Published online Jun 28, 2015. doi: 10.3748/wjg.v21.i24.7362
Peer-review started: January 8, 2015
First decision: January 22, 2015
Revised: February 3, 2015
Accepted: March 12, 2015
Article in press: March 12, 2015
Published online: June 28, 2015
Irritable bowel syndrome (IBS) is a common functional gastrointestinal disorder which is characterised by symptoms such as bloating, altered bowel habit and visceral pain. It’s generally accepted that miscommunication between the brain and gut underlies the changes in motility, absorpto-secretory function and pain sensitivity associated with IBS. However, partly due to the lack of disease-defining biomarkers, understanding the aetiology of this complex and multifactorial disease remains elusive. Anecdotally, IBS patients have noted that periods of stress can result in symptom flares and many patients exhibit co-morbid stress-related mood disorders such as anxiety and depression. However, in addition to psychosocial stressors, infection-related stress has also been linked with the initiation, persistence and severity of symptom flares. Indeed, prior gastrointestinal infection is one of the strongest predictors of developing IBS. Despite a lack of overt morphological inflammation, the importance of immune factors in the pathophysiology of IBS is gaining acceptance. Subtle changes in the numbers of mucosal immune cell infiltrates and elevated levels of circulating pro-inflammatory cytokines have been reproducibly demonstrated in IBS populations. Moreover, these immune mediators directly affect neural signalling. An exciting new area of research is the role of luminal microbiota in the modulation of neuro-immune signalling, resulting in local changes in gastrointestinal function and alterations in central neural functioning. Progress in this area has begun to unravel some of the complexities of neuroimmune and neuroendocrine interactions and how these molecular exchanges contribute to GI dysfunction
Core tip: This article assesses the importance of neuroimmune modulation of gastrointestinal function in the functional bowel disorder, irritable bowel syndrome (IBS). Recent progress in understanding the molecular mechanisms underlying the pathophysiology of IBS reveals the neuromodulatory effects of mast cell mediators, cytokines and luminal microbiota.
- Citation: O’Malley D. Immunomodulation of enteric neural function in irritable bowel syndrome. World J Gastroenterol 2015; 21(24): 7362-7366
- URL: https://www.wjgnet.com/1007-9327/full/v21/i24/7362.htm
- DOI: https://dx.doi.org/10.3748/wjg.v21.i24.7362
Although no overt morphological inflammation is evident in the functional gastrointestinal (GI) disorder Irritable bowel syndrome (IBS)[1], mounting evidence now supports the importance of immune activation in the pathophysiology of this disorder. This common condition affects between 10% and 20% of the world’s population. Symptoms, which significantly impinge on the quality of life of sufferers, include recurrent abdominal pain or discomfort coupled with bloating and disturbed bowel habits[2]. Brain-gut axis communication involving the neural, endocrine and immune systems is hypothesised to underlie IBS symptoms[3] but a thorough understanding of the disorder is still emerging, which means that therapeutic strategies are limited. Significant evidence has been garnered to demonstrate the role of stress in the initiation, exacerbation and persistence of symptoms during flares[4]. However, the contribution of the immune response, which is appropriately primed in the GI tract to protect the body’s internal milieu from potential luminal pathogens, is now also being recognised as a key player in IBS pathophysiology. Indeed, low-grade inflammation in the gastrointestinal tract, coupled with hyperactivity of the stress system, during periods of perceived environmental stress, may interact to induce and prolong symptom flares in IBS patients[5].
