy or death. C. difficile colitis is a significant and increasingly common cause of death. Surgical treatment of C. difficile colitis has a high death rate once the fulminant expression of the disease is present[165]. These authors reviewed 2334 hospitalized patients with C. difficile colitis from January 1989 to December 2000. In the setting of CDAD before the predominance of the hypervirulent strain, 64 patients died or underwent colectomy for pathology-proven C. difficile colitis. Unfortunately, those patients who underwent colectomy for C. difficile colitis had an overall death rate of 57%. Significant predictors of death after colectomy were preoperative vasopressor requirements and older age.
Fulminant C. difficile colitis is associated with a high mortality rate. As in the former study, Hall et al[166] found that the development of a vasopressor requirement and the need for intubation are ominous signs which should lead to rapid surgical intervention. From 1998 to 2006, they studied a total of 3237 consecutive patients with C. difficile cytotoxin-positive stool samples. Commonly referenced indicators for surgical intervention were gathered on the day of surgery. The preoperative characteristics of patients surviving subtotal colectomy were compared with those who did not survive. They found that 36 patients underwent colectomy. Twenty-three patients (64%) were discharged from the hospital alive. Preoperative intubation and vasopressor requirement were risk factors for in-hospital mortality (OR: 7.15; 95% CI: 1.28-39.8 and OR: 6.0; 95% CI: 1.08-33, respectively). Patients who had a recent surgical procedure experienced a lower in-hospital mortality rate (OR: 0.11; 95% CI: 0.02-0.52).
In the setting of CDAD due to the hypervirulent strain, some patients have progressed from severe disease to death in less than 48 h. Emergency colectomy has prevented mortality in some patients with fulminant CDAD. The decision to perform an emergency colectomy remains largely empirical[100]. In a retrospective observational cohort study of 165 cases of CDAD, among those patients who required ICU admission or prolongation of ICU stay between January 2003 and June 2005 at two tertiary care hospitals in Quebec, 53% died within 30 d of ICU admission, and almost half (44%) within 48 h of ICU admission. The independent predictors of 30-d mortality were: leukocytosis ≥ 50 ×109/L (AOR: 18.6; 95% CI: 3.7-94.7), lactate ≥ 5 mmol/L (AOR: 12.4; 95% CI: 2.4-63.7), age ≥ 75 years (AOR: 6.5; 95% CI: 1.7-24.3), immunosuppression (AOR: 7.9; 95% CI: 2.3-27.2) and shock requiring vasopressors (AOR: 3.4; 95% CI: 1.3-8.7). After adjustment for these confounders, patients who had an emergency colectomy were less likely to die (AOR: 0.22; 95% CI: 0.07-0.67, P = 0.008) than those treated medically. Surgical intervention is indicated in the setting of peritoneal signs, severe ileus, or toxic megacolon; but colectomy also seems more beneficial in patients aged 65 years or older, in the immune competent, and in those with a leukocytosis ≥ 20 × 109/L or serum lactate between 2.2 and 4.9 mmol/L.
The standard of care for patients undergoing emergency surgical intervention for CDAD is a total colectomy (with preservation of the rectum) and ileostomy, since primary anastomosis is not feasible acutely due to the pancolitis associated with severe disease. However, after colonic inflammation has subsided, closure of the ileostomy and ileorectal anastomosis can be performed (Table 8).
| Based upon |
| 30-d mortality |
| Leukocytosis ≥ 20 × 109/L |
| Lactate ≥ 5 mmoL/L |
| Age ≥ 75 yr |
| Immunosuppression |
| Shock requiring vasopressors |
| Especially in the presence of: |
| Toxic megacolon |
| Multi-organ system failure |
CDAD has increased in frequency and severity throughout North America and Europe over the last several years, largely due to the emergence of the NAP1 epidemic strain. This transformation of a formerly mild disease into one that can cause severe morbidity and mortality within a few days has challenged the entire approach to this suddenly serious infection. Institutions require accurate and rapid diagnostics for early detection of cases and possible outbreaks, in order to initiate specific therapy and implement early and effective infection control[167].
Aggressive diagnostic and therapeutic interventions are warranted in the setting of C. difficile infection. Bedside sigmoidoscopy or colonoscopy may be performed to make a presumptive diagnosis of C. difficile infection, by evaluating for the presence of pseudomembranes. Given the risk of perforation, care should be taken to introduce minimal amounts of air to avoid exacerbating ileus or distention. The choice of initial drug therapy depends on severity of illness, co-morbidities, and strain suspicion. Prompt surgical consultation is warranted to assess the requirement for colectomy[100].
C. difficile infection is a global problem. A comprehensive C. difficile infection infection control management rapid response team (RRT) is recommended for each health care facility throughout the world. A communications network between RRTs also is recommended, in coordination with each country’s Department of Health. It is only through the implementation of the new approaches to its diagnosis, therapy and presentation that we can help to reduce the morbidity and mortality caused by this infection.
We must address environmental reservoirs to help to limit transmission. C. difficile has been cultured not only from patient bathrooms and bedpans, but from stethoscopes, blood pressure cuffs, and hospital furniture. The initial step is identifying possible C. difficile patients, especially in long-term care facilities (LTCFs). Quinn et al[168] determined that only 111 facilities (42.2%) had a protocol to identify residents with C. difficile infection, and most (77.5%) did not test for C. difficile unless a resident had severe diarrhea. Only 58.5% of the facilities placed residents with C. difficile infection in private rooms, and 60.9% cohorted residents infected with C. difficile with other residents with C. difficile colonization or infection. Only 66 facilities (25.1%) had a program to control the use of antimicrobial agents.
Findings suggest that asymptomatic carriers of epidemic and non-epidemic C. difficile strains have the potential to contribute significantly to disease transmission in long-term care facilities. Thirty-five (51%) of 68 asymptomatic patients were carriers of toxigenic C. difficile, and 13 (37%) of these patients carried epidemic strains. Compared with non-carriers, asymptomatic carriers had higher percentages of skin (61% vs 19%; P = 0.001) and environmental contamination (59% vs 24%; P = 0.004). Eighty-seven percent of isolates found in skin samples and 58% of isolates found in environmental samples were identical to concurrent isolates found in stool samples. Spores on the skin of asymptomatic patients were transferred easily to investigators’ hands. Previous C. difficile-associated disease (P < 0.001) and previous antibiotic use (P = 0.017) were associated with asymptomatic carriage, and the combination of these two variables was predictive of asymptomatic carriage (sensitivity, 77%; specificity, 58%; PPV, 66%; NPV, 70%)[64].
In a prospective study of 27 patients with C. difficile-associated disease, it was found that C. difficile frequently contaminated multiple skin sites, including groin, chest, abdomen, forearms, and hands, and was easily acquired on investigators’ hands. Skin contamination often persisted on patients’ chest and abdomen after resolution of diarrhea. Thus, skin contact of the patient by a health-care worker is a means of C. difficile transmission[169].
It is important to emphasize that asymptomatic fecal excretion of C. difficile is transient in most patients, and treatment with metronidazole is not effective. Although treatment with vancomycin is temporarily effective in asymptomatic carriers, it is also associated with a significantly higher rate of C. difficile carriage 2 mo after treatment and, therefore, is not recommended[63].
An increase in hospital-acquired C. difficile infection rate was found at the University of Pittsburg Medical Center. A comprehensive C. difficile infection control ‘bundle’ was implemented by hospital personnel to control the outbreak of C. difficile infection. This C. difficile infection control bundle consisted of education, increased and early case-finding, expanded infection-control measures, the development of a C. difficile infection management team, and antimicrobial management. Process measures, antimicrobial usage, and hospital-acquired C. difficile infection rates were analyzed, and C. difficile infection isolates were typed. The rates of compliance with hand hygiene and isolation were 75% and 68%, respectively.
The C. difficile infection management team evaluated a mean 31 patients per month (11% were evaluated for moderate or severe disease). The use of antimicrobial therapy associated with increased C. difficile infection risk decreased by 41% during the period 2003-2005. The aggregate rate of C. difficile infection during the period 2001-2006 decreased to 4.8 infections per 1000 HDs; and, by 2006, it had decreased to 3.0 infections per 1000 HDs, a rate reduction of 71%. During the period 2000-2001, the proportion of severe C. difficile infection cases peaked at 9.4% (37 of 393 C. difficile infections were severe); this rate decreased to 3.1% in 2002 and further decreased to 1.0% in 2006, a 78% overall reduction. In 2005, 13% of C. difficile isolates were type BI (20% were hospital acquired), which represented a significant reduction from 2001. These authors concluded that the outbreak of C. difficile infection with the BI strain in hospital was controlled after implementing this infection control ‘bundle’. Thus, early identification, coupled with appropriate control measures, reduces the rate of C. difficile
infection and the frequency of adverse events. However, it requires a multipronged approach.
Methods of contact precautions and control: (1) Place patients with C. difficile in private rooms. (2) If private rooms are not available, place these patients in rooms with other patients who have C. difficile-associated disease. (3) Perform hand hygiene procedures preferably using soap and water-not alcohol. To reduce the transmission of C. difficile spores, environmental disinfection with 10% sodium hypochlorite and hand-washing with soap and water can be effective at removing the spores from hands and surfaces.
Strict antiseptic procedures should be followed by health care workers in contact with the patient, and these procedures should include the use of disposable gloves, and a mask and gown. Because alcohol is ineffective at killing C. difficile spores, health care workers must frequently wash their hands with soap and water, rather than with alcohol-based waterless hand sanitizers, especially when caring for CDAD patients. Patient-care equipment (e.g. blood-pressure cuffs, stethoscopes and thermometers) should either be used only for the infected patient or cleaned well before they are used with another patient.
