Published online Jul 21, 2012. doi: 10.3748/wjg.v18.i27.3610
Revised: April 19, 2012
Accepted: April 22, 2012
Published online: July 21, 2012
AIM: To determine the prognostic value of lymphatic and/or blood vessel invasion (LBVI) in patients with stage II gastric cancer.
METHODS: From January 2001 to December 2006, 487 patients with histologically confirmed primary gastric adenocarcinoma were diagnosed with stage II gastric cancer according to the new 7th edition American Joint Committee on Cancer stage classification at the Department of Gastric Cancer and Soft Tissue Surgery, Fudan University Shanghai Cancer Center. All patients underwent curative gastrectomy with standard lymph node (LN) dissection. Fifty-one patients who died in the postoperative period, due to various complications or other conditions, were excluded. Clinicopathological findings and clinical outcomes were analyzed. Patients were subdivided into four groups according to the status of LBVI and LN metastases. These four patient groups were characterized with regard to age, sex, tumor site, pT category, tumor grading and surgical procedure (subtotal resection vs total resection), and compared for 5-year overall survival by univariate and multivariate analysis.
RESULTS: The study was composed of 320 men and 116 women aged 58.9 ± 11.5 years (range: 23-88 years). The 5-year overall survival rates were 50.7% and the median survival time was 62 mo. Stage IIa cancer was observed in 334 patients, including 268 T3N0, 63 T2N1, and three T1N2, and stage IIb was observed in 102 patients, including 49 patients T3N1, 51 T2N2, one T1N3, and one T4aN0. The incidence of LBVI was 28.0% in stage II gastric cancer with 19.0% (51/269) and 42.5% (71/167) in LN-negative and LN-positive patients, respectively. In 218 patients (50.0%), there was neither a histopathologically detectable LBVI nor LN metastases (LBVI−/LN−, group I); in 51 patients (11.7%), LBVI with no evidence of LN metastases was detected (LBVI+/LN−, group II). In 167 patients (38.3%), LN metastases were found. Among those patients, LBVI was not determined in 96 patients (22.0%) (LBVI−/LN+, group III), and was determined in 71 patients (16.3%) (LBVI+/LN+, group IV). Correlation analysis showed that N category and the number of positive LNs were significantly associated with the presence of LBVI (P < 0.001). The overall 5-year survival was significantly longer in LN-negative patients compared with LN-positive patients (56.1% vs 42.3%, P = 0.015). There was a significant difference in the overall 5-year survival between LBVI-positive and LBVI-negative tumors (39.6% vs 54.8%, P = 0.006). Overall 5-year survival rates in each group were 58.8% (I), 45.8% (II), 45.7% (III) and 36.9% (IV), and there was a significant difference in overall survival between the four groups (P = 0.009). Multivariate analysis in stage II gastric cancer patients revealed that LBVI independently affected patient prognosis in LN-negative patients (P = 0.018) but not in LN-positive patients (P = 0.508).
CONCLUSION: In LN-negative stage II gastric cancer patients, LBVI is an additional independent prognostic marker, and may provide useful information to identify patients with poorer prognosis.
- Citation: Du CY, Chen JG, Zhou Y, Zhao GF, Fu H, Zhou XK, Shi YQ. Impact of lymphatic and/or blood vessel invasion in stage II gastric cancer. World J Gastroenterol 2012; 18(27): 3610-3616
- URL: https://www.wjgnet.com/1007-9327/full/v18/i27/3610.htm
- DOI: https://dx.doi.org/10.3748/wjg.v18.i27.3610
Gastric cancer remains a major public health issue as the fourth most common cancer and second leading cause of cancer-related death worldwide despite a decrease in its incidence level[1-3]. Surgery, including gastrectomy in combination with systemic lymph node (LN) dissection, is the current treatment of choice for gastric cancer[4-6]. A correct definition of poor prognostic factors may help to guide more aggressive adjuvant treatment protocols. Therefore, it is urgently needed to identify new biological or pathological markers related to survival in addition to well-known prognostic factors such as the tumor-node-metastasis (TNM) staging classification and clinical stage.
According to the new American Joint Committee on Cancer (AJCC) TNM staging classification (7th edition), staging of gastric cancer by TNM classification is composed of nine groups[7]. Stage II gastric cancer is an intermediate stage between stage I and stage III. Stage II gastric cancer is defined as tumor that invades into or through the muscular wall of the stomach, but not into nearby local structures, or has regional LN involvement with any extent of primary cancer, but no invasion of local structures. Surgical resection with regional lymphadenectomy is the treatment of choice for patients with stage II gastric cancer. However, preoperative and intraoperative staging to confirm stage II disease is difficult. Failure to distinguish stage II from stage I disease may lead to under- or over-treatment. Therefore, the identification of additional prognostic factors, which is timely and cost-efficient as well as available, would help in detecting those patients with poorer prognosis among the different groups of patients with stage II gastric cancer. It might be of clinical significance to select candidates for treatment considerations, such as the extent of LN dissection and further adjuvant and neoadjuvant chemotherapy. Comparisons of survival in subgroups of stage II have been reported[8]. However, to date there is no study on the subgroups of stage II gastric cancer classified by the 7th edition of the AJCC TNM, and the prognostic factors for each subgroup.
