Published online Feb 14, 2015. doi: 10.3748/wjg.v21.i6.1718
Peer-review started: August 28, 2014
First decision: September 15, 2014
Revised: October 25, 2014
Accepted: December 5, 2014
Article in press: December 8, 2014
Published online: February 14, 2015
Vitamin D through its active form 1a-25-dihydroxyvtamin D [1,25(OH)2D] is a secosteroid hormone that plays a key role in mineral metabolism. Recent years have witnessed a significant scientific interest on vitamin D and expanded its actions to include immune modulation, cell differentiation and proliferation and inflammation regulation. As our understanding of the many functions of vitamin D has grown, the presence of vitamin D deficiency has become one of the most prevalent micronutrient deficiencies worldwide. Concomitantly, non-alcoholic fatty liver disease (NAFLD) has become the most common form of chronic liver disease in western countries. NAFLD and vitamin D deficiency often coexist and epidemiologic evidence has shown that both of these conditions share several cardiometabolic risk factors. In this article we provide an overview of the epidemiology and pathophysiology linking NAFLD and vitamin D deficiency, as well as the available evidence on the clinical utility of vitamin D supplementation in NAFLD.
Core tip: Non-alcoholic fatty liver disease (NAFLD) is a multifactorial disease and its pathogenesis is closely linked to the metabolic syndrome. Vitamin D deficiency, which also shares similar associations with obesity and sedentary lifestyle, is often found together with NAFLD. As our understanding of the many functions of vitamin D has grown, emerging evidence points to a closely linked and potentially causative relationship between vitamin D deficiency and NAFLD. As such, vitamin D is now emerging as an immunomodulatory and anti-fibrotic agent. However, in order to implement clinical recommendations larger, randomized, placebo-control trials are required to better evaluate the efficacy of vitamin D replacement in NAFLD.
- Citation: Eliades M, Spyrou E. Vitamin D: A new player in non-alcoholic fatty liver disease? World J Gastroenterol 2015; 21(6): 1718-1727
- URL: https://www.wjgnet.com/1007-9327/full/v21/i6/1718.htm
- DOI: https://dx.doi.org/10.3748/wjg.v21.i6.1718
Non-alcoholic fatty liver disease (NAFLD) has become the most common form of chronic liver disease in Western countries with prevalence as high as 30%[1,2], thus exceeding that of viral hepatitis and alcoholic liver disease. NAFLD represents a continuum of hepatic injuries, which progress from simple fatty liver to steatohepatitis (NASH), cirrhosis or even hepatocellular carcinoma. The metabolic syndrome is universally considered as the key factor in the pathogenesis of NAFLD[3,4]. However, the evolution of liver inflammation in NAFLD and the progression from simple fatty liver to steatohepatitis and hepatic fibrosis is more complex[5]. As our understanding in the pathogenesis of NASH continues to evolve, vitamin D is emerging as an important player in the development and progression of NAFLD. This review will assess the role of vitamin D deficiency in the pathogenesis of NAFLD, explore the epidemiologic evidence that supports a link between vitamin D deficiency and NAFLD and provide available evidence on the clinical utility of vitamin D replacement in NAFLD subjects.
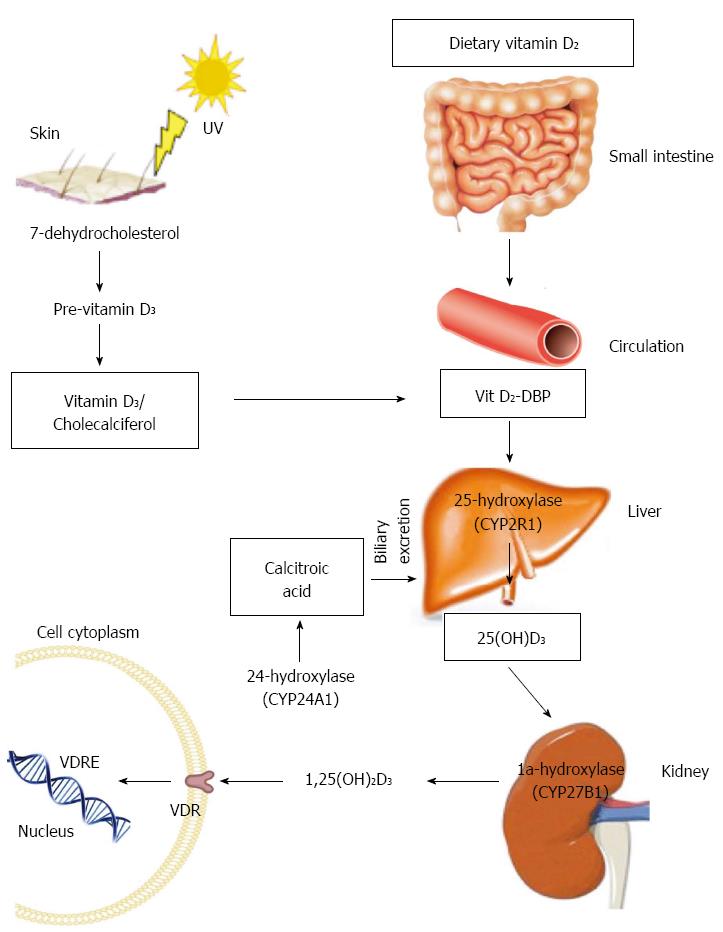
Vitamin D is a fat-soluble vitamin. Although, multiple forms of this vitamin exist, vitamin D2 (ergocalciferol) and vitamin D3 (cholecalciferol) are the two major forms. Vitamin D2 is produced by some organisms of phytoplankton, invertebrates and yeast in response to ultraviolet irradiation but it is not constitutively produced by vertebrates. Thus this form of vitamin D has been exploited commercially and is used for fortification and supplementation. Vitamin D3 on the other hand, originates in the skin of most vertebrates including humans, after irradiation of 7-dehydrocholesterol with ultraviolet light (UVB) (Figure 1). Dietary vitamin D2 is absorbed by the small intestine and incorporated into chylomicrons where it is transported to the liver bound to vitamin D-binding protein. In the liver, vitamin D from both the skin and diet is then metabolized by 25-hydroxylase (CYP2R1) to 25-hydroxyvitamin D [25(OH)D], which is the major circulating metabolite and the most widely used indicator of vitamin D stores. 25(OH)D is transported to the kidney where it undergoes hydroxylation by 1a-hydroxylase (CYP27B1) to the biologically active form 1a,25-dihydroxyvitamin D [1,25(OH)2D]. Finally, via binding to vitamin D receptor (VDR), 1,25(OH)2D is able to exert its biological actions.
The synthesis of 1,25(OH)2D is tightly regulated by the synthetic activity of 1a-hydroxylase and the catabolic activity of 24-hydroxylase (CYP24A1) which catabolizes 1,25(OH)2D to the water soluble and biologically inactive calcitoic acid which is then excreted in the bile. Parathyroid hormone, 1,25(OH)2D and Fibroblast growth factor 23 (FGF23) are the main regulators of these enzymes. 1,25(OH)2D decreases its own synthesis though negative feedback but also by way of inhibition of parathyroid hormone (PTH) which is the main stimulus for 1a-hydroxylase transcription. Fibroblast growth factor 23, secreted from osteoblasts, acts on the kidneys to suppress renal expression of 1a-hydroxylase and to promote 24-hydroxylase activity which result in reduced production of 1,25(OH)2D.
