Published online Dec 28, 2016. doi: 10.3748/wjg.v22.i48.10557
Peer-review started: June 29, 2016
First decision: October 11, 2016
Revised: October 20, 2016
Accepted: October 27, 2016
Article in press: October 27, 2016
Published online: December 28, 2016
To assess the burden of norovirus (NoV) and to determine the diversity of circulating strains among hospitalized children in Lebanon.
Stool samples were collected from children presenting with acute gastroenteritis to six major hospitals in Lebanon. A total of 739 eligible stool samples, testing negative for diarrhea caused by rotavirus as a possible viral pathogen, were collected between January 2011 and June 2013. A standardized questionnaire including demographic, epidemiological and clinical observations was used at the time of hospitalization of children presenting with diarrhea. Viral RNA was extracted from stool samples followed by reverse transcription polymerase chain reaction and nucleotide sequencing of a fragment of the viral protein 1 capsid gene. Multiple sequence alignments were carried out and phylogenetic trees were constructed using the MEGA 6 software.
Overall, 11.2% of stool samples collected from children aged < 5 years tested positive for NoV genogroups I (GI) and II (GII). GII accounted for 10.6% of the gastroenteritis cases with only five samples being positive for GI (0.7%). The majority of hospitalized children showed symptoms of diarrhea, dehydration, vomiting and fever. Upon sequencing of positive samples and based on their clustering in the phylogenetic tree, 4/5 of GI gastroenteritis cases were designated GI.3 and one case as GI.4. GII.4 was predominantly detected in stool of our study participants (68%). We report a JB-15/KOR/2008 GII.4 Apeldoorn 2008-like variant strain circulating in 2011; this strain was replaced between 2012 and 2013 by a variant sharing homology with the Sydney/NSW0514/2012/AUS GII.4 Sydney 2012 and Sydney 2012/FRA GII.4 strains. We also report the co-circulation of non-GII.4 genotypes among hospitalized children. Our data show that NoV gastroenteritis can occur throughout the year with the highest number of cases detected during the hot months.
The majority of NoV-associated viral gastroenteritis cases among our participants are attributable to GII.4, which is compatible with results reported worldwide.
Core tip: We report the results of a large study of norovirus (NoV)-associated gastroenteritis among children aged < 5 years in Lebanon. The majority of viral gastroenteritis cases were attributable to NoV GII.4, which is compatible with results reported worldwide. Our data support a peak incidence in July, while reports from other countries show peaks during the cold months. We report NoV A JB-15/KOR/2008 GII.4 Apeldoorn 2008-like variant strain circulating in 2011. This strain was replaced between 2012 and 2013 by a variant sharing homology with the Sydney/NSW0514/2012/AUS GII.4 Sydney 2012 and Sydney 2012/FRA GII.4 strains.
- Citation: Melhem NM, Zaraket H, Kreidieh K, Ali Z, Hammadi M, Ghanem S, Hajar F, Haidar A, Inati A, Rajab M, Fakhouri H, Ghanem B, Baasiri G, Dbaibo G. Clinical and epidemiological characteristics of norovirus gastroenteritis among hospitalized children in Lebanon. World J Gastroenterol 2016; 22(48): 10557-10565
- URL: https://www.wjgnet.com/1007-9327/full/v22/i48/10557.htm
- DOI: https://dx.doi.org/10.3748/wjg.v22.i48.10557
Gastroenteritis caused by norovirus (NoVs) has been recently reported to be the second most common cause of acute viral gastroenteritis worldwide following rotavirus and a major cause of foodborne illness[1,2]. NoV is the leading cause of acute gastroenteritis across all age groups seeking medical care in emergency departments, outpatient clinics, and the community[1]. Recent reviews of the literature on community, outpatient and hospital-based studies in developing and developed countries report that NoV gastroenteritis accounted for 10%-15% of severe cases in children aged < 5 years and 9%-15% of cases of mild to moderate diarrhea among individuals of all ages[3,4]. Fecal-oral spread is the primary mode of NoV transmission. The average incubation period is 24-48 h. The symptoms include vomiting (≥ 50% of cases), diarrhea, nausea, abdominal cramps, malaise and low-grade fever. Illness usually resolves in 12-72 h; however, it may last longer in young children, elderly people, and hospitalized and immunocompromised individuals. Several factors contribute to the high communicability of NoV, including, most importantly, the low infectious dose of the virus (18-100 particles); the high levels of virus shedding (> 109 particles/mL of feces during the first days following infection) known to precede illness and to be prolonged in immunosuppressed persons; stability of the virus at 0-60 °C; and finally, the high rate of mutation and recombination leading to antigenic diversity[5-7]. While 75% of NoV cases have been reported during the cooler months, geographic variability and annual fluctuations have also been described[8].
NoVs are non-enveloped, polyadenylated, single stranded, positive-sense RNA viruses of the family Caliciviridae. The RNA genome of NoVs is composed of three large open reading frames designated as ORF-1, ORF-2 and ORF-3. ORF-1 encodes six non-structural proteins including the protease and the RNA-dependent RNA-polymerase (RdRp). ORF-2 and ORF-3 encode the structural viral components viral protein 1 (VP1) (major capsid protein) and VP2 (minor capsid protein), respectively. Based on the amino-acid sequence of VP1, NoVs are divided into six genogroups (GI-GVI). GI, GII and GIV are known to infect humans[9,10]. Genogroups are further subdivided into genotypes based on the RdRp sequence or capsid sequence. At the genomic level, strains of the same genogroups are 51%-56% similar, whereas genotypes have 69%-87% similarity[11,12]. At least eight and 21 genotypes belong to GI and GII, respectively[1]. The genogroup II, genotype 4 NoVs, designated GII.4, are responsible for the majority of NoV outbreaks in the United States, Australia and many European countries[13,14]. GII.4 NoVs are continuously changing and viral variants emerge every couple of years and every 2-7 years as a result of genetic drift; an observation compatible with the immune escape mechanism observed with influenza A virus[15-19]. Globally and during the past decade, GII.3 and GII.6 were reported as the second and third most predominant genotypes after GII.4, respectively[13].
To the best of our knowledge, there have been no large studies conducted in Lebanon on NoV and its association with acute gastroenteritis. The aim of this study was to determine the prevalence of NoV gastroenteritis as well as the genotypic characterization of the virus among hospitalized children aged < 5 years.