As the cancer stage advances, tumor cells invade blood vessels and lymphatic vessels near the tumor; lymphatic and/or blood vessel tumor invasion (LBVI) is the critical step of tumor cell dissemination and metastasis in various types of cancer[4-6]. The prognostic significance of LBVI in gastric cancer has been previously investigated in a few studies, without reaching a consensus[7-11]. To date, LBVI has not been studied in patients with stage II disease alone.
In this study, we investigated the value of LBVI as a prognostic factor in patients with stage II gastric cancer who underwent curative resection. The association of LBVI with the clinicopathological factors and the effect of LBVI on survival were analyzed.
From January 2001 to December 2006, a total of 487 patients with histologically confirmed primary gastric adenocarcinoma were diagnosed with stage II gastric cancer according to new 7th edition TNM stage classification. All 487 patients underwent curative gastrectomy with standard LN dissection at the Department of Gastric Cancer and Soft Tissue Surgery, Fudan University Shanghai Cancer Center. Fifty-one patients who died in the postoperative period, due to various complications, or other conditions, were excluded from the study. Data were retrieved from operative and pathology reports, with follow-up data being obtained from the outpatient clinical database. All subjects were preoperatively diagnosed with gastric adenocarcinoma by analysis of endoscopic biopsy specimens. Standardized operative procedures were performed, such as total or subtotal gastrectomy, depending on the location of the gastric cancer, and D2 LN dissection according to the rules of the Japanese Research Society for Gastric Cancer. All chemotherapy for the enrolled patients was postoperative, and no patient with stage II gastric cancer underwent neoadjuvant chemotherapy. Patient survival was evaluated from information collected by using mail, telephone, or outpatient records.
All of the resected primary tumors and regional LNs were examined. The final diagnosis for each patient was decided by 2 pathologists to avoid misdiagnosis. Hematoxylin and eosin (HE) staining is commonly used for pathological examination. When it was difficult to identify the lymphatics or the venous structures by HE staining, either Elastica staining or Victoria-blue HE staining was performed. LBVI was defined according to the Japanese Classification of Gastric Carcinoma (absence vs presence). LBVI presence is defined as lymphovascular invasion and is detected in lymphatics or small veins in a mounted specimen containing the deepest portion of the tumor on a glass slide. The histology was grossly divided into the differentiated type (papillary and tubular adenocarcinoma) and the undifferentiated type (poorly differentiated adenocarcinoma, signet-ring cell carcinoma, mucinous carcinoma, and miscellaneous).
Gastric carcinoma was classified according to the new AJCC TNM staging criteria (7th edition). The clinical and pathological parameters evaluated included sex, age, depth of the tumor (T category), involvement of the LNs, and lymphatic and vascular invasion.
For the prognostic evaluation of isolated LBVI, the patients enrolled according to the criteria mentioned above were further subdivided into four prognostic groups: Group I: no detection of LBVI or any LN metastases (LBVI-/LN−); Group II: detection of LBVI but no LN metastases (LBVI+/LN-); Group III: detection of LN metastases but no detection of LBVI (LBVI−/LN+); Group IV: detection of LN metastases and LBVI (LBVI+/LN+). These four patient groups were characterized with regard to age, sex, tumor site, pT category, tumor grading, and surgical procedure (subtotal resection vs total resection) and compared with regard to the 5-year overall survival by univariate and multivariate analysis.
Statistical analysis was performed using SPSS 11.0 software. Survival analysis and curves were established according to the Kaplan-Meier method and compared by the log-rank test. Survival time was calculated from the month of surgery until the time of death or confirmation of survival, and survival rate was represented by the percentage of survivals at the end of the observed interval (in years and months). Multivariate analysis with Cox proportional hazard model was used to assess the role of LBVI and the other clinicopathological features as prognostic factors. P < 0.05 was considered significant.
The study was composed of 320 men and 116 women aged 58.9 ± 11.5 years (mean ± SD, range: 23-88 years). According to the new AJCC 7th edition TNM stage classification among those with stage II gastric cancers, stage IIa was observed in 334 patients, including 268 T3N0, 63 T2N1, and three T1N2, and stage IIb was observed in 102 patients, including 49 T3N1, 51 T2N2, one T1N3, and one T4aN0. Histological grade was reported as differentiated in 177 (40.6%) and differentiated in 259 (59.4%) cases. Total gastrectomy was performed in 113 (25.9%) cases, and subtotal gastrectomy in 323 (71.4%) cases. Overall follow-up ranged from 11 to 99 mo (median: 39 mo). The 5-year overall survival rates were 50.7% and the median survival time was 62 mo.
