Published online Aug 21, 2016. doi: 10.3748/wjg.v22.i31.7166
Peer-review started: April 8, 2016
First decision: May 27, 2016
Revised: June 14, 2016
Accepted: June 28, 2016
Article in press: June 28, 2016
Published online: August 21, 2016
Involvement of gastrointestinal tract by cytomegalovirus (CMV) is common. CMV infections mainly run their course without any clinical signs in immunocompetent hosts. In contrast, CMV can cause severe infections with serious consequences in a immunocompromised state typically associated with organ transplants, highly immunosuppressive cancer chemotherapy, advanced HIV infection or treatment with corticosteroids. The incidence and severity of these manifestations of CMV is directly proportional with the degree of cellular immune dysfunction, i.e., CD8+ Cytotoxic T-cell response. Clinical manifestations of CMV can become apparent in different situations including reactivation of CMV from latency, primary infection in a seronegative host, or exposure of a seropositive host to a new strain of CMV. As the clinical signs of CMV in immunodeficient patients are usually sparse, physicians should be highly vigilant about CMV infection, a treatable condition that otherwise is associated with significant mortality. Here we report a rare case of severe gastrointestinal CMV infection with sustained immunodeficiency secondary to treatment with steroids manifesting as fatal duodenal diverticular bleeding.
Core tip: Cytomegalovirus (CMV) can establish as latent infection that can lead to reactivation with immunosuppression. It can affect almost any organ system with gastrointestinal tract involvement being most common. In gastrointestinal tract, besides causing mucosal inflammation, rarely gastrointestinal perforation or hemorrhage may occur. High clinical suspicion is needed for timely diagnosis, as clinical signs are usually sparse for this fatal yet treatable CMV infection.
- Citation: Makker J, Bajantri B, Sakam S, Chilimuri S. Cytomegalovirus related fatal duodenal diverticular bleeding: Case report and literature review. World J Gastroenterol 2016; 22(31): 7166-7174
- URL: https://www.wjgnet.com/1007-9327/full/v22/i31/7166.htm
- DOI: https://dx.doi.org/10.3748/wjg.v22.i31.7166
Cytomegalovirus (CMV) infection of the gastrointestinal tract is common in immunocompromised patients. It usually presents as vague symptoms including anorexia, fever, diarrhea, abdominal pain, wasting, weight loss and rarely presents with life-threatening complications including gastrointestinal tract perforation and massive gastrointestinal bleeding[1]. Bleeding may result from severe pan-colitis, segmental colitis, isolated well-circumscribed ulcers, or diverticulitis, which in the absence of therapy or delayed diagnosis is progressive and is associated with high mortality rate. This case highlights the rare scenario of CMV infection related massive gastrointestinal bleeding from a duodenal diverticulum. Due to rare occurrence of CMV related fatal duodenal diverticular bleeding, high index of suspicion in an immunocompromised patient with gastrointestinal bleeding is needed to make an early diagnosis and possible favorable outcome.
A 77-year-old Hispanic man was hospitalized in our intensive care unit (ICU) for acute respiratory distress. The patient had been in his usual state of health until approximately 3 d prior to admission, when he developed gradually worsening shortness of breath, fever and productive cough. Patient denied paroxysmal nocturnal dyspnea, orthopnea, lower extremity swelling, chest pain and palpitations. He also denied nausea, vomiting or abdominal pain. There was no history of sick contacts, recent travel, malignancy, HIV or use of immunosuppressive drugs.
Medical history was significant for chronic obstructive pulmonary disease GOLD (Global Initiative for Chronic Obstructive Lung Disease) stage IV requiring home oxygen since 3 years, anxiety disorder, coronary artery disease and systolic congestive heart failure. He was an active cigarette smoker with history of 60 pack years, used intravenous heroin and cocaine many years ago and was receiving maintenance doses of Methadone, denied alcohol use. He had no known drug allergies. His medications at home included Albuterol, Fluticasone-Salmeterol, Tiotropium, Docusate, Senna, Mirtazapine, Zolpidem, Ibuprofen, Acetaminophen-Oxycodone, and Oxygen. He had undergone right inguinal herniorraphy many years ago. He was born in Puerto Rico, received education till ninth grade, was married and lived with his family. His family history was unremarkable.
On examination at his arrival to emergency room, he was afebrile, heart rate 120 beats per minute, respiratory rate 28 per minute, blood pressure 150/86 mmHg and oxygen saturation was low at 81 percent while breathing ambient air. General physical examination revealed anicteric conjunctivae, without petechiae or hemorrhage. The oropharynx was clear and neck was supple with no lymphadenopathy. The breath sounds were decreased bilaterally with wheezing, a grade 3/6 holosystolic murmur was heard at apex radiating to the axilla. The abdomen was non-distended with normal bowel sounds, soft, non-tender and no organomegaly. There was no costovertebral angle tenderness. There was no pedal edema in the legs bilaterally. No rash, ulcerations, or other skin lesions were present.
