Published online Dec 7, 2014. doi: 10.3748/wjg.v20.i45.17235
Revised: July 1, 2014
Accepted: July 24, 2014
Published online: December 7, 2014
AIM: To evaluate the clinical outcomes and safety of anterior- and conventional-approach hepatectomy for patients with large liver tumors.
METHODS: PubMed, EMBASE, Google Scholar and the Cochrane Library databases were searched for randomized controlled trials (RCTs) and controlled clinical trials comparing anterior-approach hepatectomy (AAH) and conventional-approach hepatectomy (CAH). Two observers independently extracted the data using a spreadsheet and assessed the studies for inclusion. Studies that fulfilled the inclusion criteria and addressed the clinical questions of this analysis were further assessed using either fixed effects or random effects models.
RESULTS: Two RCTs and six controlled clinical trials involving 807 patients met the predefined inclusion criteria. A total of 363 patients underwent AAH and 444 underwent CAH. Meta-analysis indicated that the AAH group had fewer requirements for transfusion (OR = 0.37, 95%CI: 0.21-0.63), less recurrence (OR = 0.57, 95%CI: 0.37-0.87), and lower mortality (OR = 0.29, 95%CI: 0.13-0.63). There were no significant differences between AAH and CAH with regard to perioperative complications (OR = 0.94, 95%CI: 0.58-1.51), intraoperative tumor rupture (OR = 0.98, 95%CI: 0.40-2.40), or length of hospital stay (weighted mean difference = -0.17, 95%CI: -2.36-2.02).
CONCLUSION: AAH has advantages of decreased transfusion, mortality and recurrence compared to CAH. It is a safe and effective method for large cancers requiring right hepatectomy.
Core tip: Hepatectomy remains one of the best treatments of choice for primary or metastatic liver tumors of the right hepatic lobe. Anterior and conventional approaches are the most common methods for liver resection. We conducted a systematic review and meta-analysis to evaluate their feasibility, safety and efficacy. Anterior approach hepatectomy has more advantages than the conventional approach, and no significant difference from the conventional approach for perioperative complications, intraoperative tumor rupture, and length of hospital stay.
-
Citation: Li L, Wang HQ, Wang Q, Yang J, Yang JY. Anterior
vs conventional approach hepatectomy for large liver cancer: A meta-analysis. World J Gastroenterol 2014; 20(45): 17235-17243 - URL: https://www.wjgnet.com/1007-9327/full/v20/i45/17235.htm
- DOI: https://dx.doi.org/10.3748/wjg.v20.i45.17235
Hepatectomy remains one of the best treatments of choice for primary or metastatic liver tumors of the right hepatic lobe. Most surgeons advise complete mobilization of the right lobe of the liver with the right hepatic vein controlled outside the liver before parenchymal transection[1]. This conventional approach hepatectomy (CAH) is considered to be effective in reducing intraoperative blood loss. However, this approach is difficult and dangerous when performing liver resection for large hepatocellular carcinoma (HCC) or for tumors with extrahepatic organ invasion in the right retrohepatic region[2]. An alternative method is anterior approach hepatectomy (AAH). This technique involves initial vascular inflow control, completion of parenchymal transection, and complete venous outflow control before the right liver is mobilized[1,3]. This approach avoids the squeezing of tumor cells into the circulation during mobilization of the tumor[4-6]. However, torrential bleeding can occur at the deeper plane of parenchymal transection from the right or middle hepatic vein. Bleeding can be substantial and difficult to control without prior mobilization of the right liver and control of the right hepatic vein[7].
With the refinement of surgical techniques and perioperative management in liver surgery during recent decades, outcomes after liver resection have improved substantially and the mortality of large hepatic resections has decreased to 3%-5%. However, safety and efficacy are still the current focus in liver resection. Many prospective randomized controlled trials (RCTs) and retrospective clinical trials have evaluated the feasibility, safety and efficacy of CAH and AAH, however, the clinical significance remains inconsistent. Currently, there are no meta-analyses available that evaluate the safety and efficacy of AAH. Therefore, we conducted a systematic review and meta-analysis to evaluate the feasibility, safety and efficacy of CAH and AAH in patients with large liver tumors in order to guide clinical practice.
A comprehensive systematic literature search was conducted to identify all potentially relevant publications in the following databases: Cochrane Library, Medline (Ovid), PubMed, Google Scholar and EMBASE. The date of the last search was December 2013. Articles with the following text words or medical subject headings in their titles, abstracts or keyword lists were examined: (“anterior approach hepatectomy” or “conventional approach hepatectomy”) and (“hepatocellular carcinoma” or “liver tumor” or “liver neoplasms”). The literature search was restricted to English or Chinese language publications and either RCTs or controlled clinical trials. Potentially relevant studies were extracted and their abstracts and full texts were considered for further evaluation. In case of duplicate reports, only the most recent version was considered in our analysis. Abstracts without full text or unpublished reports were excluded. When necessary, authors of the included studies were contacted to obtain clarifications.