LBVI was identified in 122 (28.0%) and absent in 314 (72.0%) cases. When the patients were classified into two groups according to LBVI, the LBVI group had a higher incidence of LN metastasis (30.6% vs 58.2%, P = 0.032) and higher tumor stage (39.6% vs 54.8%, P < 0.001). However, other clinicopathological variables, such as age, sex, tumor stage, tumor size, operative methods, and histological type showed no significant differences between the two groups (Table 1).
| Clinicopathological factors | Tumor without lymphovascular invasion | Tumor with lymphovascular invasion | P value |
| Patients | 314 | 122 | |
| Age (yr) | 58.95 ± 11.88 | 58.84 ± 10.66 | 0.927 |
| Sex | |||
| Male | 235 (74.8) | 85 (69.7) | 0.279 |
| Female | 79 (25.2) | 37 (30.3) | |
| Tumor location | |||
| Upper | 110 (35.0) | 42 (34.4) | 0.733 |
| Middle | 57 (18.2) | 20 (16.4) | |
| Lower | 140 (44.6) | 55 (45.1) | |
| Whole | 7 (2.2) | 5 (4.1) | |
| Resection type | |||
| Subtotal | 236 (75.2) | 87 (71.3) | 0.465 |
| Total | 78 (24.8 | 35 (28.7) | |
| Tumor size (cm) | |||
| < 5 cm | 206 (65.6) | 82 (67.2) | 0.822 |
| ≥ 5 cm | 108 (34.4) | 40 (32.8) | |
| Histological type | |||
| Differentiated | 123 (39.2) | 54 (44.3) | 0.331 |
| Undifferentiated | 191 (60.8) | 68 (55.7) | |
| No. of retrieved LNs | 21.5 ± 5.5 | 22.3 ± 7.0 | 0.248 |
| LN | |||
| Positive | 96 (30.6) | 51 (41.8) | 0.032 |
| Negtive | 218 (69.4) | 71 (58.2) | |
| Stage | < 0.001 | ||
| IIa | 264 (84.1) | 70 (57.4) | |
| IIb | 50 (15.9) | 52 (42.6) | |
| Survival at 5 yr (%) | 39.6 | 54.8 | 0.006 |
The incidence of LBVI was 19.0% (51/269) and 42.5% (71/167) in LN-negative patients and LN-positive patients, respectively. In 218 patients (50.0%), there was neither a histopathologically detectable LBVI nor LN metastases (LBVI−/LN−, Group I); in 51 patients (11.7%), LBVI with no evidence of LN metastases was detected (LBVI+/LN-, Group II). In 167 patients (38.3%), LN metastases were found. Among these patients, LBVI was not determined in 96 patients (22.0%) (LBVI−/LN+, Group III), and was determined in 71 patients (16.3%) (LBVI+/LN+, Group IV). An overview on the patient groups is shown in Table 2. There were no significant differences between the four patient groups with regard to age (P = 0.367), sex ratio (P = 0.160), distribution of tumor sites (P = 0.959), surgical procedures (P = 0.328), total number of retrieved LNs (P = 0.413) and tumor size (P = 0.929) (Table 2).
| Group I (LBVI-/LN-) | Group II (LBVI+/LN-) | Group III (LBVI-/LN+) | Group IV (LBVI+/LN+) | P value | |
| Patients | 218 | 51 | 96 | 71 | |
| Age (yr) | 59.7 ± 11.5 | 59.4 ± 9.1 | 57.3 ± 12.6 | 58.4 ± 11.7 | 0.367 |
| Male:female | 3.36 | 1.68 | 2.31 | 2.94 | 0.160 |
| Tumor location | |||||
| Upper | 79 (36.2) | 17 (33.3) | 31 (32.3) | 25 (35.2) | 0.959 |
| Middle | 39 (17.9) | 8 (15.7) | 18 (18.8) | 12 (16.9) | |
| Lower | 96 (44.0) | 23 (45.1) | 44 (45.8) | 32 (45.1) | |
| Whole | 4 (1.8) | 3 (5.9) | 3 (3.1) | 2 (2.8) | |
| Tumor size | |||||
| ≤ 5 cm | 141 (64.7) | 35 (68.6) | 65 (67.7) | 47 (66.2) | 0.929 |
| > 5 cm | 77 (35.3) | 16 (31.4) | 31 (32.3) | 24 (33.8) | |
| No. of retrieved LNs | 21.2 ± 5.7 | 22.1 ± 8.0 | 22.0 ± 5.0 | 22.45 ± 6.2 | 0.413 |
| Histological type | |||||
| Differentiated | 85 (39.0) | 22 (43.1) | 38 (39.6) | 32 (45.1) | 0.801 |
| Undifferentiated | 133 (61.0) | 29 (56.9) | 58 (60.4) | 39 (59.4) | |
| Resection type | |||||
| Subtotal | 167 (76.6) | 33 (64.7) | 69 (71.9) | 54 (76.1) | 0.328 |
| Total | 51 (23.4) | 18 (35.3) | 27 (28.1) | 17 (23.9) | |
| 5-year overall survival (%) | 58.8 | 45.8 | 45.7 | 36.9 | 0.009 |
Correlation analysis with the Spearman correlation coefficient showed that LBVI and LN category or the number of positive LNs were significantly correlated (correlation coefficient 0.255, 0.317; P < 0.001). There was a significantly greater incidence and number of positive LNs when LBVI was present than when LBVI was absent. Of the 122 patients with LBVI+ tumors, 71 (58.2%) had positive nodes compared with 96 (30.6%) of the 314 patients with LBVI- tumors (P < 0.001). The average number of positive nodes in patients with LBVI+ tumors was 1.59 compared with 0.62 in patients with LBVI- tumors (P < 0.001).