The study was conducted in accordance with the ethical guidelines of the Helsinki Declaration and after approval of the Institution Review Board of the American University of Beirut. Written informed consent was obtained from the legal guardians of hospitalized children, and consequently, stool samples and medical data were collected. A standardized questionnaire including demographic, epidemiological and clinical observations was used at the time of hospitalization of children presenting with diarrhea. Stool samples were collected from children presenting with acute gastroenteritis to six major hospitals in Lebanon. A total of 739 eligible stool samples, testing negative for diarrhea caused by rotavirus as a possible viral pathogen, were collected over a 2-year period (January 2011 to June 2013).
Stool specimens (0.5-1.0 mL) were suspended in 5 mL 0.89% NaCl. The fecal suspension was centrifuged at 4000 ×g; following which, the supernatant was filtered and 140 μL of the filtrate was used for viral RNA extraction. QIAamp Viral RNA Mini Kit (Qiagen, Hilden, Germany) was used for viral RNA extraction. Viral RNA was stored at -20 °C.
Reverse transcription polymerase chain reaction (RT-PCR) was performed using genogroup-specific primers as previously described[20-22]. RT-PCR targeted the 5’ end of the capsid region in ORF2 using: G1-SKF (Forward CTG CCC GAA TTY GTA AATGA) and G1-SKR (Reverse CCA ACC CAR CCA TTR TACA and primers GoG2F (Forward CAR GAR BCN ATG TTY AGR TGG ATGAG) and G2-SKR (Reverse CCR CCN GCA TRH CCR TTR TACAT) for amplifying 330- and 387-bp PCR products of GI and GII genogroups, respectively. Qiagen OneStep RT-PCR Kit was used under the following conditions: 42 °C for 30 min; initial PCR activation step at 95 °C for 15 min; denaturation at 94 °C for 30 s, annealing at 52-54 °C for 30 s, extension at 72 °C for 45 s (30 cycles); and final extension at 72 °C for 7 min. Synthetic Norovirus G1 (I) RNA (ATCC VR3199SD) and Synthetic Norovirus G2 (II) RNA (ATCC VR3200SD) were used as positive controls. The PCR products were analyzed by gel electrophoresis and stored at -20 °C until analysis. Nucleotide sequencing of NoV-positive samples was performed by Macrogen (Seoul, South Korea) using the PCR primers. A total of 19 full-length human NoV capsid protein sequences were downloaded from GenBank and used as reference strains. These included six GI and 16 GII with the following accession numbers: AAS86780.1 (GI), ACN32270.1 (GI.1), ACU56258.1 (GI.2), ACX33982.1 (GI.3), ACV41096.1 (GI.4), ADB54834.1(GI.8), aAIO11150.1 (GII), ABC96332.1 (GII), AFA55174.1 (GII.1), BAG68716.1 (GII.2), ADK23787.1 (GII.3), AEG79292.1 (GII.4), ABL74397.1 (GII.4), ABL74391.1 (GII.4), AGT95930.1 (GII.4), KM245069.1 (GII.4 Yerseke/2006a), KF361437.1 (GII.4 Minerva/2006b), KP762437.1 (GII.4 Den Haag 2006b), ADE28721.1 (GII.6), ACX85810.1 (GII.7), ADZ24003.1 (GII.12), and ACX81355.1 (GII.14). Multiple sequence alignments were carried out using CLUSTALL or BioEdit out and phylogenetic trees were constructed using the MEGA 6 software. The phylogenetic tree was generated using the neighbor-joining method validated by 1000 bootstrap replicates.
The partial nucleotide sequences determined in this study were deposited in GenBank with the following accession numbers: GI KU950315-KU950319 and GII KU963412-KU963487.
Data was analyzed using SPSS version 22. For comparisons of demographic and clinical symptoms, χ2 analysis and Pearson χ2 test were used.
Seven hundred and thirty-nine eligible rotavirus-negative stool samples were assayed for NoV by RT-PCR during January 2011 to June 2013. Stool samples were collected from children aged < 5 years presenting to six hospitals in Lebanon due to acute gastroenteritis. Tables 1 and 2 summarize the demographic and clinical characteristics of our study participants. Overall, 11.26% (n = 83/739) of the samples tested positive for NoV (Table 1). The majority of cases were NoV genogroup GII (n = 78/83) (Table 2), with a total incidence rate of 10.6%, while only five samples tested positive for NoV genogroup GI, with a total incidence rate of 0.7%. We did not have mixed infections with NoV GI and GII among our study participants. Males accounted for 55.9% (413/739) of hospitalized children and females for 44% (326/739) while 11.4% of the former and 11% of the latter were NoV positive (Table 1). Gender was not significantly associated with NoV infection (P = 0.887). The mean age of the study participants testing positive for NoV and presenting with gastroenteritis symptoms upon admission was 16.2 ± 9.5 mo. Fifteen point five percent of samples testing positive for NoV and presenting to hospitals with acute gastroenteritis symptoms were children aged 12-23 mo (35/376), followed by children aged 24-35 mo (12.7%; 10/79) (Table 1). Our results showed, however, that there was no association between age and NoV infection among our study participants (P = 0.729).
| n | NoV positive | NoV negative | |
| Participants | 739 | 83 (11.2) | 656 (88.8) |
| Gender | |||
| Male | 413 | 47 (11.4) | 366 (88.6) |
| Female | 326 | 36 (11.0) | 290 (89.0) |
| Age group (mo) | |||
| 0-11 | 376 | 34 (9.0) | 342 (91.0) |
| 12-23 | 226 | 35 (15.5) | 191 (84.5) |
| 24-35 | 79 | 10 (12.7) | 69 (87.3) |
| 36-47 | 30 | 3 (10.0) | 27 (90.0) |
| 48-59 | 27 | 1 (3.7) | 26 (96.3) |
| Region | |||
| Beirut | 217 | 20 (9.2) | 197 (90.8) |
| North Lebanon | 315 | 35 (11.1) | 280 (88.9) |
| South Lebanon | 207 | 28 (13.5) | 179 (86.5) |
| NoV positive | GI | GII | |
| Fever | |||
| Yes | 56 (67.5) | 5 (100.0) | 51 (65.4) |
| No | 27 (32.5) | 0 (0.0) | 27 (34.6) |
| Vomiting | |||
| Yes | 63 (75.9) | 3 (60.0) | 60 (76.9) |
| No | 20 (24.1) | 2 (40.0) | 18 (23.1) |
| Diarrhea | |||
| Yes | 79 (95.2) | 5 (100.0) | 74 (94.9) |
| No | 4 (4.8) | 0 (0.0) | 4 (5.1) |
| Assessed dehydration | |||
| Severe | 10 (12.0) | 1 (20.0) | 9 (11.5) |
| Mild to moderate | 64 (77.1) | 3 (60.0) | 61 (78.2) |
| No dehydration | 9 (10.8) | 1 (20.0) | 8 (10.3) |
| Vesikari score | |||
| Severe | 76 (91.6) | 4 (80.0) | 72 (92.3) |
| Mild to moderate | 7 (8.4) | 1 (20.0) | 6 (7.7) |
As expected, the majority of our study participants testing positive for NoV had symptoms of diarrhea (95%), dehydration (90%), vomiting (76%) and fever (67.5%). The Vesikari Clinical Severity Scoring System was used to assess the severity of acute gastroenteritis. Severe gastroenteritis (i.e., score > 11) was reported in 92% of NoV-positive participants (Table 2). The average hospital stay of children admitted ranged between 3 and 5 d. Ninety-five percent of NoV-positive cases received intravenous rehydration, whereas only 18% received oral rehydration during hospitalization.