The inclusion criteria were as follows: (1) RCTs or controlled clinical trials; (2) patients who were about to undergo selective hepatic resection for large liver tumor; (3) irrespective of age, sex, tumor size and nodule numbers; and (4) irrespective of primary or metastatic liver tumors.
The exclusion criteria were as follows: (1) review articles; (2) case reports, conference proceedings and abstracts; (3) studies only reported as abstracts or with incomplete data; (4) multiple publications based on the same database; (5) trials in which patients had distant metastasis or synchronous malignancy in other organs; and (6) studies with minor liver resection.
The outcomes of identified studies were summarized according to the meta-analysis of therapeutic interventions in methodology (QUOROM statement)[8]. Two investigators used a predesigned data extraction form to independently extract data from studies that met the inclusion criteria. Any disagreement during study selection and data extraction was resolved by discussion and referral to a third author for adjudication. Two review authors independently assessed the methodological quality of the trials. The Jadad score[9] was used to assess the quality of RCTs, with a cumulative score ≥ 3 indicating high quality (Table 1). The Newcastle-Ottawa scale[10] was used to assess the quality of non-randomized studies, with a score ≥ 5 indicating high quality (Table 2).
The synchronized extraction results were pooled as estimates of overall treatment effects using Review Manager for Windows version 5.2 (Microsoft Corp., Redmond, WA, United States). The estimated effect measures were OR for dichotomous data and weighted mean difference (WMD) for continuous data; both reported with 95%CIs. All results were assessed for clinical and statistical heterogeneity. Clinical heterogeneity was evaluated by assessing study populations and interventions, definition of outcome measures, concomitant treatment and perioperative management. Heterogeneity was evaluated using χ2 tests with significance set at P = 0.10, and I2 statistics were used for the evaluation of statistical heterogeneity (I2≥ 50% indicating presence of heterogeneity)[11]. We used a fixed effects model to synthesize data when heterogeneity was absent; otherwise a random effects model was used. Data are presented as forest plots and the funnel plot was used to assess publication bias.
A total of 158 titles and abstracts were identified. Of these, 45 full texts were further reviewed in detail, and ultimately a total of eight studies[1,3,7,12-16] matched the inclusion criteria, including a total of 807 patients ranging from 25 to 188 patients in each trial. Two of the eight articles were RCTs and six were controlled clinical trials. Six articles were from China including two from Taiwan and three from Hong Kong, one from Italy, and one from Hungary. The main characteristics of these studies are summarized in Tables 3 and 4. All case studies were published in full text. Only one of them was published in Chinese and seven were published in English. All the trials compared AAH (n = 363) and CAH (n = 444). Six studies only enrolled HCC patients and two studies enrolled patients with both HCC and metastatic liver tumors. All the patients in the studies underwent right hepatectomy or extended right hepatectomy.
| Ref. | Design | Country | Patients, n (AAH/CAH) | Disease | Tumor size, cm (AAH/CAH) | Tumor≥5 cm |
| Lai et al[1] | CCT | China (Hong Kong) | 25/34 | HCC | 12.8/8.4 | 85% |
| Liu et al[3] | CCT | China (Hong Kong) | 54/106 | HCC | 10.3/10.5 | 100% |