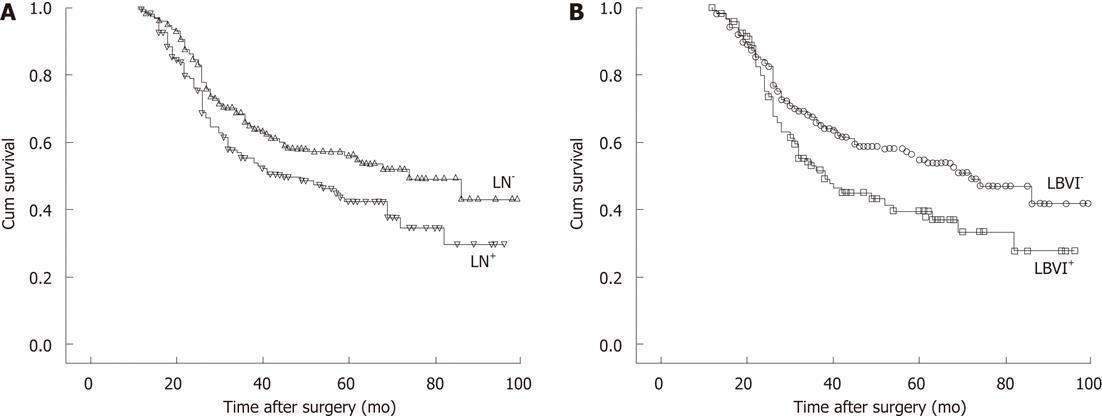
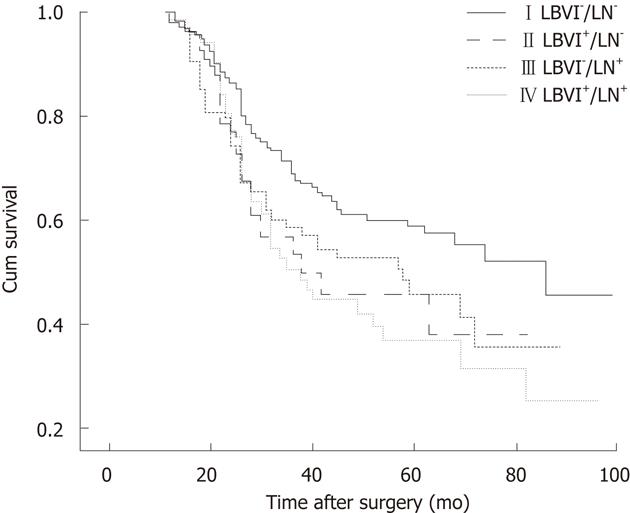
The 5-year overall survival was significantly longer in LN-negative patients compared with LN-positive patients (56.1% vs 42.3%, P = 0.015; Figure 1A). There was a significant difference in the 5-year overall survival between LBVI-positive and LBVI-negative tumors (39.6% vs 54.8%, P = 0.006; Figure 1B). Five-year overall survival rates in each group were 58.8% (I), 45.8% (II), 45.7% (III) and 36.9% (IV), and there was a significant difference in overall survival between the four groups (P = 0.009; Figure 2).
Comparison of survival time in LN-negative gastric cancer patients according to LBVI: The 5-year overall survival rate was 58.8% in LN-negative gastric cancer patients with no LBVI, and 45.8% in LN-negative patients with LBVI. There was a significant difference in overall survival between Groups I and II (P = 0.022). The 5-year overall survival rate was 42.3% in LN-positive gastric cancer patients and 45.7% and 36.9% in Groups III and IV, respectively. By the paired log rank test, the comparison of Groups III and IV revealed no significant difference (log rank test, P = 0.482).