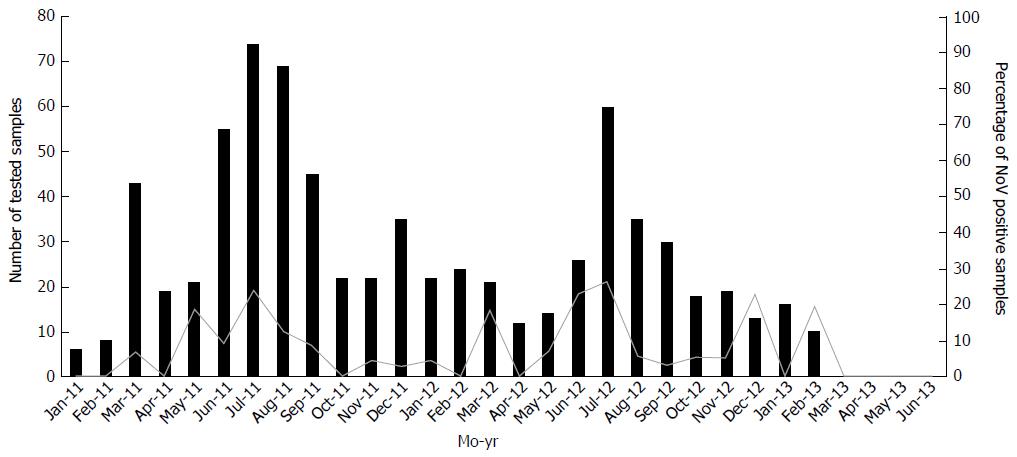
NoV incidence was similar across different geographic regions. Incidence in hospitalized children was 9%, 13% and 11% in Beirut, and the Southern and Northern parts of Lebanon, respectively (P = 0.371). Overall, 11.23% (83/739) of our study participants tested positive for NoV, of whom, 45 (54%) were detected in 2011 and 36 (43%) in 2012, and two samples tested positive during the first half of 2013. The seasonal onset of NoV cases was similar during 2011 and 2012 (Figure 1). While our data show that NoV infection can occur throughout the year, the highest percentage of NoV-positive samples was detected in July 2011 (24%) and July 2012 (27%), i.e., in the hot months. Fewer infections were observed between October and February, which are the cooler months in Lebanon.
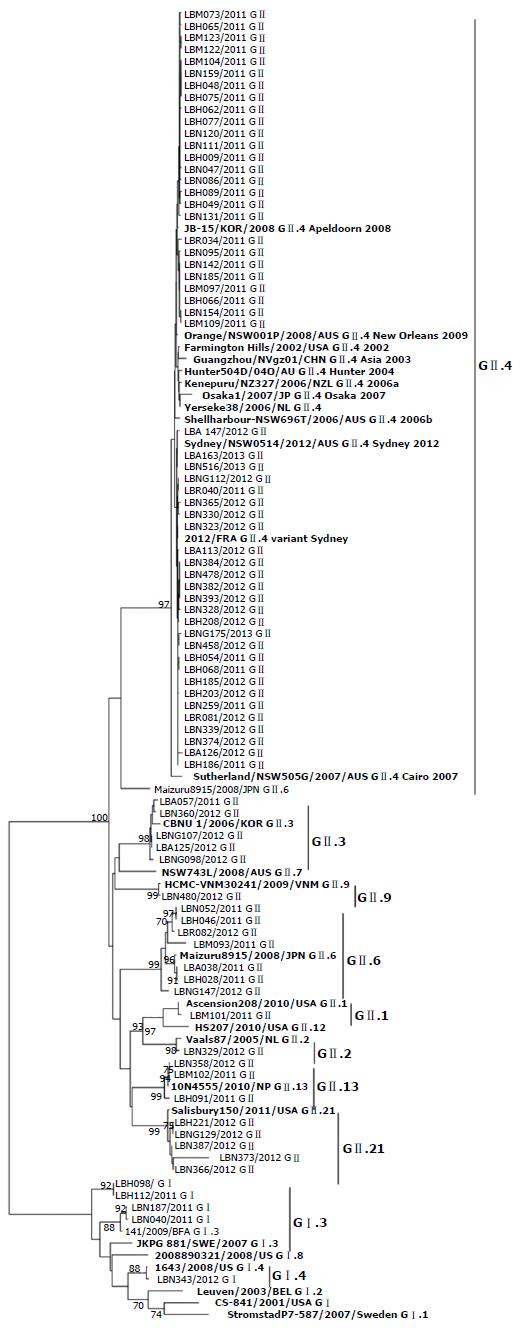
In order to analyze the extent of the genetic diversity and to designate the genotypes of NoVs detected among our cohort of children aged < 5 years, we inferred the phylogenetic relationship of the major capsid protein gene (orf2) along with subgenotype reference isolates. We sequenced 81 samples rather than the total number of NoV-positive samples (n = 83) due to the lack of sufficient volume of purified RNA for two samples. Among five GI samples, four were designated GI.3 and one as GI.4, based on their clustering in the phylogenetic tree. Eight different NoV GII genotypes were detected among our study participants, and 68% (52/76) of positive cases were attributed to GII.4. GII.4 diversified into two distinct subclusters distinguished by an A151T substitution. These subclusters co-circulated between 2011 and 2013 (Figure 2). While GII.4 was predominantly associated with gastroenteritis among our study participants, circulation of more than one sub-genotype during the same year was also recorded. The following non-GII.4 genotypes were also detected among hospitalized children during the study period: GII.6 (7/76, 9.2%), GII.21 (5/76, 6.6%), GII.3 (5/76, 6.6%), GII.13 (3/76, 3.95), GII.9 (1/76, 1.3%), GII.1 (1/76, 1.3%), and GII.2 (1/76, 1.3%) (Figure 2).
