Published online Jan 14, 2015. doi: 10.3748/wjg.v21.i2.675
Peer-review started: July 30, 2014
First decision: August 27, 2014
Revised: September 20, 2014
Accepted: December 1, 2014
Article in press: December 1, 2014
Published online: January 14, 2015
AIM: To review the literature on idiopathic sclerosing encapsulating peritonitis (SEP), also known as abdominal cocoon syndrome.
METHODS: The PubMed, MEDLINE, Google Scholar, and Google databases were searched using specific key words to identify articles related to idiopathic SEP. These key words were “sclerosing encapsulating peritonitis,”“idiopathic sclerosing encapsulating peritonitis,”“abdominal cocoon,” and “abdominal cocoon syndrome.” The search included letters to the editor, case reports, review articles, original articles, and meeting presentations published in the English-language literature from January 2000 to May 2014. Articles or abstracts containing adequate information about age, sex, symptom duration, initial diagnosis, radiological tools, and surgical approaches were included in the study. Papers with missing or inadequate data were excluded.
RESULTS: The literature search yielded 73 articles on idiopathic (primary) SEP published in 23 countries. The four countries that published the greatest number of articles were India (n = 21), Turkey (n = 14), China (n = 8) and Nigeria (n = 3). The four countries that reported the greatest number of cases were China (n = 104; 53.88%), India (n = 35; 18.13%), Turkey (n = 17; 8.80%) and Nigeria (n = 5; 2.59%). The present study included 193 patients. Data on age could be obtained for 184 patients (range: 7-87 years; mean ± SD, 34.7 ± 19.2 years), but were unavailable for nine patients. Of the 184 patients, 122 were male and 62 were female; sex data could not be accessed in the remaining nine patients. Of the 149 patients whose preoperative diagnosis information could be obtained, 65 (43.6%) underwent operations for abdominal cocoon, while the majority of the remaining patients underwent operations for a presumed diagnosis of intestinal obstruction and/or abdominal mass. Management information could be retrieved for 115 patients. Of these, 68 underwent excision + adhesiolysis (one laparoscopic); 24 underwent prophylactic appendectomy in addition to excision + adhesiolysis. Twenty patients underwent various resection and repair techniques along with excision + adhesiolysis. The remaining three patients were managed with antituberculosis therapy (n = 2) and immunosuppressive therapy (n = 1).
CONCLUSION: Idiopathic SEP is a rare disorder characterized by frequently recurring bouts of intestinal obstruction. Surgical therapy is the gold standard management strategy.
Core tip: Idiopathic sclerosing encapsulating peritonitis (SEP) is a clinical entity characterized by partial or complete encasement of the small intestines by a thick fibrocollagenous membrane. While some patients with idiopathic SEP are asymptomatic, the majority of affected individuals develop acute, subacute or chronic attacks of gastrointestinal obstruction. Preoperative diagnosis of the disease is quite difficult, and many cases are diagnosed intraoperatively. Nonetheless, recent technological advances in imaging modalities, particularly computed tomography, have made preoperative diagnosis of SEP possible. Surgery remains the best management option for patients with severe signs of intestinal obstruction.
- Citation: Akbulut S. Accurate definition and management of idiopathic sclerosing encapsulating peritonitis. World J Gastroenterol 2015; 21(2): 675-687
- URL: https://www.wjgnet.com/1007-9327/full/v21/i2/675.htm
- DOI: https://dx.doi.org/10.3748/wjg.v21.i2.675
Sclerosing encapsulating peritonitis (SEP) is a chronic inflammatory process in which the small intestines are encased by a dense fibrocollagenous membrane[1-32]. SEP was first defined nearly 100 years ago, at which time it was termed “peritonitis chronica fibrosa incapsulata”[1,6,7]. The disorder is divided into primary (idiopathic) and secondary forms according to the underlying etiological cause[1-5]. The primary form was termed “abdominal cocoon syndrome” by Foo in 1978[1]. The clinical signs and symptoms of SEP vary with the severity and duration of the disease, underlying causes, and affected person’s immunological status. SEP most commonly manifests as recurrent acute, subacute, or chronic episodes of intestinal obstruction[2,4]. However, some cases may also manifest with more uncommon, but life-threatening, complications including enterocutaneous fistula, small intestinal necrosis, and malnutrition. Preoperative diagnosis of SEP is quite difficult, and many cases are diagnosed intraoperatively[4,6]. Fortunately, preoperative diagnosis of SEP has become possible with recent technological advances in imaging modalities, particularly computed tomography (CT)[1,5-11]. Surgery remains the most effective management option for SEP[4], although controversy surrounds the indications, optimal timing, and mode of surgical operation. This is because surgical outcomes are far from satisfactory, and some patients may develop postoperative small intestinal obstruction and new adhesions[4]. The present study reviews and discusses the previously published articles on SEP.
We reviewed nearly 200 previously published articles on SEP. A serious contradiction was present between selection and classification of cases, because many authors used the term “abdominal cocoon” while actually describing cases of secondary SEP. We therefore aimed to resolve this conflict by establishing a proper definition and classification of SEP before starting the literature review. We divided SEP into primary (idiopathic; abdominal cocoon syndrome) and secondary forms. Patients with no factors explaining SEP after various examinations (history taking, blood tests, radiological imaging, and histopathological tests) performed during the preoperative, perioperative, or postoperative periods were determined to have primary SEP (idiopathic, abdominal cocoon). Patients with SEP that developed as a result of various conditions, including abdominal surgery, abdominal tuberculosis, peritoneal dialysis (PD), ventriculoperitoneal or peritoneovenous shunts, liver transplantation, recurrent peritonitis, beta-blocker treatment (practolol or propranolol), intraperitoneal chemotherapy, intraperitoneal povidone-iodine use, liver cirrhosis, gastrointestinal malignancy, fibrogenic foreign material, systemic lupus erythematosus, or parasitic infection (sometimes leading to granulomatous peritonitis) were determined to have secondary SEP. The main objective of the present study was to perform a brief review of the literature to identify studies on primary SEP (idiopathic; cocoon syndrome) published from January 2000 to May 2014. To achieve this aim, we scanned the PubMed, MEDLINE, Google Scholar, and Google databases for the key words “sclerosing encapsulating peritonitis,”“idiopathic sclerosing encapsulating peritonitis,”“abdominal cocoon,” and “abdominal cocoon syndrome” entered alone or in various combinations. Only articles published in English were included in the scanning process. Cases that met the diagnostic criteria for idiopathic SEP (abdominal cocoon) were included in the review, while cases with features of secondary SEP were excluded. The corresponding authors of some papers were e-mailed several times regarding necessary information about their articles. However, we received no effective responses from the authors of the two largest studies. We created a table with useful information about the reviewed articles, including publication year, country, number of cases, patient age, sex, history, white blood cell count, surgical approach, complications, follow-up duration and other ancillary information.
A literature review using the above mentioned inclusion criteria revealed 73 articles on idiopathic (primary) SEP from 23 countries[2-10,12-31,33-76]. The four countries with the highest numbers of published articles were India (n = 21; 28.76%), Turkey (n = 14; 19.17%), China (n = 8; 10.95%) and Nigeria (n = 3; 4.10%). The four countries reporting the highest number of cases were China (n = 104; 53.88%), India (n = 35; 18.13%), Turkey (n = 17; 8.80%) and Nigeria (n = 5; 2.59%). Other data related to the article distribution among countries are presented in Table 1. In total, 193 patients were included in this study. Their ages ranged from 7 to 87 years (mean ± SD, 34.7 ± 19.2 years) among 184 patients; this information was unavailable for the remaining 9 patients. Of the 184 patients, 122 were male and 62 were female; no sex data were available for the remaining 9 patients. The symptom duration ranged from 8 h to 210 mo among 174 patients; this information was unavailable for the remaining 19 patients. Of 149 patients with available data on preoperative diagnosis, 65 (43.6%) underwent operations for a presumed diagnosis of abdominal cocoon syndrome, while the majority of the remaining patients underwent operations for an initial diagnosis of intestinal obstruction and/or abdominal mass. Patient management data were available in 115 patients; 68 underwent excision + adhesiolysis, and 24 underwent prophylactic appendectomy in addition to excision + adhesiolysis. Twenty patients underwent various resection and anastomosis techniques in addition to excision + adhesiolysis. Two patients commenced antituberculous therapy without antecedent surgical therapy. Those patients had no signs or symptoms pertaining to tuberculosis. One patient was administered with steroids and immunosuppressive therapy. The demographic and clinical data of the 193 patients included in the present study are summarized in Table 2. Two studies were published from the same institution and used the medical data of the same patient; despite meeting the inclusion criteria for this review, one of these studies was excluded[60,77].
