Published online Feb 21, 2016. doi: 10.3748/wjg.v22.i7.2242
Peer-review started: June 3, 2015
First decision: July 17, 2015
Revised: September 28, 2015
Accepted: December 19, 2015
Article in press: December 19, 2015
Published online: February 21, 2016
Low-grade intestinal inflammation plays a key role in the pathophysiology of irritable bowel syndrome (IBS), and this role is likely to be multifactorial. The aim of this review was to summarize the evidence on the spectrum of mucosal inflammation in IBS, highlighting the relationship of this inflammation to the pathophysiology of IBS and its connection to clinical practice. We carried out a bibliographic search in Medline and the Cochrane Library for the period of January 1966 to December 2014, focusing on publications describing an interaction between inflammation and IBS. Several evidences demonstrate microscopic and molecular abnormalities in IBS patients. Understanding the mechanisms underlying low-grade inflammation in IBS may help to design clinical trials to test the efficacy and safety of drugs that target this pathophysiologic mechanism.
Core tip: Low-grade intestinal inflammation plays a key role in the pathophysiology of irritable bowel syndrome, and this influence is likely multifactorial. Several evidences showed microscopic and molecular abnormalities in large subsets of patients with irritable bowel syndrome. Understanding the mechanisms underlying the low-grade inflammation in this disease may help to design clinical trials to test the efficacy and safety of drugs that target this pathophysiologic mechanism.
- Citation: Sinagra E, Pompei G, Tomasello G, Cappello F, Morreale GC, Amvrosiadis G, Rossi F, Lo Monte AI, Rizzo AG, Raimondo D. Inflammation in irritable bowel syndrome: Myth or new treatment target? World J Gastroenterol 2016; 22(7): 2242-2255
- URL: https://www.wjgnet.com/1007-9327/full/v22/i7/2242.htm
- DOI: https://dx.doi.org/10.3748/wjg.v22.i7.2242
Irritable bowel syndrome (IBS) is a chronic, relapsing, and remitting functional disorder of the gastrointestinal tract characterized by abdominal pain, bloating, and changes in bowel habits that lack a known structural or anatomic explanation[1].
IBS consists of a set of altered bowel habits over a period of time and includes abdominal pain and discomfort. IBS is one of the most common diagnoses in primary care, accounting for approximately 12% of all visits[2]. In addition, a survey conducted by Russo et al[3] found IBS to be the most common functional gastrointestinal diagnosis, comprising 35% of all outpatient referrals to gastroenterologists[2,3]. Therefore, IBS is also the most common diagnosis for gastroenterologists, accounting for 20%-50% of patient visits[2,4].
With regard to the sex-related prevalence of IBS, in Western countries, the prevalence of IBS in female patients outnumbers that in male patients by 2:1[5,6]. Furthermore, the ratio of female to male IBS sufferers in the non-patient population is 2:1, and within the patient population who seek consultations with primary care physicians, females outnumber male patients by 3:1[5,7]. Finally, in tertiary-care settings, the number of female IBS patients is four- to five-times higher than the number of males[5-8]. This prevalence should not only be strictly attributed to sex, but also to gender-related differences in healthcare-seeking behavior and sociocultural characteristics that vary between men and women with IBS as well as among different cultures[5,6].
According to the Rome III criteria, IBS is defined based on the presence of recurrent abdominal pain or discomfort at least three days per month in the past three months associated with two or more of the following: (1) improvement with defecation; (2) onset associated with a change in frequency of stool; and (3) onset associated with a change in form (appearance) of stool. These criteria should be fulfilled for the previous three months with symptom onset at least six months before diagnosis[9].
Rome III criteria subtype IBS according to the predominant bowel habit as IBS with constipation (IBS-C), IBS with diarrhea (IBS-D), mixed type, and unclassified[9]. To this end, the definition of bowel-habit type is based on the patient’s description of the stool form by referring to the Bristol Stool Scale[10]. Furthermore, IBS patients can be divided into two categories: sporadic (nonspecific) and postinfectious (PI-IBS) inflammatory bowel disease-associated[11,12].
IBS symptoms cannot be explained by structural abnormalities, and specific laboratory tests or biomarkers are not available for IBS. Therefore, IBS is classified as a functional disorder whose diagnosis depends on the history of manifested symptoms[13].