Important laboratory findings during hospitalization are shown in Table 1. Serological tests for Hepatitis B and C were negative. CT chest with contrast ruled out pulmonary embolism but showed bilateral infiltrates.
| Parameter | Value | Parameter | Value |
| pH | 7.23 | Bicarbonate | 30 mEq/L |
| pCO2 | 74.5 mmHg | BUN | 13 mg/dL |
| pO2 | 77.2 mmHg | Creatinine | 0.9 mg/dL |
| SaO2 | 93% | INR | 1.1 |
| Hemoglobin | 11.6 g/dL | S. Protein | 6.1 g/dL |
| Hematocrit | 34.80% | S. Albumin | 2.8 g/dL |
| White count | 10.9 k/μL | AST | 41 Unit/L |
| Platelet | 205 k/μL | ALT | 35 Unit/L |
| Sodium | 141 mEq/L | Alkaline Phosphatase | 73 Unit/L |
| Potassium | 4.2 mEq/L | Bilirubin (total) | 0.4 mg/dL |
| Chloride | 103 mEq/L | Bilirubin (direct) | 0.2 mg/dL |
Initial management included noninvasive positive pressure ventilation, intravenous antibiotics, intravenous steroid and bronchodilator nebulization with an impression of chronic obstructive pulmonary disease exacerbation. Subsequently, his trachea was intubated orally with endotracheal tube due to acute hypercapneic respiratory failure. Due to lack of clinical improvement despite antibiotics and other medical therapy, bronchoscopy was performed on day 4 of hospitalization which revealed thick mucoid secretions bilaterally but more in the in the right lower lobe. Antibiotics were changed to ceftazidime and trimethroprim/sulfamethoxazole after cultures of brochoscopic lavage fluid revealed Acinetobacter baumanii and Stenotrophomas maltophilia.
He was successfully extubated and liberated of ventilator support on day 12. Hospital course was prolonged and complicated with pre-renal acute kidney injury, which responded to intravenous fluids, Acinetobacter baumanii urinary tract infection, acute pulmonary embolism with hypoxic respiratory failure requiring re-intubation of trachea on day 25 and placement of an inferior vena cava filter. Subsequently on day 28, tracheostomy was done due to prolonged mechanical ventilation and failed weaning trials. On day 40, he had a massive gastrointestinal bleed with sudden decline in hemoglobin from 7.1 g/dL to 4.8 g/dL. Despite aggressive resuscitation with packed red blood cell transfusion, he suffered a cardiac arrest after which his family members did not want to pursue any aggressive measures. Unfortunately, he expired within few hours on the same day.
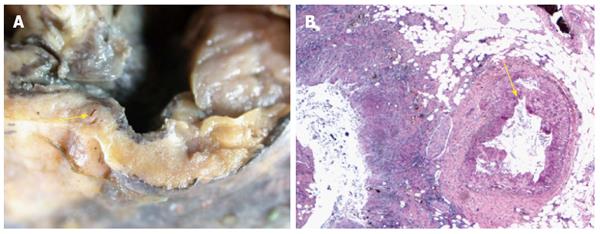
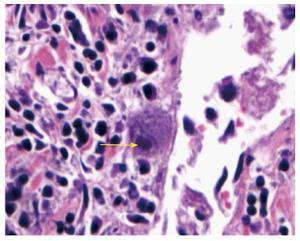
Post mortem examination showed large well-circumscribed duodenal diverticulum with ulcerated mucosa adjacent to eroded artery in posterior wall of first part of duodenum (Figure 1). Histopathological examination of the duodenal diverticulum revealed owl eyed inclusion bodies, characteristic for CMV (Figure 2).
In 1881, German pathologist Hugo Ribbert, discovered intranuclear inclusions like cytomegalovirus in newborns with syphilis and parotid gland of children and believed it to be a protozoan like structure[2]. Thereafter, for several years many researchers had similar observations and believed it be coccidia, sporozoa, embryonic epithelial cells and so forth, but not virus. It was only in 1925 when two pathologists, William C Vonglahn and Alwin M Pappenheimer from New York, considered these to be a virus[3]. Wyatt et al gave the term cytomegalic inclusion disease to the spectrum of manifestations occurring from this organism. Later Thomas H Weller, American virologist not only coined the term cytomegalovirus virus, but also isolated this virus in tissue cultures[2].
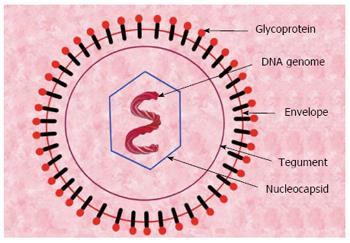
CMV is an icosahedral shaped, encapsulated, double-stranded DNA β-herpes virus, with varying size of 150 to 200 nm in diameter. The complete virion consists of an inner core of DNA genome, surrounded by capsid, which in turn is surrounded by proteinaceous tegument and an outer lipid envelope (Figure 3). The genome of CMV is a double stranded DNA of about 236 kb in size and has two segments - unique long (UL) and unique short (US) segment. These segments are flanked on their ends by terminal repeat and inverted repeat sequences. The expression of human CMV occurs in a coordinated fashion and comprises the expression of following three groups of genes - immediate early, early and late genes. Immediate early genes encodes mostly regulatory proteins, early genes code for proteins responsible for viral replication and late genes encode for proteins involved in the final assembly of the virion[4].
Virus gains entry to the host cell by fusion of the virus envelope with the host cell membrane or through phagocytosis. CMV like other herpes viruses produces two different kinds of glycoproteins gB and gH/gL which are needed for entry of CMV into human cells. Glycoprotein gB helps in interaction of CMV and the intended cell of entry, whereas glycoprotein gH/gL helps in actual cell entry of CMV. Glycoprotein gH/gL either binds with another glycoprotein gO or three proteins UL 128, UL 130 and UL 131 to facilitate the entry of CMV into cells[5]. The glycoprotein gH in envelope is highly pleomorphic, which is partially responsible for heterogeneity of CMV isolates, the concept of super infection with one strain upon another. This genetic pleomorphism also explains why immunity is not absolute, while also hindering the development of an effective vaccine against CMV.