| Countries | Published articles | Published cases |
| China | 8 (10.95) | 104 (53.88) |
| India | 21 (28.76) | 35 (18.13) |
| Turkey | 14 (19.17) | 17 (8.80) |
| Nigeria | 3 (4.10) | 5 (2.59) |
| Taiwan | 2 (2.74) | 2 (1.03) |
| Pakistan | 2 (2.74) | 2 (1.03) |
| Qatar | 2 (2.74) | 3 (1.55) |
| Saudi Arabia | 2 (2.74) | 2 (1.03) |
| Israel | 2 (2.74) | 3 (1.55) |
| Iran | 2 (2.74) | 2 (1.03) |
| Nepal | 2 (2.74) | 2 (1.03) |
| Brazil | 2 (2.74) | 3 (1.55) |
| Italy | 1 (1.37) | 1 (0.51) |
| United States | 1 (1.37) | 2 (1.03) |
| South Korea | 1 (1.37) | 2 (1.03) |
| Senegal | 1 (1.37) | 1 (0.51) |
| Iraq | 1 (1.37) | 1 (0.51) |
| Belgium | 1 (1.37) | 1 (0.51) |
| Bangladesh | 1 (1.37) | 1 (0.51) |
| Kuwait | 1 (1.37) | 1 (0.51) |
| Malaysia | 1 (1.37) | 1 (0.51) |
| New Zealand | 1 (1.37) | 1 (0.51) |
| Greece | 1 (1.37) | 1 (0.51) |
| Ref. | Year | Country | Case number | Age (yr) | Sex | Duration symptom | Preoperative diagnosis | Radiologic tools | Surgical approach |
| Rasihashemi et al[2] | 2014 | Iran | 1 | 25 | M | 2 mo | Int Obst | X-ray + Barium + CT | E + A |
| Nanwadekar et al[3] | 2014 | India | 1 | 17 | F | 4 d | Int Obst | X-ray + US + Endosc. | E + A |
| Li et al[4] | 2014 | China | 65 | 39 (14-79) | M: 57 | 3.9 ± 6.7 yr | ACS: 31 | NS | NS |
| F: 8 | NS: 34 | ||||||||
| Jovani et al[5] | 2014 | Italy | 1 | 44 | M | 60 mo | ACS | US + CT + MR | NS |
| Akbulut et al[6] | 2014 | Turkey | 1 | 87 | M | 3 mo | Int Obst + perforation | X-ray + US | E + A + resection + ileostomy |
| Sreevathsa et al[7] | 2013 | India | 3 | 43 | M | 12 mo | ACS | X-ray + CT | E + A |
| 13 | F | 12 mo | Int Obst | X-ray | Ileocecal resection | ||||
| 14 | F | 6 mo | Int Obst (Subacute) | X-ray | Ileocecal resection | ||||
| Singh et al[8] | 2013 | India | 9 | NS | NS | NS | NS: 9 | NS | NS |
| Shah et al[9] | 2013 | India | 1 | 14 | F | 6 mo | ACS | Barium + CT | E + A |
| Serter et al[10] | 2013 | Turkey | 2 | 32 | M | 2 d | Int Hernia | X-Ray + CT | E + A |
| 49 | M | 1 wk | ACS | CT | E + A | ||||
| Rahmati et al[12] | 2013 | Iran | 1 | 50 | M | 3 mo | ACS | US + CT + Endosc. | E + A |
| Patel et al[13] | 2013 | India | 1 | 45 | M | 6 mo | Int Obst | X-ray + CT | E + A + ileal resection |
| Ozkan et al[14] | 2013 | Turkey | 1 | 48 | M | 1 wk | ACS | X-ray + CT | E + A |
| Hu et al[15] | 2013 | China | 1 | 29 | F | Asympt. | Infertility | US | E + A + suturing (iatrogenic ileal injury) |
| Gupta et al[16] | 2013 | India | 1 | 40 | M | NS | ACS | X-ray + US + CT | E + A |
| Gadhire et al[17] | 2013 | India | 1 | 35 | M | 1 mo | ACS | X-ray + US + CT | E + A |
| Awe[18] | 2013 | Nigeria | 1 | 18 | F | 3 d | Int Obst | X-ray | E + A |
| Al Thani et al[19] | 2013 | Qatar | 1 | 41 | M | 7 mo | Int Obst (subacute) | CT | E + A |
| Thakur et al[20] | 2012 | India | 1 | 14 | F | 6 mo | Abd Mass | US | E + A |
| Taylor et al[21] | 2012 | N Zealand | 1 | 42 | M | 3 d | Int Obst | X-ray + CT | E + A + appendectomy |
| Solak et al[22] | 2012 | Turkey | 1 | 58 | M | 24 mo | ACS (previously operated) | X-ray + US + CT | Steroid + mycophenolate mofetil |
| Shakya et al[23] | 2012 | Nepal | 1 | 20 | M | 12 mo | Int Obst | X-ray | E + A + Ileostomy (iatrogenic ileal injury) |
| Ndiaye et al[24] | 2012 | Senegal | 1 | 15 | F | 2 mo | ACS | Barium + CT | E + A + Suturing (iatrogenic ileal injury) |
| Meshikhes et al[25] | 2012 | Saudi Arabia | 1 | 45 | M | 6 mo | Int Obst + Abd mass | CT | E + A + appendecectomy |
| Malik et al[26] | 2012 | Pakistan | 1 | 24 | F | 60 mo | Int Obst | X-ray | E + A |
| Kumar et al[27] | 2012 | India | 2 | 18 | F | 24 mo | ACS ? | Barium + US + CT | Antitubercular therapy |
| 14 | F | NS | ACS ? | CT + US | Antitubercular therapy | ||||
| Kayastha et al[28] | 2012 | Pakistan | 1 | 13 | F | 2 mo | Acute appendicitis | US | E + A |
| Kaur et al[29] | 2012 | India | 2 | 43 | M | 180 mo | ACS | X-ray + US + CT | E + A |
| 17 | F | 4 mo | ACS | X-ray + US + CT | E + A | ||||
| Araujo Filho et al[30] | 2012 | Brazil | 1 | 36 | M | 10 d | ACS | US + CT | E + A |
| Chatura et al[31] | 2012 | India | 1 | 14 | F | NS | Int Obst + Abd mass | US | E + A + ileocolectomy |
| Yeniay et al[33] | 2011 | Turkey | 2 | 26 | F | 2 d | Int Obst | X-ray + CT | E + A |
| 71 | M | 3 mo | Int Obst | X-ray + CT | E + A | ||||
| Kirshtein et al[34] | 2011 | Israel | 1 | 82 | M | 4 d | Int Obst | X-Ray + gastrografin | E + A |
| Jayant et al[35] | 2011 | India | 1 | 16 | F | NS | Int Obst | CT | E + A |
| Gupta et al[36] | 2011 | Nepal | 1 | 42 | M | 4 mo | ACS | X-ray + US + CT | E + A |
| Ertem et al[37] | 2011 | Turkey | 1 | 29 | M | 2 d | Int Obst | X-ray + US + CT | E + A - laparoscopic |
| Da Luz et al[38] | 2011 | Brazil | 2 | 30 | M | NS | Int Obst + Int Hernia | X-ray + barium | E + A + laparostomy |
| 32 | M | 6 mo | Int Obst + Chron? | X-ray + barium | E + A | ||||
| Bassiouny et al[39] | 2011 | Qatar | 2 | 7 | M | 48 mo | Int Obst + Abd mass | X-ray | E + A |
| 12 | F | 48 mo | Int Obst | X-ray + US | E + A | ||||
| Wang et al[40] | 2010 | China | 1 | 48 | M | 3 mo | ACS | CT | E + A + appendectomy |
| Tombak et al[41] | 2011 | Turkey | 1 | 36 | M | 1 mo | ACS | CT | E + A |
| Naik et al[42] | 2010 | India | 1 | 70 | M | 48 mo | Int Obst | X-ray + US + CT + Endosc. | E + A |
| Lee et al[43] | 2010 | Taiwan | 1 | 57 | F | ACS | X-ray + US + CT | E + A | |
| Gurleyik et al[44] | 2010 | Turkey | 1 | 30 | M | 36 mo | Int Obst | X-ray + US + CT | E + A |
| Al Saied et al[45] | 2010 | Saudi Arabia | 1 | 24 | M | 36 mo | ACS | X-ray + CT | E + A |
| Yang et al[46] | 2009 | China | 1 | 43 | M | NS | NS | X-ray + Endosc. | Resection (?) |
| Yang et al[47] | 2009 | China | 6 | 43.7 (39-48) | M: 4 | 3-60 mo | Int Obst: 5 | X-ray + CT | E + A: 5 |
| F: 2 | ACS: 1 | E + A + jejunal resection: 1 | |||||||
| Wu et al[48] | 2009 | Taiwan | 1 | 80 | M | 24 mo | Int Obst | X-ray + US + CT | E + A |
| Wei et al[49] | 2009 | China | 24 | 34 (15-57) | M: 9 | 3 d-216 mo | ACS: 4 | X-ay + barium + US + CT | E + A + appendectomy: 17 |
| F: 15 | Int Obst/mass: 20 | E + A + enterotomy: 2 | |||||||
| E + A + cecofixation: 2 | |||||||||
| E + A: 3 | |||||||||
| Tasdelen et al[50] | 2009 | Turkey | 1 | 85 | F | 3 d | Int Obst + Int Hernia | X-ray + CT | E + A + jejunoileal resection with anastomosis |
| Reynders et al[51] | 2009 | Belgium | 1 | 40 | M | 36 mo | Int Obst | X-ray + CT | E + A + Meckel's resection + appendectomy |