Enhanced environmental cleaning following a regular schedule with dilute bleach should be used to eliminate C. difficile spores from all patient contact surface areas. These spores may remain on infected surface areas for months or even years.
In addition, note the ability of the vegetative form of C. difficile to survive on moist surfaces. On dry surfaces, vegetative C. difficile cells die rapidly, whereas they remained viable for up to 6 h on moist surfaces in room air. This illustrates the importance of washing and drying room surfaces when cleaning contaminated rooms.
A very important method of controlling outbreaks of C. difficile-associated disease should be restricting the use of antimicrobial agents that have been implicated as risk factors for the disease, as recommended by Gerding et al[116]. Davey et al[170] documented that interventions to improve antibiotic prescribing practices to hospital inpatients can be successful, and that they can reduce antimicrobial resistance and the rates of hospital-acquired infections.
Effective surveillance of antibiotic-resistant bacteria and CDAD must be intensified in every healthcare setting, but especially in long-term care and rehabilitation facilitie. All these facilities must have easy laboratory access for prompt and active surveillance culturing and C. difficile cyto-toxin testing, for both A and B, at the earliest indication of any infection or CDAD. In addition, Furuno et al[171] advise that those patients at higher risk for carriage of antibiotic-resistant bacteria should be identified early for active surveillance targeting-culturing for methicillin-resistant S. aureus (MRSA) and vancomycin-resistant enterococci (VRE); e.g. patients who report having had antibiotics or prior hospital admissions within the past year. The authors found that this was very cost-effective, saving a projected $19 000-$26 000 relative to non-directed hospital wide screening for resistant organisms (MRSA and VRE), during the 8-mo study period at their tertiary care facility. They also found that there often is a significant delay between the onset of CDAD symptoms and the full implementation of CDC contact precautions.
Note that current Proper Hand Hygiene techniques for C. difficile differ from previous 2002 CDC Guidelines for hand hygiene in health-care settings, which were as follows.
IV.A.1. During the delivery of healthcare, avoid unnecessary touching of surfaces in close proximity to the patient to prevent both contamination of clean hands from environmental surfaces and transmission of pathogens from contaminated hands to surfaces.
IV.A.2. When hands are visibly dirty, contaminated with proteinaceous material, or visibly soiled with blood or body fluids, wash hands with either a non-antimicrobial soap and water or an antimicrobial soap and water.
IV.A.4. Wash hands with non-antimicrobial soap and water or with antimicrobial soap and water if contact with spores (e.g. C. difficile or Bacillus anthracis) is likely to have occurred. The physical action of washing and rinsing hands under such circumstances is recommended, because alcohols, chlorhexidine, iodophors, and other antiseptic agents have poor activity against spores.
Alcohol-containing hand disinfection products were recommended over soap and water in the control of most organisms of epidemiological importance[172]. Alcohol, however, does not eradicate C. difficile spores, maintain both Bettin et al[173] and Boyce et al[174]. Thus, what hand cleaning method to use in the presence of C. difficile infection is controversial. Papers conflict on the subject of alcohol eradicating C. difficile spores.
There even has been concern that the widespread use of alcohol-based hand sanitizers (instead of hand washing) has played a role in recent C. difficile outbreaks. Furthermore, because soap and water hand hygiene requires more time than alcohol-based hand hygiene, there is concern that alcohol-based hand hygiene may decrease overall effective hand hygiene compliance.
These concerns remain unproven. Overall CDAD rates have tended to decrease or remain the same after the introduction and increased use of alcohol-based sanitizers as the primary mode of hand hygiene[175176]. There is a lack of rigorous evidence, however, linking specific hand hygiene interventions with the prevention of health care associated infections (HCAIs). The varied nature of the interventions used and the diverse factors affecting the acquisition of HCAIs make it difficult to show any specific effect of hand hygiene alone. The most frequent methodologies currently used in this research area have been before-and-after observational studies without a control comparison group[177]. However, the CDC recommends soap and water hand hygiene when caring for patients with CDAD.
In summary, if a facility is experiencing a C. difficile outbreak, it is prudent to emphasize that health care workers should frequently wash their hands with soap and water, in addition to using an alcohol-based hand sanitizer[178].
The 2008 recommendations have been ambivalent, as seen below.
If your institution experiences an outbreak of C. difficile, consider using only soap and water for hand hygiene when caring for patients with C. difficile-associated disease; alcohol-based hand rubs are not as effective against spore-forming bacteria.
Current (Reviewed 3/08) CDC hand hygiene guidelines, available at http://www.cdc.gov/handhygiene/[7A] Accredited organizations are required to provide health care workers with a readily accessible alcohol-based hand rub product (CDC recommendations 8 C&D). However, use of an alcohol-based hand rub cleaner by any individual health care worker is not required. If you choose not to use it, then soap and water should be used instead.
In addition, use gloves when entering patients’ rooms and during patient care; use gowns if soiling of clothes is likely; dedicate equipment, whenever possible.
Implement an environmental cleaning and disinfection strategy. Ensure adequate cleaning and disinfection of environmental surfaces and reusable devices, especially items likely to be contaminated with feces and surfaces that are touched frequently. Use an Environmental Protection Agency (EPA)-registered hypochlorite-based disinfectant for environmental surface disinfection after cleaning, in accordance with label instructions; generic sources of hypochlorite (e.g. household chlorine bleach) also may be appropriately diluted and used. Follow the manufacturer’s instructions for the disinfection of endoscopes and other devices. Infection control practices in long-term care and home health settings are similar to those practices taken in traditional health-care settings.
How to clean and disinfect surfaces and devices according to the CDC’s evidence-based guidelines for the prevention of CDAD (as reported at http://www.cdc.gov/ncidod/dhqp/id_CdiffFAQ_HCP.html). (1) Surfaces should be kept clean, and body substance spills should be managed promptly, as outlined in the CDC’s ‘Guidelines for Environmental Infection Control in Health-Care Facilities’. (2) Hospital cleaning products can be used for routine cleaning. (3) Hypochlorite-based disinfectants have been used with some success for environmental surface disinfection in those patient-care areas where surveillance and epidemiology indicate ongoing transmission of C. difficile. (4) Consult the aforementioned guidelines for the use conditions for generic sources of hypochlorite-based products (e.g. household chlorine bleach) for disinfection of environmental surfaces. Note: EPA-registered hospital disinfectants are recommended for general use, whenever possible, in patient-care areas. At present, there are no EPA-registered products with specific claims for inactivating C. difficile spores, but there are a number of registered products that contain hypochlorite.
If an EPA-registered proprietary hypochlorite product is used, consult the label instructions for proper and safe use conditions. The literature supports the role of environmental disinfection with unbuffered hypochlorite solutions (diluted 1:10)[179].
Fawley et al[180] studied the differences between the activity of various cleaning agents and germicides against C. difficile spores and the potential for some of these products to promote sporulation. When used at recommended working concentrations, only chlorine-based germicides were able to deactivate C. difficile spores. C. difficile epidemic strains had a greater sporulation rate than non-epidemic strains. The mean sporulation rate, expressed as the proportion of a cell population that is in spore form, was 13% for all strains not exposed to any cleaning agent or germicide, and it was significantly increased by exposure to cleaning agents or germicides containing detergent alone (34%), a combination of detergent and hypochlorite (24%), or hydrogen peroxide (33%). By contrast, the mean sporulation rate did not change substantially after exposure to germicides that contain either a combination of detergent and dichloroisocyanurate (9%) or dichloroisocyanurate alone (15%).
A study by White et al[181] revealed that all floor cleaning methods reduce the overall microbial load, though high counts and bacterial pathogens occasionally persist despite cleaning. Spray cleaning yielded marginally better results than traditional mopping and vacuuming. Wet scrubbing significantly reduced levels of coagulase-positive staphylococci (P = 0.03), which, in combination with routine methods, produced an effect that persisted for at least a week. Any sudden change in CDAD incidence in any medical institution should be reported immediately to public health officials.
The use of copper surfaces within the clinical environment and the application of a germination solution in infection control procedures may offer a novel way by which to eliminate C. difficile from contaminated surfaces and reducing CDAD[182].
Three novel copper-based biocidal formulations, but not their components (copper sulfate and inorganic binders), were found by Gant et al[183] to have potent activity against organisms highly relevant to healthcare-associated infections, and all were active against C. difficile spores. This biocidal activity was not achieved by copper sulfate or the inorganic binders used in the formulations. All three copper-based formulations completely decontaminated UMF cloths containing MRSA, ACCB or C. difficile spores, suggesting that any of these copper-based formulations could be highly beneficial in the healthcare environment. None of the three copper-based formulations or copper sulfate was cytotoxic to human epithelial cells, up to concentrations of 100-200 ppm.
C. difficile infection rates are rising (Figures 3 and 4). The most recent guidelines on C. difficile infection based on the strength of recommendations and quality of evidence were issued on 9 October 2008. This was issued as part of the latest updated guidelines for hospital acquired infections by the American Hospital Association, the Joint Commission on Accreditation of Health Organizations, the Infectious Diseases Society of America, the Society for Healthcare Epidemiology of America, and the Association for Professionals in Infection Control and Epidemiology in-“A Compendium of Strategies to Prevent Healthcare-Associated Infections in Acute Care Hospitals.”Infect Control Hosp Epidemiol 2008; 29: S12-S21 (Authors-Yokoe, Mermel, Anderson, Arias, Burstin, Calfee, Coffin, Dubberke, Fraser, Gerding, Griffin, Gross, Kaye, Klompas, Lo, Marschall, Nicolle, Pegues, Perl).