Comparison of survival time between LN-negative stage II gastric cancer patients with LBVI and LN-positive stage II gastric cancer patients: No difference was observed in survival between group II and LN-positive patients (P = 0.924) or between each group (P = 0.753).
To assess the independent prognostic relevance of several parameters such as LBVI, in particular, with regard to 5-year overall survival rate, the Cox proportional hazard analysis, including lymphatic vessel invasion (LBVI-vs LBVI+), T category (T1-T4a), metastatic tumor growth within the LNs (LN-vs LN+), tumor histological type, tumor location, type of surgical procedure (subtotal vs total), sex (male vs female), age, and tumor stage (IIa vs IIb) were determined. Multivariate analysis in all stage II gastric cancer patients revealed that no factor influenced patient prognosis independently. Multivariate analysis in LN-negative patients showed that LBVI and age independently affected patient prognosis (P = 0.018, P = 0.036). However, the multivariate analysis did not detect any independent impact of LBVI on 5-year overall survival in LN-positive patients (P = 0.512).
In the present retrospective study, the effect of LBVI on survival in patients with stage II gastric carcinoma who underwent D2 curative gastrectomy was investigated. According to the study of del Casar et al[12], LBVI was defined as either the presence of neoplastic cells with fibrin clots, erythrocytes, or both within an endothelium-lined space without erythrocyte extravasation into the surrounding tissue, or by the presence of neoplastic cells within a smooth-muscle-cell-lined space. We found that 122 of the 436 patients (28%) were LBVI-positive. Multivariate analysis LN-negative patients showed that LBVI independently affected patient prognosis. However, multivariate analysis did not detect any independent impact of LBVI on 5-year overall survival in LN-positive patients. We also found that survival outcomes between LN-negative stage II gastric cancer patients with LBVI and LN-positive stage II gastric cancer patients were similar, suggesting that it may be necessary to treat these two groups as having the same malignant potential.
The incidence of LBVI in gastric cancer varies from 5.4% to 86%, with the lowest incidence of 20%-26.8% reported in patients with node-negative tumors, using routine pathological examinations with HE staining or different staining methods with endothelial markers[12-17].This could be caused, in part, by the different patient populations included in the different studies. In our analysis, 28% of stage II gastric patients who underwent curative resection were found to have evidence of LBVI. The incidence of LVBI was 19.0% (51/269) and 42.5% (71/167) in LN-negative and LN-positive patients, respectively.
In our study, the impact of LBVI on survival in patients with stage II gastric carcinoma who underwent D2 curative gastrectomy was investigated, and we found that there was a significant difference with respect to survival between LBVI-positive and LBVI-negative patients. By proportional hazards analysis, LBVI was an independent prognostic factor for survival. The mean and median overall survival interval for patients with LBVI-positive tumors was 52.9 and 38 mo, respectively, which was significantly worse than that of the patients with LBVI-negative tumors at 64.7 and 72 mo. The presence and prognostic importance of LBVI have been investigated previously in patients with gastric cancer by only a few studies and a consensus was not reached[12-19]. Several studies have shown that LBVI can be a useful marker to predict cancer recurrence and prognosis in gastric cancer patients[12,15,17]. In contrast, the presence of LBVI showed no prognostic value as an independent factor in the overall group of patients with curatively resected gastric cancer[18,19]. A possible explanation for the different results in assessing the prognostic impact of LBVI could be the methodological discrepancies in the various analyses.
In the current study, LBVI was not also an independent prognostic factor in LN-positive stage II gastric cancer. Liu et al[20] found that, in patients with T1N1M0 gastric cancer, the survival rate was not significantly different between those with and those without lymphatic vessel invasion. Therefore, LBVI may not be a prognostic factor in LN-positive gastric cancer. In contrast, our results showed that LBVI independently influenced prognosis in LN-negative stage II cancer patients, and were in agreement with previous studies examining node-negative gastric cancer, further supporting LBVI as a potential marker of biological behavior. This observation was subsequently supported in a study by Lee et al[21], which showed that LBVI was an adverse prognostic indicator, independent of clinicopathological factors in node-negative gastric cancer. The above-mentioned study concluded that LBVI may provide useful information for prognosis and clinical management in the subset of patients with node-negative gastric cancer. More recently, Kooby et al[22] showed that vascular invasion in node-negative patients was an independent predictor of poor outcome, and identified more aggressive lesions independent of tumor size and depth of invasion. This finding was consistent with our results, in which a subgroup analysis demonstrated that LBVI was independently associated with poor outcome in patients with node-negative gastric cancer. We also found that survival outcomes between stage T3N0 gastric cancer patients with LBVI and stage II gastric cancer patients with positive LN metastasis were similar, suggesting that it may be necessary to treat these two populations as having the same malignant potential.