The leading and most widely known physiological function of 1,25(OH)2D is to regulate mineral and skeletal homeostasis. However, over the last decades the functions of vitamin D have been broadened beyond those on skeletal tissue and calcium homeostasis. Indeed, the finding of VDR expression in a wide range of tissues such as the immune system (T and B cells, macrophages, and monocytes), the reproductive system (uterus, testis, ovary, prostate, placenta, and mammary glands), the endocrine system (pancreas, pituitary, thyroid and adrenal cortex), in muscles (skeletal, smooth and heart muscles), and in brain, skin, and liver has stimulated considerable interest in understating the putative pleiotropic properties of vitamin D and introduced the idea of a paracrine/autocrine role in regulating cell proliferation, differentiation and apoptosis as well as immune-cells regulation[6,7].
Numerous publications propose that low levels of 25(OH)D are strongly associated with features of the metabolic syndrome[8,9] and may play an important role in modifying the risk for cardio-metabolic outcomes including Type 2 diabetes (T2DM), hypertension and cardiovascular disease[10]. A recent systematic review found that 25(OH)D levels > 25 ng/mL were associated with 43% lower risk of T2DM compared to levels < 14 ng/mL[11]. In the same study, vitamin D treatment improved insulin resistance among patients with baseline glucose intolerance. Similarly, another meta-analysis showed that vitamin D supplementation improves insulin resistance compared to placebo, albeit the effect was weak[12]. In support of the beneficial role of vitamin D in diabetes and insulin resistance are the findings of various animal studies showing that lack of VDR in mice or vitamin D deficiency impairs insulin secretion from pancreatic beta cells[13]. In contrast to the above findings, are the results of a recent meta-analysis by Seida et al[14]. In this study, 35 randomized controlled trials were examined with a total of 43407 patients. No significant effect of vitamin D supplementation on the prevention of diabetes in individuals without diabetes, or on the reduction of insulin resistance and hyperglycemia in those with pre-diabetes or established type 2 diabetes was found. However these results are limited by the presence of moderate heterogeneity between the studies, the associated risks of bias and the short term of follow up.
Given the strong association of NAFLD with obesity and the metabolic syndrome, recent years have witnessed a significant scientific interest into the potential role of vitamin D in NAFLD. Accumulating epidemiological data suggest that low levels of serum 25(OH)D are associated with NAFLD as diagnosed either by biochemistry, imaging or biopsy. These data are summarized in a recently published meta-analysis in which NAFLD subjects were 26% more likely to be vitamin D deficient compared to controls[15]. In US the largest of these studies was by Liangpunsakul et al[16] in which the authors reported that in a subset of 1287 adult participants from the NHANES III database, those with unexplained elevation in serum alanine aminotransferase (ALT) levels - a proxy of NAFLD - had lower 25(OH)D levels than those with normal ALT levels (24.7 ± 10.4 ng/mL vs 26.8 ± 10.9 ng/mL, P < 0.01). Compared to the lowest quartile, patients with the two top quartiles of serum 25(OH)D levels had significantly lower prevalence of unexplained elevation in serum ALT, independently of metabolic syndrome features[16]. In Asia, the largest study was a cross-sectional study of 6567 Korean men which found that subjects in the lowest tertile of 25(OH)D levels had a significantly increased risk for NAFLD compared to thosein the highest tertile, even after adjusting for body mass index and metabolic syndrome (OR = 1.247 and 1.408 vs the highest tertile, P < 0.001)[17]. A more recent Korean study however, showed contradictory results. In this study the authors analyzed data from the Korean National Health and Nutrition Examination database (KNHANES IV) with more than 16000 individuals and found that obese subjects (BMI > 25) with > 2 components of the metabolic syndrome were more likely to have elevated liver enzymes compared to normal weight subjects; however there was no significant difference in vitamin D levels between the groups[18].
Targher et al[19] was the first to study the association between biopsy-proven NAFLD and vitamin D levels. The study confirmed that 25(OH)D concentrations were lower in NAFLD subjects compared to matched controls. Furthermore 25(OH)D levels predicted the histological severity of NAFLD, with NASH patients having lower 25(OH)D levels compared to those with isolated fatty liver. These findings have been confirmed by four other studies with biopsy-proven NAFLD in adults[20,21] and in children[22,23].
Collectively, the data from the published studies indicate that NAFLD subjects are more likely to be vitamin D deficient compared to controls. However, definite directionality of the results cannot be ascertained due to the nature of the above studies (i.e., cross-sectional) and the limitations observed which include the variability in the method of diagnosis of NAFLD, clinical heterogeneity among the study groups and variability in defining vitamin D deficiency.
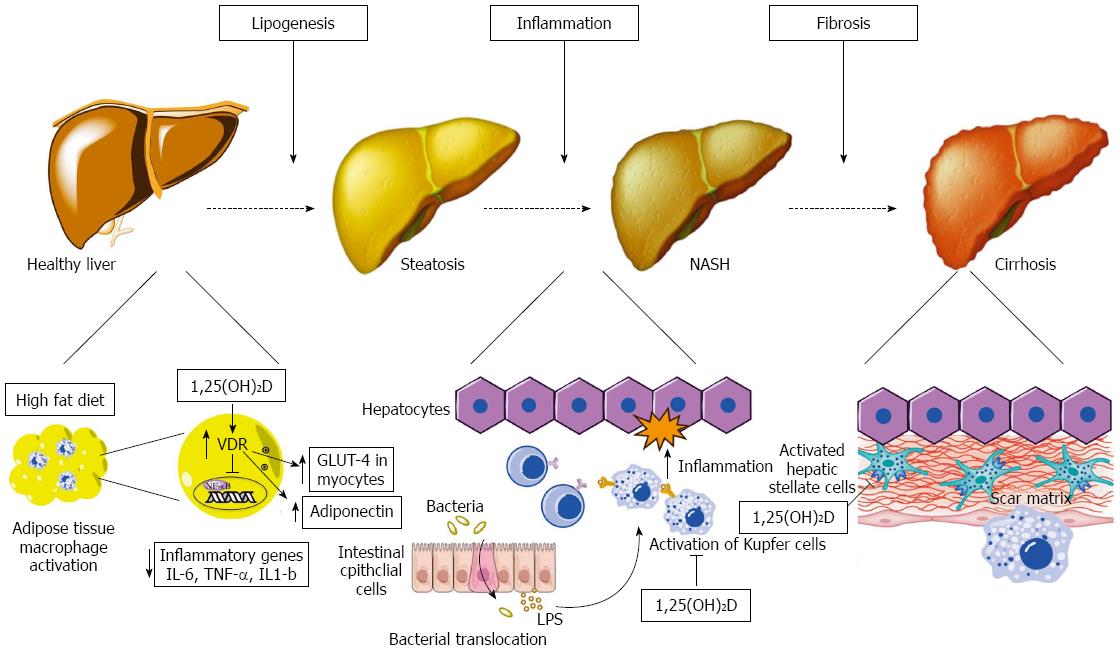
Our understanding of the pathogenesis of NAFLD has evolved from the relatively simplistic “two-hit” hypothesis to the “multiple-hits” hypothesis[5]. In this model, a number of diverse, parallel processes contribute to the development and progression of liver inflammation from simple hepatic steatosis to steatohepatitis and hepatic fibrosis. A number of these pathways can be affected by vitamin D and relate to the hormonal, immunologic and cellular differentiation “non-classical” effects of vitamin D. Below, we will discuss each of these pathways. A summary of evidence is provided in Table 1 and Figure 2 provides a schematic representation of these mechanisms.
