Published online Mar 21, 2013. doi: 10.3748/wjg.v19.i11.1820
Revised: December 4, 2012
Accepted: December 25, 2012
Published online: March 21, 2013
AIM: To assess the value of double-balloon enteroscopy (DBE) for the diagnosis of gastrointestinal mesenchymal tumors (GIMTs) in the small bowel and clarify their clinical and endoscopic characteristics.
METHODS: A retrospective review in a total of 783 patients who underwent a DBE procedure from January 2003 to December 2011 was conducted. Data from patients with pathologically confirmed GIMTs were analyzed at a single tertiary center with nine years’ experience. The primary outcomes assessed included characteristics of patients with GIMTs, indications for DBE, overall diagnostic yield of GIMTs, endoscopic morphology, positive biopsy, comparison of diagnosis with capsule endoscopy, and subsequent interventional management.
RESULTS: GIMTs were identified and analyzed in 77 patients. The mean age was 47.74 ± 14.14 years (range: 20-77 years), with 63.6% being males. The majority of individuals presented with gastrointestinal bleeding, accounting for 81.8%, followed by abdominal pain, accounting for 10.4%. Small bowel pathologies were found in 71 patients, the detection rate was 92.2%. The diagnostic yield of DBE for GIMTs was 88.3%. DBE was superior to capsule endoscopy in the diagnosis of GIMTs (P = 0.006; McNemar’s χ2 test). Gastrointestinal stromal tumor was the most frequent and leiomyoma was the second frequent GIMT. Single and focal lesions were typical of GIMTs, and masses with smooth or unsmooth surface were the most common in the small bowel. GIMTs were removed from all the patients surgically except one patient treated with endoscopic resection.
CONCLUSION: DBE is a safe and valuable procedure for patients with suspected GIMTs, and it provides an accurate position for subsequent surgical intervention.
- Citation: He Q, Bai Y, Zhi FC, Gong W, Gu HX, Xu ZM, Cai JQ, Pan DS, Jiang B. Double-balloon enteroscopy for mesenchymal tumors of small bowel: Nine years’ experience. World J Gastroenterol 2013; 19(11): 1820-1826
- URL: https://www.wjgnet.com/1007-9327/full/v19/i11/1820.htm
- DOI: https://dx.doi.org/10.3748/wjg.v19.i11.1820
Small bowel diseases (SBDs) are less common in the entire digestive tract[1]. As a result of deep anatomical location of the small bowel (SB) and nonspecific clinical manifestations of SBDs, the diagnosis and management of SBDs are frequently delayed, leading to considerable medical cost and poor prognosis[2]. Early identification, diagnosis and intervention for SBDs become extremely important in clinical practice. With the development of capsule endoscopy (CE) and balloon-assisted enteroscopy (BAE), a new era has been created for the diagnosis of SBDs.
Gastrointestinal mesenchymal tumors (GIMTs), including myogenic tumors, neurogenic tumors and gastrointestinal stromal tumors (GISTs), account for less than 10% of gastrointestinal tumors[3-5]. Radiological imaging, such as barium study, computed tomography and angiography, is usually performed to examine GIMTs without the advent of CE and double-balloon enteroscopy (DBE). Traditional examination by barium study is feasible for biggish intraluminal SBTs[6,7]. CT is used to locate the lesion, assess for invasion and detect metastasis of SBTs[8,9]. Angiography is effective for detecting SBTs with active bleeding. GIMTs are common in the SB, varying from the duodenum to the ileum[10]. Their true incidences might be higher than those reported, as novel methods such as CE and BAE are much more sensitive and specific in diagnosing GIMTs than conventional methods[7]. CE is performed to detect SBTs and produce a higher detection rate due to its advantage of invasiveness[11-14].
Several studies reported DBE for the diagnosis of SBTs, indicating that DBE is a safe and effective procedure that enables accurate diagnosis of SBTs[15-19]. To date, few studies have reported the diagnosis of GIMTs by DBE and described their clinical and endoscopic features. This study was conducted retrospectively to evaluate the usefulness and safety of DBE for the diagnosis of GIMTs and to understand their clinical and endoscopic characteristics.
