Published online Mar 14, 2011. doi: 10.3748/wjg.v17.i10.1336
Revised: December 16, 2010
Accepted: December 23, 2010
Published online: March 14, 2011
AIM: To evaluate the efficacy of non-sequential narrow band imaging (NBI) for a better recognition of gastric intestinal metaplasia (GIM).
METHODS: Previously diagnosed GIM patients underwent targeted biopsy from areas with and without GIM, as indicated by NBI, twice at an interval of 1 year. The authors compared the endoscopic criteria such as light blue crest (LBC), villous pattern (VP), and large long crest (LLC) with standard histology. The results from two surveillance endoscopies were compared with histology results for sensitivity, specificity, positive predictive value (PPV), negative predictive value (NPV), and likelihood ratio of positive test (LR+). The number of early gastric cancer cases detected was also reported.
RESULTS: NBI targeted biopsy was performed in 38 and 26 patients during the first and second surveillance endoscopies, respectively. There were 2 early gastric cancers detected in the first endoscopy. No cancer was detected from the second study. Surgical and endoscopic resections were successfully performed in each patient. Sensitivity, specificity, PPV, NPV, and LR+ of all 3 endoscopic criteria during the first/second surveillances were 78.8%/91.3%, 82.5%/89.1%, 72.8%/77.8%, 86.8%/96.1, and 4.51/8.4, respectively. LBC provided the highest LR+ over VP and LLC.
CONCLUSION: Non-sequential NBI is useful for GIM targeted biopsy. LBC provides the most sensitive reading. However, the optimal duration between two surveillances requires further study.
- Citation: Rerknimitr R, Imraporn B, Klaikeaw N, Ridtitid W, Jutaghokiat S, Ponauthai Y, Kongkam P, Kullavanijaya P. Non-sequential narrow band imaging for targeted biopsy and monitoring of gastric intestinal metaplasia. World J Gastroenterol 2011; 17(10): 1336-1342
- URL: https://www.wjgnet.com/1007-9327/full/v17/i10/1336.htm
- DOI: https://dx.doi.org/10.3748/wjg.v17.i10.1336
In Correa’s gastric cancer cascade, gastric intestinal metaplasia (GIM) is an important precancerous lesion for the development of intestinal type gastric cancer[1,2]. Although diffuse type gastric cancer can arise independently of GIM, in many cases GIM may be found nearby[3]. The benefit of annual endoscopic surveillance in these high risk patients has been shown to improve survival by enhancing the detection of gastric cancer at the early stage[4]. However, conventional white light endoscopy has a limitation in selection of the area for surveillance biopsy[5]. Multiple random biopsies under white light endoscopy to detect GIM are quite cumbersome as they are time-consuming and have the possibility of missing a small lesion. Methylene blue chromoendoscopy is the only technique that provides a good validity score for GIM targeted biopsy[6,7]. However, this technique still has a limitation because of the need for dye spraying. Moreover, it has been shown that DNA damage can occur in columnar cell-lined mucosa after chromoendoscopy with methylene blue, thus DNA damage to the GIM epithelium may also occur as it is also an intestinal type columnar cell[8]. Magnifying narrow band imaging (NBI) has been introduced which negates the need for dye spray. This technique provides better details of the mucosa and vascular patterns of minute lesions, including early gastric adenocarcinoma and GIM[9,10]. Uedo et al[11] reported on the excellent accuracy (91%) of the light blue crest (LBC) pattern detected by NBI in predicting the likelihood of GIM. Using a similar method but without magnification, a group from Missouri demonstrated that the ridge/villous pattern of the gastric mucosa detected by NBI was also useful for the diagnosis of GIM, with a sensitivity and specificity of 80% and 100%, respectively[12]. However, these pioneer investigators used the sequential NBI system that is mainly available in Japan and South Korea. This system is not widely available for other commercial users. The slight differences in color spectrum of sequential and non-sequential NBI processors provide some differences in color images[13]. Therefore the excellent results from the sequential system may not be replicable in the non-sequential system. To date there has been no study on the role of non-sequential NBI for the detection of precancerous gastric lesions. We therefore conducted a prospective endoscopic study to validate the feasibility of non-sequential NBI by using a combination of endoscopic criteria to determine the leading area for GIM biopsy.