| Mechanisms | Evidence |
| Improvement in insulin secretion and insulin resistance | Presence of VDR in pancreatic beta cells[26] |
| Expression of 1-α-hydroxylase enzyme in pancreatic beta cells[79] | |
| Impaired insulin secretory response in mice lacking a functional VDR[13] | |
| Transcriptional activation of the human insulin gene by 1,25(OH)2D[80] | |
| Vitamin D deficiency impairs glucose-mediated insulin secretion from rat pancreatic beta cells in vitro[81] and in vivo[82] | |
| Vitamin D enhances insulin responsiveness for glucose transport in muscle cells[83] | |
| Vitamin D up-regulates glucose transporter 4 (GLUT4) translocation and glucose utilization in adipocytes[44] | |
| Improvement in adipose tissue inflammation | Higher 25(OH)D concentrations were independently associated with higher adiponectin concentrations in a large cohort of men and women[40] |
| Reduction of IL-6 in adipocytes after supplementation of vitamin D in mice fed high fat diet[84] | |
| 1,25-dihydroxyvitamin D treatment in human adipocytes inhibits NF-κB pathway and reduces pro-inflammatory cytokine release[85,86] | |
| 1,25-dihydroxyvitamin D inhibits macrophage recruitment and increases adiponectin expression in preadipocytes[87] | |
| Improvement in hepatic inflammation | Vitamin D deficiency causes TLR activation and exacerbates hepatic inflammation[42] |
| Artificial sunlight therapy in rats reduced liver inflammation and apoptosis[43] | |
| VDR expression on cholangiocytes was inversely correlated with steatosis severity and nonalcoholic fatty liver disease score in NASH patients[31] | |
| Improvement in hepatic fibrosis | Presence of VDR in HSC[24] |
| Vitamin D treatment suppresses HSC proliferation in cultured HSC from rats[73] | |
| Vitamin D treatment downregulates pro-fibrotic marker TIMP-1 and collagen type I production in cultured HSCs[73,74] | |
| VDR knockout mice develop spontaneous liver injury with fibrosis[30] |
Vitamin D mediates its intracellular signals via its receptor VDR which is constitutively expressed in the liver[24,25]. It has been estimated that VDR regulates over 200 genes involved in glucose and lipid metabolism[13,26], inflammation[27], cellular proliferation and differentiation and apoptosis[28]. In a GWAS study of NAFLD subjects, four single nucleotide polymorphisms (SNPs) were found to have significant association with NAFLD. Among these four SNPs GC gene was included which is predominately expressed in hepatocytes and codes for vitamin D binding protein, the main carrier protein for vitamin D[29]. We also know from animal studies that normal functioning of VDR is crucial for liver fibrosis, as VDR knockout mice exhibit spontaneous liver injury with fibrosis[30]. In humans, Barchetta et al[31] showed that liver VDR expression is inversely correlated with severity of NAFLD on histopathology, independently from other metabolic parameters such as BMI, insulin resistance or adiponectin.
Insulin resistance, a key risk factor in the pathogenesis of NAFLD, is linked to the development of oxidative stress and lipotoxicity[32,33]. As a result, hepatic steatosis resulting from accumulation of free fatty acids is associated with a state of chronic hepatic inflammation. An important mediator in this process is nuclear factor κ-β (NF-κB) that functions as a pro-inflammatory “master switch” by upregulating the transcription of a wide range of inflammatory mediators. Accordingly, increased NF-κB activity in the livers of high fat diet mice is associated with the increased expression of pro-inflammatory cytokines, including TNF-α, IL-6, IL-1β and activation of Kupffer cells[34]. These cytokines are capable of producing all of the classical histological features of NASH including hepatocyte necrosis/apoptosis, neutrophil chemotaxis and activation of hepatic stellate cells. Moreover, human studies have demonstrated increased cytokine gene expression in the livers of patients with NASH compared to obese controls with normal livers, with the increased expression correlating with histolocical severity[35]. Adiponectin on the other hand, has been described as the prototypic adipokine by way of its function as an anti-inflammatory agent. In murine models, high levels of adiponectin have been experimentally shown to decrease necroinflammation and steatosis in alcoholic and nonalcoholic fatty liver disease[36] as well as improved insulin resistance[37]. Moreover, studies in humans showing reduced serum levels of adiponectin and reduced hepatic expression of its receptor in patients with NASH compared with body mass index-matched patients with steatosis[38,39], provide strong supportive evidence that reduced adipocyte production of adiponectin plays an important role in the pathogenesis of progressive NAFLD.
The role of vitamin D in adipokine activity is an active area of research. Vaidya et al[40] showed a positive association between 25(OH)D concentrations and levels of adiponectin in a large cohort of patients. Interestingly, this relationship was independent of BMI. Furthermore, in a double-blind, randomized, controlled trial of Iranian type 2 diabetic patients, daily intake of 1000 IU vitamin D either with or without extra calcium for 12 wk resulted in a significant increase of serum adipokines including adiponectin and decreased cellular secretion of the inflammatory cytokines IL-6 and IL-1β[41].
Additional evidence from animal studies further support the notion of an immunomodulatory role of vitamin D in NAFLD. To explore the effect of vitamin D deficiency on inflammatory markers Roth et al[42] used a rat model consistent of 4 groups; rats were fed either a low-fat diet alone (LFD) or with vitamin D depletion (LFD + VDD) or high-fat Western diet which was either replete (WD) or deficient in vitamin D (WD + VDD). In VDD groups, blood 25(OH)D levels were reduced compared to the replete diet groups. Mice fed WD/VDD showed increased hepatic steatosis comprared to LFD groups. Liver histology also showed increased lobular inflammation and NAFLD activity score in WD/VDD group compared to the WD alone group. In addition WD/VDD mice had increased hepatic mRNA levels of resistin, IL-4, IL-6 and TNF-α markers known to be implicated in oxidative stress and hepatic inflammation. Accordingly in another rat NASH model[43], phototherapy increased 25(OH)D and 1,25(OH)2D levels while reducing hepatocyte inflammation, fibrosis and apoptosis compared to controls. Phototherapy also improved insulin resistance and increased serum adiponectin in association with reduced hepatic expression of inflammatory genes TNF-α and TFG-β. In total, these findings suggest that vitamin D deficiency worsens NAFLD related to upregulation of hepatic inflammatory and oxidative stress genes.
Another interesting and plausible mechanism underlying the association of diabetes/insulin resistance with low vitamin D levels has been recently showed in an in-vitro study where 3T3L1 adipocytes were treated with high glucose in the presence or absence of 1,25-dihydroxyvitamin D. 1,25(OH)2D treatment of adipocytes caused significant up-regulation of GLUT4 receptor expression and its translocation to cell surface, and an increase in glucose uptake as well as glucose utilization[44]. Supplementation also stimulated adiponectin secretion in high glucose-treated cells, lending further weight of a beneficial effect of vitamin D in reducing adipose tissue inflammation.
The liver is positioned between the gut and systemic circulation and in addition to its synthetic function it has a key role in degrading and removing toxins, exogenous antigens, and infectious agents responding to exogenous antigenic molecules. This role makes the liver not only a metabolic organ but also a mediator of systemic and local innate and adaptive immunity[45]. The intestinal epithelial cells prevent the translocation of bacterial products to the portal circulation. When this barrier is ineffective the liver cells are exposed to bacterial products and this translocation may impair liver homeostasis and trigger liver inflammation, inducing the innate immune response[46,47]. A study from Italy showed that patients with NAFLD have increased gut permeability and small intestinal bacterial overgrowth (SIBO). Both gut permeability and the prevalence of SIBO correlated with the severity of steatosis but not with presence of NASH in this study[48].
Bacterial lipopolysaccharides (LPS) are activators of the immune system and in animal models were involved in the development of both systemic inflammation and obesity[49]. Toll Like receptor-4 (TLR-4) recognizes a diverse array of pathogen associated molecular patterns including LPS[50]. The role of TLR-4 has been studied and there is a clear association between TLR-4 activation and NAFLD[48,49,51-54]. Interestingly, the development and progression of NAFLD by western diet is exacerbated by vitamin D deficiency through the activation of TLR2 and TLR4 by way of CD14/LBP, and stimulation of downstream inflammatory signaling molecules leading to steatosis and inflammation[42].