The cause of IBS is unknown, but a single factor is not likely to be responsible for the several presentations of this complex disorder[1]; new fields of research in this area include mucosal inflammation, postinfectious low-grade inflammation, genetic and immunologic factors, alteration of the human microbiota, alterations of the intestinal permeability, and dietary and neuroendocrine factors[13]. Usually, routine histologic examinations do not show significant colonic mucosal abnormalities in the majority of IBS patients; however, recent quantitative histologic, immunohistochemical, and ultrastructural analyses have indicated subtle organic alterations in these patients.
This literature review aims to summarize the findings relating the spectrum of mucosal inflammation to IBS, highlighting their relationship to the pathophysiology of IBS and their connections, if any, to clinical practice.
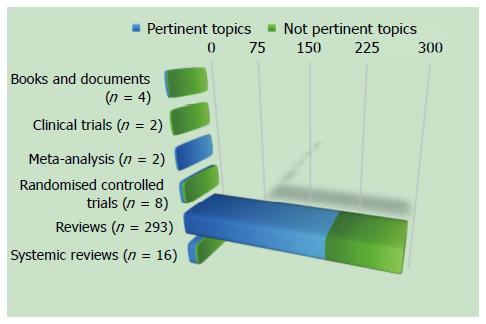
We carried out a bibliographic search in MEDLINE for the period of January 1966 to July 2015, and focused on identifying publications describing an interaction between inflammation and IBS. The keywords used were: irritable bowel syndrome, inflammation, mucosal inflammation, pathology, mast cells, neuroendocrine cells, immune cells, intestinal permeability, and enteric nerves. The inclusion criteria to select articles were based on design (systematic reviews, meta-analysis, clinical trials, and experimental studies on animals) and population (adult patients > 18 years of age). We excluded articles not pertinent to this topic.
According to the aforementioned criteria, we found 305 studies, and we excluded 100 studies because they were not pertinent to this topic (Figure 1).
According to the American Gastroenterology Association, “a colonoscopy is recommended for patients over age 50 years (due to higher pretest probability of colon cancer), but in younger patients, performing a colonoscopy or sigmoidoscopy is determined by clinical features suggestive of disease (e.g., diarrhea, weight loss), and may not be indicated”[14].
However, the British guidelines suggest that, “given the high frequency of colonic cancer in the population at large, an examination of the colon is advisable for a change in bowel habit over the age of 50”; the authors of these guidelines highlighted that, “as IBS patients have no increased risk of colon cancer, advice on screening for this is no different from the general population”[15].
More recently, Japanese guidelines suggested that “colonoscopy has a diagnostic value, not only for excluding organic diseases but also for supporting the existence of pathophysiology compatible to IBS due to visceral hypersensitivity to colonoscopic procedures and colonic spasms”[16].
A prospective, multicenter study performed by Ishihara and coworkers[17] aimed to determine the presence of organic colonic lesions in IBS patients. Their study showed that the prevalence of organic colonic diseases in IBS patients was at an acceptably low level, thus showing that the Rome III criteria are specific for the diagnosis of IBS. Conversely, another study performed by Hsiao and coworkers[18] demonstrated that IBS was not associated with the development of colon cancer in Taiwan.
Despite these recommendations, a recent Korean survey indicated that colonoscopy was the most commonly required test (79.5%) in IBS patients[19], whereas a study performed by Lieberman and coworkers[20] to evaluate trends in the utilization and outcomes of colonoscopy in the United States from 2000 to 2011 showed that the most common reason for colonoscopy in patients aged < 50 years was the evaluation of symptoms, such as IBS (28.7%), together with bleeding or anemia (35.3%).
Based on these updated data, IBS still represents the majority of colonoscopic biopsies seen by pathologists[21] that are usually considered either normal or near to normal on routine histologic examination. These findings provide valuable information to the physician who is suspecting a diagnosis of IBS. However, the pathologists must be aware of variations in normal tissue as well as artifacts that may result from bowel preparations or the biopsy procedure in order to not to report these variations as abnormal. Furthermore, the pathologists must consider subtle morphologic changes reported in the intestinal mucosa in IBS and associated with chronic inflammatory cells, mast cells, enteroendocrine cells, and enteric nerves[22].
The intestinal mucosa harbors a florid immune system that can be regarded as “physiologically inflamed”[23]. Thus low-grade inflammation can only be evaluated using quantitative assessments[24]. IBS patients have been shown to exhibit significant increases in lamina propria immune cells in the colonic mucosa compared with healthy subjects, which appears to be more predominant in the right than in the left colon[25].