After gaining entry into human cell, CMV elaborates several proteins, which help CMV evading the human immune system. It produces proteins to prevent cell apoptosis, interfere with interleukin production, inhibit natural killer cells, and block presentation of CMV to T cells. It also produces interleukins resembling human Interleukin 10, which can suppress the mononuclear cells[6]. A critical step involved in persistence of CMV in human cells is its ability to establish latency and reactivate. Recently it has been discovered that CMV can establish latency in CD34 positive myeloid progenitor cells. The key step in establishing CMV latency is to prevent the expression of viral immediate early gene, and hence preventing the cell from entering the lytic cycle. Viral major immediate early promoter (MIEP) is an important regulator, which controls the expression of viral immediate early gene. During the periods of viral latency MIEP becomes transcriptionally inactive, suppressing the expression of viral immediate early genes and hence establish latency[7].
CMV is a ubiquitous herpes virus and is prevalent worldwide. It can be transmitted intrauterine from mother to fetus during pregnancy or post-partum during breastfeeding. Besides in-utero transmission, CMV can also be acquired through person-to-person direct contact that can be sexually or by exposure to infected body fluids like urine and saliva. Transmission of CMV from these infected body fluids to human hands is an important source of CMV transmission and survival. It has been shown by Stowell et al[8,9] that CMV can survive on hands for about 15 min and on wet surfaces for as long as 6 h, hence remains a potential threat for transmission during this time. Viability of CMV on these surfaces for such a long time contributes significantly to its increased worldwide burden and seroprevalence among different populations. In a review of seroprevalence in women of reproductive age group in different countries across the world, Cannon et al[10] reported high seroprevalence in South America, Africa and Asia. CMV seroprevalence was as high as 90% or above in certain countries like India, Italy, Japan, Saudi Arabia and Spain. In a recent study evaluating seroprevalence of CMV in the United States based on the presence of antibodies, it was found that seroprevalence in the United States in the age group 6-49 years old during period 1999 to 2004 was 50.4%. Rates of CMV seropositivity in this study significantly correlated with various factors including female sex, older age, ethnic groups (more in non-Hispanic black and Mexican American), foreign birthplace and low socioeconomic status (low household income, lack of insurance, low household education, and high crowding index)[11]. According to Centers for Disease Control and Prevention estimates, about 30000 babies are born each year with congenital CMV infection[12]. In United States about 1%-4% of pregnant women acquire primary CMV infection. Transmission of CMV infection from the mother to the fetus is hugely impacted by the mode mother acquired her infection - primary versus non-primary. The transmission rate for a primarily infected mother is about 33 percent as compared to 1%-3% for a mother with reactivation (non-primary infection) of CMV[13].
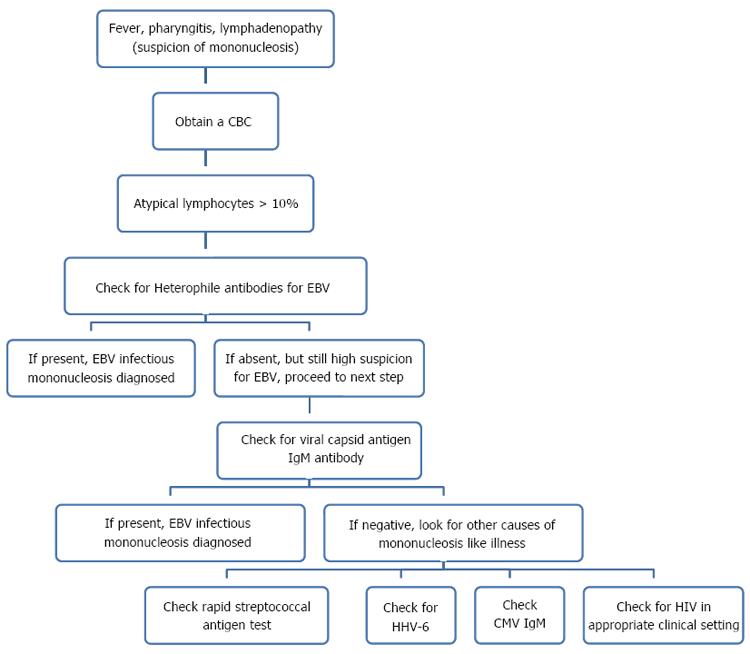
Acute infection is mostly asymptomatic in a young and healthy adult but in about 10% patients it can be associated with a transient mononucleosis like illness, which manifests as fever, lymphadenopathy and atypical lymphocytosis[14]. However, it is important to remember that various other organisms besides CMV can cause these symptoms; EBV being most common and various other pathogens like human immunodeficiency virus (HIV), human herpes virus 6 (HHV 6) and group A streptococcus. It is often not possible to differentiate these pathogens based on clinical presentation; though lymphadenopathy, splenomegaly and pharyngeal erythema are less extensive with CMV as compared EBV and elevated transaminases are more commonly seen with CMV[15]. Considering the difficulty in differentiation of these organisms on clinical grounds, it is of paramount importance to exclude EBV infection with heterophile antibody test before considering CMV. Although, no formal guidelines have been suggested till date on how to approach patients with mononucleosis, testing for heterophile antibodies, group A streptococcus rapid antigen test and CMV IgM antibody is advisable[16] (Figure 4). Further complicating the diagnostic problems, cases with coinfection of EBV and CMV have also been described. Although the effect of coinfection on the severity of disease has not been studied, longer duration of disease has been reported[17].