We report the results of a large study on NoV-associated gastroenteritis in Lebanon. Our study reflects the predominance of GII strains among children aged < 5 years who were hospitalized due to acute gastroenteritis. We detected a broad genetic diversity of NoVs causing acute gastroenteritis among our study participants. Overall, GII.4 (68%) was the most prevalent genotype isolated from hospitalized children in Lebanon during the study period. Our results are compatible with global reports in which most cases of NoV-associated gastroenteritis were attributable to GII.4[23-25], and co-circulating with other genotypes[13,26-28]. Locally, NoV GII has been previously reported in five Lebanese children less than ten years old[29]. Regionally, in the Middle East and North Africa (MENA), several studies have recently assessed the prevalence of NoV among hospitalized children aged < 5 years (hospitalized due to signs of acute gastroenteritis). These studies were performed on a variable sample size in Egypt[30], Israel[31,32], Iran[33], Jordan[34], Kuwait[35], Libya[36,37], Morocco[38], Tunisia[39,40], Turkey[41-45] and Yemen[46]. NoV was detected in stool samples of 6%-30% of hospitalized children aged < 5 years, with GII.4 and GII.3 predominantly reported in these studies.
We reported A JB-15/KOR/2008 GII.4 Apeldoorn 2008-like variant strain circulating in 2011 among children aged < 5 years in Lebanon. This strain was replaced between 2012 and 2013 by a variant sharing homology with the Sydney/NSW0514/2012/AUS GII.4 Sydney 2012 and Sydney 2012/FRA GII.4 strains. The latter emerged in Australia in March 2012 and was later isolated from the United States, Belgium, Denmark, Scotland, and Japan[47]. The co-circulation of several GII.4 lineages is well described[18,48] and is suggested to be a mechanism of positive selection of mutations to generate new NoV variants[49]. The variants of the NoV GII.4 lineage have been associated with 62%-80% of cases of NoV gastroenteritis worldwide, as well as explosive outbreaks occurring in community settings[11,50]. Global epidemics of NoV gastroenteritis have been associated with the following strains: US 1995/96 in 1996[51], Farmington Hills in 2002[52,53], Hunter in 2004[54], 2006b virus in 2007 and 2008[55], New Orleans virus during 2009-2012[56] and Sydney 2012[57]. Other GII.4 variants have also been associated with localized types of epidemics such as Henry 2001, Japan 2001, Asia 2003, and 2006a and Apeldoorn 2008[18].
While GII.3 was reported to be the second most predominant genotype in many countries, it ranked third along with GII.21 among our study participants after GII.4 and GII.6. GII.6 and GII.2 are reported to account for 5% of the globally reported strains. The prevalence of GII.6 ,the second most predominant cause of gastroenteritis among our study participants ,was similar to reports in several countries including Brazil[58], Japan[59], Africa[60] and Finland[61]. GII.21, previously reported in Brazil[62], has been described as a recombinant product between GII.4/2006b and GII.18 strains[63]. In our study, this genotype was similar to the Salisbury150/2011/United States GII.21. GII.13, previously described as an uncommon cause of gastroenteritis, is increasingly being reported in many Asian countries[13]. Our results show that GII.13 ranked fourth as a causative agent of gastroenteritis among hospitalized children in Lebanon. Among GI, GI.3 was predominantly detected, albeit less often compared to GII. Our results are compatible with global reports in which GII is the most prevalent genogroup causing approximately 96% of infections, with GI constituting the remaining genogroup and causing on average 3.6% of NoV infections[13].
Reports show that NoV infection peaks during the cold months in parts of Europe and North America, with sporadic cases detected all year round, as well as outbreaks during the summer months[59,64-66]. Similar results are reported from a few other countries[33,42,43,67]. New GII.4 variants have been reported to emerge with unusual spring/summer seasonality[52], along with a total increase in wintertime disease in Europe. The former was suggested to be due to the lack of effective immunity to the newly emergent GII.4 variant. Recently, a systematic review reported a variable global seasonality of NoV[8]. A peak in winter months was reported in the Northern Hemisphere, while cases peaks and outbreaks were reported in the hot months in the Southern Hemisphere. Little or Limited data exist from Africa, the tropical regions and the MENA region, and when present, studies report on short duration NoV infections detected in diverse settings. Consequently, the ability to associate climate and demographic factors with the seasonality of NoV in these areas is difficult. Although our data support a peak incidence in July, the seasonal pattern of NoV infection in Lebanon is to be further investigated in comparison with existing reports. In summary, the genotypic characterization of NoV-positive samples revealed a wide diversity of circulating genotypes, with GII.4 predominantly being associated with gastroenteritis. Globally, GII.4 circulating strains have been responsible for a large number of outbreaks in many countries including China, India, Japan, Egypt, Turkey and Italy[13]. The evolution of GII.4, described as being epochal (long periods of status quo followed by outbursts of variation) over time generates escape mutants that are periodically selected for by herd immunity[11]. Further studies are needed to assess the extent of NoV molecular diversity in Lebanon among different age groups.
While we partially genotyped our circulating strains, we realize that we cannot suggest any recombination event to elaborate on the diverse genotypes detected among our study participants. Nevertheless, our study confirms the significant role of NoV as a causative agent of gastroenteritis among children less than 5 years old in Lebanon. Our results are compatible with global reports in which most cases of NoV-associated gastroenteritis are attributable to GII.4. The continuous monitoring of NoV infection among different age groups is needed in Lebanon to support intervention strategies and to detect new circulating variants possibly associated with increased rates of morbidity.
In conclusion, we report the results of a large-scale study on NoV-associated gastroenteritis in Lebanon. Our results are compatible with global reports in which most cases of viral gastroenteritis among children aged < 5 years are attributable to GII.4. Moreover, our data support a peak incidence in July, whereas other reports show peak incidences during the cold months (e.g., North America, parts of Europe). The seasonal pattern of NoV in Lebanon should be further investigated. Efforts should be made to introduce the clinical diagnosis of the virus due to its impact on the community as well as health care institutions.