| Mohanty et al[52] | 2009 | India | 1 | 15 | F | 24 mo | ACS | X-ray + US + CT | E + A |
| Kumar et al[53] | 2009 | India | 3 | 45 | M | 24 mo | ACS | X-ray + CT | E + A |
| 63 | M | 216 mo | ACS | X-ray + US + CT | E + A | ||||
| 16 | F | 10 h | ACS | X-ray + US + CT | E + A | ||||
| Ibrahim et al[54] | 2009 | Nigeria | 1 | 14 | M | 72 h | Int Obst | X-ray | E + A + appendectomy |
| Choudhury et al[55] | 2009 | Bangladesh | 1 | 15 | F | 12 mo | Appendiceal mass | US | Partial ileocolic resection with anastomosis |
| Zheng et al[56] | 2008 | China | 1 | 69 | M | 1 d | ACS | X-ray + US + CT | E + A + ileal resection with anastomosis |
| Bas et al[57] | 2008 | Turkey | 1 | 42 | M | 5 mo | Int Obst | X-ray + CT | E + A |
| Singh et al[58] | 2008 | India | 1 | 38 | M | 12 mo | Int Obst | X-ray + US | E + A |
| Xu et al[59] | 2007 | China | 5 | 41 | F | 4 mo | Int Obst | X-ray + CT + Endosc. | E + A |
| 49 | F | 120 mo | Int Obst | X-ray + CT + Endosc. | E + A | ||||
| 21 | M | 36 mo | Int Obst | X-ray + CT + Endosc. | E + A | ||||
| 41 | M | 1 mo | Int Obst | X-ray + CT + Endosc. | Adhesiolysis + jejunal resection with anastomosis | ||||
| 36 | M | 2 wk | Int Obst | X-ray + CT + Endosc. | E + A | ||||
| Demir et al[60] | 2007 | Turkey | 1 | 38 | M | 6-7 mo | ACS | CT | E + A |
| Cai et al[61] | 2007 | United States | 2 | 38 | M | 2 d | Int Obst | X-ray | E + A |
| 45 | M | 8 h | Int Obst | CT | E + A | ||||
| Basu et al[62] | 2007 | India | 1 | 47 | M | 3 mo | Abd mass | X-ray + US + barium | E + A |
| Al-Ibrahim et al[63] | 2007 | Kuwait | 1 | 33 | M | 1 mo | Int Obst | X-ray + US + CT | E + A |
| Serafimidis et al[64] | 2006 | Greece | 1 | 56 | M | 48 mo | Int Obst | X-ray + US + CT + Endosc. | E + A |
| Rokade et al[65] | 2006 | India | 1 | 26 | F | 12 mo | ACS (previously operated) | US + CT | E + A |
| Pillai et al[66] | 2006 | India | 1 | 13 | F | NS | ACS | X-ray + US + CT | E + A |
| Akca et al[67] | 2006 | Turkey | 1 | 57 | M | 75 d | Int Hernia + mesenteritis | US + CT + Colonosc. | NS |
| Yucel et al[68] | 2004 | Turkey | 2 | 15 | F | NS | Int Obst | X-ray + CT | E + A |
| 38 | M | 72 mo | Int Obst | X-ray + CT | E + A | ||||
| Hur et al[69] | 2004 | South Korea | 2 | 34 | F | 120 mo | Int Obst | X-ray + barium + US + CT | NS |
| 47 | M | NS | Int Obst | X-ray + barium + CT | NS | ||||
| Vijayaraghavan et al[70] | 2003 | India | 1 | 12 | F | 3 mo | ACS + Int Hernia | US | E + A |
| Ranganathan et al[71] | 2003 | Malaysia | 1 | 25 | F | 3 mo | Large ovarian mass + ascites | US + CT | E + A |
| Hasan[72] | 2002 | Iraq | 1 | 20 | F | NS | Acute abdomen | Pregnant patient | E + A |
| Hamaloglu et al[73] | 2002 | Turkey | 1 | 38 | M | 12 mo | Int Obst | X-ray + Barium + US | E + A |
| Okobia et al[74] | 2001 | Nigeria | 3 | 18 | F | 5 mo | Pelvic collection | US | E + A + appendectomy |
| 12 | F | 1 wk | Mesenteric cyst | X-ray + US | E + A + appendectomy | ||||
| 10 | F | 2 mo | Ovarian Tm + Burkitt's Tm + uterine mass | X-ray + Urography | E + A + appendectomy | ||||
| Mordehai et al[75] | 2001 | Israel | 2 | 14 | F | 1 mo | Int Obst | X-ray + US + CT | E + A |
| 15 | F | 6 mo | Int Obst | X-ray + US | E + A | ||||
| Kumar et al[76] | 2000 | India | 1 | 12 | F | 24 h | Int Obst | X-ray + US | E + A |
The definition of SEP is associated with confusion and lack of information. The concepts of primary and secondary SEP are erroneously used interchangeably in many previously published articles on SEP[11,32]. Thus, we aimed to emphasize the correct use of the definitions of peritoneal encapsulation (PE), abdominal cocoon, idiopathic SEP, and secondary SEP in the present review.
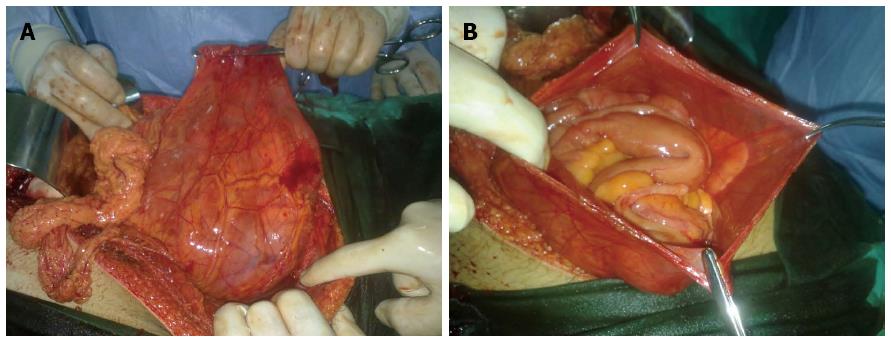
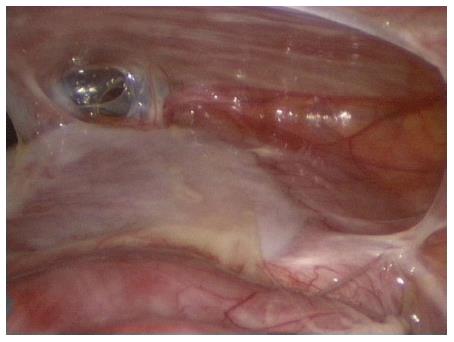
PE was first described by Cleland in 1868[32]. It is a developmental anomaly characterized by the congenital presence of an accessory peritoneal membrane, which is believed to be derived from the yolk sac peritoneum in the early stages of fetal life[10,15,29,32]. This peritoneal membrane is classically found between the mesocolon and omentum, and most of the small intestines lie posterior to this membrane[21,27,39,48,75]. In other words, PE is an anatomical anomaly unrelated to any inflammatory process. PE is typically asymptomatic and incidentally detected during laparotomy performed for other indications[29,32,62,73]. In one patient, we observed anatomical features similar to those of PE during laparotomy performed to treat a gunshot injury (Figure 1).
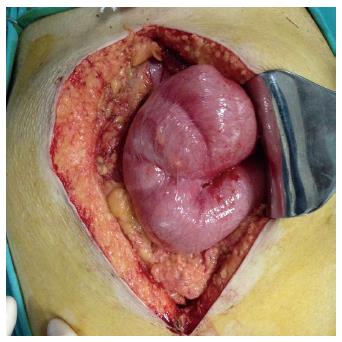
Unlike PE, SEP is an acquired condition resulting from peritoneal inflammation that may be triggered by various factors[32,38]. While the accessory peritoneal membrane is covered by mesothelium in patients with PE, the membrane that encases the intestines in patients with SEP has a dull, fibrous structure that includes inflammatory cells[33,38,39]. SEP is a clinical entity characterized by partial or complete encasement of the small intestines by a thick fibrocollagenous membrane (Figure 2)[1,4,6,10,17,24]. This membrane often encapsulates the small intestines, but it sometimes also encases other intraperitoneal organs, such as the stomach, liver, and colon[1,6,8,23,55]. This clinical entity was first defined in 1907 by Owtschinnikow, who described encasement of the intestines by a fibrocollagenous membrane[1,50,55]. Considering the morphological and histological properties of the membrane encasing the intestines, Owtschinnikow termed this condition “peritonitis chronica fibrosa incapsulata”[1,16,17,27]. Historically, SEP was classified as primary (idiopathic) or secondary, depending on its underlying cause and the pathogenetic properties of the fibrocollagenous membrane[1,23,42,49]. The idiopathic form of SEP has also been termed “abdominal cocoon syndrome,” a term that was first used by Foo in 1978[1]. Abdominal cocoon is categorized into three types according to the extent of the encasing membrane that covers the intestine. Encasement of part of the intestine by a fibrocollagenous membrane is called type 1 cocoon syndrome. Complete coverage of the intestine by the membrane is called type 2 abdominal cocoon syndrome. Type 3 cocoon syndrome refers to encasement of the whole intestine, as well as other intra-abdominal organs, such as the appendix, cecum, ascending colon, and ovaries[1,49].