Once the infection is acquired, there is a lifelong latency coupled with the risk for intermittent reactivation. Reactivation from the latency has classically been associated with immunosuppression. It is believed that monocytes and bone marrow progenitor cells are the favored sites for human CMV latency. During the period of latency the live virus is sequestered in a non-replicative state. Persons with latent infection have no symptoms but do have antibodies against CMV.
CMV primary infection involves acquisition of virus by a seronegative host. It can also occur after a seropositive host acquires a new strain of CMV or reactivation of latent virus. Significant immunosuppression, which makes an individual susceptible to CMV reactivation, can occur in a transplant recipient receiving immunosuppressive therapy, or in clinical conditions associated with immunosuppression like malignancy or infection with human immunodeficiency virus. CMV reactivation, though more common in immunosuppressed individuals, can also be seen in 33% of critically ill immunocompetent patients, in whom it is associated with prolonged hospitalization and mortality[18]. A physician has to be more vigilant to be able to diagnose CMV reactivation in an immunocompetent individual where the diagnosis of CMV is not sought as promptly as in an immunocompromised individual.
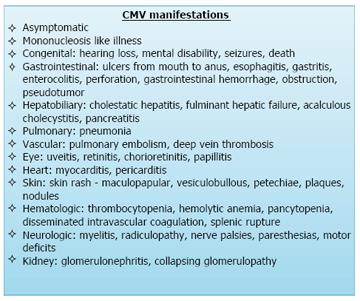
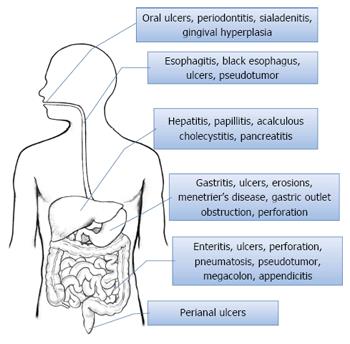
Acute manifestations of CMV disease in an immunocompetent patient, although less common as compared to an immunocompromised individual, can still manifest as a wide variety of disorders (Figure 5). CMV manifestations can infect different body systems including gastrointestinal tract, central nervous system, respiratory system, hematological system and others. Gastrointestinal tract is the most commonly involved system with CMV infection. Gastrointestinal CMV disease is an erosive or ulcerative process that can occur at any location in the gastrointestinal tract (Figure 6), from mouth to rectum, with colon being the most common and the small bowel being relatively rarely affected[1].
Oral cavity: Involvement of the oral cavity by CMV although not common, has been associated with periodontitis[19], severe sialadenitis[20], gingival hyperplasia[21] and oral ulcers[22]. Oral ulcers can involve any part of oral cavity - hard or soft palate, upper or lower lip, gingiva, buccal mucosa and tongue[23]. Patient with CMV related oral ulcerations often present with painful oral ulcers.
Esophagus: In esophagus, CMV manifestations can vary from esophagitis to severe ulcerations and acute esophageal necrosis that is also known as black esophagus[24]. Rarely presentation with pseudotumor formation has also been reported[25]. Patients can complain of odynophagia, dysphagia, substernal chest pain and hematemesis in varying combinations depending upon the severity of their esophageal involvement. Wilcox et al[26] in a review of 33 patients with CMV esophagitis reported no pathognomonic features related to CMV on endoscopy, however, in general CMV associated esophageal ulcerations were more commonly located in mid to distal esophagus, multiple in number, often shallow and more than 1 cm in size.
Stomach: Gastric involvement by CMV commonly manifests as erosions or ulcers. Ulcers can be solitary or multiple. Gastritis from CMV can rarely progress in severity and manifest as hemorrhagic necrotic gastritis[27]. Patients can present with symptoms of abdominal pain, nausea, vomiting and hematemesis. Interestingly, postural epigastric pain relieved in supine position associated with CMV gastritis has been described in literature[28]. Infrequently, CMV in stomach has been associated to Menetrier’s disease[29], gastric outlet obstruction[30] and gastric perforation[31].
Small and large intestine: Enterocolitis is the most common manifestation of CMV infection. Diarrhea is the most dominant symptom of patients with CMV enterocolitis[32], though abdominal pain, fever and hematochezia can also be present. Enterocolitis may lead to life threatening complications like fatal gastrointestinal hemorrhage[33], toxic megacolon, acute appendicitis, colonic stricture[34], intestinal obstruction[35], intestinal perforation and peritonitis. Perforation of the gastrointestinal tract is the most lethal complication and is commonly seen between ileum and splenic flexure, with colon being the most commonly involved site followed by terminal ileum[36]. Perforation of jejunum has also been reported in literature, however perforation of a duodenum affected with CMV as in our case has never been seen before. Patients requiring endoscopic work up in a suspected case of CMV colitis, should get a complete colonoscopy rather than a flexible sigmoidoscopy as about 39 percent patients may only have right sided CMV colitis[37]. CMV colonic ulcers are mostly multiple and often larger than 3 cm as reported in a case series by Lin et al[38]. Differentiating CMV enterocolitis from other infectious or non-infectious enterocolitis in the absence of histopathological confirmation of CMV can be challenging. However, mild colonic mural thickening (defined as less than 8 mm) with concomitant small bowel involvement has been suggested as a differentiating feature by Chae et al[39]. These researchers also demonstrated colonic wall thickening as the most common finding, followed by small bowel wall thickening in 30% patients. Interestingly, it was also noticed that all the patients with CMV small bowel involvement had concentric mural thickening with single halo enhancement pattern, however the appearance of involved colon was variable. Endoscopic findings of CMV enterocolitis, though not pathognomonic, are erythematous, edematous or hemorrhagic mucosa with geographic or punched out ulcers[39]. Benign and often incidental finding of pneumatosis intestinalis noticed on endoscopy or imaging study has also been attributed to CMV infection[40]. Like other parts of gastrointestinal tract, formation of pseudotumors in intestines has also been reported repeatedly in literature[41,42].