Recommended for all acute care hospitals using strength of recommendations and quality of evidence from Table 9.
| Category/grade | Definition |
| Strength of recommendation | |
| A | Good evidence to support a recommendation for use |
| B | Moderate evidence to support a recommendation for use |
| C | Poor evidence to support a recommendation |
| Quality of evidence | |
| I | Evidence from ≥ 1 properly randomized, controlled trial |
| II | Evidence from ≥ 1 well-designed clinical trial, without randomization; from cohort or case-control analytic studies (preferably from > 1 center); from multiple time series; or from dramatic results from uncontrolled experiments |
| III | Evidence from opinions of respected authorities, based on clinical experience, descriptive studies, or reports of expert committees |
A. Components of a C. difficile infection prevention program: (1) Use contact precautions for infected patients, with a single-patient room preferred (A-II for hand hygiene, A-I for gloves, B-III for gowns, and B-III for single-patient room). (2) Ensure cleaning and disinfection of equipment and the environment (B-III for equipment and B-II for the environment). (3) Implement a laboratory-based alert system to provide immediate notification to infection prevention and control personnel and clinical personnel about patients with newly diagnosed C. difficile infection (B-III). (4) Conduct C. difficile infection surveillance and analyze and report C. difficile infection data (B-III). (5) Educate healthcare personnel, housekeeping personnel, and hospital administration about C. difficile infection (B-III). (6) Educate patients and their families about C. difficile infection, as appropriate (B-III). (7) Measure compliance with CDC or World Health Organization hand-hygiene and contact precaution recommendations (B-III).
Perform a C. difficile infection risk assessment. These special approaches are recommended for use in locations and/or populations within the hospital for which outcome data and/or risk assessment suggest lack of effective control despite implementation of basic practices.
A. Approaches to minimize C. difficile transmission by healthcare personnel: (1) Intensify the assessment of compliance with process measures (B-III). (2) Perform hand hygiene with soap and water as the preferred method before exiting the room of a patient with C. difficile infection (B-III). (3) Place patients with diarrhea under contact precautions while C. difficile test results are pending (B-III). (4) Prolong the duration of contact precautions after the patient becomes asymptomatic until hospital discharge (B-III).
B. Approaches to minimize C. difficile infection transmission from the environment: (1) Assess the adequacy of room cleaning (B-III). (2) Use sodium hypochlorite (bleach)-containing cleaning agents for environmental cleaning. Implement a system to coordinate with the housekeeping department if it is determined that sodium hypochlorite is needed for environmental disinfection (B-II).
C. Approaches to reduce the risk of C. difficile infection acquisition: Initiate an antimicrobial stewardship program (A-II).
(1) Do not test patients without signs or symptoms of C. difficile infection for C. difficile (B-II). (2) Do not repeat C. difficile testing at the end of successful therapy for a patient recently treated for C. difficile infection (B-III).
In the US, The Deficit Reduction Act of 2005 (P.L. 109-171) requires the Centers for Medicare & Medicaid Services (CMS), the US federal agency which administers Medicare, Medicaid, and the State Children’s Health Insurance Program to deny the assignment of a case to a higher DRG (payment to a health care facility) based on the occurrence of one of a selected number of hospital-acquired conditions, if that condition was acquired during the hospitalization. This rule is named CMS-1390-P: Medicare Program; Proposed Changes to the Hospital Inpatient Prospective Payment Systems and Fiscal Year 2009 Rate - Provisions on Preventable Hospital-Acquired Conditions Including Infections. The US Congress requires CMS to select conditions that are high cost, high volume, or both; assigned to a higher paying DRG when present as a secondary diagnosis; and reasonably preventable through the application of evidence-based guidelines.
In its original ruling, CMS had proposed adding nine hospital-acquired conditions, including C. difficile to the list of hospital acquired infections for which it proposed to deny payment. This would have taken effect on 1 October 2008. In July 2008, however, in response to an April 2008 letter sent by all three of the US major gastroenterology organizations, CMS, in a final rule setting policies and payment rates for the hospital setting, decided not to add C. difficile to the list of ‘Hospital-Acquired Conditions for Which It Will Deny Payment’.
The gastroenterology organizations in its April 2008 letter to CMS focused on the last criterion necessary for ‘non-reimbursement’-reasonably preventable through the application of evidence-based guidelines.
US gastroenterology organizations, in the letter to CMS, made much about the alleged fact that alcohol-based products are effective against the majority of microorganisms other than (i.e. not for) C. difficile, with the statement that “Alcohol-based products, in compliance with CDC guidelines, have played a significant role in potentially complicating efforts to avoid the spread of CDAD.”
“Indeed”, stated the letter, quoting Shen et al[184] “the proportion of all hand hygiene episodes performed with soap and water dropped from 90% to 15%, three years after the introduction of alcohol hand gels in one U.S. teaching hospital”. The letter continued by stating “that the trade-off of higher overall compliance against more focused use of soap and water is one that CMS must consider given that ‘CDC guidelines have played a significant role in potentially complicating efforts to avoid the spread of CDAD’. “In conclusion”, ended the letter to CMS, “the ACG, AGA and ASGE urge CMS not to add C. difficile to its list of hospital-acquired conditions for which additional payment as a complicating condition would not be available. We strongly believe that the disease is not reasonably preventable. Adding it to the list would create a very expensive and unworkable situation for CMS, hospitals, physicians and patients.”
However, as we have seen in our above review of C. difficile infection, the fact is that the hand washing issue is controversial and basically not objectively ascertained with good RCTs. Nevertheless, one can agree that hand washing at least may be superior secondary to the mechanical shedding of C. difficile spores with vigorous hand washing.
One can agree with the arguments against adding C. difficile infection to the list of non-reimbursable hospital services because of its variable incubation period. Complicating the accurate diagnosis of CDAD is that, while symptoms typically occur within 48 h of infection, patients infected in the hospital with C. difficile usually become infected within 3 wk of admission. However, the onset of symptoms can be delayed by 2-3 mo[30]. Also, although most cases of CDAD occur on days 4-9 of antibiotic therapy[15], the subsequent diagnosis of CDAD is not always possible upon admission to the hospital, due to a variable incubation period. Therefore, one can agree that it is not reasonable to hold an inpatient hospital liable for a condition acquired in a different setting, one which is not even always detectable upon the patient’s admission into their setting.
The CMS accepted these arguments against including C. difficile infection in those nosocomial infections that are not reimbursable. However, that still does not alleviate the responsibility of each healthcare facility to make aggressive attempts to counteract this problem. One can look to successful efforts made by others.
The University of Pittsburgh Medical Center developed a program, as mentioned in our review, consisting of education, increased early case finding, expanded infection-control measures, development of a C. difficile infection management team, and microbial management. The aggregate rate of C. difficile infection decreased from 7.2 infections per 1000 (9.4 during a peak) hospital discharges to 4.8 infections per 1000 hospital discharges, and later, was 3.0 infections per 1000 hospital discharges. The rates of compliance with hand hygiene and isolation were 75% and 68%, respectively[185].