In the non-sequential NBI system, there is a rotating interference narrow band filter (R/G/B). The specific colors are transferred directly without alteration in R/G/B color pattern from the color charge-couple device (CCD) as a picture containing different mucosal depth patterns. This incomplete image is further transformed with a matrix element, coefficient K, by a computer to a more complete image that provides a better contrast[14]. The scope that we used in this study was a magnifying gastroscope (Olympus GIF Q160Z, Olympus, Tokyo, Japan) which is compatible with the non-sequential light source and processor EVIS Exera II (Olympus, Tokyo, Japan). This scope had a zoom lens placed just distal to the CCD. The CCD was located at the tip of the endoscope, and the optical power of magnification was 115 times. To maintain the optimal distance of the magnifying focus, a transparent plastic cap was attached at the scope tip.
From November 2007 to May 2009, at the King Chulalongkorn Memorial Hospital Endoscopy Unit, we recruited all patients with GIM previously diagnosed by routine endoscopic biopsy. Generally, the initial endoscopy was performed for dyspepsia, and a random biopsy was performed at that time. Patients with abnormal coagulopathy, patients who refused to give informed consent, and patients who had previous subtotal gastrectomy were excluded from the study. There were 38 eligible patients enrolled who had a prior history of GIM. All patients underwent, 1 year apart, two upper endoscopies with NBI targeted biopsy. The last procedure was performed in May 2009. The study protocol was approved by the Ethical Committee of the Faculty of Medicine, Chulalongkorn University.
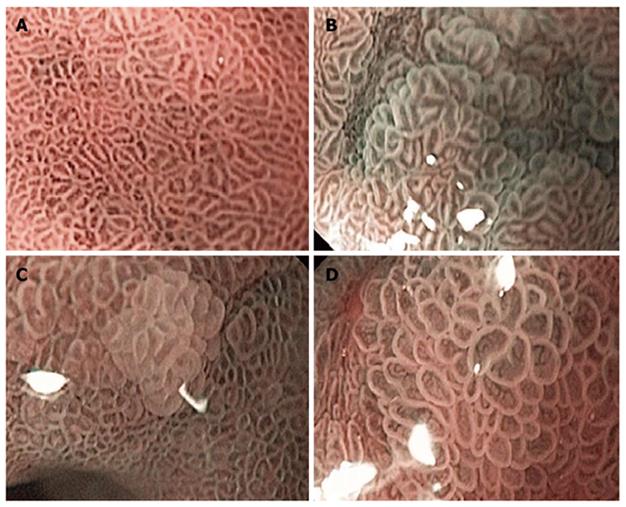
All of the endoscopic readings were performed at 115 x magnification. Based on the previous studies results[11,12], we selected three criteria as positive readings for GIM: (1) Normal gastric mucosa was defined as a uniform pattern of round pit gastric mucosa (Figure 1A); (2) Light blue crest (LBC) was defined as a fine, blue-white line on the crests of the epithelial surface, which looked like light reflection from the mirror (Figure 1B)[11]; (3) Villous pattern (VP) was defined as a raised area of villi above the gastric mucosal surface (Figure 1C)[12]; and (4) Large long crest (LLC) was defined as a combination of linear dark and light areas that differed from the normal gastric epithelium (Figure 1D)[12].
All endoscopies were performed by one gastroenterology fellow (BI) under supervision of one senior attending endoscopist (RR). Both endoscopists had experience in using NBI for other lesions, including colonic adenoma and minimal erosive reflux esophagitis, in more than 200 cases. After obtaining consent, 5 mg intravenous midazolam was injected to sedate each patient. In addition, 10 mg hyoscine was also given intravenously to decrease bowel movement for easier endoscopic visualization. Simethicone solution was used to reduce mucus and gas bubbles in the stomach. We elected to do biopsies from the antrum and incisura (area with the possible highest probability of GIM). In detail, a screening non-magnifying white light endoscopy was performed first. Then NBI was performed to target GIM. Six NBI snapshot images were obtained from four quadrants of the antrum and two areas of the gastric incisura. If there was any positive finding by one of the three criteria mentioned above (LBC or VP or LLC), that area was counted as positive for GIM and a targeted biopsy was taken from the positive lesion(s). If there was no positive finding from that area, one random biopsy was taken from that quadrant as a GIM negative specimen. Therefore, six magnifying images and their targeted biopsies were derived from each patient. Pilot cohorts (n = 10) were used for training in biopsy techniques and endoscopic readings. Helicobacter pylori infection was examined by a rapid urease test. Eradication of H. pylori was carried out if the urease test or histology was positive for infection. A standard triple therapy in this study consisted of amoxicillin, clarithromycin, and omeprazole given orally for 14 d. One year after the first endoscopy, eligible patients underwent the second surveillance gastrointestinal (GI) endoscopy, using a similar protocol to the first surveillance.