Retrospective chart review was conducted in 783 consecutive patients who were suspected to have SBDs and investigated by DBE between January 2003 and December 2011 at a single center (a university teaching hospital). The data of the patients were reviewed, including demographic data, examinations prior to DBE, indications for DBE, the locations of GIMTs, endoscopic findings, removal mode of GIMTs, histopathological findings and postoperative management.
Written informed consents were obtained from each patient and/or their guardians. The study was approved by the Institutional Review Board of Nanfang Hospital, Southern Medical University, Guangzhou, China.
All DBE procedures were performed with no absolute contraindications. A low residue and liquid diet was prescribed for the patients undergoing this procedure, and colored food was avoided at least one day prior to the procedure. All the patients completed bowel cleansing preparation by ingesting a 1.8-2 L polyethylene-glycol solution followed by an overnight fasting, at least 6-10 h prior to the start of the procedure.
The Fujinon DBE system (Fujinon Inc, Japan) introduced in our center in 2003 was used and reported previously elsewhere[20-22]. All procedures were carried out by experienced endoscopists. The selection of transoral or transanal approach was based on the clinical manifestations and/or suspected findings from prior examinations such as barium study, CT scan, and CE findings. The opposite routine was performed after making a positional mark by India ink if negative findings were detected by the peroral routine, and vice versa. If a lesion was detected by DBE, a positional mark was performed as well.
Statistical analysis was performed using the software SPSS Version 17.0 for Windows. Continuous data were presented as means, mean ± SD or range, and categorical variables were expressed as frequency or percentages. The χ2 test was used to compare differences in categorical variables examined. Agreement analysis was assessed by the Kappa statistic. A P value < 0.05 (two-sided) was considered statistically significant.
A total of 77 inpatients who underwent DBE were identified; their final diagnoses were confirmed as GIMTs by histopathology and/or surgery. Characteristics of all the patients are shown in Table 1. The mean age was 47.74 ± 14.14 years (range: 20-77 years), with 63.6% being males. The majority of patients presented with GI bleeding, accounting for 81.8%, followed by abdominal pain, accounting for 10.4%.
| Characteristics | |
| Sex (M/F) | 49/28 |
| Age (mean ± SD, range, yr) | 47.74 ± 14.14 (20-77) |
| Duration (mean ± SD, range, mo) | 25.0 ± 37.7 (0.2-156) |
| Prior blood transfusion (Y/N) | 46/31 |
| Prior abdominal/pelvic surgery (Y/N) | 8/69 |
| Previous examination before DBE | |
| Gastroduodenoscopy | 73 |
| Colonoscopy | 66 |
| Push enteroscopy | 1 |
| Barium study | 10 |
| CT | 12 |
| MRI | 2 |
| Angiography | 2 |
| Meckel’s scan | 3 |
| Bone marrow aspiration | 2 |
| Indications for DBE | |
| Melena/hematochezia | 63 |
| Abdominal pain | 8 |
| Debilitation | 1 |
| Vomiting | 1 |
| Distention | 1 |
| Weight loss | 1 |
| Physical examination | 2 |
All the patients underwent other medical examinations prior to DBE, including gastroduodenoscopy (73 cases), colonoscopy (66 cases), and push enteroscopy (1 case), and yielded negative or suspected diagnoses. Barium study was conducted in 10 patients, only one patient was suspected of having a SBT. Twelve patients received CT scan, SBT was found in two patients and suspected SBT was found in one patient. Two patients were found to have suspected SBT by magnetic resonance imaging, 2 by angiography, 3 by Meckel’s scan and 2 by bone marrow aspiration.