| Liu et al[7] | RCT | China (Hong Kong) | 60/60 | HCC | 10.5/10.0 | 100% |
| Takács et al[14] | CCT | Hungary | 52/67 | HCC or MHC | 11.6/10.0 | NR |
| Wang et al[15] | CCT | China (Taiwan) | 14/11 | HCC | 12.5/12.0 | 100% |
| Wu et al[13] | CCT | China (Taiwan) | 33/38 | HCC | 12.0/9.7 | 100% |
| Li et al[12] | CCT | China | 92/96 | HCC | 12.2/10.4 | 100% |
| Capussotti et al[16] | RCT | Italy | 33/32 | HCC or MHC | 6.0/5.2 | NR |
| Ref. | AAH/CAH | ||||||
| Mean blood loss(L) | Transfusion requirements(n:total) | Hospital stay(d) | Mortality,n (%) | Recurrence,n (%) | Complication,n (%) | Tumor rupture(n:total) | |
| Lai et al[1] | 3.2 (2.0-4.4)/3.1 (2.0-4.2)1 | NR | NR | 1 (4)/2 (5.9) | NR | 21 (84)/13 (38.2) | 3:25/1:34 |
| Liu et al[3] | 2.0 (0.6-2.0)/2.5 (0.2-2.0)1 | 31:54/86:106 | NR | 0 (0)/14 (13.2) | NR | 25 (46.3)/56 (52.8) | 1:54/7:106 |
| Liu et al[7] | 0.8 (0.5-1.4)/1.0 (0.5-2.2)2 | 4:60/17:60 | 11 (7.3-15)/12.5 (8-19)2 | 1 (1.7)/6 (0.28) | 33 (55.9)/30 (55.6) | 16 (26.7)/20 (33.3) | 1:60/1:60 |
| Takács et al[14] | NR | 30:52/49:67 | 20.4 ± 13.5/18.3 ± 6.9 | 0 (0)/2 (3.0) | NR | 20 (38.5)/24 (35.8) | NR |
| Wang et al[15] | 0.4 (0.2-0.9)/1.0 (0.5-1.2)2 | 3:14/8:11 | 15 (10.7-19.7)/16 (13-19)2 | 0 (0)/0 (0) | NR | 9 (64.3)/8 (72.7) | NR |
| Wu et al[13] | 0.5 (0.3-0.7)/0.6 (0.3-1.0)1 | 4:33/18:38 | 10 (8.0-19.5)/11 (8.0-14.0)1 | 2 (6.1)/5 (13.2) | 12 (38.7)/27 (81.8) | 8 (24.2)/16 (42.1) | 4:33/4:38 |
| Li et al[12] | 1.2 ± 1.1/2.1 ± 2.03 | 38:92/54:96 | 23 ± 11/24 ± 103 | 2 (2.2) /3 (3.1) | 40 (47.6)/50 (60.2) | 13 (14.1)/17 (17.7) | NR |
| Capussotti et al[16] | 0.4 ± 0.66/0.5 ± 0.533 | 6:33/3:32 | 10.0 ± 6.59/11.5 ± 13.63 | 1 (3.0)/1 (3.1) | NR | 17 (51.5)/18 (56.3) | 0:33/0:32 |
| Total | - | 116:338/ 235:410 | - | 7 (1.9)/33 (7.4) | 85 (48.9)/107 (63.0) | 129 (35.5)/172 (38.7) | 9:205/ 13:270 |
Data concerning blood loss were available in two RCTs and five controlled clinical trials. Many trials did not provide the mean ± SD of blood loss, and we could not obtain the original data from the authors. Therefore, we performed a meta-analysis to evaluate the only two trials[12,16] which provided these values. The analysis indicated that there was no significant difference in blood loss between the two groups (WMD = -0.43, 95%CI: -1.19-0.34; P = 0.27, I² = 87%).
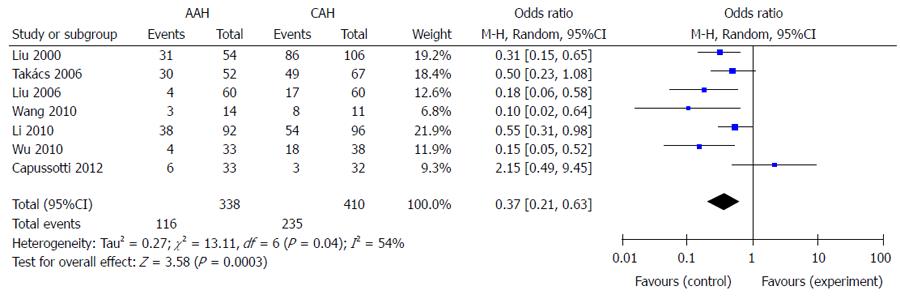
Seven trials reported the number of patients who needed transfusion in the two groups. The total rate of transfusion was 34.3% (116/338) in the AAH group and 57.3% (235/410) in the CAH group. Meta-analysis indicated that the AAH group had a significantly lower transfusion rate than the CAH group (P < 0.01) (Figure 1).
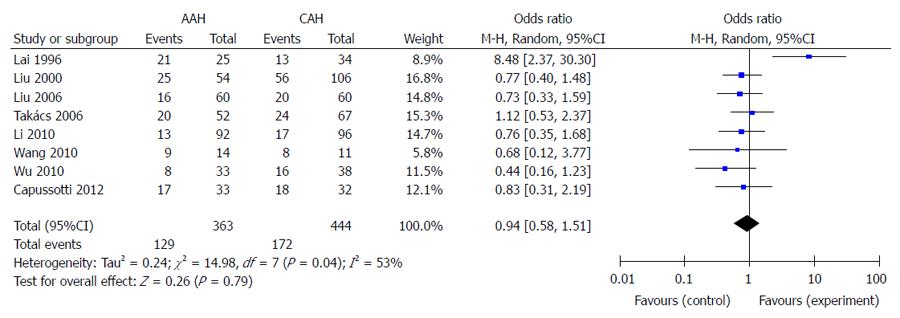
All trials provided information concerning intraoperative or postoperative complications. The pooled incidence of complications was 35.5% (129/363) in the AAH group and 38.7% (172/444) in the CAH group. There was no significant difference in the incidence of complications between the two groups (Figure 2). Four controlled clinical trials and two RCTs reported bile duct injury, with no significant difference among them (OR = 0.53, 95%CI: 0.18-1.55; P = 0.24, I² = 0%). Meta-analysis of liver failure (OR = 0.50, 95%CI: 0.21-1.15; P = 0.10, I² = 0%) and infectious complications (OR = 1.03, 95%CI: 0.28-3.76; P = 0.97, I² = 54%) also showed no difference. In addition, tumor rupture was detected in 4.4% (9/205) patients in the AAH group and 4.8% (13/270) in the CAH group, and there was no significant difference between the groups (OR = 0.98, 95%CI: 0.40-2.40; P = 0.96, I² = 5%).