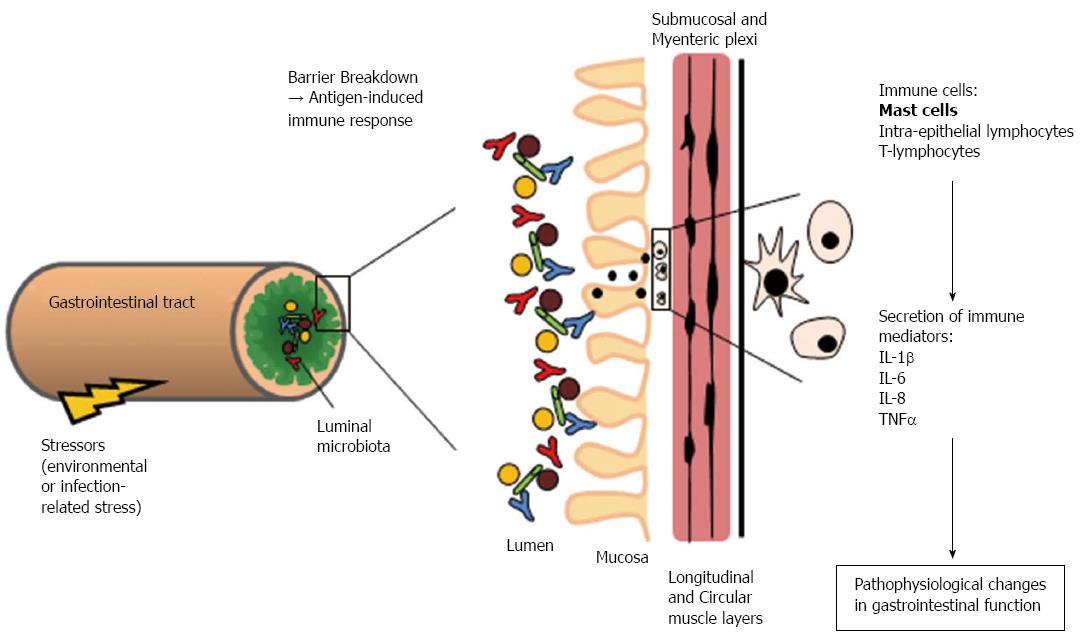
Subtle changes in mucosal immune cell populations and circulating cytokine profiles have been identified in several studies in IBS patients[6]. Biopsies from IBS patients display increased numbers of immune cells, including mast cells and lymphocytes recruited to the mucosa[7,8]. Degranulated mast cells release a range of inflammatory mediators such as proteases, prostaglandins, histamine, and cytokines. Moreover, mast cells in IBS biopsies appear to lie in closer proximity to colonic nerve endings and this strongly correlates visceral pain sensitivity[9] and IBS biopsy supernatants activate enteric neurons in a manner that is dependent on mast cell-derived mediators[10]. It has been proposed that these immune mediators (Figure 1) modulate GI motor and sensory neurons and muscle function resulting in the initiation and perpetuation of IBS symptoms[11]. Indeed, one of best predictors of developing IBS is a prior history of bacterial or viral gastroenteritis[12], with one study showing a sevenfold increase in the risk of developing the functional bowel disorder following GI infection[13].
Elevated levels of pro-inflammatory cytokines such as interleukin (IL)-6 and IL-8[14-16] have been measured in IBS plasma samples, although not all studies detected such increases[17,18]. Furthermore, in peripheral blood mononuclear cells isolated from IBS patients, abnormal secretion of pro-inflammatory cytokines in response to immune challenges was observed[16]. However, changes in circulating levels of cytokines are not merely biomarkers of the disorder, cytokines such as IL-6[19], IL-1β[20] and tumour necrosis factor α[21] can in fact have direct effects on neuronal activity, which in turn alters gut contractility[22], absorption and/or secretion[19]. Buhner et al[10] demonstrated that soluble mediators released from IBS colonic biopsies stimulate enteric neuronal activation and we found that IBS plasma stimulates rat submucosal neurons using an IL-6 dependent mechanism[23] and myenteric neurons via IL-6 and IL-8[24]. IL-6 and IL-1β also influence mucosal ion transport and epithelial permeability[19,25].
Breakdown of the mucosal barrier by IL-6 and other pro-inflammatory cytokines[25] may allow foreign particles to breach the epithelial barrier, leading to an immune response in the submucosal and myenteric neuronal plexi. In IBS, where circulating IL-6 levels are elevated and the hypothalamus-pituitary-adrenal stress axis is hyper-activated[14], a coincident compromise of the mucosal barrier is observed. Thus, increased permeability of the mucosal barrier and the subsequent initiation of an immune response may contribute to the increase in sensitivity to visceral pain in IBS patients[26]. Indeed, targeting cytokine signalling in the GI tract may relieve some of the functional symptoms of IBS such as visceral pain and altered motility[24].