Thirty-one patients underwent CE examination before DBE within an interval of two weeks. All patients successfully completed CE procedures which reached the colon. Positive diagnoses were made in 11 patients, and suspected diagnoses in 8 patients. No lesion was detected in 12 patients. No complications occurred during and after the procedure. Thirty-seven DBE procedures were performed in 31 patients, including 22 antegrade approaches, 3 retrograde approaches, and 6 combinations of the two approaches. The sensitivity of DBE and CE for the diagnosis of GIMTs was 93.5% and 61.3%, respectively. DBE for the diagnosis of GIMTs was superior to CE (P = 0.006, McNemar’s χ2 test) (Table 2).
| CE findings (n = 31) | DBE findings (n = 31) | Total | |
| Positive | Negative | ||
| Positive | 18 | 1 | 19 |
| Negative | 11 | 1 | 12 |
| Total | 29 | 2 | 31 |
A total of 93 DBE procedures were performed in 77 patients, including 49 antegrade DBE approaches, 12 retrograde DBE approaches and 16 combinations of the two approaches. Total enteroscopy (TE) was achieved in 3 patients. Lesions were found in the small bowel in 71 patients, the detection rate for GIMTs being 92.2%. Clear diagnosis was established in 68 patients, and the diagnostic yield of DBE for GIMTs was 88.3%. Multiple tissue samplings were made in 41 cases; positive diagnoses were obtained in 5 cases. Only one therapeutic procedure was performed in one patient, i.e., a leiomyoma (8 mm) was removed by DBE. All the patients successfully completed the entire DBE procedure, without any complications occurring during and after the procedure.
Among 9 patients with unclear diagnosis by DBE, one was found with overt, ongoing bleeding, and two were found with single ulcerative lesions in proximal small bowel, respectively. No abnormality was found in six patients, including two patients treated with the combination of the two approaches (neither completed TE), one with the antegrade approach, the other three with the retrograde approach. Patients with indefinite diagnoses underwent surgical procedures (laparotomy or laparoscopic exploration) because of persistent symptoms. Five patients had GIMTs with extraluminal growth confirmed by surgery; one patient undergoing the antegrade approach had a GIMT located in the ileum.
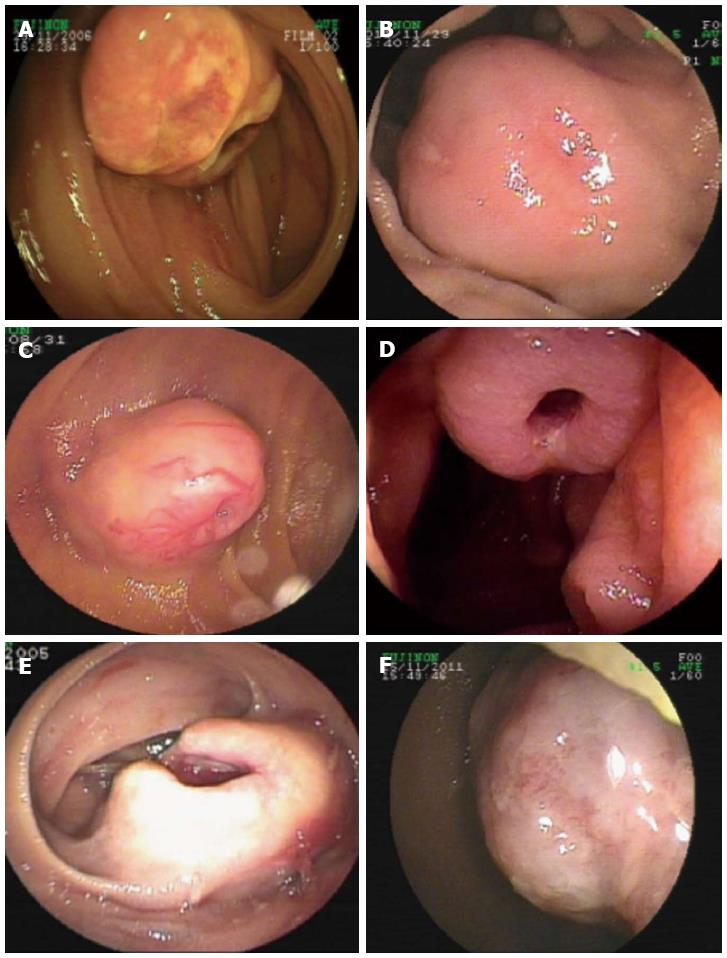
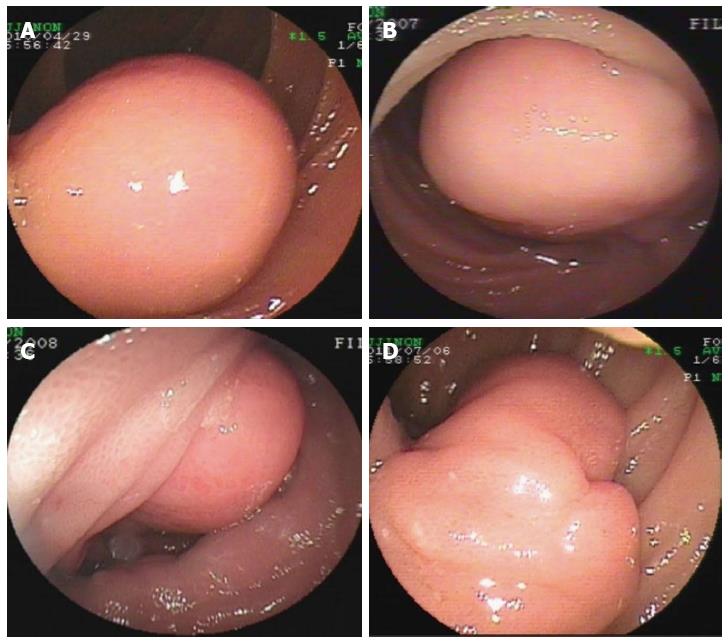
Endoscopic diagnosis was established in the overwhelming majority of the patients. Most GIMTs presented as a single lesion under the endoscopic view, protruding into the intra-luminal mass in the small bowel. The unsmooth surface of the tumor was seen most frequently, showing the appearance of erosion or ulcer (Figure 1). The second frequent morphology was a mass with smooth surface, indicating a tumor with sessile base in a rounded or oval shape (Figure 2). Rare GIMTs presented with irregular shapes under endoscopic view.
