Published online Jun 7, 2011. doi: 10.3748/wjg.v17.i21.2618
Revised: September 20, 2010
Accepted: September 27, 2010
Published online: June 7, 2011
Endoscopic submucosal dissection (ESD) is becoming a popular procedure for the diagnosis and treatment of superficial mucosal lesions, and has the advantage of en bloc resection which yields a higher complete resection and remission rate compared to endoscopic mucosal resection (EMR). However, the learning process of this advanced endoscopic procedure requires a lengthy training period and considerable experience to be proficient. A well framed training protocol which is safe, effective, easily reproducible and cost-effective is desirable to teach ESD. In addition, the training course may need to be tailored around settings such as ethnicity, culture, workload, and disease incidence. In Asian countries with a large volume of early gastric lesions which need endoscopic treatment, endoscopists would be able to learn ESD expanding their skills from EMR to ESD under the supervision of experts. Whereas, in Western countries due to the low incidence of superficial gastric tumors, trials have utilized simulator models to improve learning. In Korea, the Korean Society of Gastrointestinal Endoscopy (KSGE) is playing an important role in training many gastroenterologists who have shown an interest in performing ESD by providing an annual live demonstration and a nationwide tutoring program. The purpose of this article is to introduce our ESD tutoring experience, review the published papers related to this topic, and propose several suggestions for future directions in teaching and learning ESD.
- Citation: Kim EY, Jeon SW, Kim GH. Chicken soup for teaching and learning ESD. World J Gastroenterol 2011; 17(21): 2618-2622
- URL: https://www.wjgnet.com/1007-9327/full/v17/i21/2618.htm
- DOI: https://dx.doi.org/10.3748/wjg.v17.i21.2618
Endoscopic submucosal dissection (ESD) is an excellent procedure for the diagnosis and treatment of superficial mucosal lesions, but its clinical implementation has limitations due to technical difficulties and the risk of complications[1]. ESD has the advantage of en bloc resection, which yields a higher complete resection and remission rate compared to endoscopic mucosal resection (EMR). Because of this advantage, many gastroenterologists have shown an interest in performing ESD. Previous studies have indicated that ESD of gastric neoplasms is technically feasible when it is performed by competent endoscopists[2]. However, similar results from novice endoscopists are not expected since the learning process of this advanced endoscopic procedure is long and requires considerable experience. This is one of the reasons why a well framed training protocol is necessary for this difficult procedure. It is our understanding that the training program should be tailored around needs based on ethnicity, culture, and/or country since the incidence of disease and working environment may be different. However, the training program should focus on safety, effectiveness, easy reproducibility and cost-effectiveness regardless of conditions. In this article, we describe our teaching experience and revisit published papers related to the teaching and learning of ESD.
ESD was introduced in Korea in the late 1990s and is now widely accepted as one of the standard treatment modalities for early cancers and premalignant lesions of the stomach and colorectum. In 2008, according to the reference of the Korean Health Insurance Review and Assessment Service, ESD was performed in 70 tertiary hospitals, 3 community-based hospitals, and 1 primary physician center in Korea.