| 1. | Hookman P, Barkin JS. Review: Clostridium difficile-associated disorders/diarrhea and Clostridium difficile colitis: the emergence of a more virulent era. Dig Dis Sci. 2007;52:1071-1075. [Cited in This Article: ] |
| 2. | Hookman P, Barkin JS. Guidelines for prevention, surveillance, diagnosis and treatment in this new era of more virulent strains of antibiotic-associated diarrhea (AAD), Clostridium difficile-associ- ated diarrhea (CDAD) and Clostridium difficile colitis (CDAC). Pract Gastroenterol. 2006;30:65-82. [Cited in This Article: ] |
| 3. | Loo VG, Poirier L, Miller MA, Oughton M, Libman MD, Michaud S, Bourgault AM, Nguyen T, Frenette C, Kelly M. A predominantly clonal multi-institutional outbreak of Clostridium difficile-associated diarrhea with high morbidity and mortality. N Engl J Med. 2005;353:2442-2449. [Cited in This Article: ] |
| 4. | McDonald LC, Killgore GE, Thompson A, Owens RC Jr, Kazakova SV, Sambol SP, Johnson S, Gerding DN. An epidemic, toxin gene-variant strain of Clostridium difficile. N Engl J Med. 2005;353:2433-2441. [Cited in This Article: ] |
| 5. | Starr J. Clostridium difficile associated diarrhoea: diagnosis and treatment. BMJ. 2005;331:498-501. [Cited in This Article: ] |
| 6. | Bartlett JG. Antibiotic-associated diarrhea. Clin Infect Dis. 1992;15:573-581. [Cited in This Article: ] |
| 7. | Bartlett JG, Perl TM. The new Clostridium difficile--what does it mean? N Engl J Med. 2005;353:2503-2505. [Cited in This Article: ] |
| 8. | De Andrés S, Ibánez M, Ballesteros A, García B, Agud JL. Clostridium difficile colitis associated with valaciclovir. Pharm World Sci. 2004;26:8-9. [Cited in This Article: ] |
| 9. | Blossom DB, McDonald LC. The challenges posed by reemerging Clostridium difficile infection. Clin Infect Dis. 2007;45:222-227. [Cited in This Article: ] |
| 10. | Johal SS, Hammond J, Solomon K, James PD, Mahida YR. Clostridium difficile associated diarrhoea in hospitalised patients: onset in the community and hospital and role of flexible sigmoidoscopy. Gut. 2004;53:673-677. [Cited in This Article: ] |
| 11. | Cramer JP, Burchard GD, Lohse AW. [Old dogmas and new perspectives in antibiotic-associated diarrhea]. Med Klin (Munich). 2008;103:325-338; quiz 339-340. [Cited in This Article: ] |
| 12. | Blanckaert K, Coignard B, Grandbastien B, Astagneau P, Barbut F. [Update on Clostridium difficile infections]. Rev Med Interne. 2008;29:209-214. [Cited in This Article: ] |
| 13. | Fenger RV, Linneberg A, Tvede M, Ostergaard C. Increasing seroprevalence of Clostridium difficile in an adult Danish general population. Epidemiol Infect. 2009;137:278-283. [Cited in This Article: ] |
| 14. | Drudy D, Harnedy N, Fanning S, Hannan M, Kyne L. Emergence and control of fluoroquinolone-resistant, toxin A-negative, toxin B-positive Clostridium difficile. Infect Control Hosp Epidemiol. 2007;28:932-940. [Cited in This Article: ] |
| 15. | Schroeder MS. Clostridium difficile--associated diarrhea. Am Fam Physician. 2005;71:921-928. [Cited in This Article: ] |
| 16. | Zilberberg MD, Shorr AF, Kollef MH. Increase in adult Clostridium difficile-related hospitalizations and case-fatality rate, United States, 2000-2005. Emerg Infect Dis. 2008;14:929-931. [Cited in This Article: ] |
| 17. | Pépin J, Valiquette L, Cossette B. Mortality attributable to nosocomial Clostridium difficile-associated disease during an epidemic caused by a hypervirulent strain in Quebec. CMAJ. 2005;173:1037-1042. [Cited in This Article: ] |
| 18. | Pépin J, Saheb N, Coulombe MA, Alary ME, Corriveau MP, Authier S, Leblanc M, Rivard G, Bettez M, Primeau V. Emergence of fluoroquinolones as the predominant risk factor for Clostridium difficile-associated diarrhea: a cohort study during an epidemic in Quebec. Clin Infect Dis. 2005;41:1254-1260. [Cited in This Article: ] |
| 19. | Kuijper EJ, Coignard B, Tüll P. Emergence of Clostridium difficile-associated disease in North America and Europe. Clin Microbiol Infect. 2006;12 Suppl 6:2-18. [Cited in This Article: ] |
| 20. | Simango C, Mwakurudza S. Clostridium difficile in broiler chickens sold at market places in Zimbabwe and their antimicrobial susceptibility. Int J Food Microbiol. 2008;124:268-270. [Cited in This Article: ] |
| 21. | Koh TH, Tan AL, Tan ML, Wang G, Song KP. Epidemiology of Clostridium difficile infection in a large teaching hospital in Singapore. Pathology. 2007;39:438-442. [Cited in This Article: ] |
| 22. | Spigaglia P, Mastrantonio P. Molecular analysis of the pathogenicity locus and polymorphism in the putative negative regulator of toxin production (TcdC) among Clostridium difficile clinical isolates. J Clin Microbiol. 2002;40:3470-3475. [Cited in This Article: ] |
| 23. | Popoff MR, Rubin EJ, Gill DM, Boquet P. Actin-specific ADP-ribosyltransferase produced by a Clostridium difficile strain. Infect Immun. 1988;56:2299-2306. [Cited in This Article: ] |
| 24. | McEllistrem MC, Carman RJ, Gerding DN, Genheimer CW, Zheng L. A hospital outbreak of Clostridium difficile disease associated with isolates carrying binary toxin genes. Clin Infect Dis. 2005;40:265-272. [Cited in This Article: ] |
| 25. | Barbut F, Decré D, Lalande V, Burghoffer B, Noussair L, Gigandon A, Espinasse F, Raskine L, Robert J, Mangeol A. Clinical features of Clostridium difficile-associated diarrhoea due to binary toxin (actin-specific ADP-ribosyltransferase)-producing strains. J Med Microbiol. 2005;54:181-185. [Cited in This Article: ] |
| 26. | Warny M, Pepin J, Fang A, Killgore G, Thompson A, Brazier J, Frost E, McDonald LC. Toxin production by an emerging strain of Clostridium difficile associated with outbreaks of severe disease in North America and Europe. Lancet. 2005;366:1079-1084. [Cited in This Article: ] |
| 27. | Kuijper EJ, Barbut F, Brazier JS, Kleinkauf N, Eckmanns T, Lambert ML, Drudy D, Fitzpatrick F, Wiuff C, Brown DJ. Update of Clostridium difficile infection due to PCR ribotype 027 in Europe, 2008. Euro Surveill. 2008;366:13. [Cited in This Article: ] |
| 28. | Rupnik M. Clostridium difficile toxinotypes. University of Ljubljana: Ljubljana 2006; URL: http://www.mf.uni-mb.si/Mikro/tox/. [Cited in This Article: ] |
| 29. | Rupnik M, Avesani V, Janc M, von Eichel-Streiber C, Delmée M. A novel toxinotyping scheme and correlation of toxinotypes with serogroups of Clostridium difficile isolates. J Clin Microbiol. 1998;36:2240-2247. [Cited in This Article: ] |
| 30. | McFarland LV. Update on the changing epidemiology of Clostridium difficile-associated disease. Nat Clin Pract Gastroenterol Hepatol. 2008;5:40-48. [Cited in This Article: ] |
| 31. | Savidge TC, Pan WH, Newman P, O'brien M, Anton PM, Pothoulakis C. Clostridium difficile toxin B is an inflammatory enterotoxin in human intestine. Gastroenterology. 2003;125:413-420. [Cited in This Article: ] |
| 32. | Rupnik M, Dupuy B, Fairweather NF, Gerding DN, Johnson S, Just I, Lyerly DM, Popoff MR, Rood JI, Sonenshein AL. Revised nomenclature of Clostridium difficile toxins and associated genes. J Med Microbiol. 2005;54:113-117. [Cited in This Article: ] |
| 33. | Stabler RA, Dawson LF, Phua LT, Wren BW. Comparative analysis of BI/NAP1/027 hypervirulent strains reveals novel toxin B-encoding gene (tcdB) sequences. J Med Microbiol. 2008;57:771-775. [Cited in This Article: ] |
| 34. | Rupnik M, Kato N, Grabnar M, Kato H. New types of toxin A-negative, toxin B-positive strains among Clostridium difficile isolates from Asia. J Clin Microbiol. 2003;41:1118-1125. [Cited in This Article: ] |
| 35. | Drudy D, Quinn T, O'Mahony R, Kyne L, O'Gaora P, Fanning S. High-level resistance to moxifloxacin and gatifloxacin associated with a novel mutation in gyrB in toxin-A-negative, toxin-B-positive Clostridium difficile. J Antimicrob Chemother. 2006;58:1264-1267. [Cited in This Article: ] |
| 36. | Shin BM, Kuak EY, Yoo HM, Kim EC, Lee K, Kang JO, Whang DH, Shin JH. Multicentre study of the prevalence of toxigenic Clostridium difficile in Korea: results of a retrospective study 2000-2005. J Med Microbiol. 2008;57:697-701. [Cited in This Article: ] |
| 37. | Shin BM, Kuak EY, Yoo SJ, Shin WC, Yoo HM. Emerging toxin A-B+ variant strain of Clostridium difficile responsible for pseudomembranous colitis at a tertiary care hospital in Korea. Diagn Microbiol Infect Dis. 2008;60:333-337. [Cited in This Article: ] |
| 38. | Wershil BK, Castagliuolo I, Pothoulakis C. Direct evidence of mast cell involvement in Clostridium difficile toxin A-induced enteritis in mice. Gastroenterology. 1998;114:956-964. [Cited in This Article: ] |