SEP is considered to be primary (idiopathic) or secondary, depending on its underlying cause[1-10]. No underlying cause can be demonstrated in primary SEP, although the role of cytokines and fibroblasts in development of peritoneal fibrosis and neoangiogenesis is indisputable[40,58]. Idiopathic SEP classically presents in young adolescent girls in tropical and subtropical countries such as China, Malaysia, Singapore, Pakistan, India, Nigeria, Kenya, Saudi Arabia, and South Africa, although adult cases of idiopathic SEP in temperate zones have also been reported[1,4,7,22,23,58,61]. The present study showed that idiopathic SEP is twice as common in men than in women. Our findings on the geographical distribution of SEP coincide with those in the previously published literature. Indeed, nearly all cases presented herein occurred in tropical or subtropical regions of the world.
Many hypotheses regarding the etiology of idiopathic SEP have been proposed[55,59,64]. Some of these hypotheses involve retrograde menstruation with a superimposed viral infection, retrograde peritonitis via the fallopian tubes, and cell-mediated immunological tissue damage secondary to gynecological infection[1,4,7,13,23,28,36,39]. However, SEP also develops in men, premenopausal women, and children, reducing support for these theories[1,4,7,28,61]. In total, 66 of 89 patients included in the largest two studies on idiopathic SEP in the literature to date were male[4,49]. Some authors have argued that the fibrous membrane that encases the intestines is a result of a developmental disorder, citing vascular anomalies and omental hypoplasia as the basis of their hypothesis[1,49,59].
Secondary SEP is more common than idiopathic SEP[22,45,52]. In secondary SEP, a local or systemic factor triggers the inflammatory process in the peritoneum[52]. PD is the most common cause of secondary SEP[1]. In other words, secondary SEP is the leading cause of the most severe complications of PD. This is because once secondary SEP has developed, the ultrafiltration capacity of the peritoneal surface decreases and the risk of intestinal obstruction increases[1]. Studies have shown a direct relationship between prolonged PD and the development of secondary SEP[1,11]. Considering the number of patients undergoing PD worldwide, the importance of the relationship between PD and secondary SEP needs to be better understood. Abdominal tuberculosis continues to be a major public health issue and an important etiological agent of secondary SEP in underdeveloped countries[8]. Among the less frequent causes of secondary SEP are a history of abdominal surgery, autoimmune disorders, some drugs, peritoneal shunts, and recurrent episodes of peritonitis[1,4,7,10,17,28,32,34]. The classification and potential etiological factors of SEP are listed in detail in Table 3.
| Primary (idiopathic) sclerosing encapsulating peritonitis |
| I Adolescent form |
| II Adult form |
| Secondary sclerosing encapsulating peritonitis |
| I Systemically induced by |
| Beta adrenergic blocking agents |
| Practolol |
| Timolol |
| Propanolol |
| Other drugs |
| Methotrexate |
| Protein S deficiency |
| Exposure to asbestosis |
| II Induced by possible local and/or systemic irritants |
| Peritoneal dialysis |
| Abdominal trauma |
| Abdominal surgery |
| Liver transplantation |
| Peritoneovenous shunt |
| Ventriculoperitoneal shunt |
| Peritoneal sarcoidosis |
| Liver cirrhosis |
| Peritoneal tuberculosis |
| Sarcoidosis |
| Familial mediterranean fever |
| Systemic lupus erythematosus |
| Gastrointestinal malignancy |
| Intraperitoneal chemotherapy |
| Fibrogenic foreign body |
| Endometriosis |
| Dermoid cyst rupture |
| Luteinized ovarian thecomas |
| Cytomegalovirus peritonitis |
| Recurrent peritonitis |
| Granulomatous peritonitis related with parasitic infestation |
Idiopathic SEP is an uncommon entity, and a great majority of physicians either never encounter patients with this condition or miss the diagnosis even when they do. Achieving a correct preoperative diagnosis in affected patients is extremely difficult and requires a high index of clinical suspicion[1,4,25,38,51]. Recent advances in radiological modalities have allowed physicians to achieve a correct preoperative diagnosis of SEP in affected patients[59,71,77]. Nevertheless, preoperative diagnosis remains a clinical challenge because most patients with SEP present to emergency departments with signs and symptoms of intestinal obstruction, and many emergency departments lack advanced radiological equipment and adequate staff, and patients with this syndrome usually undergo operations on an urgent basis[38]. In one large case series, 52.3% to 100.0% of admitted patients were diagnosed during surgery and 16.7% to 48.7% were diagnosed during their preoperative examinations[4,8,50]. While some patients with SEP are asymptomatic, most affected individuals develop acute, subacute, or chronic attacks of gastrointestinal obstruction (incomplete or complete); nausea; vomiting; anorexia; appetite loss; weight loss; and malnutrition[1,4,8,10,11,26]. Although rare, a painless, soft abdominal mass can be palpated in some patients[1,4,8,29,30,76]. Additionally, abdominal ascites and distention are detectable in some patients with severe disease. Ascites may be massive enough to induce suspicion of underlying hepatic disease. Primary SEP may be considered in patients presenting with recurrent attacks of abdominal pain who are free of any disease explaining such attacks[1]. Gastrointestinal perforation is quite rare in patients with SEP; of all reported cases of SEP, only two (one secondary to tuberculosis and the other idiopathic) were associated with spontaneous perforation[6].
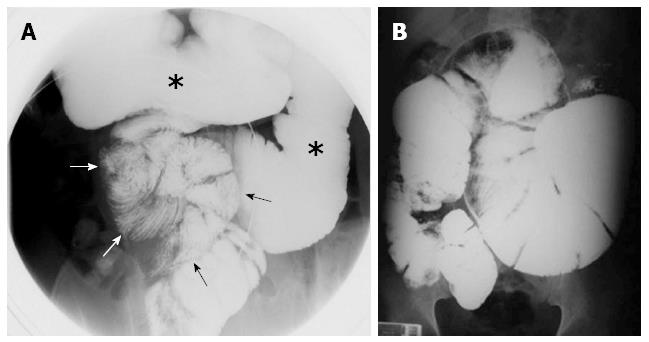
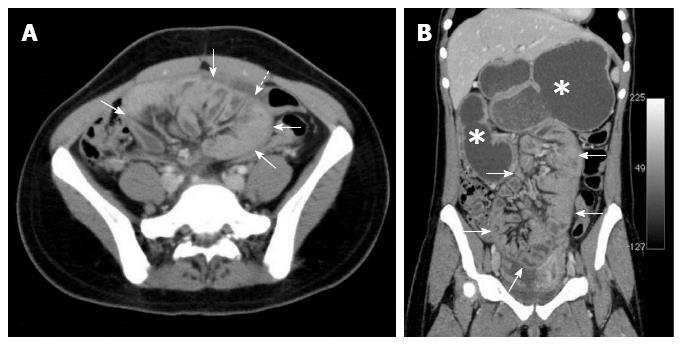
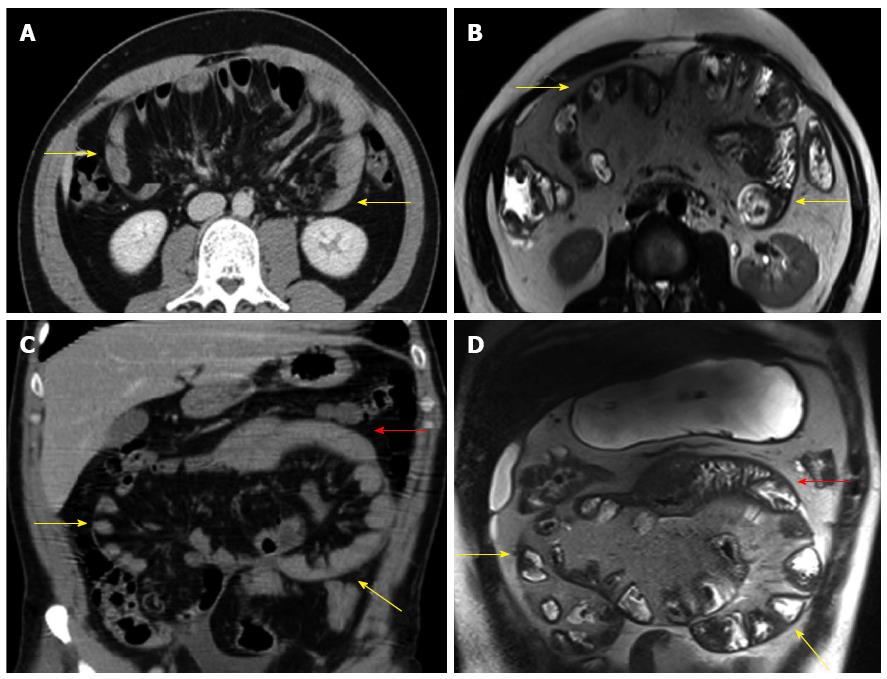
The diagnosis of SEP is often made by a combination of the medical history, a high clinical index of suspicion, various biochemical parameters, and radiological findings[18,23,26]. The patient’s medical history (tuberculosis, PD, systemic lupus erythematosus, previous abdominal operations, etc.) usually provides important clues regarding the etiology of secondary SEP. The most commonly used radiological techniques are abdominal X-rays, small intestinal barium studies, ultrasonography, abdominal CT, and occasionally contrast-enhanced magnetic resonance (MR) imaging[5,6,28,30]. Abdominal X-rays may show diffuse air-fluid levels and dilated small intestinal loops[1,3,29,35]. However, X-ray findings are not specific to idiopathic SEP; rather, they are common to many conditions characterized by intestinal obstruction[22]. In patients with SEP, small intestinal barium studies show the intestinal loops that are accumulated and conglomerated at the center of the abdomen (Figure 3A)[1,9,24,29,76]. This appearance is termed the cauliflower sign or accordion pattern and is a clue for the diagnosis of SEP[9,24,29,64,66,69]. A prolonged transit time may also aid in the diagnosis (Figure 3B)[1,23,24,38]. However, barium studies may not be possible in patients with prominent signs of intestinal obstruction. Abdominal ultrasonography may show dilated bowel segments encased by a dense fibrous membrane[1,44] or free abdominal fluid and a thickened peritoneal layer[22,29,68]. Contrast-enhanced CT is the most helpful imaging modality for the diagnosis of abdominal cocoon[3,29,36]. The characteristic sign on CT is the appearance of small bowel