According to our results, a possible indication to investigate LBVI after radical gastric cancer resection might be the case if there were no LN metastases detectable in conventional pathohistological investigation. However, analysis of various studies have shown that in 10.0%-30.1% of tumor-cell-negative LNs, revealed by conventional histopathological investigation, micrometastases were detectable with immunohistochemistry, which have been described as a negative prognostic factor[23-27]. Therefore, it is recommended to investigate LNs in a more subtle manner in patients with positive LBVI, so that detection of micrometastases is made more cost-efficient and time-saving, with selective immunohistochemical and gene-amplifying investigations of the prepared LNs in patients who have been previously classified as pN0.
However, there were some limitations in the present study due to its retrospective nature, despite our efforts to make a clinically and scientifically sound experiment design. First, due to the inherent limitations of a retrospective study and the small sample size of this study, the results require further investigation to reach a firm conclusion. Second, the presence of LVBI was identified by routine histological HE staining. The accuracy of the identification of LBVI is affected by various parameters. A crucial factor is the number of reference slices obtained from the primary tumor lesion and the level of serious attention for LBVI in the histopathological routine examination. Currently, the presence of LBVI is detected by a routine HE staining method. How novel immunohistochemical lymphangio-markers may improve the sensitivity and specificity of detection needs to be proved in further studies. The third limitation was the heterogeneity in clinical decision-making, surgical intervention and pathological evaluation. Radical gastric cancer resection was performed by several surgeons, and the specimens were evaluated by several pathologists. These doctors were trained in academic centers with experience in gastrectomy and the data are probably valid. Another limitation of our study was the choice of overall survival as the end point. In some ways this can be regarded as a powerful end point, given that overall survival is a concrete end point that we were able to ascertain reliably. Although time to recurrence would be an interesting end point to analyze, patients were not on a predefined follow-up schedule and were observed at the discretion of the treating physician. Therefore, although we tried to collect recurrence data for these patients, there were limitations to the validity of investigating this end point due to the lack of a predefined surveillance plan and schedule.
In conclusion, patients with LBVI+ tumors had a significantly increased incidence and number of positive nodes and a shorter 5-year overall survival. On multivariate analysis, LBVI was an independent prognostic factor in stage II gastric cancer patients with negative LNs. This suggests that the presence of lymphovascular invasion in stage II gastric cancer patients may provide valuable information to determine which patients would benefit from radical surgery, adjuvant chemotherapy or radiotherapy after surgery.
Surgical resection with regional lymphadenectomy is the treatment of choice for patients with stage II gastric cancer. However, preoperative and intraoperative staging to confirm stage II disease is difficult. Failure to distinguish stage II from stage I disease may lead to under- or over-treatment. Therefore, the identification of additional prognostic factors, which is timely and cost-efficient as well as available, would help in detecting those patients with poorer prognosis among the different groups of patients with stage II gastric cancer.
A retrospective study was conducted, attempting to clarify the risk factors and to investigate the effect of lymphatic and/or blood vessel invasion (LBVI) on survival in patients with stage II gastric cancer who underwent D2 curative gastrectomy.
The study revealed that LBVI independently affected patient prognosis in lymph node (LN)-negative patients but not in LN-positive patients with stage II gastric cancer.
In LN-negative stage II gastric cancer patients, LBVI is an additional independent prognostic marker, and may provide useful information to identify patients with poorer prognosis. This suggests that the presence of LBVI in stage II gastric cancer patients may provide valuable information to determine which patients would benefit from radical surgery, adjuvant chemotherapy or radiotherapy after surgery.
LBVI is the critical step of tumor cell dissemination and metastasis in various types of cancer. As the cancer stage advances, tumor cells invade blood vessels and lymphatic vessels near the tumor.
This is a retrospective clinicopathological study on the implication of LBVI in the patients who received surgical therapy for gastric cancer. The results are important and applicable to clinical practice and studies.