All gastric specimens were immersed in formalin and processed by embedding in a paraffin block. A 4 μm section of the specimen was later stained with hematoxylin-eosin, Alcian blue and Giemsa stain. A clinically blinded pathologist (NK) reviewed all the specimens. The updated Sydney classification[15] was referred to as the gold standard for gastric histological classification. Regarding GIM monitoring on each patient, a comparison was made between the first and second gastric biopsy results. Patients were classified as having persistence, regression, or progression of GIM based on the existence or absence of GIM on the first and second histologies.
The diagnostic accuracies of all three criteria (LBC or VP or LLC) were judged after the index endoscopy by comparing the endoscopic diagnosis with histology results from that site. A descriptive comparison between endoscopic and pathological readings was performed. The sensitivity, specificity, positive predictive value (PPV), negative predictive value (NPV), and likelihood ratio of positive test (LR+) for the diagnosis of GIM were calculated. The calculations were performed with SPSS statistical software (SPSS version 15, Inc., Chicago, IL, USA).
During the initial surveillance endoscopy, there were 38 patients (20 male, 18 female) with a previous diagnosis of GIM (Table 1). The mean age ± SD was 59.9 ± 11.5 years (range, 27-80 years). The median time from diagnosis of GIM to the first surveillance endoscopy was 2.2 years. The median interval between the two surveillance endoscopies was 1 year. Twenty-six patients returned for a second surveillance study. The reasons for those 12 patients not attending the second study were: being diagnosed as having another type of cancer and requiring further treatment (n = 4), withdrawal from the second study (n = 4), loss to follow-up (n = 2), and diagnosis with early gastric cancer from the first surveillance endoscopy (n = 2).
| Average age (yr), mean ± SD (range) | 59.9 ± 11.5 (27-80) |
| Gender | |
| Male:Female | 20:18 |
| Median interval duration after initial diagnosis (yr) | 1 |
| Type of GIM | All complete GIM |
| Positive rapid urease test in 1st endoscopy | 12/38 |
| Positive rapid urease test in 2nd endoscopy | 8/26 |
| Dyspeptic symptoms | 5/38 |
| Smoking | None |
| Family history of gastric cancer | None |
| Significant underlying disease | 5/38 |
From the first surveillance endoscopy, most of the background endoscopic findings indicated atrophic gastritis. Thirty one of 38 patients (81.6%) were found to have GIM. The overall number of positive specimens was 85/228 (37.3%). Each GIM positive patient had two specimens positive for GIM on average. All GIM cases were complete GIM (type I). Of these, 12 patients had a positive rapid urease test (31.6%) and all received standard triple therapy for eradication. Under standard white light endoscopy, five patients were suspected to have GIM because of a whitish color change with plaques or patches. With NBI, the sensitivity, specificity, PPV, NPV, and LR+ of the three endoscopic criteria were 78.8%, 82.5%, 72.8%, 86.8%, and 4.5, respectively, while LBC possessed the highest LR+ compared with VP and LLC (Table 2).