| 39. | He D, Hagen SJ, Pothoulakis C, Chen M, Medina ND, Warny M, LaMont JT. Clostridium difficile toxin A causes early damage to mitochondria in cultured cells. Gastroenterology. 2000;119:139-150. [Cited in This Article: ] |
| 40. | Walsh SV, Hopkins AM, Chen J, Narumiya S, Parkos CA, Nusrat A. Rho kinase regulates tight junction function and is necessary for tight junction assembly in polarized intestinal epithelia. Gastroenterology. 2001;121:566-579. [Cited in This Article: ] |
| 41. | Voth DE, Ballard JD. Clostridium difficile toxins: mechanism of action and role in disease. Clin Microbiol Rev. 2005;18:247-263. [Cited in This Article: ] |
| 42. | Zasloff M. Antimicrobial peptides of multicellular organisms. Nature. 2002;415:389-395. [Cited in This Article: ] |
| 43. | Lehrer RI, Bevins CL, Ganz T. Defensins and other antimicrobial peptides. Mucosal Immunology. 3rd ed. Academic Press: New York 2005; 95-110. [Cited in This Article: ] |
| 44. | Ganz T. Defensins: antimicrobial peptides of innate immunity. Nat Rev Immunol. 2003;3:710-720. [Cited in This Article: ] |
| 45. | Maemoto A, Qu X, Rosengren KJ, Tanabe H, Henschen-Edman A, Craik DJ, Ouellette AJ. Functional analysis of the alpha-defensin disulfide array in mouse cryptdin-4. J Biol Chem. 2004;279:44188-44196. [Cited in This Article: ] |
| 46. | Yang D, Chertov O, Bykovskaia SN, Chen Q, Buffo MJ, Shogan J, Anderson M, Schröder JM, Wang JM, Howard OM. Beta-defensins: linking innate and adaptive immunity through dendritic and T cell CCR6. Science. 1999;286:525-528. [Cited in This Article: ] |
| 47. | Yang D, Biragyn A, Hoover DM, Lubkowski J, Oppenheim JJ. Multiple roles of antimicrobial defensins, cathelicidins, and eosinophil-derived neurotoxin in host defense. Annu Rev Immunol. 2004;22:181-215. [Cited in This Article: ] |
| 48. | Salzman NH, Ghosh D, Huttner KM, Paterson Y, Bevins CL. Protection against enteric salmonellosis in transgenic mice expressing a human intestinal defensin. Nature. 2003;422:522-526. [Cited in This Article: ] |
| 49. | Ouellette AJ, Bevins CL. Paneth cell defensins and innate immunity of the small bowel. Inflamm Bowel Dis. 2001;7:43-50. [Cited in This Article: ] |
| 50. | Porter EM, Liu L, Oren A, Anton PA, Ganz T. Localization of human intestinal defensin 5 in Paneth cell granules. Infect Immun. 1997;65:2389-2395. [Cited in This Article: ] |
| 51. | Porter EM, Bevins CL, Ghosh D, Ganz T. The multifaceted Paneth cell. Cell Mol Life Sci. 2002;59:156-170. [Cited in This Article: ] |
| 52. | Hooper LV, Stappenbeck TS, Hong CV, Gordon JI. Angiogenins: a new class of microbicidal proteins involved in innate immunity. Nat Immunol. 2003;4:269-273. [Cited in This Article: ] |
| 53. | Cash HL, Whitham CV, Behrendt CL, Hooper LV. Symbiotic bacteria direct expression of an intestinal bactericidal lectin. Science. 2006;313:1126-1130. [Cited in This Article: ] |
| 54. | Bevins CL. The Paneth cell and the innate immune response. Curr Opin Gastroenterol. 2004;20:572-580. [Cited in This Article: ] |
| 55. | Hornef MW, Pütsep K, Karlsson J, Refai E, Andersson M. Increased diversity of intestinal antimicrobial peptides by covalent dimer formation. Nat Immunol. 2004;5:836-843. [Cited in This Article: ] |
| 56. | Giesemann T, Guttenberg G, Aktories K. Human alpha-defensins inhibit Clostridium difficile toxin B. Gastroenterology. 2008;134:2049-2058. [Cited in This Article: ] |
| 57. | Wang W, Mulakala C, Ward SC, Jung G, Luong H, Pham D, Waring AJ, Kaznessis Y, Lu W, Bradley KA. Retrocyclins kill bacilli and germinating spores of Bacillus anthracis and inactivate anthrax lethal toxin. J Biol Chem. 2006;281:32755-32764. [Cited in This Article: ] |
| 58. | Kim C, Slavinskaya Z, Merrill AR, Kaufmann SH. Human alpha-defensins neutralize toxins of the mono-ADP-ribosyltransferase family. Biochem J. 2006;399:225-229. [Cited in This Article: ] |
| 59. | Kim C, Kaufmann SH. Defensin: a multifunctional molecule lives up to its versatile name. Trends Microbiol. 2006;14:428-431. [Cited in This Article: ] |
| 60. | Kim C, Gajendran N, Mittrücker HW, Weiwad M, Song YH, Hurwitz R, Wilmanns M, Fischer G, Kaufmann SH. Human alpha-defensins neutralize anthrax lethal toxin and protect against its fatal consequences. Proc Natl Acad Sci USA. 2005;102:4830-4835. [Cited in This Article: ] |
| 61. | Kelly CP, LaMont JT. Clostridium difficile infection. Annu Rev Med. 1998;49:375-390. [Cited in This Article: ] |
| 62. | Lawrence SJ. Contemporary management of Clostridium difficile-associated disease. Gastroenterol Endoscopy News Spec Ed. 2007;49:35-40. [Cited in This Article: ] |
| 63. | Johnson S, Homann SR, Bettin KM, Quick JN, Clabots CR, Peterson LR, Gerding DN. Treatment of asymptomatic Clostridium difficile carriers (fecal excretors) with vancomycin or metronidazole. A randomized, placebo-controlled trial. Ann Intern Med. 1992;117:297-302. [Cited in This Article: ] |
| 64. | Riggs MM, Sethi AK, Zabarsky TF, Eckstein EC, Jump RL, Donskey CJ. Asymptomatic carriers are a potential source for transmission of epidemic and nonepidemic Clostridium difficile strains among long-term care facility residents. Clin Infect Dis. 2007;45:992-998. [Cited in This Article: ] |
| 65. | McFarland LV, Mulligan ME, Kwok RY, Stamm WE. Nosocomial acquisition of Clostridium difficile infection. N Engl J Med. 1989;320:204-210. [Cited in This Article: ] |
| 66. | Kyne L, Warny M, Qamar A, Kelly CP. Asymptomatic carriage of Clostridium difficile and serum levels of IgG antibody against toxin A. N Engl J Med. 2000;342:390-397. [Cited in This Article: ] |
| 67. | Freeman HJ. Recent developments on the role of Clostridium difficile in inflammatory bowel disease. World J Gastroenterol. 2008;14:2794-2796. [Cited in This Article: ] |
| 68. | Wanahita A, Goldsmith EA, Marino BJ, Musher DM. Clostridium difficile infection in patients with unexplained leukocytosis. Am J Med. 2003;115:543-546. [Cited in This Article: ] |
| 69. | Bulusu M, Narayan S, Shetler K, Triadafilopoulos G. Leukocytosis as a harbinger and surrogate marker of Clostridium difficile infection in hospitalized patients with diarrhea. Am J Gastroenterol. 2000;95:3137-3141. [Cited in This Article: ] |
| 70. | Triadafilopoulos G, Hallstone AE. Acute abdomen as the first presentation of pseudomembranous colitis. Gastroenterology. 1991;101:685-691. [Cited in This Article: ] |
| 71. | Dansinger ML, Johnson S, Jansen PC, Opstad NL, Bettin KM, Gerding DN. Protein-losing enteropathy is associated with Clostridium difficile diarrhea but not with asymptomatic colonization: a prospective, case-control study. Clin Infect Dis. 1996;22:932-937. [Cited in This Article: ] |
| 72. | Rybolt AH, Bennett RG, Laughon BE, Thomas DR, Greenough WB 3rd, Bartlett JG. Protein-losing enteropathy associated with Clostridium difficile infection. Lancet. 1989;1:1353-1355. [Cited in This Article: ] |
| 73. | Rubin MS, Bodenstein LE, Kent KC. Severe Clostridium difficile colitis. Dis Colon Rectum. 1995;38:350-354. [Cited in This Article: ] |
| 74. | Birnbaum J, Bartlett JG, Gelber AC. Clostridium difficile: an under-recognized cause of reactive arthritis? Clin Rheumatol. 2008;27:253-255. [Cited in This Article: ] |
| 75. | Shaikh N, Kettern MA, Hanssens Y, Elshafie SS, Louon A. A rare and unsuspected complication of Clostridium difficile infection. Intensive Care Med. 2008;34:963-966. [Cited in This Article: ] |
| 76. | De Andrés S, Ferreiro D, Ibánez M, Ballesteros A, García B, Agud JL. Clostridium difficile colitis associated with valaciclovir. Pharm World Sci. 2004;26:8-9. [Cited in This Article: ] |
| 77. | Yearsley KA, Gilby LJ, Ramadas AV, Kubiak EM, Fone DL, Allison MC. Proton pump inhibitor therapy is a risk factor for Clostridium difficile-associated diarrhoea. Aliment Pharmacol Ther. 2006;24:613-619. [Cited in This Article: ] |
| 78. | Dial S, Delaney JA, Barkun AN, Suissa S. Use of gastric acid-suppressive agents and the risk of community-acquired Clostridium difficile-associated disease. JAMA. 2005;294:2989-2995. [Cited in This Article: ] |
| 79. | Cadle RM, Mansouri MD, Logan N, Kudva DR, Musher DM. Association of proton-pump inhibitors with outcomes in Clostridium difficile colitis. Am J Health Syst Pharm. 2007;64:2359-2363. [Cited in This Article: ] |