Peer reviewers: Hitoshi Tsuda, MD, PhD, Diagnostic Pathology Section, Clinical Laboratory Division, National Cancer Center Hospital, 5-1-1 Tsukiji, Chuo-ku, Tokyo 104-0045, Japan; Dr. Paolo Aurello, Surgery 3, Second School of Medicine, Sapienza University of Rome, Sant’andrea Hospital, Via di Grottarossa, 1035, 00100 Rome, Italy
S- Editor Gou SX L- Editor Kerr C E- Editor Xiong L
| 1. | Desai AM, Pareek M, Nightingale PG, Fielding JW. Improving outcomes in gastric cancer over 20 years. Gastric Cancer. 2004;7:196-201; discussion 201-203. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 51] [Cited by in F6Publishing: 53] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 2. | Miyahara R, Niwa Y, Matsuura T, Maeda O, Ando T, Ohmiya N, Itoh A, Hirooka Y, Goto H. Prevalence and prognosis of gastric cancer detected by screening in a large Japanese population: data from a single institute over 30 years. J Gastroenterol Hepatol. 2007;22:1435-1442. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 55] [Cited by in F6Publishing: 55] [Article Influence: 3.2] [Reference Citation Analysis (0)] |
| 3. | Lin Y, Ueda J, Kikuchi S, Totsuka Y, Wei WQ, Qiao YL, Inoue M. Comparative epidemiology of gastric cancer between Japan and China. World J Gastroenterol. 2011;17:4421-4428. [PubMed] [DOI] [Cited in This Article: ] [Cited by in CrossRef: 119] [Cited by in F6Publishing: 131] [Article Influence: 10.1] [Reference Citation Analysis (0)] |
| 4. | Yokota T, Ishiyama S, Saito T, Teshima S, Shimotsuma M, Yamauchi H. Treatment strategy of limited surgery in the treatment guidelines for gastric cancer in Japan. Lancet Oncol. 2003;4:423-428. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 47] [Cited by in F6Publishing: 50] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 5. | Nakajima T. Gastric cancer treatment guidelines in Japan. Gastric Cancer. 2002;5:1-5. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 410] [Cited by in F6Publishing: 413] [Article Influence: 19.7] [Reference Citation Analysis (0)] |
| 6. | Yoshida K, Yamaguchi K, Okumura N, Osada S, Takahashi T, Tanaka Y, Tanabe K, Suzuki T. The roles of surgical oncologists in the new era: minimally invasive surgery for early gastric cancer and adjuvant surgery for metastatic gastric cancer. Pathobiology. 2011;78:343-352. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 30] [Cited by in F6Publishing: 36] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 7. | Washington K. 7th edition of the AJCC cancer staging manual: stomach. Ann Surg Oncol. 2010;17:3077-3079. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 702] [Cited by in F6Publishing: 801] [Article Influence: 61.6] [Reference Citation Analysis (0)] |
| 8. | Park JM, Kim JH, Park SS, Kim SJ, Mok YJ, Kim CS. Prognostic factors and availability of D2 lymph node dissection for the patients with stage II gastric cancer: comparative analysis of subgroups in stage II. World J Surg. 2008;32:1037-1044. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 4] [Cited by in F6Publishing: 5] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 9. | Liang P, Nakada I, Hong JW, Tabuchi T, Motohashi G, Takemura A, Nakachi T, Kasuga T, Tabuchi T. Prognostic significance of immunohistochemically detected blood and lymphatic vessel invasion in colorectal carcinoma: its impact on prognosis. Ann Surg Oncol. 2007;14:470-477. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 57] [Cited by in F6Publishing: 60] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 10. | Brücher BL, Stein HJ, Werner M, Siewert JR. Lymphatic vessel invasion is an independent prognostic factor in patients with a primary resected tumor with esophageal squamous cell carcinoma. Cancer. 2001;92:2228-2233. [PubMed] [DOI] [Cited in This Article: ] [Cited by in F6Publishing: 1] [Reference Citation Analysis (0)] |
| 11. | Schmid K, Birner P, Gravenhorst V, End A, Geleff S. Prognostic value of lymphatic and blood vessel invasion in neuroendocrine tumors of the lung. Am J Surg Pathol. 2005;29:324-328. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 17] [Cited by in F6Publishing: 19] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 12. | del Casar JM, Corte MD, Alvarez A, García I, Bongera M, González LO, García-Muñiz JL, Allende MT, Astudillo A, Vizoso FJ. Lymphatic and/or blood vessel invasion in gastric cancer: relationship with clinicopathological parameters, biological factors and prognostic significance. J Cancer Res Clin Oncol. 2008;134:153-161. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 34] [Cited by in F6Publishing: 37] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 13. | Yonemura Y, Endou Y, Tabachi K, Kawamura T, Yun HY, Kameya T, Hayashi I, Bandou E, Sasaki T, Miura M. Evaluation of lymphatic invasion in primary gastric cancer by a new monoclonal antibody, D2-40. Hum Pathol. 2006;37:1193-1199. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 56] [Cited by in F6Publishing: 66] [Article Influence: 3.7] [Reference Citation Analysis (0)] |