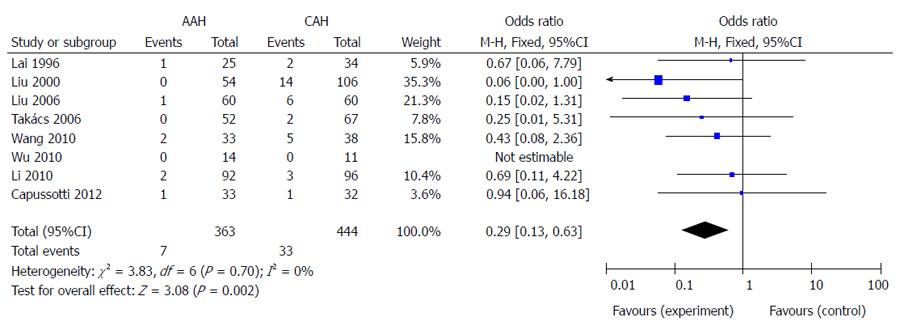
The mortality was 1.9% (7/363) in the AAH group and 7.4% (33/444) in the CAH group. Meta-analysis indicated that the mortality in the CAH group was significantly higher than in the AAH group (P < 0.01) (Figure 3).
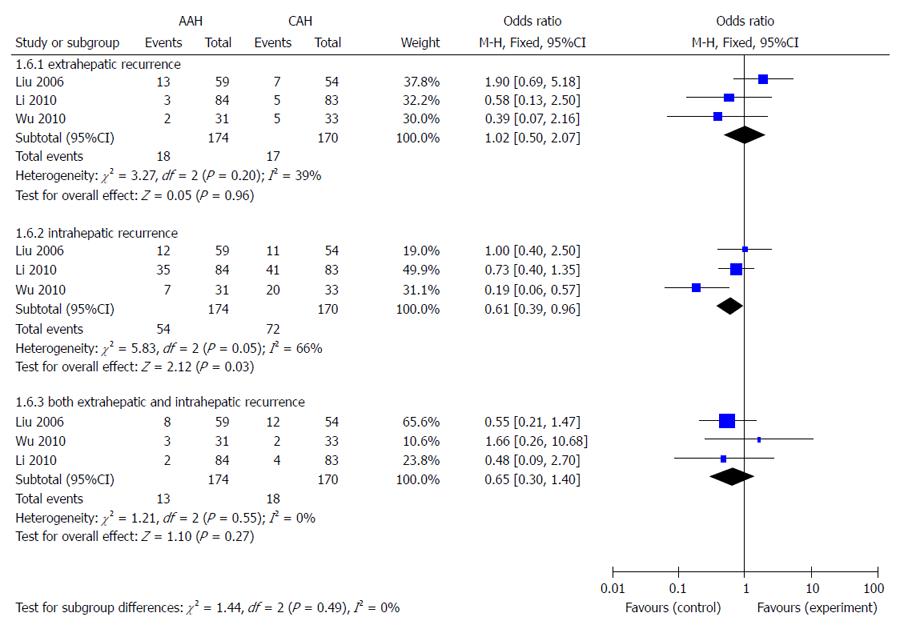
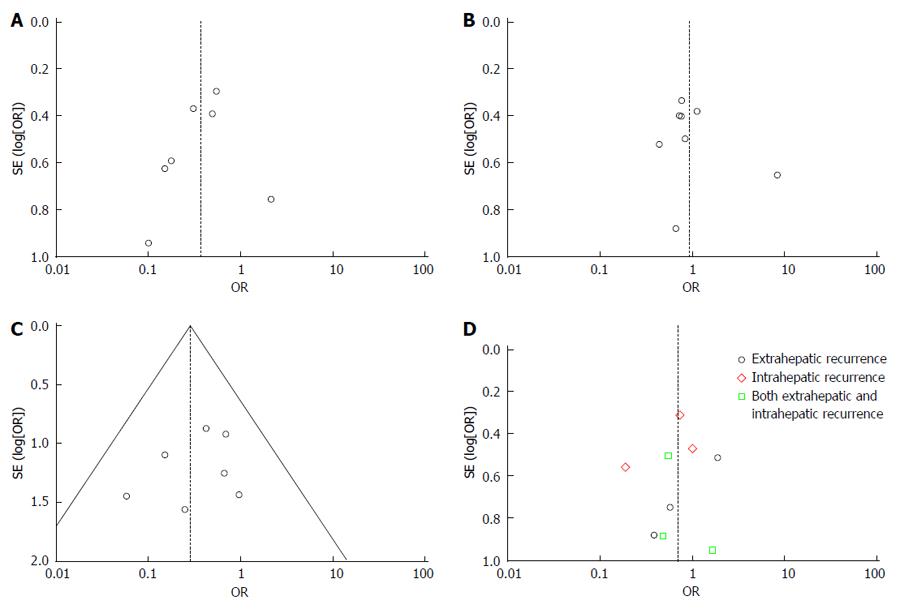
Three of the eight trials provided information about tumor recurrence[7,13,14]. The total recurrence rate was 48.9% (85/174) in the AAH group and 62.9% (107/170) in the CAH group. The meta-analysis showed that the recurrence rate of the AAH group was significantly lower than in the CAH group (OR = 0.57, 95%CI: 0.37-0.87, P = 0.01, I² = 75%). Patients were divided into three subgroups based on the recurrence location, including intrahepatic, extrahepatic, or both. As shown in Figure 4, the meta-analyses indicated that the AAH group had significantly less intrahepatic recurrence than the CAH group (P = 0.03), with no differences in extrahepatic recurrence or both intrahepatic and extrahepatic recurrence.
Six trials reported data on hospital stay, but only three provided the mean ± SD. Meta-analysis of the three trials with these values showed no significant difference in hospital stay between the two groups (WMD = -0.17, 95%CI: -2.36-2.02; P = 0.88, I² = 0%).
The funnel plots did not suggest the presence of significant publication bias in our meta-analysis (Figure 5).
For conventional hepatic resection of liver tumors, complete mobilization of the liver is necessary prior to parenchymal transection. During the course of liver mobilization, CAH can result in excessive blood loss and hemodynamic instability due to laceration of the inferior vena cava (IVC) wall, rupture or ligation slippage of the short hepatic veins. At the same time, injudicious mobilization of the liver may carry theoretical risks of prolonged ischemia of the liver remnant from rotation of the hepatoduodenal ligament, iatrogenic tumor rupture, and spillage of cancer cells into the systemic circulation[2]. These potential disadvantages of mobilization of the right liver with a large tumor using the conventional approach are well recognized. The anterior approach was first described by Ozawa, as one of the non-conventional approaches to advanced liver cancer in an attempt to avoid causing impairment. The “no-touch” isolation technique[17] has been reported to reduce intraoperative