Microbiota-host interactions are an additional consideration in understanding IBS-mediated immune activation[27]. The intestinal epithelium is exposed to the bacterial antigens of both commensal and pathogenic microbiota which aids in sustaining the function and integrity of the epithelial barrier and its blood supply. This host-microbiota interaction also promotes the development of gut associated lymphoid tissue and is essential for normal gut motility as evidenced by impaired gut function in microbiota-deficient germ-free mice[28]. Changes in the balance and composition of commensal microbiota strains in the human gut have been reported in several IBS studies[29,30]. Such dysbiosis of the microbiota could allow opportunistic pathogens to breach the innate immune defences. The luminal microbiota represents a virtual organ which is also integrated into the bi-directional communication system between the gut and the brain. Indeed, manipulation of the microbial environment with probiotics has been shown to improve symptoms in IBS patients[31] by suppressing pro-inflammatory cytokines[32], maintaining intestinal barrier integrity[33] and causing down-regulation of T cells[34]. Moreover, probiotics have been shown to prevent adhesion of enteric pathogens to the wall of the GI tract[35]. That said, more recent studies did not detect an improvement in IBS symptoms following longer-term treatment with probiotics[36,37].
Expression of pattern recognition receptors such as toll-like receptors (TLRs), which form part of the innate immune system, are also altered in IBS. TLRs recognise and respond to a variety of pathogens. Thus, altered expression of TLR4, 5, 7 and 8 in mucosal biopsies from IBS patients further supports the importance of interactions between the luminal microbes and the host in this disorder[38]. Although this is an exciting new area of research, the mechanisms by which changes in luminal microbiota modulate neuro-immune signalling, both locally in the GI tract and also in the central nervous system, are far from clear. Studies are challenged by the need to account for a number of variables including the inter-individual heterogeneity of IBS, environmental factors such as stress and diet and the malleable nature of human bacterial composition.
Despite the complexity and inter-individual heterogeneity of this functional GI disorder, recent progress in the field of IBS research has begun to unravel some of the intricacies of neuroimmune and neuroendocrine interactions and how molecular crosstalk between these systems may contribute to GI dysfunction. These advances in our understanding of IBS pathophysiology give hope for the identification and development of new targeted therapeutic strategies.
P- Reviewer: Chen Z, Maria Streba LA, Ranieri G S- Editor: Qi Y L- Editor: A E- Editor: Zhang DN
| 1. | Schoepfer AM, Trummler M, Seeholzer P, Seibold-Schmid B, Seibold F. Discriminating IBD from IBS: comparison of the test performance of fecal markers, blood leukocytes, CRP, and IBD antibodies. Inflamm Bowel Dis. 2008;14:32-39. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 183] [Cited by in F6Publishing: 187] [Article Influence: 11.7] [Reference Citation Analysis (0)] |
| 2. | El-Serag HB, Olden K, Bjorkman D. Health-related quality of life among persons with irritable bowel syndrome: a systematic review. Aliment Pharmacol Ther. 2002;16:1171-1185. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 213] [Cited by in F6Publishing: 225] [Article Influence: 10.2] [Reference Citation Analysis (0)] |
| 3. | Collins SM, Bercik P. The relationship between intestinal microbiota and the central nervous system in normal gastrointestinal function and disease. Gastroenterology. 2009;136:2003-2014. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 390] [Cited by in F6Publishing: 377] [Article Influence: 25.1] [Reference Citation Analysis (0)] |