In this study, GIMTs with confirmed diagnoses included GIST (60 cases), leiomyoma (6 cases), lipoma (3 cases), hemangioma (3 cases), lymphangioma (3 cases), fibrous histiocytoma (1 case), and angiosarcoma (1 case). Based on the primary sites of tumors, GIMTs in our study were all primary tumors verified surgically and pathologically. Two kinds of GIMTs were detected on the basis of site, including intra- and extra-luminal tumors. Intra-luminal GIMTs were detected most frequently and verified by endoscopy and surgery (Figures 1, 2). A single lesion was most frequently examined, except in two patients who had multiple lymphangiomas. GIMTs were detected most frequently in the jejunum (60 cases), and next in the ileum (16 cases) and duodenum (1 case). No spread and metastasis was investigated and confirmed after surgical removal.
The findings of DBE changed the therapeutic plan and enabled all the patients to receive early intervention. The clinical symptoms disappeared after surgery and all the patients felt an improvement in their conditions. They received an average follow-up time of 14.5 mo after intervention, and important improvements were obtained in the patients after DBE and surgical intervention. No complication was reported.
Since the introduction of CE and DBE, the blind spot of the entire GI tract has been revealed and investigated thoroughly. Our study reported the diagnosis of GIMTs using the DBE technique and their clinical characteristics. We analyzed the data of all the subjects registered in the DBE database in China, which represented more than 780 patients investigated by DBE for a variety of indications after the introduction of this modality. DBE produced a higher detection rate because GIMTs were diagnosed in 77 (9.8%) of 783 subjects who were suspected to have SBDs.
Other diagnostic modalities such as barium study and CT scan were used to detect SBTs prior to the introduction of CE and DBE. However, confirmative analysis is unfeasible for GIMTs. Moreover, surgical intervention should be performed cautiously in patients with indefinite diagnosis by these examinations. Small lesions are difficult to examine by these traditional examinations. DBE is performed to permit real-time visualization of the tumors and make a positional mark, which helps the surgeons to reveal the lesions. In our study, prior examinations only established clear diagnoses in a few patients. This may result in delayed interventions for GIMTs in patients with negative diagnoses. Most GIMTs in the small bowel may be malignant and invasive. Early detection and diagnosis of GIMTs using DBE would be conducive to early intervention and improvement of prognosis. Therefore, DBE is a reliable method as a complementary tool for traditional methods (such as barium meal and CT) or as a direct means for detecting GIMTs in the small bowel.