shedding of tumor cells into the portal vein during resection of colorectal cancer. However, uncontrolled massive bleeding at the deeper parenchymal transection plane is a well-known problem in AAH. To reduce the risk of bleeding from the anterior wall of the IVC and the transection surface, Belghiti et al[18] proposed the liver-hanging maneuver using a tape passed between the anterior surface of the IVC and the liver parenchyma. The liver is lifted up with a tape during parenchymal transection, and the risk of massive venous bleeding is minimized. However, there is a potential risk of bleeding from the caudate hepatic veins induced by the blind passage of an instrument anterior to the IVC. Bleeding from these branches can be substantial and difficult to stop, especially in patients with liver cirrhosis and portal hypertension[7].
Although the precise long-term prognoses in patients who undergo AAH or CAH remain unclear, many surgeons from various countries have conducted a series of controlled trials. However, any single study may be affected by potential confounding factors. Therefore, we systematically combined those related studies using meta-analysis in order to evaluate more precisely the safety and efficacy of AAH and CAH. In our study, AAH was associated with fewer transfusion requirements, mortality and recurrence after right hepatectomy. However, AAH has no advantage over CAH in relation to complications, tumor rupture and hospital stay. Our results indicate that AAH is safe and effective to reduce mortality and tumor recurrence.
Although other studies have reported that AAH has significantly less intraoperative blood loss than CAH for large tumors during right hepatectomy[3,7,12,13,15,16], our meta-analysis found no difference in blood loss between the two groups. The unavailability of blood loss data in the studies included in our meta-analysis may have contributed to this discrepancy. Therefore, studies with large samples and more RCTs are needed to resolve this conflict. The median intraoperative blood loss was lower during AAH. Consequently, the rate of perioperative blood transfusions was low. That might have led to a reduction in tumor recurrence and benefit to short-term prognosis, because excessive intraoperative bleeding has been reported to have a detrimental effect on postoperative liver function and to increase perioperative mortality[19,20].