| 4. | Stengel A, Taché Y. Neuroendocrine control of the gut during stress: corticotropin-releasing factor signaling pathways in the spotlight. Annu Rev Physiol. 2009;71:219-239. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 100] [Cited by in F6Publishing: 114] [Article Influence: 7.6] [Reference Citation Analysis (0)] |
| 5. | O’Malley D, Quigley EM, Dinan TG, Cryan JF. Do interactions between stress and immune responses lead to symptom exacerbations in irritable bowel syndrome? Brain Behav Immun. 2011;25:1333-1341. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 85] [Cited by in F6Publishing: 98] [Article Influence: 7.5] [Reference Citation Analysis (1)] |
| 6. | Clarke G, Quigley EM, Cryan JF, Dinan TG. Irritable bowel syndrome: towards biomarker identification. Trends Mol Med. 2009;15:478-489. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 125] [Cited by in F6Publishing: 147] [Article Influence: 9.8] [Reference Citation Analysis (0)] |
| 7. | Chadwick VS, Chen W, Shu D, Paulus B, Bethwaite P, Tie A, Wilson I. Activation of the mucosal immune system in irritable bowel syndrome. Gastroenterology. 2002;122:1778-1783. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 564] [Cited by in F6Publishing: 562] [Article Influence: 25.5] [Reference Citation Analysis (0)] |
| 8. | Goral V, Kucukoner M, Buyukbayram H. Mast cells count and serum cytokine levels in patients with irritable bowel syndrome. Hepatogastroenterology. 2010;57:751-754. [PubMed] [Cited in This Article: ] |
| 9. | Barbara G, Stanghellini V, De Giorgio R, Cremon C, Cottrell GS, Santini D, Pasquinelli G, Morselli-Labate AM, Grady EF, Bunnett NW. Activated mast cells in proximity to colonic nerves correlate with abdominal pain in irritable bowel syndrome. Gastroenterology. 2004;126:693-702. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 1004] [Cited by in F6Publishing: 971] [Article Influence: 48.6] [Reference Citation Analysis (0)] |
| 10. | Buhner S, Li Q, Vignali S, Barbara G, De Giorgio R, Stanghellini V, Cremon C, Zeller F, Langer R, Daniel H. Activation of human enteric neurons by supernatants of colonic biopsy specimens from patients with irritable bowel syndrome. Gastroenterology. 2009;137:1425-1434. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 254] [Cited by in F6Publishing: 262] [Article Influence: 17.5] [Reference Citation Analysis (0)] |
| 11. | Barbara G, Wang B, Stanghellini V, de Giorgio R, Cremon C, Di Nardo G, Trevisani M, Campi B, Geppetti P, Tonini M. Mast cell-dependent excitation of visceral-nociceptive sensory neurons in irritable bowel syndrome. Gastroenterology. 2007;132:26-37. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 531] [Cited by in F6Publishing: 540] [Article Influence: 31.8] [Reference Citation Analysis (0)] |
| 12. | Spiller R, Garsed K. Postinfectious irritable bowel syndrome. Gastroenterology. 2009;136:1979-1988. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 489] [Cited by in F6Publishing: 524] [Article Influence: 34.9] [Reference Citation Analysis (0)] |
| 13. | Halvorson HA, Schlett CD, Riddle MS. Postinfectious irritable bowel syndrome--a meta-analysis. Am J Gastroenterol. 2006;101:1894-189; quiz 1942. [PubMed] [Cited in This Article: ] |
| 14. | Dinan TG, Quigley EM, Ahmed SM, Scully P, O’Brien S, O’Mahony L, O’Mahony S, Shanahan F, Keeling PW. Hypothalamic-pituitary-gut axis dysregulation in irritable bowel syndrome: plasma cytokines as a potential biomarker? Gastroenterology. 2006;130:304-311. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 440] [Cited by in F6Publishing: 440] [Article Influence: 24.4] [Reference Citation Analysis (0)] |
| 15. | Dinan TG, Clarke G, Quigley EM, Scott LV, Shanahan F, Cryan J, Cooney J, Keeling PW. Enhanced cholinergic-mediated increase in the pro-inflammatory cytokine IL-6 in irritable bowel syndrome: role of muscarinic receptors. Am J Gastroenterol. 2008;103:2570-2576. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 89] [Cited by in F6Publishing: 108] [Article Influence: 6.8] [Reference Citation Analysis (0)] |