Toll-like receptor 5 (expressed in the gut mucosa helping defense against infection) is implicated in the development of metabolic syndrome and alterations in gut microbiota. In a study published in 2010, TLR-5-deficient mice developed hyperphagia, obesity, insulin resistance and hepatic steatosis. In this study, transfer of microbiota from TLR-5-deficient mice to healthy mice led to development of de novo disease, indicating a possible connection between TLR-5 and intestinal microbiota in NAFLD[55]. In contrast to this study, Kanuri et al[54] found that TLR-5 is significantly overexpressed in patients with NAFLD compared to controls thus making the role of TLR-5 in the development of NAFLD unclear.
A different subtype of TLR, TLR-9 is activated by bacterial/viral DNA and has been implicated in the development of steatohepatitis in animal models[56]. Miura et al[57] showed that TLR-9-deficient mice failed to develop inflammation vs controls when exposed to IL-1b. In another study mentioned earlier the investigators showed that vitamin D deficient mice following Western Diet had increased levels of messenger RNA of TLR-2, TLR-4, and TLR-9[42]. However, there are no randomized controlled trials studying whether vitamin D replacement is beneficial in suppressing the effects of TLR-4 and TLR-9.
The role of the major components of the innate immunity like macrophages and Kuppfer cells[58-61], neutrophils[62-64], eosinophils[65] and dendritic cells (DC)[66,67] in the pathogenesis of NAFLD and insulin resistance has been studied but a detailed report extends beyond the goals of the current review. It is however interesting to note the immunomodulatory effect of vitamin D in DC. Dendritic cells express VDR[68] and treatment with 1,25(OH)2D inhibits DC maturation and promotes a tolerogenic phenotype in some studies[69,70]. However Henning et al[66] showed that DC depletion markedly exacerbates intrahepatic fibroinflammation. Thus the role of vitamin D in the regulation of this pathway is not clear yet.
The development of liver fibrosis in NAFLD indicates advanced disease and is in fact the strongest predictor for disease-specific mortality[71]. It is characterized by an accumulation of extracellular matrix (ECM) with subsequent destruction of the normal liver architecture, leading to liver cell dysfunction. Hepatic stellate cells (HSCs) play a critical role in the development of liver fibrosis, since they are responsible for excessive deposition of ECM proteins, predominantly type I collagen. Two main processes lead to liver fibrosis. First, HSCs become activated, resulting in increased cellular proliferation and biotransformation to an activated myofibroblast-like cell. Second, there is an increase in ECM protein synthesis and deposition, predominantly type I collagen. TGF-β1 signaling pathway plays a central role in this process, as it is the main stimulating factor for profibrotic ECM synthesis[72].
Although it has been known for some time that vitamin D has anti-proliferative and anti-fibrotic properties and plays an important role in the regulation of ECM, little has been known until recently about the effects of vitamin D on HSCs. Indeed, the finding of a robust VDR expression in HSCs[24] has led to the discovery that VDR signaling can suppress fibrotic gene expression and inhibit proliferation of HSCs[30,73]. Elegant mechanistic studies with VDR knockout mice confirmed the development of spontaneous liver injury with fibrosis in these mice[30]. Moreover, in an experimental mouse model of liver injury, co-treatment with VDR ligand attenuated the progression of liver fibrosis[30]. Consistent with the above studies are the findings of another in-vitro study, this time in human HSCs, in which treatment with 1,25(OH)2D caused inhibition of fibrogenesis by inhibiting type 1 collagen formation[74]. The underlying mechanism by which VDR prevents this pathological process has only been recently elucidated and involves the antagonistic action of VDR on SMAD transcription factor - a key transcription factor in the pro-fibrotic process linking TGF-β1[30].
In total, the evidence to date suggests that vitamin D may be beneficial in preventing the progression of NAFLD. Therefore, clinical trials that directly evaluate the effect of vitamin D supplementation on disease progression in NAFLD subjects are warranted. Only recently were the results of a small double-blind, placebo-control study in NAFLD subjects published. In this study[75], the investigators randomly assigned NAFLD participants in the vitamin D group (50000 IU every 14 d for 4 mo) and the control group (no vitamin D supplementation). After a follow-up period of 4 mo, there were no significant differences in the serum levels of liver enzymes, HOMA-IR or grades of hepatic steatosis, measured by ultrasound, between the two groups. However there was a significant decrease in the levels of hsCRP and MDA (malondialdehyde) - a marker of lipid peroxidation - in the subjects treated with vitamin D. The negative results of this study on the markers of steatosis and liver injury have to be interpreted carefully given the limited number of participants (n = 53) and the relatively short term of follow up. On the contrary, the confirmation that vitamin D treatment resulted in improvement of inflammatory biomarkers in NAFLD subjects suggests that vitamin D supplementation may be considered as an adjunctive therapy to attenuate systemic inflammation in NAFLD.
Vitamin D deficiency is commonly associated with NAFLD and has even been correlated with disease severity. The metabolic, anti-inflammatory and anti-fibrotic properties of vitamin D provide plausible mechanisms by which vitamin D may impact on the various steps of disease progression and severity. Cumulatively, this would suggest that vitamin D replacement might be effective in the treatment of NAFLD. However, controversies exist in the field given the limited number of prospective randomized studies in humans examining the role of vitamin D supplementation in NAFLD, the presence of variability on the methodologies used for detecting vitamin D levels[76] as well as the lack of consensus in the scientific community on defining the optimal levels of vitamin D (> 20 ng/mL vs > 30 ng/mL)[77,78].
In conclusion, larger, randomized, placebo-controlled trials are required to better evaluate the efficacy of vitamin D replacement and parameters of therapy in NAFLD. Until then, it is premature to recommend vitamin D supplementation for the specific treatment of NAFLD even though its role seems to be promising.