segments that are conglomerated at the midline and encased by a dense capsule with a contrast-free periphery (Figure 4)[1,4,14,19,35,36]. CT may also show intestinal obstruction, ascites, localized fluid collections, peritoneal or mesenteric thickening, mural or peritoneal calcifications, and lymphadenopathy[1,60]. Multidetector CT technology has greater accuracy because it allows for multiplanar (axial, sagittal, and coronal) reconstruction. It thus provides valuable information about the severity and level of intestinal obstruction[22,40,41]. Multiplanar reformatted images provided by multidetector CT are very helpful for both exclusion of other potential causes of intestinal obstruction and planning of the surgical operation[22,29,37,41,69]. To the best of our knowledge, only one report to date has described the use of contrast-enhanced MR imaging in a patient with idiopathic SEP. Jovani et al[5] performed MR enterography of their patients and compared MR images with CT images after oral administration of 1.5 L of polyethylene glycol and intravenous administration of gadolinium. The authors concluded that MR-acquired images were similar to or even better than CT-acquired images in patients with SEP (Figure 5). In summary, contrast-enhanced CT (multidetector CT with multiplanar reformation) is the most helpful radiological tool for confirming the diagnosis, planning therapy, and avoiding unnecessary resection in patients with SEP.
Most patients with symptomatic SEP present to an emergency department or general surgery clinic with recurrent acute, subacute, or chronic episodes of gastrointestinal obstruction[8,34]. Postoperative adhesions are detectable in approximately 60% to 80% of patients who present with small intestinal obstruction, while unusual conditions are diagnosed in about 6% of affected individuals[1,26,31,34,36,43,50]. Idiopathic SEP is one of the more unusual conditions that lead to intestinal obstruction[36,52,53]. Internal herniation and congenital PE are the two pathological conditions that should be primarily considered as differential diagnoses in such patients[10,16,29,43,70]. Less common conditions to be considered as differential diagnoses are voluminous invagination, intestinal malrotation, secondary peritonitis, and other causes of peritoneal adhesion[1,10,60]. Tuberculous peritonitis should be definitively excluded in patients who live in tuberculosis-prevalent regions[17,23]. Tuberculosis is so common in some regions that antituberculosis therapy is empirically administered to some patients with intestinal obstruction[23,25,27]. The medical history of the patient (e.g., pulmonary or genital tuberculosis), adenosine deaminase level in ascitic fluid, culture of sputum and ascitic fluid, and erythrocyte sedimentation rate should be evaluated to avoid erroneous administration of clinical therapies[17,65]. Laparoscopic or open surgical biopsy of the peritoneum may be performed to rule in a diagnosis of SEP[25]. An accurate preoperative diagnosis is vital for both accurate treatment planning and prognosis prediction[1,4]. The surgeon may avoid complications more effectively when he or she knows what to expect during laparotomy[1,4,10,22,43]. However, reaching a preoperative diagnosis for many patients is a challenging task, despite the performance of an extensive preoperative radiological and clinical workup; the correct diagnosis can only be achieved by intraoperative observation and histopathological examination[36,47,57,65].
The peritoneum of patients with SEP is characterized histopathologically by fibroconnective tissue proliferation, inflammatory infiltration, and dilated lymphatics. No evidence of foreign body granulomas, giant cells, or birefringent material is present. The term “sclerosing” refers to the progressive formation of sheets of dense collagenous tissue, the term “encapsulating” describes the sheath of new fibrous tissue that covers and constricts the small bowel and restricts its motility, and the term “peritonitis” implies an ongoing inflammatory process and the presence of a mononuclear inflammatory infiltrate within the new fibrosing tissue[1,41]. Although not pathognomonic, these findings are useful for the diagnosis of SEP when combined with characteristic surgical findings.
There is no evidence-based consensus regarding the optimal treatment approach in patients with idiopathic SEP[8], because 97.7% of the papers on idiopathic SEP to date are case reports (1 to 6 cases). Administration of conservative treatment for as long as possible is the best approach in patients with mild abdominal symptoms. In such patients, bowel rest, nasogastric decompression, and nutritional support (enteral or parenteral) are the most appropriate treatment options[22,78]. Appetite loss, malnutrition, and weight loss are the most common symptoms in patients with idiopathic SEP[4,78]. This is because recurrent bouts of intestinal obstruction, nausea, and vomiting limit patients’ oral intake, leading to weight loss and malnutrition. Li et al[4] showed that preoperative nutritional support is a statistically significant independent factor for preventing postoperative complications. Based on the results of their study, the authors recommended enteral nutritional support in patients who are able to eat and parenteral nutritional support in those unable to eat. Studies have indicated that enteral or parenteral nutritional support is key to avoiding complications and malnutrition, as well as to guarantee satisfaction among patients who undergo either medical or surgical management[4,78]. Patients with symptoms resistant to conservative therapy may be treated with drug therapies comprising tamoxifen, steroids, colchicine, azathioprine and mycophenolate mofetil[1,22,38,47,79]. Corticosteroids are thought to inhibit collagen synthesis and maturation by suppressing the inflammatory process within the peritoneal membrane. They also completely eliminate the thickened membrane[78,79]. Tamoxifen is a selective estrogen receptor modulator that inhibits fibroblastic production of transforming growth factor beta, a probiotic cytokine. This drug is therefore commonly used to treat certain fibrosclerotic disorders, such as retroperitoneal fibrosis and Riedel’s thyroiditis[1,26,78,79]. Many articles have described the use of tamoxifen in patients with SEP[1,26,78]. Colchicine inhibits mRNA expression of transforming growth factor beta, thereby exhibiting an anti-inflammatory action. It has a low side effect profile and cost, but a strong antifibrogenic effect[22]. Cornelis et al[79] reported that corticosteroids and tamoxifen are useful in preventing and/or treating SEP. However, the authors concluded that data on other agents are quite limited. Many previous studies have evaluated anti-inflammatory/antifibrogenic medical therapy in patients with SEP undergoing PD[38]. However, there are almost no data, apart from a few case reports, on the use of such medications in patients with idiopathic SEP[78,79]. Solak et al[22] reported the successful use of a steroid+mycophenolate mofetil in a patient with recurrent symptoms after a surgical operation for idiopathic SEP. Malik et al[26] similarly administered postoperative steroids. Based on the aforementioned study data, we can conclude that medical therapy may be of benefit in patients with type II and III cocoon syndrome in whom adequate excision + adhesiolysis cannot be achieved or in patients with recurrent postoperative symptoms.