Norovirus (NoV) is one of the most common causes of acute gastroenteritis among children. To the best of our knowledge, there have been no large scale studies conducted in Lebanon on NoV and its association with acute gastroenteritis among children aged < 5 years. Moreover, the authors have no data on the genotypic characterization of the predominantly circulating NoV strains in Lebanon as compared to other countries. This study is important to support intervention strategies.
NoV is the leading cause of acute gastroenteritis across all age groups seeking medical care in emergency departments, outpatient clinics and the community. Recent reviews of the literature on community, outpatient and hospital-based studies in developing and developed countries report that NoV gastroenteritis accounts for 10%-15% of severe cases in children aged < 5 years and 9%-15% of cases of mild to moderate diarrhea among individuals of all ages. This data are compatible with global reports in which NoV Genogroup 2 genotype 4 are the most prevalent strains associated with gastroenteritis.
To the best of our knowledge, there have been no large studies conducted in Lebanon on NoV and its association with acute gastroenteritis. The aim of this study was to determine the prevalence of NoV gastroenteritis, as well as the genotypic characterization of the virus among hospitalized children < 5 years old. The authors believe that this study is the first in Lebanon to report on the circulating strains of NoV GI and GII among children hospitalized due to acute gastroenteritis.
This study is believed to be the first to report on the clinical epidemiology, seasonality and genotypic characterization of NoV as a causative agent of acute gastroenteritis leading to hospitalization among children < 5 years old in Lebanon. This study is important to guide intervention strategies in Lebanon as well as the national introduction of clinical diagnosis of the virus as a major cause of gastroenteritis.
Important work on an interesting topic, the clinical and epidemiologic characteristics of NoV gastroenteritis among hospitalized children in Lebanon.
Manuscript source: Invited manuscript
Specialty type: Gastroenterology and hepatology
Country of origin: Lebanon
Peer-review report classification
Grade A (Excellent): 0
Grade B (Very good): B
Grade C (Good): 0
Grade D (Fair): 0
Grade E (Poor): 0
P- Reviewer: Solano R S- Editor: Yu J L- Editor: Kerr C E- Editor: Zhang FF
| 1. | Division of Viral Diseases; National Center for Immunization and Respiratory Diseases; Centers for Disease Control and Prevention. Updated norovirus outbreak management and disease prevention guidelines. MMWR Recomm Rep. 2011;60:1-18. [PubMed] [Cited in This Article: ] |
| 2. | Lanata CF, Fischer-Walker CL, Olascoaga AC, Torres CX, Aryee MJ, Black RE. Global causes of diarrheal disease mortality in children & lt; 5 years of age: a systematic review. PLoS One. 2013;8:e72788. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 423] [Cited by in F6Publishing: 438] [Article Influence: 39.8] [Reference Citation Analysis (0)] |
| 3. | Patel MM, Hall AJ, Vinjé J, Parashar UD. Noroviruses: a comprehensive review. J Clin Virol. 2009;44:1-8. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 557] [Cited by in F6Publishing: 519] [Article Influence: 32.4] [Reference Citation Analysis (0)] |
| 4. | Patel MM, Widdowson MA, Glass RI, Akazawa K, Vinjé J, Parashar UD. Systematic literature review of role of noroviruses in sporadic gastroenteritis. Emerg Infect Dis. 2008;14:1224-1231. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 739] [Cited by in F6Publishing: 728] [Article Influence: 45.5] [Reference Citation Analysis (0)] |
| 5. | Lee BE, Pang XL. New strains of norovirus and the mystery of viral gastroenteritis epidemics. CMAJ. 2013;185:1381-1382. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 16] [Cited by in F6Publishing: 16] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 6. | Barclay L, Park GW, Vega E, Hall A, Parashar U, Vinjé J, Lopman B. Infection control for norovirus. Clin Microbiol Infect. 2014;20:731-740. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 115] [Cited by in F6Publishing: 99] [Article Influence: 9.9] [Reference Citation Analysis (0)] |
| 7. | Glass RI, Parashar UD, Estes MK. Norovirus gastroenteritis. N Engl J Med. 2009;361:1776-1785. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 842] [Cited by in F6Publishing: 778] [Article Influence: 51.9] [Reference Citation Analysis (0)] |
| 8. | Ahmed SM, Lopman BA, Levy K. A systematic review and meta-analysis of the global seasonality of norovirus. PLoS One. 2013;8:e75922. [PubMed] [Cited in This Article: ] |
| 9. | Zheng DP, Ando T, Fankhauser RL, Beard RS, Glass RI, Monroe SS. Norovirus classification and proposed strain nomenclature. Virology. 2006;346:312-323. [PubMed] [Cited in This Article: ] |
| 10. | Vinjé J, Green J, Lewis DC, Gallimore CI, Brown DW, Koopmans MP. Genetic polymorphism across regions of the three open reading frames of “Norwalk-like viruses”. Arch Virol. 2000;145:223-241. [PubMed] [Cited in This Article: ] |
| 11. | Donaldson EF, Lindesmith LC, Lobue AD, Baric RS. Viral shape-shifting: norovirus evasion of the human immune system. Nat Rev Microbiol. 2010;8:231-241. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 206] [Cited by in F6Publishing: 204] [Article Influence: 14.6] [Reference Citation Analysis (0)] |
| 12. | Katayama K, Shirato-Horikoshi H, Kojima S, Kageyama T, Oka T, Hoshino F, Fukushi S, Shinohara M, Uchida K, Suzuki Y. Phylogenetic analysis of the complete genome of 18 Norwalk-like viruses. Virology. 2002;299:225-239. [PubMed] [Cited in This Article: ] |
| 13. | Hoa Tran TN, Trainor E, Nakagomi T, Cunliffe NA, Nakagomi O. Molecular epidemiology of noroviruses associated with acute sporadic gastroenteritis in children: global distribution of genogroups, genotypes and GII.4 variants. J Clin Virol. 2013;56:185-193. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 196] [Cited by in F6Publishing: 208] [Article Influence: 17.3] [Reference Citation Analysis (0)] |