| 16. | Liebregts T, Adam B, Bredack C, Röth A, Heinzel S, Lester S, Downie-Doyle S, Smith E, Drew P, Talley NJ. Immune activation in patients with irritable bowel syndrome. Gastroenterology. 2007;132:913-920. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 452] [Cited by in F6Publishing: 493] [Article Influence: 29.0] [Reference Citation Analysis (0)] |
| 17. | Kindt S, Van Oudenhove L, Broekaert D, Kasran A, Ceuppens JL, Bossuyt X, Fischler B, Tack J. Immune dysfunction in patients with functional gastrointestinal disorders. Neurogastroenterol Motil. 2009;21:389-398. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 105] [Cited by in F6Publishing: 111] [Article Influence: 7.4] [Reference Citation Analysis (0)] |
| 18. | Chang L, Adeyemo M, Karagiannides I, Videlock EJ, Bowe C, Shih W, Presson AP, Yuan PQ, Cortina G, Gong H. Serum and colonic mucosal immune markers in irritable bowel syndrome. Am J Gastroenterol. 2012;107:262-272. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 111] [Cited by in F6Publishing: 108] [Article Influence: 9.0] [Reference Citation Analysis (0)] |
| 19. | O’Malley D, Liston M, Hyland NP, Dinan TG, Cryan JF. Colonic soluble mediators from the maternal separation model of irritable bowel syndrome activate submucosal neurons via an interleukin-6-dependent mechanism. Am J Physiol Gastrointest Liver Physiol. 2011;300:G241-G252. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 51] [Cited by in F6Publishing: 57] [Article Influence: 4.4] [Reference Citation Analysis (0)] |
| 20. | Xia Y, Hu HZ, Liu S, Ren J, Zafirov DH, Wood JD. IL-1beta and IL-6 excite neurons and suppress nicotinic and noradrenergic neurotransmission in guinea pig enteric nervous system. J Clin Invest. 1999;103:1309-1316. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 90] [Cited by in F6Publishing: 102] [Article Influence: 4.1] [Reference Citation Analysis (0)] |
| 21. | Rehn M, Hübschle T, Diener M. TNF-alpha hyperpolarizes membrane potential and potentiates the response to nicotinic receptor stimulation in cultured rat myenteric neurones. Acta Physiol Scand. 2004;181:13-22. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 26] [Cited by in F6Publishing: 28] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 22. | Zhang L, Hu L, Chen M, Yu B. Exogenous interleukin-6 facilitated the contraction of the colon in a depression rat model. Dig Dis Sci. 2013;58:2187-2196. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 13] [Cited by in F6Publishing: 15] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 23. | O’Malley D, Buckley MM, McKernan DP, Quigley EM, Cryan JF, Dinan TG. Soluble mediators in plasma from irritable bowel syndrome patients excite rat submucosal neurons. Brain Behav Immun. 2015;44:57-67. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 13] [Cited by in F6Publishing: 12] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 24. | Buckley MM, O’Halloran KD, Rae MG, Dinan TG, O’Malley D. Modulation of enteric neurons by interleukin-6 and corticotropin-releasing factor contributes to visceral hypersensitivity and altered colonic motility in a rat model of irritable bowel syndrome. J Physiol. 2014;592:5235-5250. [PubMed] [DOI] [Cited in This Article: ] [Cited by in F6Publishing: 5] [Reference Citation Analysis (0)] |
| 25. | Natale L, Piepoli AL, De Salvia MA, De Salvatore G, Mitolo CI, Marzullo A, Portincasa P, Moschetta A, Palasciano G, Mitolo-Chieppa D. Interleukins 1 beta and 6 induce functional alteration of rat colonic motility: an in vitro study. Eur J Clin Invest. 2003;33:704-712. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 43] [Cited by in F6Publishing: 49] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 26. | Zhou Q, Zhang B, Verne GN. Intestinal membrane permeability and hypersensitivity in the irritable bowel syndrome. Pain. 2009;146:41-46. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 246] [Cited by in F6Publishing: 262] [Article Influence: 17.5] [Reference Citation Analysis (0)] |