Unlike asymptomatic/mildly symptomatic patients, those with severe signs of intestinal obstruction or who have been intraoperatively diagnosed with SEP may have several surgical options. Partial membrane excision + adhesiolysis, resection + anastomosis, resection + anastomosis + protective enterostomy, and explorative laparotomy may be used alone or in combination, depending on the patient-related factors involved[1,2,12,20,68,74]. In patients with idiopathic SEP, the most suitable procedure includes peeling the membrane off of the intestinal surface and excising the dense adhesions between the intestinal loops[4,8,75]. Membrane excision + adhesiolysis should be applied to all encased intestinal segments when there are no other contraindications for this procedure. The risk of recurrence is quite low when the membrane on the intestinal surface can be totally excised[4]. Instilling an antiadhesive substance with between the intestinal loops before closing the abdomen may prevent the development of postoperative adhesive small bowel obstruction[25,49]. Whether administration of an antifibrogenic/anti-inflammatory agent during the postoperative period is beneficial in patients in whom the membrane that encapsulates the intestinal loops cannot be completely excised is debatable. To avoid complications, such as anastomosis leakage and short bowel syndrome, in patients with idiopathic SEP, bowel resection is indicated only when necrosis has developed[1,2,4,8,63]. Resection is usually unnecessary, and, when performed without a solid indication, may increase patient morbidity and mortality[1,4,26].
The most common postoperative complications are early postoperative small bowel obstruction (EPSBO), intra-abdominal infection, enterocutaneous fistula, short bowel syndrome, and bowel perforation[4,25,34,45,56]. EPSBO usually develops within 30 d postoperatively in patients who have undergone extensive adhesiolysis and excision[56]. EPSBO is secondary to excessive manipulation of the intestinal loops, prolonged operation times, and intestinal edema[4,17,56]. It is a temporary form of intestinal obstruction that usually has no sequelae after treatment with bowel rest and total parenteral nutrition[4,17,56]. Some authors have recommended the performance of small bowel intubation through the orifice of the appendix in patients with type II and III cocoon syndrome to reduce the risk of developing postoperative EPBSO[4,49]. Li et al[4] reported that EPSBO (P = 0.0001) and adhesive intestinal obstructions (P = 0.005) were less common in SEP patients undergoing intestinal intubation. The same authors also reported that they administered nutritional support combined with somatostatin and, when necessary, low-dose steroids in patients with EPSBO[4,56]. Such a treatment approach both reduces intestinal edema and minimizes bacterial translocation caused by stasis. Spontaneous development of enterocutaneous fistulas and perforation are rare, and only one such case has been reported to date; this case was characterized by idiopathic SEP-induced spontaneous perforation[6]. Postoperative fistula and perforation, on the other hand, are secondary to iatrogenic injury or anastomosis leakage. Long-term outcomes are quite impressive in patients who have undergone appropriate membrane excision + adhesiolysis[4,8,34].
Laparoscopy is not part of the standard surgical approach in patients with SEP. A limited number of case reports have described successful laparoscopic membrane excision and adhesiolysis[37]. An advantage of laparoscopy is that it can be used for both diagnostic and therapeutic purposes in patients with an unclear diagnosis after appropriate testing (Figure 6)[17,25]. However, Hu et al[15] reported that when they attempted laparoscopic exploration in one patient, the trocar directly entered the bowel because of the presence of adhesions. According to both our personal experience and impressions gained from the literature, it is best to first insert the trocar into the abdomen via the open technique when laparoscopy is planned for treatment of intestinal obstruction or intra-abdominal space-occupying lesions[36]. This rule also applies to patients with peritoneal fibrosis secondary to SEP or other causes, as well as to patients with a history of abdominal surgery. Moreover, it is vital that the laparoscopic procedure is performed by an experienced operator to avoid iatrogenic bowel perforation[15,37].
In conclusion, idiopathic SEP is a clinical entity of unknown cause that is characterized by encasement of the intestines by a fibrocollagenous cocoon-like membrane. Most affected patients present to emergency departments with frequently recurring signs and symptoms of intestinal obstruction. Although recent advances in CT devices that allow for multiplanar imaging have enabled preoperative diagnosis of SEP, most cases are still incidentally diagnosed during laparotomy. Surgery remains the gold standard treatment for symptomatic idiopathic SEP. The most commonly used surgical method is membrane excision coupled with adhesiolysis. Minimally invasive management strategies help to avoid complications. Bowel rest, nasogastric decompression, and nutritional support may provide successful outcomes in asymptomatic or minimally symptomatic patients. Although various immunosuppressive, anti-inflammatory, and antifibrogenic agents reportedly provide satisfactory results in patients with secondary SEP, data on their use in patients with idiopathic SEP are limited. How those medications affect patients with idiopathic SEP remains unclear.
Figure 3A and Figure 4 are used with permission from Copyright © 2012 Elsevier Masson SAS. All rights reserved. Figure 3B is used with permission from Copyright © 2011 Springer Japan. All rights reserved. Figure 5 is used with permission from Copyright © 2014 Elsevier Netherlands. All rights reserved. Figure 6 is used with permission of Dr Volkan Ozben.
Sclerosing encapsulating peritonitis (SEP) is a chronic inflammatory process in which the small intestines are encased by a dense fibrocollagenous membrane. SEP was first described in 1907 by Owtschinnikow, who termed this condition “peritonitis chronica fibrosa incapsulata.” SEP is characterized as either primary (idiopathic) or secondary, according to its underlying cause.
The primary aim of this review was to screen the literature on idiopathic SEP, also known as abdominal cocoon syndrome. To the best of our knowledge, no studies on the use of correct terminology regarding SEP, primary SEP, and secondary SEP have been performed.
SEP is characterized by a thick, grayish-white fibrocollagenous membrane that partially or totally encases the small bowel and that can extend to involve other organs. Patients with no factors explaining the condition are considered to have primary SEP, while patients with SEP that has developed as a result of various surgical or medical conditions are considered to have secondary SEP. Based on the extent of the encasing membrane that covers the intestine, SEP is categorized into three types. Encasement of part of the intestine by a fibrocollagenous membrane is called type 1 SEP. Complete coverage of the intestine by the membrane is called type 2 SEP. Type 3 SEP refers to encasement of the whole intestine as well as other intra-abdominal organs such as the appendix, cecum, ascending colon, and ovaries.
The study is interesting, in which authors review the literature on idiopathic SEP, also known as abdominal cocoon syndrome. The results are interesting and suggest that idiopathic SEP is a rare disorder characterized by frequently recurring bouts of intestinal obstruction.