| 14. | Desai R, Hembree CD, Handel A, Matthews JE, Dickey BW, McDonald S, Hall AJ, Parashar UD, Leon JS, Lopman B. Severe outcomes are associated with genogroup 2 genotype 4 norovirus outbreaks: a systematic literature review. Clin Infect Dis. 2012;55:189-193. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 129] [Cited by in F6Publishing: 130] [Article Influence: 10.8] [Reference Citation Analysis (0)] |
| 15. | Siebenga JJ, Vennema H, Renckens B, de Bruin E, van der Veer B, Siezen RJ, Koopmans M. Epochal evolution of GGII.4 norovirus capsid proteins from 1995 to 2006. J Virol. 2007;81:9932-9941. [PubMed] [Cited in This Article: ] |
| 16. | Allen DJ, Gray JJ, Gallimore CI, Xerry J, Iturriza-Gómara M. Analysis of amino acid variation in the P2 domain of the GII-4 norovirus VP1 protein reveals putative variant-specific epitopes. PLoS One. 2008;3:e1485. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 103] [Cited by in F6Publishing: 108] [Article Influence: 6.8] [Reference Citation Analysis (0)] |
| 17. | Allen DJ, Noad R, Samuel D, Gray JJ, Roy P, Iturriza-Gómara M. Characterisation of a GII-4 norovirus variant-specific surface-exposed site involved in antibody binding. Virol J. 2009;6:150. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 59] [Cited by in F6Publishing: 61] [Article Influence: 4.1] [Reference Citation Analysis (0)] |
| 18. | Eden JS, Tanaka MM, Boni MF, Rawlinson WD, White PA. Recombination within the pandemic norovirus GII.4 lineage. J Virol. 2013;87:6270-6282. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 217] [Cited by in F6Publishing: 205] [Article Influence: 18.6] [Reference Citation Analysis (0)] |
| 19. | Lindesmith LC, Donaldson EF, Lobue AD, Cannon JL, Zheng DP, Vinje J, Baric RS. Mechanisms of GII.4 norovirus persistence in human populations. PLoS Med. 2008;5:e31. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 432] [Cited by in F6Publishing: 419] [Article Influence: 26.2] [Reference Citation Analysis (0)] |
| 20. | Chen Y, Li Z, Han D, Cui D, Chen X, Zheng S, Yu F, Liu J, Lai S, Yan Y. Viral agents associated with acute diarrhea among outpatient children in southeastern China. Pediatr Infect Dis J. 2013;32:e285-e290. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 29] [Cited by in F6Publishing: 29] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 21. | Gao Y, Jin M, Cong X, Duan Z, Li HY, Guo X, Zuo Y, Zhang Y, Zhang Y, Wei L. Clinical and molecular epidemiologic analyses of norovirus-associated sporadic gastroenteritis in adults from Beijing, China. J Med Virol. 2011;83:1078-1085. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 23] [Cited by in F6Publishing: 23] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 22. | Kojima S, Kageyama T, Fukushi S, Hoshino FB, Shinohara M, Uchida K, Natori K, Takeda N, Katayama K. Genogroup-specific PCR primers for detection of Norwalk-like viruses. J Virol Methods. 2002;100:107-114. [PubMed] [Cited in This Article: ] |
| 23. | Bull RA, White PA. Mechanisms of GII.4 norovirus evolution. Trends Microbiol. 2011;19:233-240. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 122] [Cited by in F6Publishing: 123] [Article Influence: 9.5] [Reference Citation Analysis (0)] |
| 24. | Siebenga JJ, Vennema H, Duizer E, Koopmans MP. Gastroenteritis caused by norovirus GGII.4, The Netherlands, 1994-2005. Emerg Infect Dis. 2007;13:144-146. [PubMed] [Cited in This Article: ] |
| 25. | Guo L, Song J, Xu X, Ren L, Li J, Zhou H, Wang M, Qu J, Wang J, Hung T. Genetic analysis of norovirus in children affected with acute gastroenteritis in Beijing, 2004-2007. J Clin Virol. 2009;44:94-98. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 38] [Cited by in F6Publishing: 41] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 26. | Pang XL, Preiksaitis JK, Wong S, Li V, Lee BE. Influence of novel norovirus GII.4 variants on gastroenteritis outbreak dynamics in Alberta and the Northern Territories, Canada between 2000 and 2008. PLoS One. 2010;5:e11599. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 48] [Cited by in F6Publishing: 50] [Article Influence: 3.6] [Reference Citation Analysis (0)] |
| 27. | Siebenga JJ, Lemey P, Kosakovsky Pond SL, Rambaut A, Vennema H, Koopmans M. Phylodynamic reconstruction reveals norovirus GII.4 epidemic expansions and their molecular determinants. PLoS Pathog. 2010;6:e1000884. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 113] [Cited by in F6Publishing: 118] [Article Influence: 8.4] [Reference Citation Analysis (0)] |
| 28. | Puustinen L, Blazevic V, Huhti L, Szakal ED, Halkosalo A, Salminen M, Vesikari T. Norovirus genotypes in endemic acute gastroenteritis of infants and children in Finland between 1994 and 2007. Epidemiol Infect. 2012;140:268-275. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 35] [Cited by in F6Publishing: 34] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 29. | Al-Ali RM, Chehadeh W, Hamze M, Dabboussi F, Hlais S, Mallat H. First description of gastroenteritis viruses in Lebanese children: a pilot study. J Infect Public Health. 2011;4:59-64. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 14] [Cited by in F6Publishing: 14] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 30. | El-Mohammady H, Mansour A, Shaheen HI, Henien NH, Motawea MS, Raafat I, Moustafa M, Adib-Messih IA, Sebeny PJ, Young SY. Increase in the detection rate of viral and parasitic enteric pathogens among Egyptian children with acute diarrhea. J Infect Dev Ctries. 2012;6:774-781. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 14] [Cited by in F6Publishing: 15] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 31. | Muhsen K, Kassem E, Rubinstein U, Schachter Y, Kremer A, Goren S, Zilberstein I, Ephros M, Cohen D, Shulman LM. Incidence and characteristics of sporadic norovirus gastroenteritis associated with hospitalization of children less than 5 years of age in Israel. Pediatr Infect Dis J. 2013;32:688-690. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 12] [Cited by in F6Publishing: 12] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 32. | Leshem E, Givon-Lavi N, Vinjé J, Gregoricus N, Parashar U, Dagan R. Differences in Norovirus-Associated Hospital Visits Between Jewish and Bedouin Children in Southern Israel. Pediatr Infect Dis J. 2015;34:1036-1038. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 14] [Cited by in F6Publishing: 14] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 33. | Najafi A, Najafi S, Vahdat K, Kargar M, Javdani N. Importance of viral pathogens in children with acute gastroenteritis in the south of Iran. Ann Saudi Med. 2013;33:124-129. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 15] [Cited by in F6Publishing: 14] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 34. | Kaplan NM, Kirby A, Abd-Eldayem SA, Dove W, Nakagomi T, Nakagomi O, Cunliffe NA. Detection and molecular characterisation of rotavirus and norovirus infections in Jordanian children with acute gastroenteritis. Arch Virol. 2011;156:1477-1480. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 29] [Cited by in F6Publishing: 27] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 35. | Al-Rashidi A, Chehadeh W, Szücs GG, Albert MJ. Different norovirus genotypes in patients with gastroenteritis in Kuwait. J Med Virol. 2013;85:1611-1618. [PubMed] [Cited in This Article: ] |
| 36. | Abugalia M, Cuevas L, Kirby A, Dove W, Nakagomi O, Nakagomi T, Kara M, Gweder R, Smeo M, Cunliffe N. Clinical features and molecular epidemiology of rotavirus and norovirus infections in Libyan children. J Med Virol. 2011;83:1849-1856. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 46] [Cited by in F6Publishing: 50] [Article Influence: 4.2] [Reference Citation Analysis (0)] |
| 37. | Rahouma A, Klena JD, Krema Z, Abobker AA, Treesh K, Franka E, Abusnena O, Shaheen HI, El Mohammady H, Abudher A. Enteric pathogens associated with childhood diarrhea in Tripoli-Libya. Am J Trop Med Hyg. 2011;84:886-891. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 29] [Cited by in F6Publishing: 38] [Article Influence: 2.9] [Reference Citation Analysis (0)] |
| 38. | El Qazoui M, Oumzil H, Baassi L, El Omari N, Sadki K, Amzazi S, Benhafid M, El Aouad R. Rotavirus and norovirus infections among acute gastroenteritis children in Morocco. BMC Infect Dis. 2014;14:300. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 32] [Cited by in F6Publishing: 36] [Article Influence: 3.6] [Reference Citation Analysis (0)] |
| 39. | Sdiri-Loulizi K, Hassine M, Gharbi-Khelifi H, Aouni Z, Chouchane S, Sakly N, Neji-Guédiche M, Pothier P, Ambert-Balay K, Aouni M. Molecular detection of genogroup I sapovirus in Tunisian children suffering from acute gastroenteritis. Virus Genes. 2011;43:6-12. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 24] [Cited by in F6Publishing: 24] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 40. | Ben Salem-Ben Nejma I, Hassine Zaafrane M, Hassine F, Sdiri-Loulizi K, Ben Said M, Aouni M, Mzoughi R. Etiology of Acute Diarrhea in Tunisian Children with Emphasis on Diarrheagenic Escherichia coli: Prevalence and Identification of E. coli Virulence Markers. Iran J Public Health. 2014;43:947-960. [PubMed] [Cited in This Article: ] |
| 41. | Bicer S, Col D, Erdag GC, Giray T, Gurol Y, Yilmaz G, Vitrinel A, Ozelgun B. A retrospective analysis of acute gastroenteritis agents in children admitted to a university hospital pediatric emergency unit. Jundishapur J Microbiol. 2014;7:e9148. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 6] [Cited by in F6Publishing: 11] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 42. | Cöl D, Biçer S, Ciler Erdağ G, Giray T, Gürol Y, Yilmaz G, Küçük Ö, Vitrinel A. Annual report on norovirus in children with acute gastroenteritis in 2009 and their genotypes in Turkey. Infez Med. 2013;21:261-269. [PubMed] [Cited in This Article: ] |
| 43. | Altay A, Bozdayı G, Meral M, Dallar Bilge Y, Dalgıç B, Ozkan S, Ahmed K. [Investigation of norovirus infection incidence among 0-5 years old children with acute gastroenteritis admitted to two different hospitals in ankara, Turkey]. Mikrobiyol Bul. 2013;47:98-108. [PubMed] [Cited in This Article: ] |
| 44. | Mitui MT, Bozdayi G, Ahmed S, Matsumoto T, Nishizono A, Ahmed K. Detection and molecular characterization of diarrhea causing viruses in single and mixed infections in children: a comparative study between Bangladesh and Turkey. J Med Virol. 2014;86:1159-1168. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 20] [Cited by in F6Publishing: 20] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 45. | Ozkul AA, Kocazeybek BS, Turan N, Reuter G, Bostan K, Yilmaz A, Altan E, Uyunmaz G, Karaköse AR, Muratoglu K. Frequency and phylogeny of norovirus in diarrheic children in Istanbul, Turkey. J Clin Virol. 2011;51:160-164. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 14] [Cited by in F6Publishing: 14] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 46. | Kirby A, Al-Eryani A, Al-Sonboli N, Hafiz T, Beyer M, Al-Aghbari N, Al-Moheri N, Dove W, Cunliffe NA, Cuevas LE. Rotavirus and norovirus infections in children in Sana’a, Yemen. Trop Med Int Health. 2011;16:680-684. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 20] [Cited by in F6Publishing: 22] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 47. | Rahman M, Nahar S, Afrad MH, Faruque AS, Azim T. Norovirus variant GII.4/Sydney/2012, Bangladesh. Emerg Infect Dis. 2013;19:1347-1348. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 10] [Cited by in F6Publishing: 13] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 48. | Eden JS, Hewitt J, Lim KL, Boni MF, Merif J, Greening G, Ratcliff RM, Holmes EC, Tanaka MM, Rawlinson WD. The emergence and evolution of the novel epidemic norovirus GII.4 variant Sydney 2012. Virology. 2014;450-451:106-113. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 100] [Cited by in F6Publishing: 96] [Article Influence: 8.7] [Reference Citation Analysis (0)] |