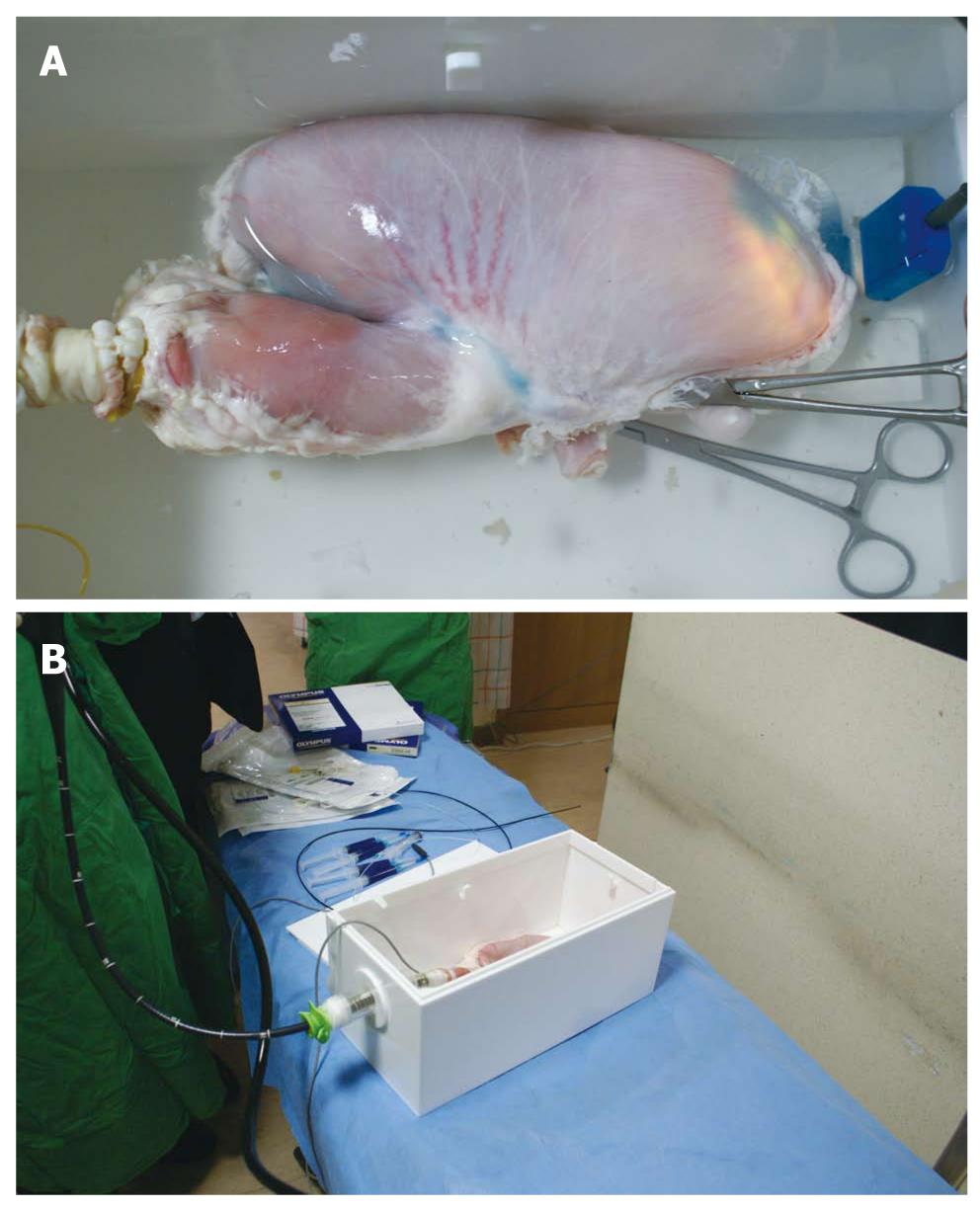
Live demonstrations have been held annually by the Korean Society of Gastrointestinal Endoscopy (KSGE) since 2004. It is a good opportunity for beginners to observe procedures performed by experts and to obtain information on ESD. However, we have noticed that simple observation of these procedures does not serve the needs of the endoscopists who are in the fellowship training course. For this reason, the ESD study group of KSGE held a nationwide tutoring program (7 provinces, 8 sessions) from January through October 2007. This program was divided into two sections and included a live demonstration of ESD in an ex vivo porcine stomach model and a hands-on training course using the same model. The well-irrigated porcine stomach was used in the experiment 24 h after the animal was slaughtered. The outlet portion of the stomach was closed with Kelly. A schematized frame was used for electrical patch attachment and water immersion was used for detection of air leakage. The inlet portion of the stomach was connected to the overtube and fixed within the frame (Figure 1). Before demonstrating the procedure, a mini lecture on the procedure was given to explain the procedure to the attendees. In the first section, tutors performed ESD with elaboration of the procedure. The endoscope was introduced through the overtube and the stomach was inflated with the endoscope. After selecting a target portion, the imaginary lesion was dissected after marking, injection and circumferential precutting. After completion of ESD, the integrity of the stomach wall was investigated by air insufflations with detection of leakage. In the second session, every participant performed this procedure once or twice under the supervision of the tutors. Although this program was not connected to the next step, such as in-vivo animal models or human studies, it permitted the trainee endoscopists to gain initial experience in performing this complex procedure and to accumulate knowledge on this therapeutic endoscopic technique. The previously mentioned annual live demonstrations by the KSGE provided valuable information for those who attended the training sessions and for those who led the courses.