As a noninvasive and pain-free tool for investigating the small bowel, these advantages have made CE more competitive than DBE. Previous studies have shown that using CE to diagnose SBTs produced a higher accuracy in suspected patients[2,11,14,23]. Even though a small proportion of subjects received CE examinations before DBE, clear diagnosis for GIMTs established by CE was significantly lower than that by DBE in this study. This may be because of the nature of CE and confirmation of previous reports. CE is performed to visualize the GI tract according to bowel movement, this feature is both an advantage and a disadvantage. False positive or false negative findings for SBTs are the significant limitations of CE[24,25]. Missed diagnosis may occur during the examination because of only forward movement[26]. An intra-luminal tumor without mucosal damage or with less protuberance into the lumen is a significant challenge for CE to establish a clear diagnosis. In diagnosis of GIMTs in this study, CE failed to detect the presence of tumors in some patients. This shortcoming can be overcome by DBE through straightening the intestinal tube, which can reduce greatly the possibility of missed tumors. Moreover, severe complications, such as intestinal obstruction or CE retention, may occur during the procedure[27-31]. The most important aspect is that biopsy and histopathological establishment are unavailable for CE. These shortcomings of CE can be overcome by BAE.
Although previous studies have reported the diagnosis of DBE for SBTs and characterized the features of SBTs,[15,17-19,32] there are differences in distinct types of tumors arising from different tissues. Furthermore, a few patients with confirmed GIMTs were detected in these studies. The present study exclusively focused on the diagnosis and characteristics of GIMTs investigated by DBE. In theory, tumors from mesenchymal tissues have similar clinical and endoscopic characteristics. As reported in the literature[17-19,32], most SBTs are detected in adult patients and GI bleeding is a major indication for the DBE procedure. We found that patients with GIMTs were all adults. Males predominantly accounted for more than half of the patients. GI hemorrhage is the most frequent symptom of GIMTs. The main site of SBTs reported in the literature is the ileum[33]. Confirmed GIMTs from the current findings were almost exclusively located in the jejunum. Moreover, our rate of GIMTs (77/783) is higher than that previously reported[18]. In fact, GISTs represented 77.9% of GIMTs in our series. It is reported that this type of tumor is more frequently seen in the proximal small bowel[18,19].
We found that a single and focal lesion is typical of GIMTs, and that masses with a smooth or unsmooth surface are the most common in the small bowel. As far as GIMTs were concerned, precise diagnosis is readily concluded using the DBE procedure before histopathological analysis, which is judged by the endoscopic characteristics of GIMTs. According to our experiences and the findings of the present study, preoperative endoscopic diagnosis is consistent with the final histological diagnosis. As reported by Mitsui et al[18] 40.9% of patients with GISTs were positively diagnosed by the biopsy specimen. In our series, the rate of positive diagnosis by biopsy pathology was lower in patients with GIMTs (12.2%) than that reported previously. Biopsy diagnosis for GIMTs is not as effective as postoperative pathology of the excised specimen. Therefore, endoscopic diagnosis by BAE for GIMTs is effective and significant in clinical practice.
In conclusion, DBE is a safe and valuable procedure for patients with suspected GIMTs, and provides accurate position for subsequent surgical intervention.
We are grateful to our colleagues from the Department of General Surgery, Nanfang Hospital, Southern Medical University for their help with the study; and to the Department of Pathology, Nanfang Hospital, Southern Medical University for providing us the pathological images.
Previous studies reported that double balloon enteroscopy (DBE) is a safe and effective procedure for the diagnosis of small bowel tumors. To date, few reports have focused on the diagnosis of gastrointestinal mesenchymal tumors (GIMTs) detected by DBE and described their clinical and endoscopic features.