P- Reviewer: Karaca CA, Yilmaz M S- Editor: Gou SX L- Editor: Stewart GJ E- Editor: Ma S
| 1. | Tannoury JN, Abboud BN. Idiopathic sclerosing encapsulating peritonitis: abdominal cocoon. World J Gastroenterol. 2012;18:1999-2004. [PubMed] [DOI] [Cited in This Article: ] [Cited by in CrossRef: 69] [Cited by in F6Publishing: 79] [Article Influence: 6.6] [Reference Citation Analysis (1)] |
| 2. | Rasihashemi SZ, Ramouz A, Ebrahimi F. An unusual small bowel obstruction (abdominal cocoon): a case report. Arq Bras Cir Dig. 2014;27:82-83. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 6] [Cited by in F6Publishing: 6] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 3. | Naniwadekar RG, Kulkarni SR, Bane P, Agrarwal S, Garje A. Abdominal cocoon: an unusual presentation of small bowel obstruction. J Clin Diagn Res. 2014;8:173-174. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 3] [Cited by in F6Publishing: 8] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 4. | Li N, Zhu W, Li Y, Gong J, Gu L, Li M, Cao L, Li J. Surgical treatment and perioperative management of idiopathic abdominal cocoon: single-center review of 65 cases. World J Surg. 2014;38:1860-1867. [PubMed] [Cited in This Article: ] |
| 5. | Jovani M, Baticci F, Bonifacio C, Omodei PD, Malesci A. Abdominal cocoon or idiopathic encapsulating peritoneal sclerosis: magnetic resonance imaging. Dig Liver Dis. 2014;46:192-193. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 9] [Cited by in F6Publishing: 11] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 6. | Akbulut S, Yagmur Y, Babur M. Coexistence of abdominal cocoon, intestinal perforation and incarcerated Meckel’s diverticulum in an inguinal hernia: A troublesome condition. World J Gastrointest Surg. 2014;6:51-54. [PubMed] [DOI] [Cited in This Article: ] [Cited by in CrossRef: 16] [Cited by in F6Publishing: 17] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 7. | Sreevathsa MR, Harsha AH. Chronic encapsulating peritonitis or cocoon abdomen. Trop Gastroenterol. 2013;34:204-206. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 5] [Cited by in F6Publishing: 5] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 8. | Singh B, Gupta S. Abdominal cocoon: a case series. Int J Surg. 2013;11:325-328. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 29] [Cited by in F6Publishing: 32] [Article Influence: 2.9] [Reference Citation Analysis (0)] |
| 9. | Shah MY, Gedam BS, Sonarkar R, Gopinath KS. Abdominal cocoon: an unusual cause of subacute intestinal obstruction. Indian J Surg. 2013;75:391-393. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 7] [Cited by in F6Publishing: 8] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 10. | Serter A, Kocakoç E, Çipe G. Supposed to be rare cause of intestinal obstruction; abdominal cocoon: report of two cases. Clin Imaging. 2013;37:586-589. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 10] [Cited by in F6Publishing: 12] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 11. | Salamone G, Atzeni J, Agrusa A, Gulotta G. A rare case of abdominal cocoon. Ann Ital Chir. 2013;84. [PubMed] [Cited in This Article: ] |
| 12. | Rahmati A, Shakeri R, Ajhdarkosh H, Rakhshani N, Almasi A, Zamani F. Photoclinic. Arch Iran Med. 2013;16:371-372. [PubMed] [Cited in This Article: ] |
| 13. | Patel S, Jindal S, Singh M. Abdominal cocoon: A rare cause of intestinal obstruction: A case report. JIMSA. 2013;26:110. [Cited in This Article: ] |
| 14. | Ozkan F, Goksu M, Ozcan N. A Rare Cause of Intestinal Obstruction: Partial Abdominal Cocoon. JCAM. 2013; Available from: http://www.jcam.com.tr/files/KATD-973.pdf. [DOI] [Cited in This Article: ] [Cited by in Crossref: 1] [Cited by in F6Publishing: 1] [Article Influence: 0.1] [Reference Citation Analysis (0)] |
| 15. | Hu D, Wang R, Xiong T, Zhang HW. Successful delivery after IVF-ET in an abdominal cocoon patient: case report and literature review. Int J Clin Exp Pathol. 2013;6:994-997. [PubMed] [Cited in This Article: ] |
| 16. | Gupta S, Gupta A, Yadav C, Dwiverdi A. Abdominal Cocoon: Case Report and Literature Review. Sch J App Med Sci. 2013;1:748-52. [Cited in This Article: ] |
| 17. | Gadhire M, Singh MB, Jshi M. Abdominal cocoon syndrome. JEMDS. 2013;2:1857-1861. [Cited in This Article: ] |
| 18. | Awe JA. Abdominal cocoon syndrome (idiopathic sclerosing encapsulating peritonitis): how easy is its diagnosis preoperatively? A case report. Case Rep Surg. 2013;2013:604061. [PubMed] [DOI] [Cited in This Article: ] [Cited by in F6Publishing: 1] [Reference Citation Analysis (0)] |
| 19. | Al-Thani H, El Mabrok J, Al Shaibani N, El-Menyar A. Abdominal cocoon and adhesiolysis: a case report and a literature review. Case Rep Gastrointest Med. 2013;2013:381950. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 3] [Cited by in F6Publishing: 6] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 20. | Thakur SK, Agrawal T. The abdominal cocoon. J Indian Med Assoc. 2012;110:192. [PubMed] [Cited in This Article: ] |
| 21. | Taylor M, Clarke MG, Jarvis J, Booth M. A mystery wrapped in an enigma: the abdominal cocoon syndrome. N Z Med J. 2012;125:77-80. [PubMed] [Cited in This Article: ] |
| 22. | Solak A, Solak İ. Abdominal cocoon syndrome: preoperative diagnostic criteria, good clinical outcome with medical treatment and review of the literature. Turk J Gastroenterol. 2012;23:776-779. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 13] [Cited by in F6Publishing: 28] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 23. | Shakya VC, Agrawal CS, Rajbanshi SK, Pradhan A, Khaniya S, Adhikary S. Abdominal cocoon in an adolescent male. Kathmandu Univ Med J (KUMJ). 2012;10:83-86. [PubMed] [Cited in This Article: ] |
| 24. | Ndiaye AR, Mbengue A, Soko TO, Diémé EP, Diagne NM, Diouf CT, Fall A, Fall F, Diop Y, Diakhaté IC. Idiopathic sclerosing encapsulating peritonitis: a case in an adolescent girl. Diagn Interv Imaging. 2012;93:629-631. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 7] [Cited by in F6Publishing: 10] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 25. | Meshikhes AW, Bojal S. A rare cause of small bowel obstruction: Abdominal cocoon. Int J Surg Case Rep. 2012;3:272-274. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 5] [Cited by in F6Publishing: 9] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 26. | Malik SA, Javed MA, Mian MA. Abdominal cocoon (sclerosing encapsulating peritonitis): a rare cause of intestinal obstruction. J Coll Physicians Surg Pak. 2012;22:171-173. [PubMed] [Cited in This Article: ] |
| 27. | Kumar J, Garg A, Chowdhury V, Prakash A, Singh S. Abdominal cocoon--a rare case of intestinal obstruction. A report of two cases. Arab J Gastroenterol. 2012;13:188-190. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 2] [Cited by in F6Publishing: 3] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 28. | Kayastha K, Mirza B. Abdominal cocoon simulating acute appendicitis. APSP J Case Rep. 2012;3:8. [PubMed] [Cited in This Article: ] |
| 29. | Kaur R, Chauhan D, Dalal U, Khurana U. Abdominal cocoon with small bowel obstruction: two case reports. Abdom Imaging. 2012;37:275-278. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 12] [Cited by in F6Publishing: 12] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 30. | Araujo Filho JAB, Martines JAS, Martines BMR, Silva AF, Lovisolo SM, Castro CC. Idiopathic sclerosing encapsulating peritonitis: an uncommon cause of intestinal obstruction. Autopsy Case Rep. 2012;2:51-56. [DOI] [Cited in This Article: ] [Cited by in Crossref: 1] [Cited by in F6Publishing: 1] [Article Influence: 0.1] [Reference Citation Analysis (0)] |
| 31. | Chatura RK, Nayak VJ. Abdominal cocoon: case report of a rare cause of intestinal obstruction. Indian J Pathol Microbiol. 2012;55:379-380. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 5] [Cited by in F6Publishing: 5] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 32. | Browne LP, Patel J, Guillerman RP, Hanson IC, Cass DL. Abdominal cocoon: a unique presentation in an immunodeficient infant. Pediatr Radiol. 2012;42:263-266. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 11] [Cited by in F6Publishing: 12] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 33. | Yeniay L, Karaca CA, Calışkan C, Fırat O, Ersin SM, Akgün E. Abdominal cocoon syndrome as a rare cause of mechanical bowel obstruction: report of two cases. Ulus Travma Acil Cerrahi Derg. 2011;17:557-560. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 12] [Cited by in F6Publishing: 14] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 34. | Kirshtein B, Mizrahi S, Sinelnikov I, Lantsberg L. Abdominal cocoon as a rare cause of small bowel obstruction in an elderly man: report of a case and review of the literature. Indian J Surg. 2011;73:73-75. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 16] [Cited by in F6Publishing: 20] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 35. | Jayant M, Kaushik R. Cocoon within an abdominal cocoon. JSCR. 2011;5:7. [Cited in This Article: ] |
| 36. | Gupta RK, Chandra AS, Bajracharya A, Sah PL. Idiopathic sclerosing encapsulating peritonitis in an adult male with intermittent subacute bowel obstruction, preoperative multidetector-row CT (MDCT) diagnosis. BMJ Case Rep. 2011;2011:pii bcr0720114448. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 3] [Cited by in F6Publishing: 3] [Article Influence: 0.2] [Reference Citation Analysis (0)] |