| Criteria | Sensitivity (%) | Specificity (%) | PPV (%) | NPV (%) | LR + (%) |
| LBC | 70.59 (59.57-79.72) | 83.22 (75.75-88.75) | 71.43 (60.37-80.49) | 82.64 (75.24-88.25) | 4.21 (2.85-6.21) |
| VP | 29.41 (20.28-40.43) | 97.90 (93.51-99.45) | 89.28 (70.63-97.19) | 70.00 (63.06-76.16) | 14.02 (4.36-45.03) |
| LLC | 17.65 (10.53-27.75) | 95.10 (89.79-97.84) | 68.18 (45.12-85.26) | 66.02 (59.06-72.36) | 3.60 (1.53-8.48) |
| All criteria | 78.82 (68.34-86.64) | 82.51 (75.08-88.16) | 72.83 (62.38-81.33) | 86.76 (79.63-98.73) | 4.51 (3.11-6.55) |
The first surveillance study was able to detect early gastric cancer in two patients. The first patient was diagnosed with GIM a year before recruitment. NBI was able to demonstrate a focal area of depressed mucosa with loss of the normal vascular pattern. At that time, the technique of endoscopic submucosal dissection (ESD) was not available in Thailand, hence the patient was sent for a subtotal gastrectomy. The gastric pathology confirmed a minute area of signet ring carcinoma involving only the submucosal area. The patient has been doing well without evidence of recurrence after a 2-year follow-up. The second patient had been treated as having a Helicobacter-associated antral ulcer, and found to have diffuse GIM. Six months later, at the first surveillance endoscopy, H. pylori eradication and healing of the previous ulcer were confirmed but a new elevated lesion with abnormal subepithelial vascular network in the lesser curvature was discovered. Pathology confirmed it as an intestinal type gastric adenocarcinoma. This patient underwent a successful ESD. The resected specimen showed malignant cells confined to the submucosal level without any vascular invasion.
During the second surveillance endoscopy, two patients still had persistent H. pylori infection confirmed by a rapid urease test. Six other patients became positive for H. pylori infection in the second rapid urease test. All these patients, including those with a persistent positive rapid urease result, received standard triple therapy for eradication. Clarithromycin was replaced with metronidazole for the second round of triple therapy in the two patients with persistent infection. During the second surveillance endoscopy, 17/26 patients were persistently positive for GIM, 5/26 had disappearance of GIM, and 4/26 were persistently negative for GIM. No new GIM was detected in those who were previously negative for GIM. No gastric cancer was found in this second study. The sensitivity, specificity, PPV, NPV, and LR+ from the second endoscopy were 91.3%, 89.1%, 77.8%, 96.1%, and 8.4, respectively (Table 3).
| Criteria | Sensitivity (%) | Specificity (%) | PPV (%) | NPV (%) | LR + (%) |
| LBC | 82.61 (68.05-91.68) | 92.73 (85.74-96.58) | 82.61 (68.05-91.68) | 92.73 (85.74-96.58) | 11.36 (5.75-22.42) |
| VP | 21.74 (11.45-36.76) | 98.15 (92.81-99.67) | 83.33 (50.88-97.06) | 74.65 (66.53-81.40) | 11.74 (2.67-51.48) |
| LLC | 43.48 (29.25-58.79) | 99.07 (94.20-99.95) | 95.24 (74.13-99.75) | 80.45 (72.49-86.61) | 46.95 (6.49-339.58) |
| ALL | 91.30 (78.31-97.17) | 89.09 (81.36-93.98) | 77.78 (64.05-87.52) | 96.07 (89.69-98.73) | 8.37 (4.87-14.38) |
Sequential NBI has been well recognized as an excellent tool to detect abnormal mucosal GI lesions in certain Asian countries such as Japan, Korea, and Hong Kong, and in some US/Europe research centers[16-18]. However, most GI endoscopists worldwide have no access to this system and instead, they are more familiar with the non-sequential system. Many endoscopists are impressed with the difference in the contrast and the brightness of the non-sequential image compared with those images published in the literature using sequential NBI[13,19].
Studies of the feasibility of NBI to detect a minute abnormality in the GI tract mucosa have been widely published[10-12,16-18]. Certain patterns of abnormal gastric mucosa have been described[16-18]. LBC is an important endoscopic marker for GIM described by distinctive sharp blue and white on the gastric epithelial crest. This lesion can be best assessed by at least 80 × magnification with NBI. When sequential NBI was applied in 107 consecutive GIM patients, Uedo et al[11] reported that LBC criteria had a sensitivity of 89% [95% confidence interval (CI): 83-96], a specificity of 93% (95% CI: 88-97), a PPV of 91% (95% CI: 85-96), a NPV of 92% (95% CI: 87-97), and an accuracy of 91% (95%CI: 88-95).
VP and LLC represent the thickness of the abnormal GIM epithelium on top of the regular gastric surface. In one non-magnifying sequential NBI study, Bansal et al[12] reported that the sensitivity, specificity, and PPV of the VP and LLC criteria (the original series naming villous and ridge patterns) to diagnose GIM were 80%, 100%, and 100%, respectively.