In Korea, a large volume of cases which need endoscopic therapy, especially in the stomach, enables endoscopists to extend their procedure from EMR to ESD step by step. Some early frontiers of ESD in Korea have accumulated their expertise as the EMR technique has evolved. EMR after circumferential precutting (EMR-P), which was first described as endoscopic resection with local injection of hypertonic saline-epinephrine (ERHSE) by Hirao et al[3] for superficial lesions less than 2 cm, was a common challenge towards the next step. With the EMR-P method, lesions are resected by a snare after circumferential precutting and en bloc resection of the lesion with low risk of complications is possible. For lesions less than 2 cm in diameter, the rates of en bloc resection and complications are comparable for EMR-P and ESD[4]. Choi et al[5] demonstrated a learning curve for the EMR-P technique, reporting an increase in the en bloc resection rate from 45% to 85% after 40 cases by summarizing their evolving self-taught experience on the EMR-P technique. There were three perforations in the first 20 procedures (15%) and only one in the remaining 60 procedures (1.7%). They concluded that the trainee would need to perform 20-40 procedures to be able to use the technique safely and effectively. However, in the era of many ESD experts, the self-taught method of learning the EMR/ESD technique described in this study is unlikely to be acceptable as a good model for learning ESD[6].
In recognition of the complexity of ESD, the National Cancer Center Hospital in Japan, which is one of the highest-volume centers of ESD, has developed a rigorous training program. In this hospital, ESD is performed under the close supervision of an experienced endoscopist who offers advice and can complete the procedure when it is necessary for the benefit of the patient. In that setting, Gotoda et al[6] reported that experience of at least 30 cases is required for a beginner to gain early proficiency in this technique. In addition, they suggested that a major portion of the ESD training must be devoted to avoiding and managing its potential complications such as bleeding[7]. Recently, Yamamoto et al[8] reported a study on the assessment of the feasibility and learning curve in ESD performed by supervised residents. Before entry into this study, three supervised residents had experience of at least 1500 regular esophagogastroduodenal procedures and more than 10 EMRs. In addition, they had assisted ESD procedures performed by senior doctors for at least 1 year, and then attended a lecture on ESD techniques, using a manual and videos, by an experienced endoscopist. Each of them performed 30 consecutive ESD procedures for differentiated-type mucosal early gastric cancer without ulcers or scars, and smaller than 2 cm. Among the 90 procedures, there was a good overall complete resection rate of 93%, with an acceptable complication rate of 4.4%. The distribution of complete resection and complication rates were similar between operators. The self-completion rate and operation time were significantly worse for submucosal dissection than mucosal incision, which was mostly related to uncontrolled hemorrhage. Median operation time for mucosal incision did not change markedly and remained around 30 min for all operators. The median operation time for submucosal dissection became shorter than 30 min for one operator whose self-completion rate increased, but did not decrease for the other two operators. In this study, the authors stressed the importance of the assisting period. Trainees acquire the skills needed to troubleshoot various situations while assisting experienced endoscopists. Moreover, since most of the difficulties surrounding the procedure were related to uncontrollable hemorrhage, obtaining expertise in hemostasis before starting ESD is recommended. Kakushima et al[9] reported a learning curve for ESD for gastric epithelial neoplasms on the basis of the clinicopathological data from 383 ESD procedures by thirteen endoscopists. In study 1, the performance of the two principal operators (one performed 188 ESDs and the other performed 118 ESDs) was assessed every 25 cases. The endoscopist’s experience did not affect either the treatment efficacy or the safety profile. However, the procedure time decreased with experience, regardless of the increase in lesion size and the increase in resected specimen size. In study 2, the performance of all thirteen operators (11 operators experienced fewer than 30 cases) was assessed according to their experience. There was no significant difference in treatment efficacy and complication rates between the operators throughout the study period. The lesions were mainly located in the lower part of the stomach in the procedures performed by the 11 less experienced endoscopists. The procedure times shortened as experience in the method increased. In this study, the authors were not able to demonstrate an optimal number of cases required to gain adequate experience. However, they suggested that a beginner could start to treat lesions in the lower part of the stomach independently after performing about 30 supervised ESD procedures.