Perianal: Involvement of dermis is not a common phenomenon, excluding severely immunocompromised individuals where CMV can manifest as painful chronic perianal ulcers. Lesions other than ulcers, namely maculopapular eruptions, vesiculobullous lesions, petechiae, plaques and nodules have also been described[43].
Liver: Features of CMV infection of liver can range from minimally elevated bilirubin and/or liver enzymes to fulminant hepatic failure[44]. First case of liver involvement by CMV was described by Lamb and Stern[45], and since then numerous cases have been brought into light. Cholestatic hepatitis is the most common presentation. Immunocompetent patients with CMV infection of liver may remain asymptomatic, however immunosuppressed individuals suffer severe morbidity and mortality from this infection. In post transplant immunosuppressed patients; CMV besides causing damage to different organs of the body poses a threat of acute and chronic graft rejection. Two most important risk factors identified for graft rejection are transplant from a seropositive donor to seronegative recipient and intensity of immunosuppression, which is most intense in first three months. Two different strategies namely prophylactic and preemptive therapy have been utilized by different transplant centers to prevent CMV infection after liver transplant[46].
Biliary and pancreatic: Pancreatobiliary involvement of CMV infection is very rare. CMV leading to complications like duodenal papillitis, acute pancreatitis[47] and acalculous cholecystitis[48] have been described. Acalculous cholecystitis caused by CMV is undifferentiable from other varied causes of acalculous cholecystitis and presents as epigastric pain associated with nausea or vomiting. Fatal and extremely uncommon complication like perforation of gall bladder has also been reported in medical literature.
Pathogenesis: The pathogenesis of gastrointestinal lesions caused by CMV is a complex process. CMV affects columnar epithelial cells, endothelial cells and myofibroblasts within the lamina propria causing mucosal inflammation and ulceration. Vascular endothelial involvement in submucosa is strongly implicated in the pathogenesis of ulceration causing subsequent ischemic mucosal necrosis[49]. Endothelial cell invasion leads to enlarged swollen cells with cytomegalic viral inclusions, luminal blockage, and fibrin thrombi, resulting in small vessel vasculitis and damage to the tissues supplied by the affected vessels or sometimes the ulcer eroding the vessel surface causing sudden GI bleeding.
Diagnosis: Diagnosis of CMV gastrointestinal infection often needs an endoscopic work up. Ulcerations, erosions, and mucosal hemorrhage are the primary endoscopic findings. Diagnosis of gastrointestinal CMV disease is confirmed by demonstration of erosive process in gut wall with positive mucosal biopsy that shows the presence of CMV. CMV can be demonstrated by histopathologic (Owl’s eye inclusion bodies) or other techniques including staining for CMV antigen, DNA assay by real time polymerase chain reaction and culture[50]. These quantitative assays may be particularly useful for early detection of CMV disease and for the monitoring of therapy.
Management: Management of patient’s with CMV infection should include supportive measures, nutritional support, decreased immunosuppression if possible, and empiric treatment with Ganciclovir, Valganciclovir[51] or Foscarnet. Although CMV infection is systemic and medical therapy effective, surgery may be indicated for life threatening gastrointestinal hemorrhage and/or perforation. However in case of a massive diverticular bleed, despite immediate surgery and antiviral therapy, mortality remains high due to high operative mortality and increased postoperative complications.
In conclusion, CMV, a worldwide prevalent herpes virus, is acquired through intrauterine infection or by contact with infected body fluids. Infected individual may have no symptoms or present as mononucleosis like illness. CMV can establish as latent infection that can lead to reactivation with immunosuppression. It can affect almost any organ system with gastrointestinal tract involvement being most common. In gastrointestinal tract, besides causing mucosal inflammation, rarely gastrointestinal perforation or hemorrhage may occur. High clinical suspicion is needed for timely diagnosis, as clinical signs are usually sparse for this fatal yet treatable CMV infection.
A 77-year-old Hispanic man was hospitalized in the intensive care unit for acute respiratory distress. The patient had been in his usual state of health until approximately 3 d prior to admission, when he developed gradually worsening shortness of breath, fever and productive cough. Patient denied paroxysmal nocturnal dyspnea, orthopnea, lower extremity swelling, chest pain and palpitations.
Acute respiratory distress.
Serological tests for hepatitis B and C were negative.
Computed tomography chest with contrast ruled out pulmonary embolism but showed bilateral infiltrates.