We found that LBC was the most sensitive recognized endoscopic pattern (71.2% and 82.6% during the first and second endoscopic surveillances, respectively). It seems that our LBC sensitivity was lower than the results reported by Uedo et al[11]. We speculate that different types of NBI may have had a bearing on the difference in the results. In addition, from our observation that the background gastric mucosa in Thais with GIM contained some gastric inflammation, determining LBC from an unclear gastric mucosal background can be quite difficult. Despite magnifying the images, we found less impressive results using VP and LLC criteria when compared with the original series results (46.2% during the first endoscopy and 65.2% during the second endoscopy). When we combined all three criteria together (LBC, VP and LLC), the overall sensitivities went up to 78.8% and 91.3% during the first and second surveillances respectively. In addition, our VP and LLC criteria provided comparable results on the specificity (82.7%-99.1%), PPV (71.4%-88.2%), NPV (69.7%-96.1%), and LR+ (69.2%-89.7%) with those of LBC.
When compared with the first surveillance results, the second surveillance in our study provided higher levels of all validity scores (sensitivity, specificity, PPV, NPV, and LR+). However, these did not reach statistical difference.
During the present study, we were able to detect two early gastric cancers. Both were detected during the first surveillance and underwent successful resection. After resection both patients have been doing well.
Although a gastric cancer screening and surveillance program is only available in certain countries such as Japan and Korea, many countries have seen an increase in the prevalence of this cancer[20]. In Thailand, gastric cancer screening has not yet become a national health policy due to an insufficient number of the endoscopists, and a lower prevalence of gastric cancer in Thailand[21].
H. pylori infection, smoking, chronic atrophic gastritis, heavy alcohol use, and several dietary factors have been recognized as risk factors for gastric cancer[22]. The potential factors for gastric cancer in our population are chronic/atrophic gastritis and H. pylori infection. The majority of our patients had chronic or atrophic gastritis as their background. H. pylori infection rates were found in one third. No other significant risk factors could be determined in our cohorts. None of our patients were smokers and none had a family history of gastric cancer. They were in middle age and the youngest patient was only 32 years old. Despite few risk factors being identified, we were able to detect two early gastric cancers from these cohorts.
To put this into a more practical surveillance protocol, we may have to look for patients with atrophic gastritis by standard white light endoscopy first and later do a targeted GIM biopsy by NBI. However, cost effectiveness may be an issue for a country with low gastric cancer prevalence, such as Thailand.
GIM can either progress or regress depending on its nature and environmental stimuli[23]. H. pylori eradication may help the regression of GIM. All patients infected with H. pylori (12/38; 31.6%) received an eradication regimen after the first surveillance. During the second surveillance, we found disappearance of GIM in only 5/26 patients (19.2%). Interestingly, those were patients with positive for H. pylori infection from the first study and GIM disappeared after H. pylori eradication (data not shown).
Furthermore, we identified two patients who had persistent infection with H. pylori during the follow-up endoscopy. These two received different triple therapy. In addition, six more patients had newly discovered H. pylori infections during the second endoscopy. All were patients persistently positive for GIM. The explanation for the finding of new infection was uncertain, but we speculate that these were false negative cases in the first urease tests. It had been our routine to discontinue proton pump inhibitor, H2 blocker, and antibiotics at least 2 wk prior to the test for H. pylori. Thus prior medication use was not a cause for our false negative. Gastric mucosa has dynamic regeneration. This regenerated mucosa may be a factor in the re-growth of the occult H. pylori infection.
The limitations of the present study are mainly the small number of patients and the short 1-year duration of follow-up. Some patients dropped out from the second surveillance endoscopy for a variety of reasons. Despite these limitations, we were able to identify two early gastric cancers (5.3%) from this high risk cohort, and this was the first time in Thailand that we were able to select a group that may gain benefit from this intensive endoscopic surveillance.
In conclusion, non-sequential NBI is an interesting technique that can facilitate GIM detection by targeted biopsy. It helps the detection of early gastric cancer in patients with atrophic gastritis and GIM. However, the precise protocol regarding the frequency and duration of follow-up, and how to identify this target group still requires further study.