P- Reviewer: Balaban YH, Calamita G, Chen LZ, Guo HH, Hyogo H, Mikolasevic I, Sertoglu E S- Editor: Ma YJ L- Editor: A E- Editor: Ma S
| 1. | Lazo M, Clark JM. The epidemiology of nonalcoholic fatty liver disease: a global perspective. Semin Liver Dis. 2008;28:339-350. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 521] [Cited by in F6Publishing: 503] [Article Influence: 31.4] [Reference Citation Analysis (0)] |
| 2. | Browning JD, Szczepaniak LS, Dobbins R, Nuremberg P, Horton JD, Cohen JC, Grundy SM, Hobbs HH. Prevalence of hepatic steatosis in an urban population in the United States: impact of ethnicity. Hepatology. 2004;40:1387-1395. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 2633] [Cited by in F6Publishing: 2593] [Article Influence: 129.7] [Reference Citation Analysis (3)] |
| 3. | Moore JB. Non-alcoholic fatty liver disease: the hepatic consequence of obesity and the metabolic syndrome. Proc Nutr Soc. 2010;69:211-220. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 148] [Cited by in F6Publishing: 157] [Article Influence: 11.2] [Reference Citation Analysis (0)] |
| 4. | Fabbrini E, Sullivan S, Klein S. Obesity and nonalcoholic fatty liver disease: biochemical, metabolic, and clinical implications. Hepatology. 2010;51:679-689. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 1303] [Cited by in F6Publishing: 1370] [Article Influence: 97.9] [Reference Citation Analysis (1)] |
| 5. | Tilg H, Moschen AR. Evolution of inflammation in nonalcoholic fatty liver disease: the multiple parallel hits hypothesis. Hepatology. 2010;52:1836-1846. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 1543] [Cited by in F6Publishing: 1629] [Article Influence: 116.4] [Reference Citation Analysis (0)] |
| 6. | Holick MF. Vitamin D deficiency. N Engl J Med. 2007;357:266-281. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 9399] [Cited by in F6Publishing: 8986] [Article Influence: 528.6] [Reference Citation Analysis (1)] |
| 7. | Verstuyf A, Carmeliet G, Bouillon R, Mathieu C. Vitamin D: a pleiotropic hormone. Kidney Int. 2010;78:140-145. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 223] [Cited by in F6Publishing: 224] [Article Influence: 16.0] [Reference Citation Analysis (0)] |
| 8. | Ju SY, Jeong HS, Kim do H. Blood vitamin D status and metabolic syndrome in the general adult population: a dose-response meta-analysis. J Clin Endocrinol Metab. 2014;99:1053-1063. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 70] [Cited by in F6Publishing: 68] [Article Influence: 6.8] [Reference Citation Analysis (0)] |
| 9. | Pittas AG, Lau J, Hu FB, Dawson-Hughes B. The role of vitamin D and calcium in type 2 diabetes. A systematic review and meta-analysis. J Clin Endocrinol Metab. 2007;92:2017-2029. [PubMed] [Cited in This Article: ] |
| 10. | Pittas AG, Chung M, Trikalinos T, Mitri J, Brendel M, Patel K, Lichtenstein AH, Lau J, Balk EM. Systematic review: Vitamin D and cardiometabolic outcomes. Ann Intern Med. 2010;152:307-314. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 496] [Cited by in F6Publishing: 476] [Article Influence: 34.0] [Reference Citation Analysis (0)] |
| 11. | Mitri J, Muraru MD, Pittas AG. Vitamin D and type 2 diabetes: a systematic review. Eur J Clin Nutr. 2011;65:1005-1015. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 356] [Cited by in F6Publishing: 351] [Article Influence: 27.0] [Reference Citation Analysis (0)] |
| 12. | George PS, Pearson ER, Witham MD. Effect of vitamin D supplementation on glycaemic control and insulin resistance: a systematic review and meta-analysis. Diabet Med. 2012;29:e142-e150. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 227] [Cited by in F6Publishing: 242] [Article Influence: 20.2] [Reference Citation Analysis (0)] |
| 13. | Zeitz U, Weber K, Soegiarto DW, Wolf E, Balling R, Erben RG. Impaired insulin secretory capacity in mice lacking a functional vitamin D receptor. FASEB J. 2003;17:509-511. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 296] [Cited by in F6Publishing: 271] [Article Influence: 12.9] [Reference Citation Analysis (0)] |
| 14. | Seida JC, Mitri J, Colmers IN, Majumdar SR, Davidson MB, Edwards AL, Hanley DA, Pittas AG, Tjosvold L, Johnson JA. Clinical review: Effect of vitamin D3 supplementation on improving glucose homeostasis and preventing diabetes: a systematic review and meta-analysis. J Clin Endocrinol Metab. 2014;99:3551-3560. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 179] [Cited by in F6Publishing: 188] [Article Influence: 18.8] [Reference Citation Analysis (0)] |
| 15. | Eliades M, Spyrou E, Agrawal N, Lazo M, Brancati FL, Potter JJ, Koteish AA, Clark JM, Guallar E, Hernaez R. Meta-analysis: vitamin D and non-alcoholic fatty liver disease. Aliment Pharmacol Ther. 2013;38:246-254. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 169] [Cited by in F6Publishing: 192] [Article Influence: 17.5] [Reference Citation Analysis (0)] |
| 16. | Liangpunsakul S, Chalasani N. Serum vitamin D concentrations and unexplained elevation in ALT among US adults. Dig Dis Sci. 2011;56:2124-2129. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 49] [Cited by in F6Publishing: 47] [Article Influence: 3.6] [Reference Citation Analysis (0)] |
| 17. | Rhee EJ, Kim MK, Park SE, Park CY, Baek KH, Lee WY, Kang MI, Park SW, Kim SW, Oh KW. High serum vitamin D levels reduce the risk for nonalcoholic fatty liver disease in healthy men independent of metabolic syndrome. Endocr J. 2013;60:743-752. [PubMed] [Cited in This Article: ] |
| 18. | Hong HC, Lee JS, Choi HY, Yang SJ, Yoo HJ, Seo JA, Kim SG, Kim NH, Baik SH, Choi DS. Liver enzymes and vitamin D levels in metabolically healthy but obese individuals: Korean National Health and Nutrition Examination Survey. Metabolism. 2013;62:1305-1312. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 33] [Cited by in F6Publishing: 33] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 19. | Targher G, Bertolini L, Scala L, Cigolini M, Zenari L, Falezza G, Arcaro G. Associations between serum 25-hydroxyvitamin D3 concentrations and liver histology in patients with non-alcoholic fatty liver disease. Nutr Metab Cardiovasc Dis. 2007;17:517-524. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 284] [Cited by in F6Publishing: 309] [Article Influence: 17.2] [Reference Citation Analysis (0)] |
| 20. | Abawi MBA, Baranova A, Afendy A, Page S, Stepanova M. Vitamin D levels in non-alcoholic fatty liver disease (NAFLD) patients correlate with apoptosis and serum levels of M30. Am J Gastroenterol. 2011;106:S121. [Cited in This Article: ] |
| 21. | Dasarathy JPP, Allampati S, Hawkins CA, Brandt PT, Khiyami A, McCullough AJ, Dasarathy S. Hypovitaminosis D associated with more advanced non alcoholic fatty liver disease. Hepatology. 2012;56:889A-890A. [Cited in This Article: ] |
| 22. | Manco M, Ciampalini P, Nobili V. Low levels of 25-hydroxyvitamin D(3) in children with biopsy-proven nonalcoholic fatty liver disease. Hepatology. 2010;51:2229; author reply 2230. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 54] [Cited by in F6Publishing: 62] [Article Influence: 4.4] [Reference Citation Analysis (0)] |
| 23. | Nobili V, Giorgio V, Liccardo D, Bedogni G, Morino G, Alisi A, Cianfarani S. Vitamin D levels and liver histological alterations in children with nonalcoholic fatty liver disease. Eur J Endocrinol. 2014;170:547-553. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 72] [Cited by in F6Publishing: 75] [Article Influence: 7.5] [Reference Citation Analysis (0)] |