A growing number of studies have been conducted on the diagnosis of small bowel diseases (SBDs) since the introduction of double-balloon enteroscopy. The current study exclusively focuses on the diagnosis of mesenchymal tumors in the small bowel and their clinical characteristics.
This study reported the diagnosis of GIMTs using the DBE technique and their clinical characteristics in 780 patients investigated by DBE for a variety of indications. DBE produced a higher detection rate because GIMTs were diagnosed in 77 (9.8%) out of 783 subjects who were suspected to have SDBs.
DBE is a safe and valuable procedure for patients with suspected GIMTs, and provides an accurate position for subsequent surgical intervention.
This study is interesting, representing one of the largest DBE series ever reported and provides very good information for gastroenterologists. DBE is a very useful diagnostic tool for SBDs, particularly for the diagnosis of GIMTs for both gastroenterologists and surgeons.
P- Reviewers Goral V, Racz I S- Editor Gou SX L- Editor A E- Editor Zhang DN
| 1. | Siegel R, Ward E, Brawley O, Jemal A. Cancer statistics, 2011: the impact of eliminating socioeconomic and racial disparities on premature cancer deaths. CA Cancer J Clin. 2011;61:212-236. [PubMed] [Cited in This Article: ] |
| 2. | Cobrin GM, Pittman RH, Lewis BS. Increased diagnostic yield of small bowel tumors with capsule endoscopy. Cancer. 2006;107:22-27. [PubMed] [DOI] [Cited in This Article: ] [Cited by in F6Publishing: 1] [Reference Citation Analysis (0)] |
| 3. | Morgan BK, Compton C, Talbert M, Gallagher WJ, Wood WC. Benign smooth muscle tumors of the gastrointestinal tract. A 24-year experience. Ann Surg. 1990;211:63-66. [PubMed] [Cited in This Article: ] |
| 4. | Miettinen M, Lasota J. Gastrointestinal stromal tumors--definition, clinical, histological, immunohistochemical, and molecular genetic features and differential diagnosis. Virchows Arch. 2001;438:1-12. [PubMed] [Cited in This Article: ] |
| 5. | Nishida T, Hirota S. Biological and clinical review of stromal tumors in the gastrointestinal tract. Histol Histopathol. 2000;15:1293-1301. [PubMed] [Cited in This Article: ] |
| 6. | Hara AK, Leighton JA, Sharma VK, Heigh RI, Fleischer DE. Imaging of small bowel disease: comparison of capsule endoscopy, standard endoscopy, barium examination, and CT. Radiographics. 2005;25:697-711; discussion 711-718. [PubMed] [DOI] [Cited in This Article: ] [Cited by in F6Publishing: 1] [Reference Citation Analysis (0)] |
| 7. | Târcoveanu E, Georgescu S, Vasilescu A, Dănilă N, Lupaşcu C, Dimofte G, Neacşu CN, Moldovanu R. [Small bowel tumours from barium meal to capsule endoscopy and from open to laparoscopic approach]. Chirurgia (Bucur). 2011;106:451-464. [PubMed] [Cited in This Article: ] |
| 8. | Orjollet-Lecoanet C, Ménard Y, Martins A, Crombé-Ternamian A, Cotton F, Valette PJ. [CT enteroclysis for detection of small bowel tumors]. J Radiol. 2000;81:618-627. [PubMed] [Cited in This Article: ] |
| 9. | Chen LH, Cao HJ, Zhang H, Shan GD, Zhang BL, Jiang LL, Li L, Chen HT, Fang Y, Cheng Y. [Diagnostic values of double-balloon enteroscopy and abdominal computed tomography in small bowel disease]. Zhonghua Yi Xue Zazhi. 2008;88:3305-3308. [PubMed] [Cited in This Article: ] |
| 10. | Crosby JA, Catton CN, Davis A, Couture J, O’Sullivan B, Kandel R, Swallow CJ. Malignant gastrointestinal stromal tumors of the small intestine: a review of 50 cases from a prospective database. Ann Surg Oncol. 2001;8:50-59. [PubMed] [Cited in This Article: ] |
| 11. | Bailey AA, Debinski HS, Appleyard MN, Remedios ML, Hooper JE, Walsh AJ, Selby WS. Diagnosis and outcome of small bowel tumors found by capsule endoscopy: a three-center Australian experience. Am J Gastroenterol. 2006;101:2237-2243. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 121] [Cited by in F6Publishing: 133] [Article Influence: 7.4] [Reference Citation Analysis (0)] |