Post mortem examination showed large well-circumscribed duodenal diverticulum with ulcerated mucosa adjacent to eroded artery in posterior wall of first part of duodenum. Histopathological examination of the duodenal diverticulum revealed owl eyed inclusion bodies, characteristic for cytomegalovirus (CMV).
Initial management included noninvasive positive pressure ventilation, intravenous antibiotics,intravenous steroid and bronchodilator nebulization with an impression of chronic obstructive pulmonary disease exacerbation. Subsequently, Antibiotics were changed to ceftazidime and trimethroprim/sulfamethoxazole.
High clinical suspicion is needed for timely diagnosis, as clinical signs are usually sparse for this fatal yet treatable CMV infection.
Excellent clinical description of the case report and excellent overview of this interesting and very important clinical condition.
Manuscript source: Unsolicited manuscript
Specialty type: Gastroenterology and hepatology
Country of origin: United States
Peer-review report classification
Grade A (Excellent): 0
Grade B (Very good): B
Grade C (Good): C
Grade D (Fair): 0
Grade E (Poor): 0
P- Reviewer: Antonini F, Rodrigo L S- Editor: Qi Y L- Editor: A E- Editor: Wang CH
| 1. | Rafailidis PI, Mourtzoukou EG, Varbobitis IC, Falagas ME. Severe cytomegalovirus infection in apparently immunocompetent patients: a systematic review. Virol J. 2008;5:47. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 332] [Cited by in F6Publishing: 344] [Article Influence: 21.5] [Reference Citation Analysis (0)] |
| 2. | Ho M. The history of cytomegalovirus and its diseases. Med Microbiol Immunol. 2008;197:65-73. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 133] [Cited by in F6Publishing: 121] [Article Influence: 7.6] [Reference Citation Analysis (0)] |
| 3. | Vonglahn WC, Pappenheimer AM. Intranuclear Inclusions in Visceral Disease. Am J Pathol. 1925;1:445-466.3. [PubMed] [Cited in This Article: ] |
| 4. | Ma Y, Wang N, Li M, Gao S, Wang L, Zheng B, Qi Y, Ruan Q. Human CMV transcripts: an overview. Future Microbiol. 2012;7:577-593. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 18] [Cited by in F6Publishing: 19] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 5. | Vanarsdall AL, Johnson DC. Human cytomegalovirus entry into cells. Curr Opin Virol. 2012;2:37-42. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 110] [Cited by in F6Publishing: 114] [Article Influence: 9.5] [Reference Citation Analysis (0)] |
| 6. | Boeckh M, Geballe AP. Cytomegalovirus: pathogen, paradigm, and puzzle. J Clin Invest. 2011;121:1673-1680. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 267] [Cited by in F6Publishing: 243] [Article Influence: 18.7] [Reference Citation Analysis (0)] |
| 7. | Sinclair J, Sissons P. Latency and reactivation of human cytomegalovirus. J Gen Virol. 2006;87:1763-1779. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 287] [Cited by in F6Publishing: 282] [Article Influence: 15.7] [Reference Citation Analysis (0)] |
| 8. | Stowell JD, Forlin-Passoni D, Radford K, Bate SL, Dollard SC, Bialek SR, Cannon MJ, Schmid DS. Cytomegalovirus survival and transferability and the effectiveness of common hand-washing agents against cytomegalovirus on live human hands. Appl Environ Microbiol. 2014;80:455-461. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 18] [Cited by in F6Publishing: 19] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 9. | Stowell JD, Forlin-Passoni D, Din E, Radford K, Brown D, White A, Bate SL, Dollard SC, Bialek SR, Cannon MJ. Cytomegalovirus survival on common environmental surfaces: opportunities for viral transmission. J Infect Dis. 2012;205:211-214. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 32] [Cited by in F6Publishing: 33] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 10. | Cannon MJ, Schmid DS, Hyde TB. Review of cytomegalovirus seroprevalence and demographic characteristics associated with infection. Rev Med Virol. 2010;20:202-213. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 863] [Cited by in F6Publishing: 908] [Article Influence: 64.9] [Reference Citation Analysis (0)] |
| 11. | Bate SL, Dollard SC, Cannon MJ. Cytomegalovirus seroprevalence in the United States: the national health and nutrition examination surveys, 1988-2004. Clin Infect Dis. 2010;50:1439-1447. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 516] [Cited by in F6Publishing: 446] [Article Influence: 31.9] [Reference Citation Analysis (0)] |
| 12. | Congenital CMV Infection Trends and Statistics. Cytomegalovirus. Available from: http://www.cdc.gov/cmv/trends-stats.html. [Cited in This Article: ] |
| 13. | Swanson EC, Schleiss MR. Congenital cytomegalovirus infection: new prospects for prevention and therapy. Pediatr Clin North Am. 2013;60:335-349. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 138] [Cited by in F6Publishing: 145] [Article Influence: 13.2] [Reference Citation Analysis (0)] |
| 14. | Horwitz CA, Henle W, Henle G, Snover D, Rudnick H, Balfour HH, Mazur MH, Watson R, Schwartz B, Muller N. Clinical and laboratory evaluation of cytomegalovirus-induced mononucleosis in previously healthy individuals. Report of 82 cases. Medicine (Baltimore). 1986;65:124-134. [PubMed] [Cited in This Article: ] |