| 24. | Gascon-Barré M, Demers C, Mirshahi A, Néron S, Zalzal S, Nanci A. The normal liver harbors the vitamin D nuclear receptor in nonparenchymal and biliary epithelial cells. Hepatology. 2003;37:1034-1042. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 187] [Cited by in F6Publishing: 185] [Article Influence: 8.8] [Reference Citation Analysis (0)] |
| 25. | Han S, Li T, Ellis E, Strom S, Chiang JY. A novel bile acid-activated vitamin D receptor signaling in human hepatocytes. Mol Endocrinol. 2010;24:1151-1164. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 103] [Cited by in F6Publishing: 106] [Article Influence: 7.6] [Reference Citation Analysis (0)] |
| 26. | Maestro B, Dávila N, Carranza MC, Calle C. Identification of a Vitamin D response element in the human insulin receptor gene promoter. J Steroid Biochem Mol Biol. 2003;84:223-230. [PubMed] [Cited in This Article: ] |
| 27. | Chun RF, Liu PT, Modlin RL, Adams JS, Hewison M. Impact of vitamin D on immune function: lessons learned from genome-wide analysis. Front Physiol. 2014;5:151. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 246] [Cited by in F6Publishing: 233] [Article Influence: 23.3] [Reference Citation Analysis (0)] |
| 28. | Jiang YJ, Teichert AE, Fong F, Oda Y, Bikle DD. 1α,25(OH)2-dihydroxyvitamin D3/VDR protects the skin from UVB-induced tumor formation by interacting with the β-catenin pathway. J Steroid Biochem Mol Biol. 2013;136:229-232. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 38] [Cited by in F6Publishing: 41] [Article Influence: 3.7] [Reference Citation Analysis (0)] |
| 29. | Adams LA, White SW, Marsh JA, Lye SJ, Connor KL, Maganga R, Ayonrinde OT, Olynyk JK, Mori TA, Beilin LJ. Association between liver-specific gene polymorphisms and their expression levels with nonalcoholic fatty liver disease. Hepatology. 2013;57:590-600. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 62] [Cited by in F6Publishing: 67] [Article Influence: 6.1] [Reference Citation Analysis (0)] |
| 30. | Ding N, Yu RT, Subramaniam N, Sherman MH, Wilson C, Rao R, Leblanc M, Coulter S, He M, Scott C. A vitamin D receptor/SMAD genomic circuit gates hepatic fibrotic response. Cell. 2013;153:601-613. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 425] [Cited by in F6Publishing: 478] [Article Influence: 43.5] [Reference Citation Analysis (0)] |
| 31. | Barchetta I, Carotti S, Labbadia G, Gentilucci UV, Muda AO, Angelico F, Silecchia G, Leonetti F, Fraioli A, Picardi A. Liver vitamin D receptor, CYP2R1, and CYP27A1 expression: relationship with liver histology and vitamin D3 levels in patients with nonalcoholic steatohepatitis or hepatitis C virus. Hepatology. 2012;56:2180-2187. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 156] [Cited by in F6Publishing: 164] [Article Influence: 13.7] [Reference Citation Analysis (0)] |
| 32. | Albano E, Mottaran E, Occhino G, Reale E, Vidali M. Review article: role of oxidative stress in the progression of non-alcoholic steatosis. Aliment Pharmacol Ther. 2005;22 Suppl 2:71-73. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 102] [Cited by in F6Publishing: 95] [Article Influence: 5.0] [Reference Citation Analysis (0)] |
| 33. | Bechmann LP, Hannivoort RA, Gerken G, Hotamisligil GS, Trauner M, Canbay A. The interaction of hepatic lipid and glucose metabolism in liver diseases. J Hepatol. 2012;56:952-964. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 593] [Cited by in F6Publishing: 633] [Article Influence: 52.8] [Reference Citation Analysis (0)] |
| 34. | Cai D, Yuan M, Frantz DF, Melendez PA, Hansen L, Lee J, Shoelson SE. Local and systemic insulin resistance resulting from hepatic activation of IKK-beta and NF-kappaB. Nat Med. 2005;11:183-190. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 1612] [Cited by in F6Publishing: 1671] [Article Influence: 87.9] [Reference Citation Analysis (0)] |
| 35. | Crespo J, Cayón A, Fernández-Gil P, Hernández-Guerra M, Mayorga M, Domínguez-Díez A, Fernández-Escalante JC, Pons-Romero F. Gene expression of tumor necrosis factor alpha and TNF-receptors, p55 and p75, in nonalcoholic steatohepatitis patients. Hepatology. 2001;34:1158-1163. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 485] [Cited by in F6Publishing: 485] [Article Influence: 21.1] [Reference Citation Analysis (0)] |
| 36. | Xu A, Wang Y, Keshaw H, Xu LY, Lam KS, Cooper GJ. The fat-derived hormone adiponectin alleviates alcoholic and nonalcoholic fatty liver diseases in mice. J Clin Invest. 2003;112:91-100. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 36] [Cited by in F6Publishing: 377] [Article Influence: 18.0] [Reference Citation Analysis (0)] |
| 37. | Liu Y, Palanivel R, Rai E, Park M, Gabor TV, Scheid MP, Xu A, Sweeney G. Adiponectin stimulates autophagy and reduces oxidative stress to enhance insulin sensitivity during high-fat diet feeding in mice. Diabetes. 2015;64:36-48. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 160] [Cited by in F6Publishing: 160] [Article Influence: 17.8] [Reference Citation Analysis (0)] |
| 38. | Hui JM, Hodge A, Farrell GC, Kench JG, Kriketos A, George J. Beyond insulin resistance in NASH: TNF-alpha or adiponectin? Hepatology. 2004;40:46-54. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 671] [Cited by in F6Publishing: 668] [Article Influence: 33.4] [Reference Citation Analysis (0)] |
| 39. | Kaser S, Moschen A, Cayon A, Kaser A, Crespo J, Pons-Romero F, Ebenbichler CF, Patsch JR, Tilg H. Adiponectin and its receptors in non-alcoholic steatohepatitis. Gut. 2005;54:117-121. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 323] [Cited by in F6Publishing: 300] [Article Influence: 15.8] [Reference Citation Analysis (0)] |
| 40. | Vaidya A, Williams JS, Forman JP. The independent association between 25-hydroxyvitamin D and adiponectin and its relation with BMI in two large cohorts: the NHS and the HPFS. Obesity (Silver Spring). 2012;20:186-191. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 64] [Cited by in F6Publishing: 67] [Article Influence: 5.6] [Reference Citation Analysis (0)] |
| 41. | Neyestani TR, Nikooyeh B, Alavi-Majd H, Shariatzadeh N, Kalayi A, Tayebinejad N, Heravifard S, Salekzamani S, Zahedirad M. Improvement of vitamin D status via daily intake of fortified yogurt drink either with or without extra calcium ameliorates systemic inflammatory biomarkers, including adipokines, in the subjects with type 2 diabetes. J Clin Endocrinol Metab. 2012;97:2005-2011. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 86] [Cited by in F6Publishing: 87] [Article Influence: 7.3] [Reference Citation Analysis (0)] |
| 42. | Roth CL, Elfers CT, Figlewicz DP, Melhorn SJ, Morton GJ, Hoofnagle A, Yeh MM, Nelson JE, Kowdley KV. Vitamin D deficiency in obese rats exacerbates nonalcoholic fatty liver disease and increases hepatic resistin and Toll-like receptor activation. Hepatology. 2012;55:1103-1111. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 193] [Cited by in F6Publishing: 203] [Article Influence: 16.9] [Reference Citation Analysis (0)] |
| 43. | Nakano T, Cheng YF, Lai CY, Hsu LW, Chang YC, Deng JY, Huang YZ, Honda H, Chen KD, Wang CC. Impact of artificial sunlight therapy on the progress of non-alcoholic fatty liver disease in rats. J Hepatol. 2011;55:415-425. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 107] [Cited by in F6Publishing: 116] [Article Influence: 8.9] [Reference Citation Analysis (0)] |
| 44. | Manna P, Jain SK. Vitamin D up-regulates glucose transporter 4 (GLUT4) translocation and glucose utilization mediated by cystathionine-γ-lyase (CSE) activation and H2S formation in 3T3L1 adipocytes. J Biol Chem. 2012;287:42324-42332. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 103] [Cited by in F6Publishing: 109] [Article Influence: 9.1] [Reference Citation Analysis (0)] |