| 80. | Jump RL, Pultz MJ, Donskey CJ. Vegetative Clostridium difficile survives in room air on moist surfaces and in gastric contents with reduced acidity: a potential mechanism to explain the association between proton pump inhibitors and C. difficile-associated diarrhea? Antimicrob Agents Chemother. 2007;51:2883-2887. [Cited in This Article: ] |
| 81. | Rouphael NG, O'Donnell JA, Bhatnagar J, Lewis F, Polgreen PM, Beekmann S, Guarner J, Killgore GE, Coffman B, Campbell J. Clostridium difficile-associated diarrhea: an emerging threat to pregnant women. Am J Obstet Gynecol. 2008;198:635.e1-635.e6. [Cited in This Article: ] |
| 82. | Elixhauser A (AHRQ), Jhung MA. (Centers for Disease Control and Prevention). Clostridium Difficile-Associated Disease in U.S. Hospitals, 1993-2005. HCUP Statistical Brief #50. April 2008. Agency for Healthcare Research and Quality, Rockville, MD.. Available from: URL: http://www.hcup-us.ahrq.gov/reports/statbriefs/sb50.pdf. . [Cited in This Article: ] |
| 83. | Kurd MF, Pulido L, Joshi A, Purtill JJ, Parvizi J. Clostridium difficile infection after total joint arthroplasty: who is at risk? J Arthroplasty. 2008;23:839-842. [Cited in This Article: ] |
| 84. | Muñoz P, Giannella M, Alcalá L, Sarmiento E, Fernandez Yañez J, Palomo J, Catalán P, Carbone J, Bouza E. Clostridium difficile-associated diarrhea in heart transplant recipients: is hypogammaglobulinemia the answer? J Heart Lung Transplant. 2007;26:907-914. [Cited in This Article: ] |
| 85. | Stelzmueller I, Goegele H, Biebl M, Wiesmayr S, Berger N, Tabarelli W, Ruttmann E, Albright J, Margreiter R, Fille M. Clostridium difficile colitis in solid organ transplantation--a single-center experience. Dig Dis Sci. 2007;52:3231-3236. [Cited in This Article: ] |
| 86. | Kawecki D, Chmura A, Pacholczyk M, Lagiewska B, Adadynski L, Wasiak D, Malkowski P, Sawicka-Grzelak A, Rokosz A, Szymanowska A. Detection of Clostridium difficile in stool samples from patients in the early period after liver transplantation. Transplant Proc. 2007;39:2812-2815. [Cited in This Article: ] |
| 87. | Carignan A, Allard C, Pépin J, Cossette B, Nault V, Valiquette L. Risk of Clostridium difficile infection after perioperative antibacterial prophylaxis before and during an outbreak of infection due to a hypervirulent strain. Clin Infect Dis. 2008;46:1838-1843. [Cited in This Article: ] |
| 88. | Zerey M, Paton BL, Lincourt AE, Gersin KS, Kercher KW, Heniford BT. The burden of Clostridium difficile in surgical patients in the United States. Surg Infect (Larchmt). 2007;8:557-566. [Cited in This Article: ] |
| 89. | Issa M, Vijayapal A, Graham MB, Beaulieu DB, Otterson MF, Lundeen S, Skaros S, Weber LR, Komorowski RA, Knox JF. Impact of Clostridium difficile on inflammatory bowel disease. Clin Gastroenterol Hepatol. 2007;5:345-351. [Cited in This Article: ] |
| 90. | Adams SD, Mercer DW. Fulminant Clostridium difficile colitis. Curr Opin Crit Care. 2007;13:450-455. [Cited in This Article: ] |
| 91. | Ananthakrishnan AN, McGinley EL, Binion DG. Excess hospitalisation burden associated with Clostridium difficile in patients with inflammatory bowel disease. Gut. 2008;57:205-210. [Cited in This Article: ] |
| 92. | Rodemann JF, Dubberke ER, Reske KA, Seo da H, Stone CD. Incidence of Clostridium difficile infection in inflammatory bowel disease. Clin Gastroenterol Hepatol. 2007;5:339-344. [Cited in This Article: ] |
| 93. | Scheurer D. Diagnostic and treatment delays in recurrent Clostridium difficile-associated disease. J Hosp Med. 2008;3:156-159. [Cited in This Article: ] |
| 94. | Fekety R, Shah AB. Diagnosis and treatment of Clostridium difficile colitis. JAMA. 1993;269:71-75. [Cited in This Article: ] |
| 95. | Song HJ, Shim KN, Jung SA, Choi HJ, Lee MA, Ryu KH, Kim SE, Yoo K. Antibiotic-associated diarrhea: candidate organisms other than Clostridium difficile. Korean J Intern Med. 2008;23:9-15. [Cited in This Article: ] |
| 96. | Riegler M, Sedivy R, Pothoulakis C, Hamilton G, Zacherl J, Bischof G, Cosentini E, Feil W, Schiessel R, LaMont JT. Clostridium difficile toxin B is more potent than toxin A in damaging human colonic epithelium in vitro. J Clin Invest. 1995;95:2004-2011. [Cited in This Article: ] |
| 97. | Seppälä K, Hjelt L, Sipponen P. Colonoscopy in the diagnosis of antibiotic-associated colitis. A prospective study. Scand J Gastroenterol. 1981;16:465-468. [Cited in This Article: ] |
| 98. | Tedesco FJ. Antibiotic associated pseudomembranous colitis with negative proctosigmoidoscopy examination. Gastroenterology. 1979;77:295-297. [Cited in This Article: ] |
| 99. | Shen BO, Jiang ZD, Fazio VW, Remzi FH, Rodriguez L, Bennett AE, Lopez R, Queener E, Dupont HL. Clostridium difficile infection in patients with ileal pouch-anal anastomosis. Clin Gastroenterol Hepatol. 2008;6:782-788. [Cited in This Article: ] |
| 100. | Lamontagne F, Labbé AC, Haeck O, Lesur O, Lalancette M, Patino C, Leblanc M, Laverdière M, Pépin J. Impact of emergency colectomy on survival of patients with fulminant Clostridium difficile colitis during an epidemic caused by a hypervirulent strain. Ann Surg. 2007;245:267-272. [Cited in This Article: ] |
| 101. | Hayetian FD, Read TE, Brozovich M, Garvin RP, Caushaj PF. Ileal perforation secondary to Clostridium difficile enteritis: report of 2 cases. Arch Surg. 2006;141:97-99. [Cited in This Article: ] |
| 102. | Vesoulis Z, Williams G, Matthews B. Pseudomembranous enteritis after proctocolectomy: report of a case. Dis Colon Rectum. 2000;43:551-554. [Cited in This Article: ] |
| 103. | Gooding IR, Springall R, Talbot IC, Silk DB. Idiopathic small-intestinal inflammation after colectomy for ulcerative colitis. Clin Gastroenterol Hepatol. 2008;6:707-709. [Cited in This Article: ] |
| 104. | Lundeen SJ, Otterson MF, Binion DG, Carman ET, Peppard WJ. Clostridium difficile enteritis: an early postoperative complication in inflammatory bowel disease patients after colectomy. J Gastrointest Surg. 2007;11:138-142. [Cited in This Article: ] |
| 105. | Tremaine WJ. Inflammatory Bowel Disease and Clostridium difficile-associated diarrhea: a growing problem. Clin Gastroenterol Hepatol. 2007;5:310-311. [Cited in This Article: ] |
| 106. | Verdoorn BP, Orenstein R, Wilson JW, Estes LL, Wendt RF, Schleck CD, Harmsen WS, Nyre LM, Patel R. Effect of telephoned notification of positive Clostridium difficile test results on the time to the ordering of antimicrobial therapy. Infect Control Hosp Epidemiol. 2008;29:658-660. [Cited in This Article: ] |
| 107. | Kuijper EJ, van Dissel JT, Wilcox MH. Clostridium difficile: changing epidemiology and new treatment options. Curr Opin Infect Dis. 2007;20:376-383. [Cited in This Article: ] |
| 108. | Peterson LR, Manson RU, Paule SM, Hacek DM, Robicsek A, Thomson RB Jr, Kaul KL. Detection of toxigenic Clostridium difficile in stool samples by real-time polymerase chain reaction for the diagnosis of C. difficile-associated diarrhea. Clin Infect Dis. 2007;45:1152-1160. [Cited in This Article: ] |
| 109. | O'Connor D, Hynes P, Cormican M, Collins E, Corbett-Feeney G, Cassidy M. Evaluation of methods for detection of toxins in specimens of feces submitted for diagnosis of Clostridium difficile-associated diarrhea. J Clin Microbiol. 2001;39:2846-2849. [Cited in This Article: ] |
| 110. | Turgeon DK, Novicki TJ, Quick J, Carlson L, Miller P, Ulness B, Cent A, Ashley R, Larson A, Coyle M. Six rapid tests for direct detection of Clostridium difficile and its toxins in fecal samples compared with the fibroblast cytotoxicity assay. J Clin Microbiol. 2003;41:667-670. [Cited in This Article: ] |
| 111. | Vanpoucke H, De Baere T, Claeys G, Vaneechoutte M, Verschraegen G. Evaluation of six commercial assays for the rapid detection of Clostridium difficile toxin and/or antigen in stool specimens. Clin Microbiol Infect. 2001;7:55-64. [Cited in This Article: ] |
| 112. | Yücesoy M, McCoubrey J, Brown R, Poxton IR. Detection of toxin production in Clostridium difficile strains by three different methods. Clin Microbiol Infect. 2002;8:413-418. [Cited in This Article: ] |
| 113. | Killgore G, Thompson A, Johnson S, Brazier J, Kuijper E, Pepin J, Frost EH, Savelkoul P, Nicholson B, van den Berg RJ. Comparison of seven techniques for typing international epidemic strains of Clostridium difficile: restriction endonuclease analysis, pulsed-field gel electrophoresis, PCR-ribotyping, multilocus sequence typing, multilocus variable-number tandem-repeat analysis, amplified fragment length polymorphism, and surface layer protein A gene sequence typing. J Clin Microbiol. 2008;46:431-437. [Cited in This Article: ] |