| 14. | Arigami T, Natsugoe S, Uenosono Y, Arima H, Mataki Y, Ehi K, Yanagida S, Ishigami S, Hokita S, Aikou T. Lymphatic invasion using D2-40 monoclonal antibody and its relationship to lymph node micrometastasis in pN0 gastric cancer. Br J Cancer. 2005;93:688-693. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 75] [Cited by in F6Publishing: 78] [Article Influence: 4.1] [Reference Citation Analysis (0)] |
| 15. | Dicken BJ, Graham K, Hamilton SM, Andrews S, Lai R, Listgarten J, Jhangri GS, Saunders LD, Damaraju S, Cass C. Lymphovascular invasion is associated with poor survival in gastric cancer: an application of gene-expression and tissue array techniques. Ann Surg. 2006;243:64-73. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 87] [Cited by in F6Publishing: 100] [Article Influence: 5.6] [Reference Citation Analysis (0)] |
| 16. | Kunisaki C, Makino H, Kimura J, Takagawa R, Kosaka T, Ono HA, Akiyama H, Fukushima T, Nagahori Y, Takahashi M. Impact of lymphovascular invasion in patients with stage I gastric cancer. Surgery. 2010;147:204-211. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 44] [Cited by in F6Publishing: 50] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 17. | Hyung WJ, Lee JH, Choi SH, Min JS, Noh SH. Prognostic impact of lymphatic and/or blood vessel invasion in patients with node-negative advanced gastric cancer. Ann Surg Oncol. 2002;9:562-567. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 21] [Cited by in F6Publishing: 25] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 18. | Kim JP, Lee JH, Kim SJ, Yu HJ, Yang HK. Clinicopathologic characteristics and prognostic factors in 10 783 patients with gastric cancer. Gastric Cancer. 1998;1:125-133. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 178] [Cited by in F6Publishing: 202] [Article Influence: 7.8] [Reference Citation Analysis (0)] |
| 19. | Du C, Zhou Y, Cai H, Zhao G, Fu H, Shi YQ. Poor prognostic factors in patients with stage I gastric cancer according to the seventh edition TNM classification: a comparative analysis of three subgroups. J Surg Oncol. 2012;105:323-328. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 12] [Cited by in F6Publishing: 14] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 20. | Liu C, Zhang R, Lu Y, Li H, Lu P, Yao F, Jin F, Xu H, Wang S, Chen J. Prognostic role of lymphatic vessel invasion in early gastric cancer: a retrospective study of 188 cases. Surg Oncol. 2010;19:4-10. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 28] [Cited by in F6Publishing: 31] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 21. | Lee CC, Wu CW, Lo SS, Chen JH, Li AF, Hsieh MC, Shen KH, Lui WY. Survival predictors in patients with node-negative gastric carcinoma. J Gastroenterol Hepatol. 2007;22:1014-1018. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 18] [Cited by in F6Publishing: 20] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 22. | Kooby DA, Suriawinata A, Klimstra DS, Brennan MF, Karpeh MS. Biologic predictors of survival in node-negative gastric cancer. Ann Surg. 2003;237:828-35; discussion 835-7. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 40] [Cited by in F6Publishing: 76] [Article Influence: 3.6] [Reference Citation Analysis (0)] |
| 23. | Arigami T, Natsugoe S, Uenosono Y, Yanagita S, Arima H, Hirata M, Ishigami S, Aikou T. CCR7 and CXCR4 expression predicts lymph node status including micrometastasis in gastric cancer. Int J Oncol. 2009;35:19-24. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 34] [Cited by in F6Publishing: 43] [Article Influence: 2.9] [Reference Citation Analysis (0)] |
| 24. | Kim JJ, Song KY, Hur H, Hur JI, Park SM, Park CH. Lymph node micrometastasis in node negative early gastric cancer. Eur J Surg Oncol. 2009;35:409-414. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 48] [Cited by in F6Publishing: 55] [Article Influence: 3.4] [Reference Citation Analysis (0)] |
| 25. | Shimizu Y, Takeuchi H, Sakakura Y, Saikawa Y, Nakahara T, Mukai M, Kitajima M, Kitagawa Y. Molecular detection of sentinel node micrometastases in patients with clinical N0 gastric carcinoma with real-time multiplex reverse transcription-polymerase chain reaction assay. Ann Surg Oncol. 2012;19:469-477. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 41] [Cited by in F6Publishing: 48] [Article Influence: 3.7] [Reference Citation Analysis (0)] |
| 26. | Muto Y, Matubara H, Tanizawa T, Nabeya Y, Kawahira H, Akai T, Hoshino I, Hayashi H. Rapid diagnosis of micrometastasis of gastric cancer using reverse transcription loop-mediated isothermal amplification. Oncol Rep. 2011;26:789-794. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 2] [Cited by in F6Publishing: 7] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 27. | Kim JH, Park SS, Park SH, Kim SJ, Mok YJ, Kim CS, Lee JH, Kim YS. Clinical significance of immunohistochemically-identified lymphatic and/or blood vessel tumor invasion in gastric cancer. J Surg Res. 2010;162:177-183. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 29] [Cited by in F6Publishing: 35] [Article Influence: 2.3] [Reference Citation Analysis (0)] |