| 15. | Hurt C, Tammaro D. Diagnostic evaluation of mononucleosis-like illnesses. Am J Med. 2007;120:911.e1-911.e8. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 127] [Cited by in F6Publishing: 135] [Article Influence: 7.9] [Reference Citation Analysis (0)] |
| 16. | Luzuriaga K, Sullivan JL. Infectious mononucleosis. N Engl J Med. 2010;362:1993-2000. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 391] [Cited by in F6Publishing: 325] [Article Influence: 23.2] [Reference Citation Analysis (0)] |
| 17. | Ito Y, Shibata-Watanabe Y, Kawada J, Maruyama K, Yagasaki H, Kojima S, Kimura H. Cytomegalovirus and Epstein-Barr virus coinfection in three toddlers with prolonged illnesses. J Med Virol. 2009;81:1399-1402. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 14] [Cited by in F6Publishing: 16] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 18. | Limaye AP, Kirby KA, Rubenfeld GD, Leisenring WM, Bulger EM, Neff MJ, Gibran NS, Huang ML, Santo Hayes TK, Corey L. Cytomegalovirus reactivation in critically ill immunocompetent patients. JAMA. 2008;300:413-422. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 195] [Cited by in F6Publishing: 304] [Article Influence: 19.0] [Reference Citation Analysis (0)] |
| 19. | Chalabi M, Moghim S, Mogharehabed A, Najafi F, Rezaie F. EBV and CMV in chronic periodontitis: a prevalence study. Arch Virol. 2008;153:1917-1919. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 25] [Cited by in F6Publishing: 24] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 20. | Guntinas-Lichius O, Wagner M, Krueger GR, Streppel M, Voessing M, Stennert E. Severe acute cytomegalovirus sialadenitis in an immunocompetent adult: case report. Clin Infect Dis. 1996;22:1117-1118. [PubMed] [Cited in This Article: ] |
| 21. | Epstein JB, Sherlock CH, Wolber RA. Oral manifestations of cytomegalovirus infection. Oral Surg Oral Med Oral Pathol. 1993;75:443-451. [PubMed] [Cited in This Article: ] |
| 22. | Leimola-Virtanen R, Happonen RP, Syrjänen S. Cytomegalovirus (CMV) and Helicobacter pylori (HP) found in oral mucosal ulcers. J Oral Pathol Med. 1995;24:14-17. [PubMed] [Cited in This Article: ] |
| 23. | Jones AC, Freedman PD, Phelan JA, Baughman RA, Kerpel SM. Cytomegalovirus infections of the oral cavity. A report of six cases and review of the literature. Oral Surg Oral Med Oral Pathol. 1993;75:76-85. [PubMed] [Cited in This Article: ] |
| 24. | Trappe R, Pohl H, Forberger A, Schindler R, Reinke P. Acute esophageal necrosis (black esophagus) in the renal transplant recipient: manifestation of primary cytomegalovirus infection. Transpl Infect Dis. 2007;9:42-45. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 20] [Cited by in F6Publishing: 26] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 25. | Laguna F, Garcia-Samaniego J, Alonso MJ, Alvarez I, Gonzalez-Lahoz JM. Pseudotumoral appearance of cytomegalovirus esophagitis and gastritis in AIDS patients. Am J Gastroenterol. 1993;88:1108-1111. [PubMed] [Cited in This Article: ] |
| 26. | Wilcox CM, Straub RF, Schwartz DA. Prospective endoscopic characterization of cytomegalovirus esophagitis in AIDS. Gastrointest Endosc. 1994;40:481-484. [PubMed] [Cited in This Article: ] |
| 27. | Ruiz AR, Borum ML. Cytomegalovirus hemorrhagic gastritis. AIDS Patient Care STDS. 2001;15:1-5. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 10] [Cited by in F6Publishing: 11] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 28. | Giladi M, Lembo A, Johnson BL. Postural epigastric pain: a unique symptom of primary cytomegalovirus gastritis? Infection. 1998;26:234-235. [PubMed] [Cited in This Article: ] |
| 29. | Megged O, Schlesinger Y. Cytomegalovirus-associated protein-losing gastropathy in childhood. Eur J Pediatr. 2008;167:1217-1220. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 44] [Cited by in F6Publishing: 73] [Article Influence: 4.6] [Reference Citation Analysis (0)] |
| 30. | Mohammed AA, Benmousa A, Almeghaiseeb I, Alkarawi M. Gastric outlet obstruction. Hepatogastroenterology. 2007;54:2415-2420. [PubMed] [Cited in This Article: ] |
| 31. | Mégarbane B, Résière D, Ferrand J, Raskine L, Vahedi K, Baud FJ. Difficulties in assessing cytomegalovirus-associated gastric perforation in an HIV-infected patient. BMC Infect Dis. 2005;5:28. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 6] [Cited by in F6Publishing: 6] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 32. | June L, Chin N, Chatterjee D. Cytomegalovirus colitis presenting as massive lower gastrointestinal bleeding in an immunocompetent patient. Indian J Surg. 2008;70:28-31. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 5] [Cited by in F6Publishing: 6] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 33. | Le ST, Lee SS, Prideaux L, Block AA, Moore GT. Primary cytomegalovirus ileitis complicated by massive gastrointestinal haemorrhage in a patient with steroid refractory Crohn’s disease. Intern Med J. 2010;40:788-791. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 4] [Cited by in F6Publishing: 4] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 34. | Ekema G, Pedersini P, Milianti S, Ubertazzi M, Minoli D, Manciana A. Colonic stricture mimicking Hirschsprung’s disease: a localized cytomegalovirus infection. J Pediatr Surg. 2006;41:850-852. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 22] [Cited by in F6Publishing: 23] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 35. | Shah SK, Kreiner LA, Walker PA, Klein KL, Bajwa KS, Robinson EK, Millas SG, Souchon EA, Wray CJ. Cytomegalovirus enteritis manifesting as recurrent bowel obstruction and jejunal perforation in patient with acquired immunodeficiency syndrome: rare report of survival and review of the literature. Surg Infect (Larchmt). 2012;13:121-124. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 6] [Cited by in F6Publishing: 6] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 36. | Kram HB, Shoemaker WC. Intestinal perforation due to cytomegalovirus infection in patients with AIDS. Dis Colon Rectum. 1990;33:1037-1040. [PubMed] [Cited in This Article: ] |
| 37. | Dieterich DT, Rahmin M. Cytomegalovirus colitis in AIDS: presentation in 44 patients and a review of the literature. J Acquir Immune Defic Syndr. 1991;4 Suppl 1:S29-S35. [PubMed] [Cited in This Article: ] |
| 38. | Lin WR, Su MY, Hsu CM, Ho YP, Ngan KW, Chiu CT, Chen PC. Clinical and endoscopic features for alimentary tract cytomegalovirus disease: report of 20 cases with gastrointestinal cytomegalovirus disease. Chang Gung Med J. 2005;28:476-484. [PubMed] [Cited in This Article: ] |
| 39. | Chae EY, Lee SS, Chung JW, Kim HJ, Park SH, Kim AY, Ha HK. Cytomegalovirus enterocolitis in apparently immunocompetent hosts: evaluation of the radiologic findings and clinical features. J Comput Assist Tomogr. 2010;34:892-898. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 6] [Cited by in F6Publishing: 6] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 40. | Mannes GP, de Boer WJ, van der Jagt EJ, Meinesz AF, Meuzelaar JJ, van der Bij W. Pneumatosis intestinalis and active cytomegaloviral infection after lung transplantation. Groningen Lung Transplant Group. Chest. 1994;105:929-930. [PubMed] [Cited in This Article: ] |
| 41. | Swansiger B, Orchard JL. A colonic mass lesion due to cytomegalovirus in an immunocompromised patient. J Clin Gastroenterol. 1996;22:41-44. [PubMed] [Cited in This Article: ] |
| 42. | Tan CB, Vardaros M, Prasad A, Rashid S, Dahl K, Moise D, Gebre W, Rizvon K, Mustacchia P. Cytomegalovirus infection of the colon presenting as a mass-like lesion. Case Rep Gastroenterol. 2012;6:266-272. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 5] [Cited by in F6Publishing: 6] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 43. | Kaisar MO, Kirwan RM, Strutton GM, Hawley CM, Mudge DW, Campbell SB, Johnson DW, Isbel NM. Cutaneous manifestations of cytomegalovirus disease in renal transplant recipients: a case series. Transpl Infect Dis. 2008;10:209-213. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 26] [Cited by in F6Publishing: 27] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 44. | Yu YD, Park GC, Park PJ, Choi YI, Hwang S, Song GW, Jung DH, Ahn CS, Kim KH, Moon DB. Cytomegalovirus infection-associated fulminant hepatitis in an immunocompetent adult requiring emergency living-donor liver transplantation: report of a case. Surg Today. 2013;43:424-428. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 20] [Cited by in F6Publishing: 23] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 45. | Lamb SG, Stern H. Cytomegalovirus mononucleosis with jaundice as presenting sign. Lancet. 1966;2:1003-1006. [PubMed] [Cited in This Article: ] |
| 46. | Lautenschlager I. CMV infection, diagnosis and antiviral strategies after liver transplantation. Transpl Int. 2009;22:1031-1040. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 46] [Cited by in F6Publishing: 47] [Article Influence: 3.1] [Reference Citation Analysis (0)] |
| 47. | Kamalkumar BS, Agarwal SK, Garg P, Dinda A, Tiwari SC. Acute pancreatitis with CMV papillitis and cholangiopathy in a renal transplant recipient. Clin Exp Nephrol. 2009;13:389-391. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 8] [Cited by in F6Publishing: 10] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 48. | Riediger C, Beimler J, Weitz J, Zeier M, Sauer P. Cytomegalovirus infection of the major duodenal papilla in a renal allograft recipient with severe biliary obstruction and acalculous cholecystitis. Transpl Infect Dis. 2013;15:E129-E133. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 6] [Cited by in F6Publishing: 7] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 49. | Buckner FS, Pomeroy C. Cytomegalovirus disease of the gastrointestinal tract in patients without AIDS. Clin Infect Dis. 1993;17:644-656. [PubMed] [Cited in This Article: ] |
| 50. | You DM, Johnson MD. Cytomegalovirus infection and the gastrointestinal tract. Curr Gastroenterol Rep. 2012;14:334-342. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 55] [Cited by in F6Publishing: 56] [Article Influence: 4.7] [Reference Citation Analysis (0)] |
| 51. | Vaziri S, Pezhman Z, Sayyad B, Mansouri F, Janbakhsh A, Afsharian M, Najafi F. Efficacy of valganciclovir and ganciclovir for cytomegalovirus disease in solid organ transplants: A meta-analysis. J Res Med Sci. 2014;19:1185-1192. [PubMed] [Cited in This Article: ] |