One prospective study on ESD using a porcine model was reported in Hong Kong[10]. Before entry into the study, an ESD training workshop was held. It consisted of three components: (1) three days of an advanced intensive endoscopic course providing lectures on the basics of ESD; (2) a live demonstration of ESD performed for gastric, esophageal, and colonic lesions; and (3) a hands-on practical session of ESD using a porcine model under the supervision of local and overseas faculties. Twenty-four endoscopists were included and performed gastric and esophageal ESD using a porcine model. The mean procedural times were 52.1 ± 24.7 min for gastric ESD and 32.5 ± 8.5 min for esophageal ESD. Surprisingly, during gastric ESD, 15 participants (65.2%) encountered perforations, whereas bleeding occurred during 13 ESDs (56.5%). There were two procedure-related mortalities. The initial performances of ESD were associated with a high incidence of complications, the risk of which was not dependent on previous experience in endoscopy. The majority of the participants in this study agreed that the porcine model would be an appropriate simulation of human ESDs. Live porcine ex vivo or computer simulation models have gained much acceptance for training in upper endoscopy, and some evidence suggests that prior training with these simulation models enhances skill acquisition[11,12]. The use of simulation models may be helpful in attaining the skills required for safe performance of ESD, especially in the areas of a low volume of ESDs. From our experience and that of endoscopists in Palo Alto, California, USA[6] and Hong Kong[10], harvested porcine organs and live porcine models seem to provide a potential solution for learning ESD. Harvested porcine organs are a ready-to-use and inexpensive means of becoming proficient in a novel technique. Multiple large resections of the esophagus and the stomach may be practiced before the use of a live porcine model. The live model simulates a more realistic setting for endoscopic procedures and provides the opportunity to respond to and to treat potential complications such as bleeding and perforation. The use of models allows endoscopists to obtain knowledge in a relatively short time period, with a tutor on site. Further mentoring during the subsequent initial clinical experience would complement the animal model experience. Nevertheless, it should be recognized that these simulation workshops are a means of augmenting training in skills in low-volume centers but will not replace patient-based training[11].
Vázquez-Sequeiros et al[13] from Spain reported their experience of learning and performing ESD in the absence of experts on ESD in their country. Four endoscopists with no experience in ESD underwent a four-step training program: (1) review of the literature and acquisition of theoretical concepts of ESD; (2) training in an ex-vivo animal model; (3) training in an in-vivo animal model; and (4) ESD of a gastric tumor in a patient. The four participants performed a total of 6 experiments using 6 porcine stomachs and esophagus for ex-vivo training. Six supervised ESDs were performed in a live porcine model under general anesthesia. After that, an ESD procedure in a patient was performed under general anesthesia in the operating room with a surgical team available. The procedure was successful but took quite a long time (210 min) and the resected specimen was 35 mm in size.