| 114. | Merz CS, Kramer C, Forman M, Gluck L, Mills K, Senft K, Steiman I, Wallace N, Charache P. Comparison of four commercially available rapid enzyme immunoassays with cytotoxin assay for detection of Clostridium difficile toxin(s) from stool specimens. J Clin Microbiol. 1994;32:1142-1147. [Cited in This Article: ] |
| 115. | Altindiş M, Usluer S, Ciftçi H, Tunç N, Cetinkaya Z, Aktepe OC. [Investigation of the presence of Clostridium difficile in antibiotic associated diarrhea patients by culture and toxin detection methods]. Mikrobiyol Bul. 2007;41:29-37. [Cited in This Article: ] |
| 116. | Gerding DN, Muto CA, Owens RC Jr. Measures to control and prevent Clostridium difficile infection. Clin Infect Dis. 2008;46 Suppl 1:S43-S49. [Cited in This Article: ] |
| 117. | Modena S, Gollamudi S, Friedenberg F. Continuation of antibiotics is associated with failure of metronidazole for Clostridium difficile-associated diarrhea. J Clin Gastroenterol. 2006;40:49-54. [Cited in This Article: ] |
| 118. | Teasley DG, Gerding DN, Olson MM, Peterson LR, Gebhard RL, Schwartz MJ, Lee JT Jr. Prospective randomised trial of metronidazole versus vancomycin for Clostridium-difficile-associated diarrhoea and colitis. Lancet. 1983;2:1043-1046. [Cited in This Article: ] |
| 119. | Zar FA, Bakkanagari SR, Moorthi KM, Davis MB. A comparison of vancomycin and metronidazole for the treatment of Clostridium difficile-associated diarrhea, stratified by disease severity. Clin Infect Dis. 2007;45:302-307. [Cited in This Article: ] |
| 120. | Al-Nassir WN, Sethi AK, Nerandzic MM, Bobulsky GS, Jump RL, Donskey CJ. Comparison of clinical and microbiological response to treatment of Clostridium difficile-associated disease with metronidazole and vancomycin. Clin Infect Dis. 2008;47:56-62. [Cited in This Article: ] |
| 121. | Bartlett JG. Narrative review: the new epidemic of Clostridium difficile-associated enteric disease. Ann Intern Med. 2006;145:758-764. [Cited in This Article: ] |
| 122. | Freeman J, Baines SD, Saxton K, Wilcox MH. Effect of metronidazole on growth and toxin production by epidemic Clostridium difficile PCR ribotypes 001 and 027 in a human gut model. J Antimicrob Chemother. 2007;60:83-91. [Cited in This Article: ] |
| 123. | McFarland LV, Elmer GW, Surawicz CM. Breaking the cycle: treatment strategies for 163 cases of recurrent Clostridium difficile disease. Am J Gastroenterol. 2002;97:1769-1775. [Cited in This Article: ] |
| 124. | Johnson S, Schriever C, Galang M, Kelly CP, Gerding DN. Interruption of recurrent Clostridium difficile-associated diarrhea episodes by serial therapy with vancomycin and rifaximin. Clin Infect Dis. 2007;44:846-848. [Cited in This Article: ] |
| 125. | Fernandez A, Anand G, Friedenberg F. Factors associated with failure of metronidazole in Clostridium difficile-associated disease. J Clin Gastroenterol. 2004;38:414-418. [Cited in This Article: ] |
| 126. | Kyne L, Kelly CP. Recurrent Clostridium difficile diarrhoea. Gut. 2001;49:152-153. [Cited in This Article: ] |
| 127. | Wenisch C, Parschalk B, Hasenhündl M, Hirschl AM, Graninger W. Comparison of vancomycin, teicoplanin, metronidazole, and fusidic acid for the treatment of Clostridium difficile-associated diarrhea. Clin Infect Dis. 1996;22:813-818. [Cited in This Article: ] |
| 128. | Pillai A, Nelson R. Probiotics for treatment of Clostridium difficile-associated colitis in adults. Cochrane Database Syst Rev. 2008;22:CD004611. [Cited in This Article: ] |
| 129. | McFarland LV, Surawicz CM, Greenberg RN, Fekety R, Elmer GW, Moyer KA, Melcher SA, Bowen KE, Cox JL, Noorani Z. A randomized placebo-controlled trial of Saccharomyces boulardii in combination with standard antibiotics for Clostridium difficile disease. JAMA. 1994;271:1913-1918. [Cited in This Article: ] |
| 130. | McFarland LV, Dublin S. Meta-analysis of probiotics for the treatment of irritable bowel syndrome. World J Gastroenterol. 2008;14:2650-2661. [Cited in This Article: ] |
| 131. | Surawicz CM, McFarland LV, Greenberg RN, Rubin M, Fekety R, Mulligan ME, Garcia RJ, Brandmarker S, Bowen K, Borjal D. The search for a better treatment for recurrent Clostridium difficile disease: use of high-dose vancomycin combined with Saccharomyces boulardii. Clin Infect Dis. 2000;31:1012-1017. [Cited in This Article: ] |
| 132. | Segarra-Newnham M. Probiotics for Clostridium difficile-associated diarrhea: focus on Lactobacillus rhamnosus GG and Saccharomyces boulardii. Ann Pharmacother. 2007;41:1212-1221. [Cited in This Article: ] |
| 133. | Borody TJ, Warren EF, Leis SM, Surace R, Ashman O, Siarakas S. Bacteriotherapy using fecal flora: toying with human motions. J Clin Gastroenterol. 2004;38:475-483. [Cited in This Article: ] |
| 134. | Borody TJ. "Flora Power"-- fecal bacteria cure chronic C. difficile diarrhea. Am J Gastroenterol. 2000;95:3028-3029. [Cited in This Article: ] |
| 135. | Aas J, Gessert CE, Bakken JS. Recurrent Clostridium difficile colitis: case series involving 18 patients treated with donor stool administered via a nasogastric tube. Clin Infect Dis. 2003;36:580-585. [Cited in This Article: ] |
| 136. | Bowden TA Jr, Mansberger AR Jr, Lykins LE. Pseudomembraneous enterocolitis: mechanism for restoring floral homeostasis. Am Surg. 1981;47:178-183. [Cited in This Article: ] |
| 137. | Eiseman B, Silen W, Bascom GS, Kauvar AJ. Fecal enema as an adjunct in the treatment of pseudomembranous enterocolitis. Surgery. 1958;44:854-859. [Cited in This Article: ] |
| 138. | Fløtterød O, Hopen G. [Refractory Clostridium difficile infection. Untraditional treatment of antibiotic-induced colitis]. Tidsskr Nor Laegeforen. 1991;111:1364-1365. [Cited in This Article: ] |
| 139. | Gustafsson A, Lund-Tønnesen S, Berstad A, Midtvedt T, Norin E. Faecal short-chain fatty acids in patients with antibiotic-associated diarrhoea, before and after faecal enema treatment. Scand J Gastroenterol. 1998;33:721-727. [Cited in This Article: ] |
| 140. | Lund-Tønnesen S, Berstad A, Schreiner A, Midtvedt T. [Clostridium difficile-associated diarrhea treated with homologous feces]. Tidsskr Nor Laegeforen. 1998;118:1027-1030. [Cited in This Article: ] |
| 141. | Persky SE, Brandt LJ. Treatment of recurrent Clostridium difficile-associated diarrhea by administration of donated stool directly through a colonoscope. Am J Gastroenterol. 2000;95:3283-3285. [Cited in This Article: ] |
| 142. | Schwan A, Sjölin S, Trottestam U, Aronsson B. Relapsing Clostridium difficile enterocolitis cured by rectal infusion of normal faeces. Scand J Infect Dis. 1984;16:211-215. [Cited in This Article: ] |
| 143. | Tvede M, Rask-Madsen J. Bacteriotherapy for chronic relapsing Clostridium difficile diarrhoea in six patients. Lancet. 1989;1:1156-1160. [Cited in This Article: ] |
| 144. | You DM, Franzos MA, Holman RP. Successful treatment of fulminant Clostridium difficile infection with fecal bacteriotherapy. Ann Intern Med. 2008;148:632-633. [Cited in This Article: ] |
| 145. | Borody TJ, Warren EF, Leis S, Surace R, Ashman O. Treatment of ulcerative colitis using fecal bacteriotherapy. J Clin Gastroenterol. 2003;37:42-47. [Cited in This Article: ] |
| 146. | McVay CS, Rolfe RD. In vitro and in vivo activities of nitazoxanide against Clostridium difficile. Antimicrob Agents Chemother. 2000;44:2254-2258. [Cited in This Article: ] |
| 147. | Musher DM, Logan N, Hamill RJ, Dupont HL, Lentnek A, Gupta A, Rossignol JF. Nitazoxanide for the treatment of Clostridium difficile colitis. Clin Infect Dis. 2006;43:421-427. [Cited in This Article: ] |
| 148. | Pankuch GA, Appelbaum PC. Activities of tizoxanide and nitazoxanide compared to those of five other thiazolides and three other agents against anaerobic species. Antimicrob Agents Chemother. 2006;50:1112-1117. [Cited in This Article: ] |
| 149. | Citron DM, Tyrrell KL, Warren YA, Fernandez H, Merriam CV, Goldstein EJ. In vitro activities of tinidazole and metronidazole against Clostridium difficile, Prevotella bivia and Bacteroides fragilis. Anaerobe. 2005;11:315-317. [Cited in This Article: ] |
| 150. | Fung HB, Doan TL. Tinidazole: a nitroimidazole antiprotozoal agent. Clin Ther. 2005;27:1859-1884. [Cited in This Article: ] |
| 151. | Gerber M, Ackermann G. OPT-80, a macrocyclic antimicrobial agent for the treatment of Clostridium difficile infections: a review. Expert Opin Investig Drugs. 2008;17:547-553. [Cited in This Article: ] |