Perioperative transfusion has also been found to promote recurrence of HCC after hepatic resection, resulting in short disease-free and overall survivals[20,21]. According to the conclusions of most of the included studies[3,7,12-16], the overall survival in the AAH group was significantly better than that of the CAH group, and the results for mortality also support this. Our meta-analysis indicated that the total complications, bile duct injury, infectious complications, and liver failure did not differ significantly between the two groups. The observation was not consistent with the suggestion by Ozawa in his initial proposal that AAH could contribute to better preservation of postoperative liver function by avoiding prolonged rotation and displacement of the hepatic lobes causing impairment of the afferent and efferent circulation of the liver remnant[2]. The studies in our analysis[1,3,7,12-16] had no uniform definition for complications, which may have influenced the results of the analysis. Thus additional studies are required to confirm the results.
The liver tumor is known to be soft, friable, and highly vascular, therefore, major right hepatectomy during difficult mobilization may result in rupture of the tumor. This usually leads to excessive bleeding and tumor cell spillage into the peritoneal cavity, and hematogenous dissemination of malignant tumor cells has been reported during surgical resection of biliary-pancreatic, colorectal and prostate cancers[3]. Modification of the surgical technique is associated with improved operative and survival outcomes of patients undergoing cancer surgery[7], hepatectomy for HCC, and surgery for other malignancies[22]. The “no-touch” isolation technique has been reported to reduce intraoperative shedding of tumor cells into the portal vein during resection of colorectal cancer[18]. This implies that the potential risk of tumor cell dissemination can theoretically be minimized with use of AAH. However, just one clinical study by Liu et al[22] reported that AAH can significantly reduce intraoperative tumor rupture, but others showed no difference. In our meta-analysis, AAH was not superior to CAH for reducing intraoperative tumor rupture. However, not all the included studies reported detailed intraoperative information. Therefore, whether tumor rupture differs significantly between the two surgical approaches should be subjected to further evaluation. Through our analysis, the incidence of extrahepatic tumor recurrence also did not differ significantly, and this result seems to support no difference in iatrogenic tumor rupture between the two groups.
In patients with liver tumors, venous permeation and vascular invasion are common. Excessive compression during mobilization may enhance the spread of tumor cells into the systemic circulation or the portal venous system. We found that tumor recurrence in the AAH group was lower than in the CAH group. Subgroup analysis found that the AAH group had less intrahepatic recurrence than the CAH group, but there was no difference in patients with both intrahepatic and extrahepatic recurrence. Thus, application of AAH in routine right hepatic resection of liver tumors influenced operative and survival outcomes, as well as recurrence of malignant tumors. However, α-fetoprotein mRNA, nucleic acid in the blood[23], and cell-free plasma albumin mRNA[24] need to be examined in follow-up clinical studies to evaluate these results further. Excessive intraoperative bleeding, perioperative transfusion and postoperative morbidity have been reported to increase perioperative mortality. Meta-analysis also verified that the AAH group had significantly less mortality than the CAH group. Thus, we found that AAH can indeed reduce tumor recurrence and improve survival outcome.
This study had a number of limitations that should be acknowledged. First, some of the trials included were RCTs while others were not, and the number of RCTs was small. Hence, the evidence from some of these studies is not of the highest possible quality. Second, there was a lack of available data on postoperative outcome, such as blood loss and tumor recurrence. Third, the heterogeneity of the patients in the included trials may have influenced the conclusions. Some trials included major and complex central liver resections, whereas others only included right hepatectomy. Most liver resections in our study were performed in HCC patients with underlying liver parenchyma damage from cirrhosis, but some were performed in normal liver parenchyma because of metastatic liver tumors. Cirrhosis is indeed recognized as a clinically relevant feature influencing morbidity and mortality after major hepatic resection[25,26]. Additionally, liver mobilization in cirrhotic patients is more technically demanding, especially in those with portal hypertension, because of a greater bleeding tendency and the presence of adhesions. Unfortunately, the included trials did not provide detailed data regarding cirrhosis, thus, we could not conduct a subgroup analysis between patients with and without cirrhosis.
In conclusion, patients in the AAH group had fewer transfusion requirements, tumor recurrences and reduced mortality compared to those in the CAH group. However, the anterior approach had no advantages over the conventional approach in relation to blood loss, morbidity or hospital stay after right hepatectomy. The current evidence from the available clinical trials demonstrated that AAH is a safe and effective method of liver resection for large tumors needing right hepatectomy. However, further well-designed, multicenter trials are needed to evaluate the role of AAH.
Resection of a large liver tumor is difficult and is associated with poor outcome. In the past two decades there have been significant developments in hepatobiliary surgery. During surgery for major right hepatectomy, conventional and anterior approaches have been widely recommended. However, their beneficial effects on the operative and postoperative outcomes have been controversial.