| 49. | Medici MC, Tummolo F, De Grazia S, Calderaro A, De Conto F, Terio V, Chironna M, Bonura F, Pucci M, Bányai K. Epidemiological dynamics of norovirus GII.4 variant New Orleans 2009. J Gen Virol. 2015;96:2919-2927. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 5] [Cited by in F6Publishing: 7] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 50. | Siebenga JJ, Vennema H, Zheng DP, Vinjé J, Lee BE, Pang XL, Ho EC, Lim W, Choudekar A, Broor S. Norovirus illness is a global problem: emergence and spread of norovirus GII.4 variants, 2001-2007. J Infect Dis. 2009;200:802-812. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 542] [Cited by in F6Publishing: 523] [Article Influence: 34.9] [Reference Citation Analysis (0)] |
| 51. | White PA, Hansman GS, Li A, Dable J, Isaacs M, Ferson M, McIver CJ, Rawlinson WD. Norwalk-like virus 95/96-US strain is a major cause of gastroenteritis outbreaks in Australia. J Med Virol. 2002;68:113-118. [PubMed] [Cited in This Article: ] |
| 52. | Lopman B, Vennema H, Kohli E, Pothier P, Sanchez A, Negredo A, Buesa J, Schreier E, Reacher M, Brown D. Increase in viral gastroenteritis outbreaks in Europe and epidemic spread of new norovirus variant. Lancet. 2004;363:682-688. [PubMed] [Cited in This Article: ] |
| 53. | Widdowson MA, Cramer EH, Hadley L, Bresee JS, Beard RS, Bulens SN, Charles M, Chege W, Isakbaeva E, Wright JG. Outbreaks of acute gastroenteritis on cruise ships and on land: identification of a predominant circulating strain of norovirus--United States, 2002. J Infect Dis. 2004;190:27-36. [PubMed] [Cited in This Article: ] |
| 54. | Bull RA, Tu ET, McIver CJ, Rawlinson WD, White PA. Emergence of a new norovirus genotype II.4 variant associated with global outbreaks of gastroenteritis. J Clin Microbiol. 2006;44:327-333. [PubMed] [Cited in This Article: ] |
| 55. | Eden JS, Bull RA, Tu E, McIver CJ, Lyon MJ, Marshall JA, Smith DW, Musto J, Rawlinson WD, White PA. Norovirus GII.4 variant 2006b caused epidemics of acute gastroenteritis in Australia during 2007 and 2008. J Clin Virol. 2010;49:265-271. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 69] [Cited by in F6Publishing: 71] [Article Influence: 5.5] [Reference Citation Analysis (0)] |
| 56. | Yen C, Wikswo ME, Lopman BA, Vinje J, Parashar UD, Hall AJ. Impact of an emergent norovirus variant in 2009 on norovirus outbreak activity in the United States. Clin Infect Dis. 2011;53:568-571. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 90] [Cited by in F6Publishing: 88] [Article Influence: 6.8] [Reference Citation Analysis (0)] |
| 57. | van Beek J, Ambert-Balay K, Botteldoorn N, Eden JS, Fonager J, Hewitt J, Iritani N, Kroneman A, Vennema H, Vinjé J. Indications for worldwide increased norovirus activity associated with emergence of a new variant of genotype II.4, late 2012. Euro Surveill. 2013;18:8-9. [PubMed] [Cited in This Article: ] |
| 58. | de Andrade Jda S, Rocha MS, Carvalho-Costa FA, Fioretti JM, Xavier Mda P, Nunes ZM, Cardoso J, Fialho AM, Leite JP, Miagostovich MP. Noroviruses associated with outbreaks of acute gastroenteritis in the State of Rio Grande do Sul, Brazil, 2004-2011. J Clin Virol. 2014;61:345-352. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 34] [Cited by in F6Publishing: 34] [Article Influence: 3.4] [Reference Citation Analysis (0)] |
| 59. | Thongprachum A, Khamrin P, Maneekarn N, Hayakawa S, Ushijima H. Epidemiology of gastroenteritis viruses in Japan: Prevalence, seasonality, and outbreak. J Med Virol. 2016;88:551-570. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 56] [Cited by in F6Publishing: 56] [Article Influence: 6.2] [Reference Citation Analysis (0)] |
| 60. | Mans J, Armah GE, Steele AD, Taylor MB. Norovirus Epidemiology in Africa: A Review. PLoS One. 2016;11:e0146280. [PubMed] [DOI] [Cited in This Article: ] [Cited by in F6Publishing: 454] [Reference Citation Analysis (0)] |
| 61. | Puustinen L, Blazevic V, Salminen M, Hämäläinen M, Räsänen S, Vesikari T. Noroviruses as a major cause of acute gastroenteritis in children in Finland, 2009-2010. Scand J Infect Dis. 2011;43:804-808. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 57] [Cited by in F6Publishing: 57] [Article Influence: 4.4] [Reference Citation Analysis (0)] |
| 62. | Ferreira MS, Cubel Garcia Rde C, Xavier Mda P, Ribeiro RL, Assis RM, Mota Mdo C, Leite JP, Miagostovich MP, de Oliveira SA. Genotyping of gastroenteric viruses in hospitalised children: first report of norovirus GII.21 in Brazil. Mem Inst Oswaldo Cruz. 2012;107:1064-1067. [PubMed] [Cited in This Article: ] |
| 63. | Chhabra P, Walimbe AM, Chitambar SD. Molecular characterization of three novel intergenotype norovirus GII recombinant strains from western India. Virus Res. 2010;147:242-246. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 23] [Cited by in F6Publishing: 24] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 64. | Mounts AW, Ando T, Koopmans M, Bresee JS, Noel J, Glass RI. Cold weather seasonality of gastroenteritis associated with Norwalk-like viruses. J Infect Dis. 2000;181 Suppl 2:S284-S287. [PubMed] [Cited in This Article: ] |
| 65. | Rohayem J. Norovirus seasonality and the potential impact of climate change. Clin Microbiol Infect. 2009;15:524-527. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 75] [Cited by in F6Publishing: 77] [Article Influence: 5.1] [Reference Citation Analysis (0)] |
| 66. | Verhoef L, Depoortere E, Boxman I, Duizer E, van Duynhoven Y, Harris J, Johnsen C, Kroneman A, Le Guyader S, Lim W. Emergence of new norovirus variants on spring cruise ships and prediction of winter epidemics. Emerg Infect Dis. 2008;14:238-243. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 86] [Cited by in F6Publishing: 90] [Article Influence: 5.6] [Reference Citation Analysis (0)] |
| 67. | Ayukekbong JA, Andersson ME, Vansarla G, Tah F, Nkuo-Akenji T, Lindh M, Bergström T. Monitoring of seasonality of norovirus and other enteric viruses in Cameroon by real-time PCR: an exploratory study. Epidemiol Infect. 2014;142:1393-1402. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 17] [Cited by in F6Publishing: 17] [Article Influence: 1.5] [Reference Citation Analysis (0)] |