| 12. | van Tuyl SA, van Noorden JT, Timmer R, Stolk MF, Kuipers EJ, Taal BG. Detection of small-bowel neuroendocrine tumors by video capsule endoscopy. Gastrointest Endosc. 2006;64:66-72. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 55] [Cited by in F6Publishing: 46] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 13. | Schwartz GD, Barkin JS. Small-bowel tumors detected by wireless capsule endoscopy. Dig Dis Sci. 2007;52:1026-1030. [PubMed] [DOI] [Cited in This Article: ] [Cited by in F6Publishing: 1] [Reference Citation Analysis (0)] |
| 14. | Cheung DY, Lee IS, Chang DK, Kim JO, Cheon JH, Jang BI, Kim YS, Park CH, Lee KJ, Shim KN. Capsule endoscopy in small bowel tumors: a multicenter Korean study. J Gastroenterol Hepatol. 2010;25:1079-1086. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 46] [Cited by in F6Publishing: 52] [Article Influence: 3.7] [Reference Citation Analysis (0)] |
| 15. | Almeida N, Figueiredo P, Lopes S, Gouveia H, Leitão MC. Double-balloon enteroscopy and small bowel tumors: a South-European single-center experience. Dig Dis Sci. 2009;54:1520-1524. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 30] [Cited by in F6Publishing: 61] [Article Influence: 4.1] [Reference Citation Analysis (0)] |
| 16. | Yeh TS, Liu KH, Su MY, Lin CH, Chiu CT, Tseng JH. Laparoscopically assisted bowel surgery in an era of double-balloon enteroscopy: from inside to outside. Surg Endosc. 2009;23:739-744. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 12] [Cited by in F6Publishing: 14] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 17. | Imaoka H, Higaki N, Kumagi T, Miyaike J, Ohmoto M, Yamauchi K, Murakami T, Murakami H, Ikeda Y, Yokota T. Characteristics of small bowel tumors detected by double balloon endoscopy. Dig Dis Sci. 2011;56:2366-2371. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 18] [Cited by in F6Publishing: 25] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 18. | Mitsui K, Tanaka S, Yamamoto H, Kobayashi T, Ehara A, Yano T, Goto H, Nakase H, Tanaka S, Matsui T. Role of double-balloon endoscopy in the diagnosis of small-bowel tumors: the first Japanese multicenter study. Gastrointest Endosc. 2009;70:498-504. [PubMed] [DOI] [Cited in This Article: ] [Cited by in F6Publishing: 1] [Reference Citation Analysis (0)] |
| 19. | Lee BI, Choi H, Choi KY, Byeon JS, Jang HJ, Eun CS, Cheon JH, Shin SJ, Kim JO, Lee MS. Clinical characteristics of small bowel tumors diagnosed by double-balloon endoscopy: KASID multi-center study. Dig Dis Sci. 2011;56:2920-2927. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 25] [Cited by in F6Publishing: 32] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 20. | Yamamoto H, Yano T, Kita H, Sunada K, Ido K, Sugano K. New system of double-balloon enteroscopy for diagnosis and treatment of small intestinal disorders. Gastroenterology. 2003;125:1556; author reply 1556-1557. [PubMed] [Cited in This Article: ] |
| 21. | May A, Nachbar L, Wardak A, Yamamoto H, Ell C. Double-balloon enteroscopy: preliminary experience in patients with obscure gastrointestinal bleeding or chronic abdominal pain. Endoscopy. 2003;35:985-991. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 149] [Cited by in F6Publishing: 163] [Article Influence: 7.8] [Reference Citation Analysis (0)] |
| 22. | Yamamoto H, Kita H, Sunada K, Hayashi Y, Sato H, Yano T, Iwamoto M, Sekine Y, Miyata T, Kuno A. Clinical outcomes of double-balloon endoscopy for the diagnosis and treatment of small-intestinal diseases. Clin Gastroenterol Hepatol. 2004;2:1010-1016. [PubMed] [Cited in This Article: ] |