Several trials have compared anterior approach hepatectomy (AAH) and conventional approach hepatectomy (CAH) to evaluate their safety and efficacy in major right liver resection; however, the results of these trials were inconsistent. This study was a meta-analysis to evaluate the safety, efficacy and clinical outcome of the two methods for liver resection.
This is the first meta-analysis of the efficacy, safety and clinical outcome after CAH for the treatment of large right liver tumor compared with AAH. The authors conducted a comprehensive search for relevant studies. Several important conclusions might be used to guide future clinical practice.
This meta-analysis shows that AAH is associated with reduced transfusion requirements, tumor recurrence and mortality in comparison with CAH. AAH may be a better treatment method for patients who need major right hepatectomy.
AAH is defined as liver resection, which involves initial completion of the parenchymal transection before the right hepatic lobe is mobilized, and it can be used in patients with difficult right hepatic tumors. After hilar control of the inflow vessels, the liver parenchyma is transected using an ultrasonic dissector until the anterior surface of the inferior vena cava is exposed. The right hepatic lobe is then mobilized laterally by securing all venous tributaries, including the right hepatic vein.
This meta-analysis evaluated the clinical outcomes and safety of liver tumor surgery in patients undergoing hepatectomy with the anterior compared with conventional approach. This manuscript is easy to understand and well reviewed.
P- Reviewer: Bao BY, Giraldi G S- Editor: Gou SX L- Editor: AmEditor E- Editor: Ma S
| 1. | Lai EC, Fan ST, Lo CM, Chu KM, Liu CL. Anterior approach for difficult major right hepatectomy. World J Surg. 1996;20:314-317; discussion 318. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 129] [Cited by in F6Publishing: 138] [Article Influence: 4.9] [Reference Citation Analysis (0)] |
| 2. | Ozawa K. Hepatic function and liver resection. J Gastroenterol Hepatol. 1990;5:296-309. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 76] [Cited by in F6Publishing: 75] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 3. | Liu CL, Fan ST, Lo CM, Tung-Ping Poon R, Wong J. Anterior approach for major right hepatic resection for large hepatocellular carcinoma. Ann Surg. 2000;232:25-31. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 153] [Cited by in F6Publishing: 156] [Article Influence: 6.5] [Reference Citation Analysis (0)] |
| 4. | Imamura H, Seyama Y, Kokudo N, Maema A, Sugawara Y, Sano K, Takayama T, Makuuchi M. One thousand fifty-six hepatectomies without mortality in 8 years. Arch Surg. 2003;138:1198-1206; discussion 1206. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 597] [Cited by in F6Publishing: 585] [Article Influence: 29.3] [Reference Citation Analysis (0)] |
| 5. | Miyazono F, Takao S, Natsugoe S, Uchikura K, Kijima F, Aridome K, Shinchi H, Aikou T. Molecular detection of circulating cancer cells during surgery in patients with biliary-pancreatic cancer. Am J Surg. 1999;177:475-479. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 51] [Cited by in F6Publishing: 51] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 6. | Rahbari NN, Wente MN, Schemmer P, Diener MK, Hoffmann K, Motschall E, Schmidt J, Weitz J, Büchler MW. Systematic review and meta-analysis of the effect of portal triad clamping on outcome after hepatic resection. Br J Surg. 2008;95:424-432. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 109] [Cited by in F6Publishing: 116] [Article Influence: 7.3] [Reference Citation Analysis (0)] |
| 7. | Liu CL, Fan ST, Cheung ST, Lo CM, Ng IO, Wong J. Anterior approach versus conventional approach right hepatic resection for large hepatocellular carcinoma: a prospective randomized controlled study. Ann Surg. 2006;244:194-203. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 184] [Cited by in F6Publishing: 198] [Article Influence: 11.0] [Reference Citation Analysis (0)] |
| 8. | Moher D, Cook DJ, Eastwood S, Olkin I, Rennie D, Stroup DF. Improving the quality of reports of meta-analyses of randomised controlled trials: the QUOROM statement. Quality of Reporting of Meta-analyses. Lancet. 1999;354:1896-1900. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 3364] [Cited by in F6Publishing: 3285] [Article Influence: 131.4] [Reference Citation Analysis (0)] |
| 9. | Moher D, Liberati A, Tetzlaff J, Altman DG. Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. BMJ. 2009;339:b2535. [PubMed] [Cited in This Article: ] |
| 10. | Jadad AR, Enkin MW. Computers: transcending our limits? BMJ. 2007;334 Suppl 1:s8. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 12] [Cited by in F6Publishing: 12] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 11. | Deeks JJ, Higgins JPT, Altman DG. Chapter 9: Analysing data and undertaking meta-analyses. In: Higgins JPT, Green S, editors. Cochrane Handbook for Systematic Reviews of Interventions Version 510 (updated March 2011). The Cochrane Collaboration 2011; Available from: http://www.cochranehandbood.org. [Cited in This Article: ] |