| 45. | Kita H, Mackay IR, Van De Water J, Gershwin ME. The lymphoid liver: considerations on pathways to autoimmune injury. Gastroenterology. 2001;120:1485-1501. [PubMed] [Cited in This Article: ] |
| 46. | Crispe IN. The liver as a lymphoid organ. Annu Rev Immunol. 2009;27:147-163. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 686] [Cited by in F6Publishing: 702] [Article Influence: 46.8] [Reference Citation Analysis (0)] |
| 47. | Seki E, Brenner DA. Toll-like receptors and adaptor molecules in liver disease: update. Hepatology. 2008;48:322-335. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 515] [Cited by in F6Publishing: 522] [Article Influence: 32.6] [Reference Citation Analysis (0)] |
| 48. | Miele L, Valenza V, La Torre G, Montalto M, Cammarota G, Ricci R, Mascianà R, Forgione A, Gabrieli ML, Perotti G. Increased intestinal permeability and tight junction alterations in nonalcoholic fatty liver disease. Hepatology. 2009;49:1877-1887. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 938] [Cited by in F6Publishing: 982] [Article Influence: 65.5] [Reference Citation Analysis (0)] |
| 49. | Cani PD, Bibiloni R, Knauf C, Waget A, Neyrinck AM, Delzenne NM, Burcelin R. Changes in gut microbiota control metabolic endotoxemia-induced inflammation in high-fat diet-induced obesity and diabetes in mice. Diabetes. 2008;57:1470-1481. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 3224] [Cited by in F6Publishing: 3232] [Article Influence: 202.0] [Reference Citation Analysis (0)] |
| 50. | Alisi A, Carsetti R, Nobili V. Pathogen- or damage-associated molecular patterns during nonalcoholic fatty liver disease development. Hepatology. 2011;54:1500-1502. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 36] [Cited by in F6Publishing: 39] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 51. | Spruss A, Kanuri G, Wagnerberger S, Haub S, Bischoff SC, Bergheim I. Toll-like receptor 4 is involved in the development of fructose-induced hepatic steatosis in mice. Hepatology. 2009;50:1094-1104. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 403] [Cited by in F6Publishing: 428] [Article Influence: 28.5] [Reference Citation Analysis (0)] |
| 52. | Li L, Chen L, Hu L, Liu Y, Sun HY, Tang J, Hou YJ, Chang YX, Tu QQ, Feng GS. Nuclear factor high-mobility group box1 mediating the activation of Toll-like receptor 4 signaling in hepatocytes in the early stage of nonalcoholic fatty liver disease in mice. Hepatology. 2011;54:1620-1630. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 168] [Cited by in F6Publishing: 191] [Article Influence: 14.7] [Reference Citation Analysis (0)] |
| 53. | Csak T, Ganz M, Pespisa J, Kodys K, Dolganiuc A, Szabo G. Fatty acid and endotoxin activate inflammasomes in mouse hepatocytes that release danger signals to stimulate immune cells. Hepatology. 2011;54:133-144. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 442] [Cited by in F6Publishing: 472] [Article Influence: 36.3] [Reference Citation Analysis (0)] |
| 54. | Kanuri G, Ladurner R, Skibovskaya J, Spruss A, Königsrainer A, Bischoff SC, Bergheim I. Expression of toll-like receptors 1-5 but not TLR 6-10 is elevated in livers of patients with non-alcoholic fatty liver disease. Liver Int. 2015;35:562-568. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 37] [Cited by in F6Publishing: 41] [Article Influence: 4.6] [Reference Citation Analysis (0)] |
| 55. | Vijay-Kumar M, Aitken JD, Carvalho FA, Cullender TC, Mwangi S, Srinivasan S, Sitaraman SV, Knight R, Ley RE, Gewirtz AT. Metabolic syndrome and altered gut microbiota in mice lacking Toll-like receptor 5. Science. 2010;328:228-231. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 1496] [Cited by in F6Publishing: 1501] [Article Influence: 107.2] [Reference Citation Analysis (0)] |
| 56. | Miura K, Seki E, Ohnishi H, Brenner DA. Role of toll-like receptors and their downstream molecules in the development of nonalcoholic Fatty liver disease. Gastroenterol Res Pract. 2010;2010:362847. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 106] [Cited by in F6Publishing: 118] [Article Influence: 9.1] [Reference Citation Analysis (0)] |
| 57. | Miura K, Kodama Y, Inokuchi S, Schnabl B, Aoyama T, Ohnishi H, Olefsky JM, Brenner DA, Seki E. Toll-like receptor 9 promotes steatohepatitis by induction of interleukin-1beta in mice. Gastroenterology. 2010;139:323-34.e7. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 527] [Cited by in F6Publishing: 558] [Article Influence: 39.9] [Reference Citation Analysis (0)] |
| 58. | Lumeng CN, Bodzin JL, Saltiel AR. Obesity induces a phenotypic switch in adipose tissue macrophage polarization. J Clin Invest. 2007;117:175-184. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 3164] [Cited by in F6Publishing: 3299] [Article Influence: 194.1] [Reference Citation Analysis (0)] |
| 59. | Vats D, Mukundan L, Odegaard JI, Zhang L, Smith KL, Morel CR, Wagner RA, Greaves DR, Murray PJ, Chawla A. Oxidative metabolism and PGC-1beta attenuate macrophage-mediated inflammation. Cell Metab. 2006;4:13-24. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 922] [Cited by in F6Publishing: 1002] [Article Influence: 55.7] [Reference Citation Analysis (0)] |
| 60. | Fujisaka S, Usui I, Bukhari A, Ikutani M, Oya T, Kanatani Y, Tsuneyama K, Nagai Y, Takatsu K, Urakaze M. Regulatory mechanisms for adipose tissue M1 and M2 macrophages in diet-induced obese mice. Diabetes. 2009;58:2574-2582. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 538] [Cited by in F6Publishing: 533] [Article Influence: 35.5] [Reference Citation Analysis (0)] |
| 61. | Lumeng CN, DelProposto JB, Westcott DJ, Saltiel AR. Phenotypic switching of adipose tissue macrophages with obesity is generated by spatiotemporal differences in macrophage subtypes. Diabetes. 2008;57:3239-3246. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 674] [Cited by in F6Publishing: 642] [Article Influence: 40.1] [Reference Citation Analysis (0)] |
| 62. | Elgazar-Carmon V, Rudich A, Hadad N, Levy R. Neutrophils transiently infiltrate intra-abdominal fat early in the course of high-fat feeding. J Lipid Res. 2008;49:1894-1903. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 315] [Cited by in F6Publishing: 329] [Article Influence: 20.6] [Reference Citation Analysis (0)] |
| 63. | Talukdar S, Oh da Y, Bandyopadhyay G, Li D, Xu J, McNelis J, Lu M, Li P, Yan Q, Zhu Y. Neutrophils mediate insulin resistance in mice fed a high-fat diet through secreted elastase. Nat Med. 2012;18:1407-1412. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 614] [Cited by in F6Publishing: 675] [Article Influence: 56.3] [Reference Citation Analysis (0)] |
| 64. | Rensen SS, Bieghs V, Xanthoulea S, Arfianti E, Bakker JA, Shiri-Sverdlov R, Hofker MH, Greve JW, Buurman WA. Neutrophil-derived myeloperoxidase aggravates non-alcoholic steatohepatitis in low-density lipoprotein receptor-deficient mice. PLoS One. 2012;7:e52411. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 80] [Cited by in F6Publishing: 97] [Article Influence: 8.1] [Reference Citation Analysis (0)] |
| 65. | Wu D, Molofsky AB, Liang HE, Ricardo-Gonzalez RR, Jouihan HA, Bando JK, Chawla A, Locksley RM. Eosinophils sustain adipose alternatively activated macrophages associated with glucose homeostasis. Science. 2011;332:243-247. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 957] [Cited by in F6Publishing: 995] [Article Influence: 76.5] [Reference Citation Analysis (0)] |
| 66. | Henning JR, Graffeo CS, Rehman A, Fallon NC, Zambirinis CP, Ochi A, Barilla R, Jamal M, Deutsch M, Greco S. Dendritic cells limit fibroinflammatory injury in nonalcoholic steatohepatitis in mice. Hepatology. 2013;58:589-602. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 113] [Cited by in F6Publishing: 126] [Article Influence: 11.5] [Reference Citation Analysis (0)] |