| 37. | Ertem M, Ozben V, Gok H, Aksu E. An unusual case in surgical emergency: Abdominal cocoon and its laparoscopic management. J Minim Access Surg. 2011;7:184-186. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 11] [Cited by in F6Publishing: 19] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 38. | Da Luz MM, Barral SM, Barral CM, Bechara Cde S, Lacerda-Filho A. Idiopathic encapsulating peritonitis: report of two cases. Surg Today. 2011;41:1644-1648. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 11] [Cited by in F6Publishing: 16] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 39. | Bassiouny IE, Abbas TO. Small bowel cocoon: a distinct disease with a new developmental etiology. Case Rep Surg. 2011;2011:940515. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 4] [Cited by in F6Publishing: 7] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 40. | Wang Q, Wang D. Abdominal cocoon: multi-detector row CT with multiplanar reformation and review of literatures. Abdom Imaging. 2010;35:92-94. [PubMed] [Cited in This Article: ] |
| 41. | Tombak MC, Apaydin FD, Colak T, Duce MN, Balci Y, Yazici M, Kara E. An unusual cause of intestinal obstruction: abdominal cocoon. AJR Am J Roentgenol. 2010;194:W176-W178. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 25] [Cited by in F6Publishing: 30] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 42. | Naik RP, Joshipura VP, Patel NR, Chavda HJ. Encapsulating sclerosing peritonitis. Trop Gastroenterol. 2010;31:235-237. [PubMed] [Cited in This Article: ] |
| 43. | Lee T, Lee MD, Hsu MH, Lee SA, Malik U. Computed tomographic findings of an abdominal cocoon with Intestinal obstruction: a case report. Chin J Radiol. 2010;35:61-5. [Cited in This Article: ] |
| 44. | Gurleyik G, Emir S, Saglam A. The abdominal cocoon: a rare cause of intestinal obstruction. Acta Chir Belg. 2010;110:396-398. [PubMed] [Cited in This Article: ] |
| 45. | Al Saied G, Hassan AZ, Ossip M, Hassan AZ. Idiopathic sclerosing encapsulating peritonitis. Case report and review of literature. Eur Surg. 2010;42:103-106. [DOI] [Cited in This Article: ] [Cited by in Crossref: 4] [Cited by in F6Publishing: 3] [Article Influence: 0.2] [Reference Citation Analysis (0)] |
| 46. | Yang XY, Chen CX, Zhang BL, Yang LP, Su HJ, Teng LS, Li YM. Diagnostic effect of capsule endoscopy in 31 cases of subacute small bowel obstruction. World J Gastroenterol. 2009;15:2401-2405. [PubMed] [DOI] [Cited in This Article: ] [Cited by in CrossRef: 25] [Cited by in F6Publishing: 26] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 47. | Yang W, Ding J, Jin X, Wu H, Kuang J, Tao Z, Chu PG, Yen Y, Qiu W. The plication and splinting procedure for idiopathic sclerosing encapsulating peritonitis. J Invest Surg. 2009;22:286-291. [PubMed] [Cited in This Article: ] |
| 48. | Wu JJ, Wu YC, Chang WY, Chang TH, Kung WC, Hsu JW. Abdominal cocoon: A rare case in elderly male. Int J Gerontol. 2009;3:126-128. [Cited in This Article: ] |
| 49. | Wei B, Wei HB, Guo WP, Zheng ZH, Huang Y, Hu BG, Huang JL. Diagnosis and treatment of abdominal cocoon: a report of 24 cases. Am J Surg. 2009;198:348-353. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 54] [Cited by in F6Publishing: 64] [Article Influence: 4.3] [Reference Citation Analysis (0)] |
| 50. | Tasdelen N, Demirag A, Kalayci M, Gurses M, Kilickesmez NO, Comunoglu N, Gurmen AN. Intestinal obstruction due to abdominal cocoon: CT findings. Eur J Radiol Extra. 2009;70:79-e81. [Cited in This Article: ] |
| 51. | Reynders D, Van der Stighelen Y. The abdominal cocoon. A case report. Acta Chir Belg. 2009;109:772-774. [PubMed] [Cited in This Article: ] |
| 52. | Mohanty D, Jain BK, Agrawal J, Gupta A, Agrawal V. Abdominal cocoon: clinical presentation, diagnosis, and management. J Gastrointest Surg. 2009;13:1160-1162. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 21] [Cited by in F6Publishing: 22] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 53. | Kumar A, Ramakrishnan TS, Sahu S, Mishra KB. Idiopathic sclerosing encapsulating peritonitis--is a preoperative diagnosis possible? Report of three cases. Surg Today. 2009;39:610-614. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 7] [Cited by in F6Publishing: 8] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 54. | Ibrahim NA, Oludara MA. Abdominal cocoon in an adolescent male patient. Trop Doct. 2009;39:254-256. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 8] [Cited by in F6Publishing: 9] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 55. | Choudhury T, Kamal M. Abdominal Cocoon - A Case Report with Short Review of Literature. BSMMU J. 2009;2:81-84. [Cited in This Article: ] |
| 56. | Zheng YB, Zhang PF, Ma S, Tong SL. Abdominal cocoon complicated with early postoperative small bowel obstruction. Ann Saudi Med. 2008;28:294-296. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 1] [Cited by in F6Publishing: 2] [Article Influence: 0.1] [Reference Citation Analysis (0)] |
| 57. | Bas G, Eryilmaz R, Okan I, Somay A, Sahin M. Idiopathic abdominal cocoon: report of a case. Acta Chir Belg. 2008;108:266-268. [PubMed] [Cited in This Article: ] |
| 58. | Singh O, Gupta S, Shukla S, Mathur R. Idiopathic sclerosing encapsulating peritonitis; rare cause of intestinal obstruction. Int J Surg. 2008;20:e169-171. [Cited in This Article: ] |
| 59. | Xu P, Chen LH, Li YM. Idiopathic sclerosing encapsulating peritonitis (or abdominal cocoon): a report of 5 cases. World J Gastroenterol. 2007;13:3649-3651. [PubMed] [Cited in This Article: ] |
| 60. | Demir MK, Akinci O, Onur E, Koksal N. Case 108: sclerosing encapsulating peritonitis. Radiology. 2007;242:937-939. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 13] [Cited by in F6Publishing: 16] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 61. | Cai J, Wang Y, Xuan Z, Hering J, Helton S, Espat NJ. The abdominal cocoon: a rare cause of intestinal obstruction in two patients. Am Surg. 2007;73:1133-1135. [PubMed] [Cited in This Article: ] |
| 62. | Basu A, Sukumar R, Sistla SC, Jagdish S. “Idiopathic” abdominal cocoon. Surgery. 2007;141:277-278. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 11] [Cited by in F6Publishing: 12] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 63. | Al-Ebrahim E, Khalifah A, Al-Hajry F. Idiopathic sclerosing encapsulated peritonitis (abdominal cocoon): A case report and literature review. Bas J Surg. 2007;13:42-46. [Cited in This Article: ] |
| 64. | Serafimidis C, Katsarolis I, Vernadakis S, Rallis G, Giannopoulos G, Legakis N, Peros G. Idiopathic sclerosing encapsulating peritonitis (or abdominal cocoon). BMC Surg. 2006;6:3. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 24] [Cited by in F6Publishing: 30] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 65. | Rokade ML, Ruparel M, Agrawal JB. Abdominal cocoon. J Clin Ultrasound. 2007;35:204-206. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 9] [Cited by in F6Publishing: 8] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 66. | Pillai JR, Kumar SN. Idiopathic abdominal cocoon. Ind J Radiol Imag. 2006;16:483-485. [Cited in This Article: ] |
| 67. | Akca T, Ocal K, Turkmenoglu O, Bilgin O, Aydin S. Image of the month: Abdominal cocoon. Arch Surg. 2006;141:943. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 4] [Cited by in F6Publishing: 5] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 68. | Yucel AF, Kocakusak A, Arikan S, Koyuncu A. Abdominal cocoon: A rare cause of intestinal obstruction in two patients. Indian J Surg. 2004;66:241. [Cited in This Article: ] |
| 69. | Hur J, Kim KW, Park MS, Yu JS. Abdominal cocoon: preoperative diagnostic clues from radiologic imaging with pathologic correlation. AJR Am J Roentgenol. 2004;182:639-641. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 61] [Cited by in F6Publishing: 73] [Article Influence: 3.7] [Reference Citation Analysis (0)] |
| 70. | Vijayaraghavan SB, Palanivelu C, Sendhilkumar K, Parthasarathi R. Abdominal cocoon: sonographic features. J Ultrasound Med. 2003;22:719-721. [PubMed] [Cited in This Article: ] |
| 71. | Ranganathan S, Abdullah BJJ, Sivanesaratnam V. Abdominal cocoon syndrome. J HK Coll Radiol. 2003;6:201-203. [Cited in This Article: ] |
| 73. | Hamaloglu E, Altun H, Ozdemir A, Ozenc A. The abdominal cocoon: a case report. Dig Surg. 2002;19:422-424. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 17] [Cited by in F6Publishing: 22] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 74. | Okobia MN, Evbuomwan I, Osime U, Okonofua FE. The abdominal cocoon- a rare of three cases and literature review. Nigerian J Clin Practice. 2001;4:100-103. [Cited in This Article: ] |
| 75. | Mordehai J, Kleiner O, Kirshtein B, Barki Y, Mares AJ. Peritoneal encapsulation: a rare cause of bowel obstruction in children. J Pediatr Surg. 2001;36:1059-1061. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 13] [Cited by in F6Publishing: 15] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 76. | Kumar M, Deb M, Parshad R. Abdominal cocoon: report of a case. Surg Today. 2000;30:950-953. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 21] [Cited by in F6Publishing: 22] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 77. | Altinli E, Sumer A, Celik A. Abdominal Cocoon: A Rare Cause of Intestinal Obstruction. Israel J Emergency Med. 2007;7:42-44. [Cited in This Article: ] |
| 78. | Habib SM, Betjes MG, Fieren MW, Boeschoten EW, Abrahams AC, Boer WH, Struijk DG, Ruger W, Krikke C, Westerhuis R. Management of encapsulating peritoneal sclerosis: a guideline on optimal and uniform treatment. Neth J Med. 2011;69:500-507. [PubMed] [Cited in This Article: ] |
| 79. | Cornelis T, Oreopoulos DG. Update on potential medical treatments for encapsulating peritoneal sclerosis; human and experimental data. Int Urol Nephrol. 2011;43:147-156. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 21] [Cited by in F6Publishing: 20] [Article Influence: 1.5] [Reference Citation Analysis (0)] |