| 12. | Li SQ, Liang LJ, Peng BG, Yin XY, Lü MD, Kuang M, Li DM, Fu SJ. [A comparative study of anterior versus conventional approach right hepatectomy for large hepatocellular carcinoma]. Zhonghua Yixue Zazhi. 2010;90:1670-1673. [PubMed] [Cited in This Article: ] |
| 13. | Wu TJ, Wang F, Lin YS, Chan KM, Yu MC, Lee WC. Right hepatectomy by the anterior method with liver hanging versus conventional approach for large hepatocellular carcinomas. Br J Surg. 2010;97:1070-1078. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 38] [Cited by in F6Publishing: 42] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 14. | Takács I, Furka A, Kotán R, Boland Mehrdad G, Pósán J, Vágvölgyi A, Hallay J, Sápy P. [Anterior approach for liver resection in the cases of the treatment of large liver tumors]. Magy Seb. 2006;59:362-368. [PubMed] [Cited in This Article: ] |
| 15. | Wang CC, Jawade K, Yap AQ, Concejero AM, Lin CY, Chen CL. Resection of large hepatocellular carcinoma using the combination of liver hanging maneuver and anterior approach. World J Surg. 2010;34:1874-1878. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 29] [Cited by in F6Publishing: 29] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 16. | Capussotti L, Ferrero A, Russolillo N, Langella S, Lo Tesoriere R, Viganò L. Routine anterior approach during right hepatectomy: results of a prospective randomised controlled trial. J Gastrointest Surg. 2012;16:1324-1332. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 18] [Cited by in F6Publishing: 19] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 17. | Hayashi N, Egami H, Kai M, Kurusu Y, Takano S, Ogawa M. No-touch isolation technique reduces intraoperative shedding of tumor cells into the portal vein during resection of colorectal cancer. Surgery. 1999;125:369-374. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 86] [Cited by in F6Publishing: 87] [Article Influence: 3.5] [Reference Citation Analysis (0)] |
| 18. | Belghiti J, Guevara OA, Noun R, Saldinger PF, Kianmanesh R. Liver hanging maneuver: a safe approach to right hepatectomy without liver mobilization. J Am Coll Surg. 2001;193:109-111. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 385] [Cited by in F6Publishing: 344] [Article Influence: 15.0] [Reference Citation Analysis (0)] |
| 19. | Matsumata T, Ikeda Y, Hayashi H, Kamakura T, Taketomi A, Sugimachi K. The association between transfusion and cancer-free survival after curative resection for hepatocellular carcinoma. Cancer. 1993;72:1866-1871. [PubMed] [Cited in This Article: ] |
| 20. | Yamamoto J, Kosuge T, Takayama T, Shimada K, Yamasaki S, Ozaki H, Yamaguchi N, Mizuno S, Makuuchi M. Perioperative blood transfusion promotes recurrence of hepatocellular carcinoma after hepatectomy. Surgery. 1994;115:303-309. [PubMed] [Cited in This Article: ] |
| 21. | Poon RT, Fan ST, Lo CM, Ng IO, Liu CL, Lam CM, Wong J. Improving survival results after resection of hepatocellular carcinoma: a prospective study of 377 patients over 10 years. Ann Surg. 2001;234:63-70. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 451] [Cited by in F6Publishing: 454] [Article Influence: 19.7] [Reference Citation Analysis (0)] |
| 22. | Liu CL, Fan ST. Anterior approach for right hepatectomy for hilar cholangiocarcinoma. J Hepatobiliary Pancreat Surg. 2003;10:292-294. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 12] [Cited by in F6Publishing: 12] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 23. | Paterlini-Bréchot P, Vona G, Bréchot C. Circulating tumorous cells in patients with hepatocellular carcinoma. Clinical impact and future directions. Semin Cancer Biol. 2000;10:241-249. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 21] [Cited by in F6Publishing: 23] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 24. | Kopreski MS, Gocke CD. Cellular- versus extracellular-based assays. Comparing utility in DNA and RNA molecular marker assessment. Ann N Y Acad Sci. 2000;906:124-128. [PubMed] [Cited in This Article: ] |
| 25. | Capussotti L, Muratore A, Amisano M, Polastri R, Bouzari H, Massucco P. Liver resection for hepatocellular carcinoma on cirrhosis: analysis of mortality, morbidity and survival--a European single center experience. Eur J Surg Oncol. 2005;31:986-993. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 89] [Cited by in F6Publishing: 83] [Article Influence: 4.4] [Reference Citation Analysis (0)] |
| 26. | Schneider PD. Preoperative assessment of liver function. Surg Clin North Am. 2004;84:355-373. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 172] [Cited by in F6Publishing: 182] [Article Influence: 9.1] [Reference Citation Analysis (0)] |