| 23. | Sîngeap AM, Trifan A, Cojocariu C, Sfarti C, Stanciu C. [Capsule endoscopy role in diagnosis of small bowel tumors]. Rev Med Chir Soc Med Nat Iasi. 2010;114:988-992. [PubMed] [Cited in This Article: ] |
| 24. | Urgesi R, Riccioni ME, Bizzotto A, Cianci R, Spada C, Pelecca G, Ricci R, Costamagna G. Increased diagnostic yield of small bowel tumors with PillCam: the role of capsule endoscopy in the diagnosis and treatment of gastrointestinal stromal tumors (GISTs). Italian single-center experience. Tumori. 2012;98:357-363. [PubMed] [DOI] [Cited in This Article: ] [Cited by in F6Publishing: 1] [Reference Citation Analysis (0)] |
| 25. | Baichi MM, Arifuddin RM, Mantry PS. Small-bowel masses found and missed on capsule endoscopy for obscure bleeding. Scand J Gastroenterol. 2007;42:1127-1132. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 38] [Cited by in F6Publishing: 45] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 26. | Chong AK, Chin BW, Meredith CG. Clinically significant small-bowel pathology identified by double-balloon enteroscopy but missed by capsule endoscopy. Gastrointest Endosc. 2006;64:445-449. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 96] [Cited by in F6Publishing: 108] [Article Influence: 6.0] [Reference Citation Analysis (0)] |
| 27. | Magdeburg R, Riester T, Hummel F, Löhr M, Post S, Sturm J. Ileus secondary to wireless capsule enteroscopy. Int J Colorectal Dis. 2006;21:610-613. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 5] [Cited by in F6Publishing: 5] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 28. | Rondonotti E, Pennazio M, Toth E, Menchen P, Riccioni ME, De Palma GD, Scotto F, De Looze D, Pachofsky T, Tacheci I. Small-bowel neoplasms in patients undergoing video capsule endoscopy: a multicenter European study. Endoscopy. 2008;40:488-495. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 155] [Cited by in F6Publishing: 142] [Article Influence: 8.9] [Reference Citation Analysis (0)] |
| 29. | Ghoshal UC, Lakshmi CP, Kumar S, Das K, Misra A, Rai P, Mohindra S, Saraswat VA, Kumar A, Choudhuri G. Capsule endoscopy for obscure gastrointestinal bleeding in the tropics: report from India. Dig Endosc. 2011;23:17-23. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 9] [Cited by in F6Publishing: 11] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 30. | Van Weyenberg SJ, Van Turenhout ST, Bouma G, Van Waesberghe JH, Van der Peet DL, Mulder CJ, Jacobs MA. Double-balloon endoscopy as the primary method for small-bowel video capsule endoscope retrieval. Gastrointest Endosc. 2010;71:535-541. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 55] [Cited by in F6Publishing: 60] [Article Influence: 4.3] [Reference Citation Analysis (0)] |
| 31. | Lin OS, Brandabur JJ, Schembre DB, Soon MS, Kozarek RA. Acute symptomatic small bowel obstruction due to capsule impaction. Gastrointest Endosc. 2007;65:725-728. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 33] [Cited by in F6Publishing: 43] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 32. | Yamagami H, Oshitani N, Hosomi S, Suekane T, Kamata N, Sogawa M, Okazaki H, Watanabe K, Tominaga K, Watanabe T. Usefulness of double-balloon endoscopy in the diagnosis of malignant small-bowel tumors. Clin Gastroenterol Hepatol. 2008;6:1202-1205. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 15] [Cited by in F6Publishing: 20] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 33. | Moglia A, Menciassi A, Dario P, Cuschieri A. Clinical update: endoscopy for small-bowel tumours. Lancet. 2007;370:114-116. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 17] [Cited by in F6Publishing: 16] [Article Influence: 0.9] [Reference Citation Analysis (0)] |