Gastric intestinal metaplasia (GIM) is considered as a precancerous lesion. The benefit of annual endoscopic surveillance in patients with GIM has been shown to improve survival by enhancing the detection of gastric cancer at the early stage. However, conventional white light endoscopy has a limitation in its sensitivity. In addition, this technique is quite cumbersome because it is time consuming and may miss small lesions.
Previously, chromoendoscopy has been proven to provide a good validity score for GIM targeted biopsy. Without a need of dye spray and only switching on a button, magnifying NBI has become a good alternative for the detection of GI precancerous lesions including colonic polyps, Barrett esophagus, and GIM. However, these pioneer results were based on the sequential NBI system and there may be slight differences in color spectrum between sequential and non- sequential NBI.
The present study supports the majority of practices that use the non-sequential system. It showed a higher accuracy for targeted biopsy in GIM suspicious lesions over previously reports with conventional white light endoscopy.
Instead of using a dye spray, the non-sequential NBI system can be more practical in routine practice. With comparable accuracy to the sequential system, it provides high diagnostic yields for GIM detection.
Light blue crest (LBC) is defined as a fine, blue-white line on the crests of the epithelial surface, which looks like light reflection from a mirror. Villous pattern (VP) is defined as a raised area of villi above the gastric mucosal surface. Large long crest (LLC) is defined as a combination of linear dark and light areas that are different from normal gastric epithelium.
The present study described the usefulness of non-sequential NBI, that is much more available worldwide, for targeted biopsy of GIM and surveillance for early gastric cancer. This system showed high sensitivity, specificity, positive predictive value, negative predictive value, and likelihood ratio of positive test for GIM prediction. Among the three criteria purposed by the authors, LBC seems to provide the best diagnostic value. Although the number of patients was limited, these patients with GIM provided a good set for this observation.
Peer reviewer: Gyorgy Baffy, MD, PhD, Assistant Professor of Medicine, Harvard Medical School, Chief, Gastroenterology, VA Boston Healthcare System, 150 S, Huntington Ave, Rm A6-46, Boston, MA 02130, United States
S- Editor Sun H L- Editor Cant MR E- Editor Ma WH
| 1. | Correa P, Haenszel W, Cuello C, Zavala D, Fontham E, Zarama G, Tannenbaum S, Collazos T, Ruiz B. Gastric precancerous process in a high risk population: cross-sectional studies. Cancer Res. 1990;50:4731-4736. [Cited in This Article: ] |
| 2. | Correa P, Piazuelo MB. Natural history of Helicobacter pylori infection. Dig Liver Dis. 2008;40:490-496. [Cited in This Article: ] |
| 3. | Arista-Nasr J, Jiménez-Rosas F, Uribe-Uribe N, Herrera-Goepfert R, Lazos-Ochoa M. Pathological disorders of the gastric mucosa surrounding carcinomas and primary lymphomas. Am J Gastroenterol. 2001;96:1746-1750. [Cited in This Article: ] |
| 4. | Whiting JL, Sigurdsson A, Rowlands DC, Hallissey MT, Fielding JW. The long term results of endoscopic surveillance of premalignant gastric lesions. Gut. 2002;50:378-381. [Cited in This Article: ] |
| 5. | Sauerbruch T, Schreiber MA, Schüssler P, Permanetter W. Endoscopy in the diagnosis of gastritis. Diagnostic value of endoscopic criteria in relation to histological diagnosis. Endoscopy. 1984;16:101-104. [Cited in This Article: ] |
| 6. | Dinis-Ribeiro M, da Costa-Pereira A, Lopes C, Lara-Santos L, Guilherme M, Moreira-Dias L, Lomba-Viana H, Ribeiro A, Santos C, Soares J. Magnification chromoendoscopy for the diagnosis of gastric intestinal metaplasia and dysplasia. Gastrointest Endosc. 2003;57:498-504. [Cited in This Article: ] |