| 152. | Finegold SM, Molitoris D, Vaisanen ML, Song Y, Liu C, Bolaños M. In vitro activities of OPT-80 and comparator drugs against intestinal bacteria. Antimicrob Agents Chemother. 2004;48:4898-902. [Cited in This Article: ] |
| 153. | Kokkotou E, Moss AC, Michos A, Espinoza D, Cloud JW, Mustafa N, O'Brien M, Pothoulakis C, Kelly CP. Comparative efficacies of rifaximin and vancomycin for treatment of Clostridium difficile-associated diarrhea and prevention of disease recurrence in hamsters. Antimicrob Agents Chemother. 2008;52:1121-1126. [Cited in This Article: ] |
| 154. | Boero M, Berti E, Morgando A, Verma G. Treatment for colitis caused by Clostridium difficile: results of a randomized open study of rifaximin versus vancomycin. Microbiologia Medica. 1990;5:74-77. [Cited in This Article: ] |
| 155. | Pullman J, Prieto J, Leach TS. Ramoplanin versus vancomycin in the treatment of Clostridium difficile diarrhea: a phase 2 study. Abstr. 44th Intersci. Conf.. : Antimicrob Agents Chemother 2004; abstract K-985a. [Cited in This Article: ] |
| 156. | Taylor CP, Tummala S, Molrine D, Davidson L, Farrell RJ, Lembo A, Hibberd PL, Lowy I, Kelly CP. Open-label, dose escalation phase I study in healthy volunteers to evaluate the safety and pharmacokinetics of a human monoclonal antibody to Clostridium difficile toxin A. Vaccine. 2008;26:3404-3409. [Cited in This Article: ] |
| 157. | Hinkson PL, Dinardo C, DeCiero D, Klinger JD, Barker RH Jr. Tolevamer, an anionic polymer, neutralizes toxins produced by the BI/027 strains of Clostridium difficile. Antimicrob Agents Chemother. 2008;52:2190-2195. [Cited in This Article: ] |
| 158. | Jank T, Ziegler MO, Schulz GE, Aktories K. Inhibition of the glucosyltransferase activity of clostridial Rho/Ras-glucosylating toxins by castanospermine. FEBS Lett. 2008;582:2277-2282. [Cited in This Article: ] |
| 159. | Sougioultzis S, Kyne L, Drudy D, Keates S, Maroo S, Pothoulakis C, Giannasca PJ, Lee CK, Warny M, Monath TP. Clostridium difficile toxoid vaccine in recurrent C. difficile-associated diarrhea. Gastroenterology. 2005;128:764-770. [Cited in This Article: ] |
| 160. | Aronsson B, Granström M, Möllby R, Nord CE. Serum antibody response to Clostridium difficile toxins in patients with Clostridium difficile diarrhoea. Infection. 1985;13:97-101. [Cited in This Article: ] |
| 161. | Leung DY, Kelly CP, Boguniewicz M, Pothoulakis C, LaMont JT, Flores A. Treatment with intravenously administered gamma globulin of chronic relapsing colitis induced by Clostridium difficile toxin. J Pediatr. 1991;118:633-637. [Cited in This Article: ] |
| 162. | Warny M, Vaerman JP, Avesani V, Delmée M. Human antibody response to Clostridium difficile toxin A in relation to clinical course of infection. Infect Immun. 1994;62:384-389. [Cited in This Article: ] |
| 163. | Kotloff KL, Wasserman SS, Losonsky GA, Thomas W Jr, Nichols R, Edelman R, Bridwell M, Monath TP. Safety and immunogenicity of increasing doses of a Clostridium difficile toxoid vaccine administered to healthy adults. Infect Immun. 2001;69:988-995. [Cited in This Article: ] |
| 164. | McPherson S, Rees CJ, Ellis R, Soo S, Panter SJ. Intravenous immunoglobulin for the treatment of severe, refractory, and recurrent Clostridium difficile diarrhea. Dis Colon Rectum. 2006;49:640-645. [Cited in This Article: ] |
| 165. | Dallal RM, Harbrecht BG, Boujoukas AJ, Sirio CA, Farkas LM, Lee KK, Simmons RL. Fulminant Clostridium difficile: an underappreciated and increasing cause of death and complications. Ann Surg. 2002;235:363-372. [Cited in This Article: ] |
| 166. | Hall JF, Berger D. Outcome of colectomy for Clostridium difficile colitis: a plea for early surgical management. Am J Surg. 2008;196:384-388. [Cited in This Article: ] |
| 167. | Oughton MT, Miller MA. Clinical and Epidemiological Aspects of Clostridium difficile. Clin Microbiol Newsletter. 2008;30:87-95. [Cited in This Article: ] |
| 168. | Quinn LK, Chen Y, Herwaldt LA. Infection control policies and practices for Iowa long-term care facility residents with Clostridium difficile infection. Infect Control Hosp Epidemiol. 2007;28:1228-1232. [Cited in This Article: ] |
| 169. | Bobulsky GS, Al-Nassir WN, R iggs MM, Sethi AK, Donskey CJ. Clostridium difficile skin contamination in patients with C. difficile-associated disease. Clin Infect Dis. 2008;46:447-450. [Cited in This Article: ] |
| 170. | Davey P, Brown E, Fenelon L, Finch R, Gould I, Hartman G, Holmes A, Ramsay C, Taylor E, Wilcox M. Interventions to improve antibiotic prescribing practices for hospital inpatients. Cochrane Database Syst Rev. 2005;46:CD003543. [Cited in This Article: ] |
| 171. | Furuno JP, Harris AD, Wright MO, Hartley DM, McGregor JC, Gaff HD, Hebden JN, Standiford HC, Perencevich EN. Value of performing active surveillance cultures on intensive care unit discharge for detection of methicillin-resistant Staphylococcus aureus. Infect Control Hosp Epidemiol. 2007;28:666-670. [Cited in This Article: ] |
| 172. | Siegel JD, Rhinehart E, Jackson M, Chiarello L. 2007 Guideline for Isolation Precautions: Preventing Transmission of Infectious Agents in Healthcare Settings 2007. Centers for Disease Control and Prevention: Atlanta (GA) 2007; . [Cited in This Article: ] |
| 173. | Bettin K, Clabots C, Mathie P, Willard K, Gerding DN. Effectiveness of liquid soap vs. chlorhexidine gluconate for the removal of Clostridium difficile from bare hands and gloved hands. Infect Control Hosp Epidemiol. 1994;15:697-702. [Cited in This Article: ] |
| 174. | Boyce JM, Pittet D. Guideline for Hand Hygiene in Health-Care Settings. Recommendations of the Healthcare Infection Control Practices Advisory Committee and the HICPAC/SHEA/APIC/IDSA Hand Hygiene Task Force. Society for Healthcare Epidemiology of America/Association for Professionals in Infection Control/Infectious Diseases Society of America. MMWR Recomm Rep. 2002;51:1-45, quiz CE1-CE4. [Cited in This Article: ] |
| 175. | Boyce JM, Ligi C, Kohan C, Dumigan D, Havill NL. Lack of association between the increased incidence of Clostridium difficile-associated disease and the increasing use of alcohol-based hand rubs. Infect Control Hosp Epidemiol. 2006;27:479-483. [Cited in This Article: ] |
| 176. | Gordin FM, Schultz ME, Huber RA, Gill JA. Reduction in nosocomial transmission of drug-resistant bacteria after introduction of an alcohol-based handrub. Infect Control Hosp Epidemiol. 2005;26:650-653. [Cited in This Article: ] |
| 177. | Backman C, Zoutman DE, Marck PB. An integrative review of the current evidence on the relationship between hand hygiene interventions and the incidence of health care-associated infections. Am J Infect Control. 2008;36:333-348. [Cited in This Article: ] |
| 178. | McDonald LC. Clostridium difficile: responding to a new threat from an old enemy. Infect Control Hosp Epidemiol. 2005;26:672-675. [Cited in This Article: ] |
| 179. | Surawicz CM. Treatment of recurrent Clostridium difficile-associated disease. Nat Clin Pract Gastroenterol Hepatol. 2004;1:32-38. [Cited in This Article: ] |
| 180. | Fawley WN, Underwood S, Freeman J, Baines SD, Saxton K, Stephenson K, Owens RC Jr, Wilcox MH. Efficacy of hospital cleaning agents and germicides against epidemic Clostridium difficile strains. Infect Control Hosp Epidemiol. 2007;28:920-925. [Cited in This Article: ] |
| 181. | White LF, Dancer SJ, Robertson C. A microbiological evaluation of hospital cleaning methods. Int J Environ Health Res. 2007;17:285-295. [Cited in This Article: ] |
| 182. | Wheeldon LJ, Worthington T, Lambert PA, Hilton AC, Lowden CJ, Elliott TS. Antimicrobial efficacy of copper surfaces against spores and vegetative cells of Clostridium difficile: the germination theory. J Antimicrob Chemother. 2008;62:522-525. [Cited in This Article: ] |
| 183. | Gant VA, Wren MW, Rollins MS, Jeanes A, Hickok SS, Hall TJ. Three novel highly charged copper-based biocides: safety and efficacy against healthcare-associated organisms. J Antimicrob Chemother. 2007;60:294-299. [Cited in This Article: ] |
| 184. | Shen EP, Surawicz CM. The changing face of Clostridium difficile: what treatment options remain? Am J Gastroenterol. 2007;102:2789-2792. [Cited in This Article: ] |
| 185. | Muto CA, Blank MK, Marsh JW, Vergis EN, O'Leary MM, Shutt KA, Pasculle AW, Pokrywka M, Garcia JG, Posey K. Control of an outbreak of infection with the hypervirulent Clostridium difficile BI strain in a university hospital using a comprehensive "bundle" approach. Clin Infect Dis. 2007;45:1266-1273. [Cited in This Article: ] |