It has been reported that closely supervised trainees can perform advanced surgery such as esophagogastrectomy, hepatectomy[14], or pancreatectomy[15] with similar outcomes to consultant surgeons. In those studies, surgeons with a large workload encouraged trainees to accept more opportunities to participate in such complex operations, with appropriate supervision, because this improved their learning of the surgical methods and did not jeopardize patient care. Similarly, proficiency in ESD cannot be achieved without the availability of a highly experienced supervisor[8], because a significant number of cases were not completed by the trainee alone and complications such as perforations were generally managed by the supervisor.
In Asian countries such as Korea and Japan where there is a large volume of early gastric lesions which need endoscopic treatment, endoscopists can learn ESD expanding their skill from EMR to ESD under the supervision of experts. Initial experience in ESD with a simulator model and accumulation of experience during the assisting period and supervised EMR-P and ESD procedures for easier sites such as the gastric antrum would be right step.
On the other hand, it is difficult to overcome a flat learning curve due to a low case volume in Western countries. According to previously published data, Western countries report a lower rate of complete resection and higher morbidity following ESD compared to most trials in Asian countries[14]. In these countries more time should be devoted to indirect learning with literature and videos. ESD videos are available at some web sites such as DAVE Project (http://daveproject.org/) and published papers with videos. When available, unedited full length videos of other operators may be more valuable for learning purposes. Attending live demonstrations or learning from a DVD which explains ESD technique (such as the one edited by the American Society of Gastrointestinal Endoscopy) is also helpful. In addition, the target lesion for ESD is different in Western countries and ESD could be more frequently used for resection of neoplasia in Barrett’s esophagus or the colon due to their higher incidence compared with early gastric cancer[16,17]. Hence, ex-vivo and in-vivo training for esophageal and/or colonic ESD may be essential for Western trainees since ESD of these lesions is more difficult than gastric ESD[18].
ESD is a beneficial procedure which achieves higher rates of en bloc resection and complete resection for early cancer. However, training with a high enough volume to become proficient in ESD requires considerable time and patience especially in Western countries. A well structured training program is essential for the trainee, because the outcome of ESD is dependent on the experience of the endoscopist. Novice endoscopists can learn ESD by utilizing a simulation model, observing and/or assisting procedures performed by experts. The training course would be designed differently for Asian and Western countries according to the workload and incidence of disease. Close collaboration between Western and Asian countries will be helpful to improve ESD technique for various sites and to benefit patients who are suffering from early gastric, esophageal or colorectal cancer.
Peer reviewers: Yutaka Saito, Professor, Division of Endoscopy, National Cancer Center Hospital, 5-1-1, Tsukiji, Chuo-ku, Tokyo, 104-0045, Japan; Javier San Martín, MD, Chief, Gastroenterology and Endoscopy, Sanatorio Cantegril, Av. Roosevelt y P 13, Punta del Este 20100, Uruguay
S- Editor Wang JL L- Editor Webster JR E- Editor Ma WH
| 1. | Chung IK, Lee JH, Lee SH, Kim SJ, Cho JY, Cho WY, Hwangbo Y, Keum BR, Park JJ, Chun HJ. Therapeutic outcomes in 1000 cases of endoscopic submucosal dissection for early gastric neoplasms: Korean ESD Study Group multicenter study. Gastrointest Endosc. 2009;69:1228-1235. [Cited in This Article: ] |