| 67. | Bertola A, Ciucci T, Rousseau D, Bourlier V, Duffaut C, Bonnafous S, Blin-Wakkach C, Anty R, Iannelli A, Gugenheim J. Identification of adipose tissue dendritic cells correlated with obesity-associated insulin-resistance and inducing Th17 responses in mice and patients. Diabetes. 2012;61:2238-2247. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 267] [Cited by in F6Publishing: 264] [Article Influence: 22.0] [Reference Citation Analysis (0)] |
| 68. | Brennan A, Katz DR, Nunn JD, Barker S, Hewison M, Fraher LJ, O’Riordan JL. Dendritic cells from human tissues express receptors for the immunoregulatory vitamin D3 metabolite, dihydroxycholecalciferol. Immunology. 1987;61:457-461. [PubMed] [Cited in This Article: ] |
| 69. | Penna G, Adorini L. 1 Alpha,25-dihydroxyvitamin D3 inhibits differentiation, maturation, activation, and survival of dendritic cells leading to impaired alloreactive T cell activation. J Immunol. 2000;164:2405-2411. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 962] [Cited by in F6Publishing: 944] [Article Influence: 39.3] [Reference Citation Analysis (0)] |
| 70. | Adorini L, Penna G, Giarratana N, Uskokovic M. Tolerogenic dendritic cells induced by vitamin D receptor ligands enhance regulatory T cells inhibiting allograft rejection and autoimmune diseases. J Cell Biochem. 2003;88:227-233. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 179] [Cited by in F6Publishing: 182] [Article Influence: 8.7] [Reference Citation Analysis (0)] |
| 71. | Ekstedt M, Hagström H, Nasr P, Fredrikson M, Stål P, Kechagias S, Hultcrantz R. Fibrosis stage is the strongest predictor for disease-specific mortality in NAFLD after up to 33 years of follow-up. Hepatology. 2014;Epub ahead of print. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 1353] [Cited by in F6Publishing: 1467] [Article Influence: 163.0] [Reference Citation Analysis (0)] |
| 72. | Inagaki Y, Okazaki I. Emerging insights into Transforming growth factor beta Smad signal in hepatic fibrogenesis. Gut. 2007;56:284-292. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 339] [Cited by in F6Publishing: 379] [Article Influence: 22.3] [Reference Citation Analysis (0)] |
| 73. | Abramovitch S, Dahan-Bachar L, Sharvit E, Weisman Y, Ben Tov A, Brazowski E, Reif S. Vitamin D inhibits proliferation and profibrotic marker expression in hepatic stellate cells and decreases thioacetamide-induced liver fibrosis in rats. Gut. 2011;60:1728-1737. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 190] [Cited by in F6Publishing: 210] [Article Influence: 16.2] [Reference Citation Analysis (0)] |
| 74. | Potter JJ, Liu X, Koteish A, Mezey E. 1,25-dihydroxyvitamin D3 and its nuclear receptor repress human α1 (I) collagen expression and type I collagen formation. Liver Int. 2013;33:677-686. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 46] [Cited by in F6Publishing: 45] [Article Influence: 4.1] [Reference Citation Analysis (0)] |
| 75. | Sharifi N, Amani R, Hajiani E, Cheraghian B. Does vitamin D improve liver enzymes, oxidative stress, and inflammatory biomarkers in adults with non-alcoholic fatty liver disease? A randomized clinical trial. Endocrine. 2014;47:70-80. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 162] [Cited by in F6Publishing: 159] [Article Influence: 15.9] [Reference Citation Analysis (0)] |
| 76. | Singh RJ. Quantitation of 25-OH-vitamin D (25OHD) using liquid tandem mass spectrometry (LC-MS-MS). Methods Mol Biol. 2010;603:509-517. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 31] [Cited by in F6Publishing: 36] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 77. | Holick MF, Binkley NC, Bischoff-Ferrari HA, Gordon CM, Hanley DA, Heaney RP, Murad MH, Weaver CM. Guidelines for preventing and treating vitamin D deficiency and insufficiency revisited. J Clin Endocrinol Metab. 2012;97:1153-1158. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 402] [Cited by in F6Publishing: 389] [Article Influence: 32.4] [Reference Citation Analysis (0)] |
| 78. | Rosen CJ, Abrams SA, Aloia JF, Brannon PM, Clinton SK, Durazo-Arvizu RA, Gallagher JC, Gallo RL, Jones G, Kovacs CS. IOM committee members respond to Endocrine Society vitamin D guideline. J Clin Endocrinol Metab. 2012;97:1146-1152. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 385] [Cited by in F6Publishing: 394] [Article Influence: 32.8] [Reference Citation Analysis (0)] |
| 79. | Bland R, Markovic D, Hills CE, Hughes SV, Chan SL, Squires PE, Hewison M. Expression of 25-hydroxyvitamin D3-1alpha-hydroxylase in pancreatic islets. J Steroid Biochem Mol Biol. 2004;89-90:121-125. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 245] [Cited by in F6Publishing: 216] [Article Influence: 10.8] [Reference Citation Analysis (0)] |
| 80. | Maestro B, Molero S, Bajo S, Dávila N, Calle C. Transcriptional activation of the human insulin receptor gene by 1,25-dihydroxyvitamin D(3). Cell Biochem Funct. 2002;20:227-232. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 191] [Cited by in F6Publishing: 188] [Article Influence: 8.5] [Reference Citation Analysis (0)] |
| 81. | Norman AW, Frankel JB, Heldt AM, Grodsky GM. Vitamin D deficiency inhibits pancreatic secretion of insulin. Science. 1980;209:823-825. [PubMed] [Cited in This Article: ] |
| 82. | Cade C, Norman AW. Vitamin D3 improves impaired glucose tolerance and insulin secretion in the vitamin D-deficient rat in vivo. Endocrinology. 1986;119:84-90. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 193] [Cited by in F6Publishing: 203] [Article Influence: 5.3] [Reference Citation Analysis (0)] |
| 83. | Alkharfy KM, Al-Daghri NM, Yakout SM, Hussain T, Mohammed AK, Krishnaswamy S. Influence of vitamin D treatment on transcriptional regulation of insulin-sensitive genes. Metab Syndr Relat Disord. 2013;11:283-288. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 36] [Cited by in F6Publishing: 38] [Article Influence: 3.5] [Reference Citation Analysis (0)] |
| 84. | Lira FS, Rosa JC, Cunha CA, Ribeiro EB, do Nascimento CO, Oyama LM, Mota JF. Supplementing alpha-tocopherol (vitamin E) and vitamin D3 in high fat diet decrease IL-6 production in murine epididymal adipose tissue and 3T3-L1 adipocytes following LPS stimulation. Lipids Health Dis. 2011;10:37. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 54] [Cited by in F6Publishing: 57] [Article Influence: 4.4] [Reference Citation Analysis (0)] |
| 85. | Marcotorchino J, Gouranton E, Romier B, Tourniaire F, Astier J, Malezet C, Amiot MJ, Landrier JF. Vitamin D reduces the inflammatory response and restores glucose uptake in adipocytes. Mol Nutr Food Res. 2012;56:1771-1782. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 97] [Cited by in F6Publishing: 100] [Article Influence: 8.3] [Reference Citation Analysis (0)] |
| 86. | Ding C, Wilding JP, Bing C. 1,25-dihydroxyvitamin D3 protects against macrophage-induced activation of NFκB and MAPK signalling and chemokine release in human adipocytes. PLoS One. 2013;8:e61707. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 75] [Cited by in F6Publishing: 78] [Article Influence: 7.1] [Reference Citation Analysis (0)] |
| 87. | Gao D, Trayhurn P, Bing C. 1,25-Dihydroxyvitamin D3 inhibits the cytokine-induced secretion of MCP-1 and reduces monocyte recruitment by human preadipocytes. Int J Obes (Lond). 2013;37:357-365. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 98] [Cited by in F6Publishing: 91] [Article Influence: 8.3] [Reference Citation Analysis (0)] |