| 7. | Ojetti V, Persiani R, Nista EC, Rausei S, Lecca G, Migneco A, Cananzi FC, Cammarota G, D'Ugo D, Gasbarrini G. A case-control study comparing methylene blue directed biopsies and random biopsies for detecting pre-cancerous lesions in the follow-up of gastric cancer patients. Eur Rev Med Pharmacol Sci. 2007;11:291-296. [Cited in This Article: ] |
| 8. | Olliver JR, Wild CP, Sahay P, Dexter S, Hardie LJ. Chromoendoscopy with methylene blue and associated DNA damage in Barrett's oesophagus. Lancet. 2003;362:373-374. [Cited in This Article: ] |
| 9. | Kadowaki S, Tanaka K, Toyoda H, Kosaka R, Imoto I, Hamada Y, Katsurahara M, Inoue H, Aoki M, Noda T. Ease of early gastric cancer demarcation recognition: a comparison of four magnifying endoscopy methods. J Gastroenterol Hepatol. 2009;24:1625-1630. [Cited in This Article: ] |
| 10. | Nakayoshi T, Tajiri H, Matsuda K, Kaise M, Ikegami M, Sasaki H. Magnifying endoscopy combined with narrow band imaging system for early gastric cancer: correlation of vascular pattern with histopathology (including video). Endoscopy. 2004;36:1080-1084. [Cited in This Article: ] |
| 11. | Uedo N, Ishihara R, Iishi H, Yamamoto S, Yamamoto S, Yamada T, Imanaka K, Takeuchi Y, Higashino K, Ishiguro S. A new method of diagnosing gastric intestinal metaplasia: narrow-band imaging with magnifying endoscopy. Endoscopy. 2006;38:819-824. [Cited in This Article: ] |
| 12. | Bansal A, Ulusarac O, Mathur S, Sharma P. Correlation between narrow band imaging and nonneoplastic gastric pathology: a pilot feasibility trial. Gastrointest Endosc. 2008;67:210-216. [Cited in This Article: ] |
| 13. | Emura F, Saito Y, Ikematsu H. Narrow-band imaging optical chromocolonoscopy: advantages and limitations. World J Gastroenterol. 2008;14:4867-4872. [Cited in This Article: ] |
| 14. | Kuznetsov K, Lambert R, Rey JF. Narrow-band imaging: potential and limitations. Endoscopy. 2006;38:76-81. [Cited in This Article: ] |
| 15. | Dixon MF, Genta RM, Yardley JH, Correa P. Classification and grading of gastritis. The updated Sydney System. International Workshop on the Histopathology of Gastritis, Houston 1994. Am J Surg Pathol. 1996;20:1161-1181. [Cited in This Article: ] |
| 16. | Tahara T, Shibata T, Nakamura M, Yoshioka D, Okubo M, Arisawa T, Hirata I. Gastric mucosal pattern by using magnifying narrow-band imaging endoscopy clearly distinguishes histological and serological severity of chronic gastritis. Gastrointest Endosc. 2009;70:246-253. [Cited in This Article: ] |
| 17. | Kaise M, Kato M, Urashima M, Arai Y, Kaneyama H, Kanzazawa Y, Yonezawa J, Yoshida Y, Yoshimura N, Yamasaki T. Magnifying endoscopy combined with narrow-band imaging for differential diagnosis of superficial depressed gastric lesions. Endoscopy. 2009;41:310-315. [Cited in This Article: ] |
| 18. | Kanao H, Tanaka S, Oka S, Hirata M, Yoshida S, Chayama K. Narrow-band imaging magnification predicts the histology and invasion depth of colorectal tumors. Gastrointest Endosc. 2009;69:631-636. [Cited in This Article: ] |
| 19. | Muto M, Horimatsu T, Ezoe Y, Morita S, Miyamoto S. Improving visualization techniques by narrow band imaging and magnification endoscopy. J Gastroenterol Hepatol. 2009;24:1333-1346. [Cited in This Article: ] |
| 20. | Crew KD, Neugut AI. Epidemiology of gastric cancer. World J Gastroenterol. 2006;12:354-362. [Cited in This Article: ] |
| 21. | Suwanrungruang K, Wiangnon S, Sriamporn S, Sookprasert A, Rangsrikajee D, Kamsa-Ard S, Horsith S, Usantia P. Trends in incidences of stomach and colorectal cancer in Khon Kaen, Thailand 1985-2004. Asian Pac J Cancer Prev. 2006;7:623-626. [Cited in This Article: ] |
| 22. | Layke JC, Lopez PP. Gastric cancer: diagnosis and treatment options. Am Fam Physician. 2004;69:1133-1140. [Cited in This Article: ] |