| 27. | Bravo JA, Forsythe P, Chew MV, Escaravage E, Savignac HM, Dinan TG, Bienenstock J, Cryan JF. Ingestion of Lactobacillus strain regulates emotional behavior and central GABA receptor expression in a mouse via the vagus nerve. Proc Natl Acad Sci USA. 2011;108:16050-16055. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 2500] [Cited by in F6Publishing: 2275] [Article Influence: 175.0] [Reference Citation Analysis (0)] |
| 28. | Thompson GR, Trexler PC. Gastrointestinal structure and function in germ-free or gnotobiotic animals. Gut. 1971;12:230-235. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 103] [Cited by in F6Publishing: 104] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 29. | Kassinen A, Krogius-Kurikka L, Mäkivuokko H, Rinttilä T, Paulin L, Corander J, Malinen E, Apajalahti J, Palva A. The fecal microbiota of irritable bowel syndrome patients differs significantly from that of healthy subjects. Gastroenterology. 2007;133:24-33. [PubMed] [Cited in This Article: ] |
| 30. | Jeffery IB, O’Toole PW, Öhman L, Claesson MJ, Deane J, Quigley EM, Simrén M. An irritable bowel syndrome subtype defined by species-specific alterations in faecal microbiota. Gut. 2012;61:997-1006. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 577] [Cited by in F6Publishing: 592] [Article Influence: 49.3] [Reference Citation Analysis (0)] |
| 31. | Yoon JS, Sohn W, Lee OY, Lee SP, Lee KN, Jun DW, Lee HL, Yoon BC, Choi HS, Chung WS. Effect of multispecies probiotics on irritable bowel syndrome: a randomized, double-blind, placebo-controlled trial. J Gastroenterol Hepatol. 2014;29:52-59. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 106] [Cited by in F6Publishing: 111] [Article Influence: 11.1] [Reference Citation Analysis (0)] |
| 32. | O’Mahony L, McCarthy J, Kelly P, Hurley G, Luo F, Chen K, O’Sullivan GC, Kiely B, Collins JK, Shanahan F. Lactobacillus and bifidobacterium in irritable bowel syndrome: symptom responses and relationship to cytokine profiles. Gastroenterology. 2005;128:541-551. [PubMed] [Cited in This Article: ] |
| 33. | Prakash S, Rodes L, Coussa-Charley M, Tomaro-Duchesneau C. Gut microbiota: next frontier in understanding human health and development of biotherapeutics. Biologics. 2011;5:71-86. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 70] [Cited by in F6Publishing: 107] [Article Influence: 8.2] [Reference Citation Analysis (0)] |
| 34. | O’Mahony C, Scully P, O’Mahony D, Murphy S, O’Brien F, Lyons A, Sherlock G, MacSharry J, Kiely B, Shanahan F. Commensal-induced regulatory T cells mediate protection against pathogen-stimulated NF-kappaB activation. PLoS Pathog. 2008;4:e1000112. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 249] [Cited by in F6Publishing: 262] [Article Influence: 16.4] [Reference Citation Analysis (0)] |
| 35. | Lee BJ, Bak YT. Irritable bowel syndrome, gut microbiota and probiotics. J Neurogastroenterol Motil. 2011;17:252-266. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 122] [Cited by in F6Publishing: 137] [Article Influence: 10.5] [Reference Citation Analysis (0)] |
| 36. | Begtrup LM, de Muckadell OB, Kjeldsen J, Christensen RD, Jarbøl DE. Long-term treatment with probiotics in primary care patients with irritable bowel syndrome--a randomised, double-blind, placebo controlled trial. Scand J Gastroenterol. 2013;48:1127-1135. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 49] [Cited by in F6Publishing: 55] [Article Influence: 5.0] [Reference Citation Analysis (0)] |
| 37. | Roberts LM, McCahon D, Holder R, Wilson S, Hobbs FD. A randomised controlled trial of a probiotic ‘functional food’ in the management of irritable bowel syndrome. BMC Gastroenterol. 2013;13:45. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 50] [Cited by in F6Publishing: 57] [Article Influence: 5.2] [Reference Citation Analysis (0)] |
| 38. | Bloom JR, Stewart SL, Chang S, Banks PJ. Then and now: quality of life of young breast cancer survivors. Psychooncology. 2004;13:147-160. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 277] [Cited by in F6Publishing: 284] [Article Influence: 14.2] [Reference Citation Analysis (0)] |