| 2. | Takeuchi Y, Uedo N, Iishi H, Yamamoto S, Yamamoto S, Yamada T, Higashino K, Ishihara R, Tatsuta M, Ishiguro S. Endoscopic submucosal dissection with insulated-tip knife for large mucosal early gastric cancer: a feasibility study (with videos). Gastrointest Endosc. 2007;66:186-193. [Cited in This Article: ] |
| 3. | Hirao M, Masuda K, Nakamura M. [Endoscopic resection with local injection of HSE (ERHSE) in early gastric carcinomas]. Gan No Rinsho. 1986;32:1180-1184. [Cited in This Article: ] |
| 4. | Min BH, Lee JH, Kim JJ, Shim SG, Chang DK, Kim YH, Rhee PL, Kim KM, Park CK, Rhee JC. Clinical outcomes of endoscopic submucosal dissection (ESD) for treating early gastric cancer: comparison with endoscopic mucosal resection after circumferential precutting (EMR-P). Dig Liver Dis. 2009;41:201-209. [Cited in This Article: ] |
| 5. | Choi IJ, Kim CG, Chang HJ, Kim SG, Kook MC, Bae JM. The learning curve for EMR with circumferential mucosal incision in treating intramucosal gastric neoplasm. Gastrointest Endosc. 2005;62:860-865. [Cited in This Article: ] |
| 6. | Gotoda T, Friedland S, Hamanaka H, Soetikno R. A learning curve for advanced endoscopic resection. Gastrointest Endosc. 2005;62:866-867. [Cited in This Article: ] |
| 7. | Jeon SW, Jung MK, Cho CM, Tak WY, Kweon YO, Kim SK, Choi YH. Predictors of immediate bleeding during endoscopic submucosal dissection in gastric lesions. Surg Endosc. 2009;23:1974-1979. [Cited in This Article: ] |
| 8. | Yamamoto S, Uedo N, Ishihara R, Kajimoto N, Ogiyama H, Fukushima Y, Yamamoto S, Takeuchi Y, Higashino K, Iishi H. Endoscopic submucosal dissection for early gastric cancer performed by supervised residents: assessment of feasibility and learning curve. Endoscopy. 2009;41:923-928. [Cited in This Article: ] |
| 9. | Kakushima N, Fujishiro M, Kodashima S, Muraki Y, Tateishi A, Omata M. A learning curve for endoscopic submucosal dissection of gastric epithelial neoplasms. Endoscopy. 2006;38:991-995. [Cited in This Article: ] |
| 10. | Teoh AY, Chiu PW, Wong SK, Sung JJ, Lau JY, Ng EK. Difficulties and outcomes in starting endoscopic submucosal dissection. Surg Endosc. 2010;24:1049-1054. [Cited in This Article: ] |
| 11. | Nelson DB, Bosco JJ, Curtis WD, Faigel DO, Kelsey PB, Leung JW, Mills MR, Smith P, Tarnasky PR, VanDam J. ASGE technology evaluation report. Endoscopy simulators. May 1999. American Society for Gastrointestinal Endoscopy. Gastrointest Endosc. 1999;50:935-937. [Cited in This Article: ] |
| 12. | Sedlack R, Petersen B, Binmoeller K, Kolars J. A direct comparison of ERCP teaching models. Gastrointest Endosc. 2003;57:886-890. [Cited in This Article: ] |
| 13. | Vázquez-Sequeiros E, de Miquel DB, Olcina JR, Martín JA, García M, Lucas DJ, Garrido E, González C, Blanco AP, Arnau MR. Training model for teaching endoscopic submucosal dissection of gastric tumors. Rev Esp Enferm Dig. 2009;101:546-552. [Cited in This Article: ] |
| 14. | Paisley AM, Madhavan KK, Paterson-Brown S, Praseedom RK, Garden OJ. Role of the surgical trainee in upper gastrointestinal resectional surgery. Ann R Coll Surg Engl. 1999;81:40-45. [Cited in This Article: ] |
| 15. | Praseedom RK, Paisley A, Madhavan KK, Garden OJ, Carter DC, Paterson-Brown S. Supervised surgical trainees can perform pancreatic resections safely. J R Coll Surg Edinb. 1999;44:16-18. [Cited in This Article: ] |
| 16. | Neuhaus H. Endoscopic submucosal dissection in the upper gastrointestinal tract: present and future view of Europe. Dig Endosc. 2009;21 Suppl 1:S4-S6. [Cited in This Article: ] |
| 17. | Verna EC, Larghi A. Endoscopic submucosal dissection: learning from the Japanese experience. Dig Liver Dis. 2009;41:210-211. [Cited in This Article: ] |