More than ten years ago, O’Sullivan et al[26] evaluated the number of plasma cells, lymphocytes, eosinophils, neutrophils, and macrophages in a case-control study. Specifically, each cell type was semiquantitatively graded in hematoxylin-and-eosin-stained sections of the entire colon, and possible increases in the number of mast cells (MCs) in the colon of IBS patients compared with controls were examined using a monoclonal mouse antibody for human MC tryptase (AA1). Other than MCs, increases in cellular infiltrate were not observed in the IBS group, and the number of MCs was significantly increased in the cecum of IBS patients compared with controls.
Similarly, in 2008, Piche and coworkers[27] aimed to examine associations between fatigue, depression, and the MCs of the colonic mucosa in IBS by comparing the numbers of CD3-positive intraepithelial T lymphocytes, MCs, plasma cells, eosinophils, and neutrophils in cecal biopsies taken during colonoscopy. There was not a significant difference in the numbers of intraepithelial lymphocytes, plasma cells, eosinophils, or neutrophils between IBS patients and healthy controls, but the MC numbers per high-power field were significantly higher in IBS patients than in healthy controls (9.3 vs 4.0, P = 0.001). Furthermore, the number of MCs correlated with the severity of fatigue and depression scores in IBS patients but not in healthy controls[28].
With regard to the small bowel, Walker et al[29] examined the MC, eosinophil, and intraepithelial lymphocyte populations in duodenal biopsies of subjects with IBS and functional dyspepsia. Their study showed a significant increase in the number of intraepithelial lymphocytes in biopsies from the duodenum in patients with IBS-C. However, this increase was not observed in the second part of the duodenum. Nevertheless, MC counts were also higher in IBS cases in both the first and second parts of the duodenum, but this difference was only significant for constipation-predominant IBS[28]. Interestingly, the eosinophil counts in this study did not differ between IBS patients and controls in either the first or second part of the duodenum[28].
To date, a significant difference in the numbers of plasma cells, neutrophils, or eosinophils has not been demonstrated among IBS cases[28]. Importantly, increases in eosinophils were not identified in IBS, but the eosinophil counts were elevated in individuals with functional dyspepsia[29]. Functional dyspepsia and IBS demonstrate significant overlap in cross-sectional surveys[30], despite attempts to classify them separately, and a biomarker to predict the presence of IBS remains elusive[31]. Therefore, this histopathologic marker may serve to distinguish the two conditions[28].
MCs are innate immune cells involved in food allergies, wound healing, and protection against pathogens[32]. Their functional activation consists of a degranulation process, leading to the release of various compounds, such as histamine, tryptase, and chymase[32].
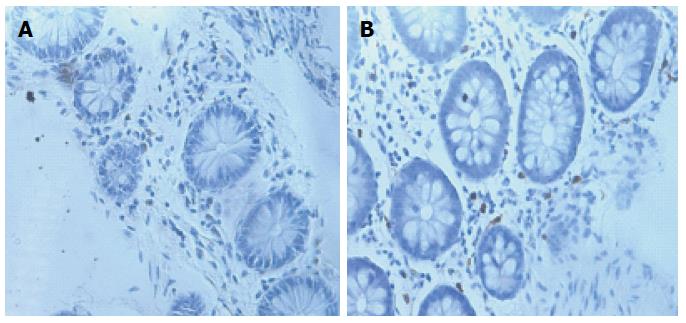
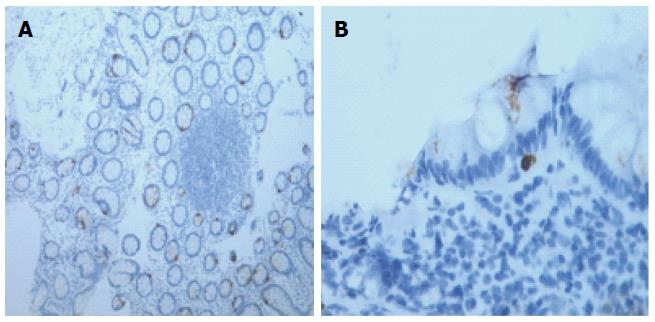
More than 40 years ago, Hiatt and Katz[33] were the first to demonstrate MC infiltration in the muscular layer of full-thickness colonic biopsy samples from four patients with “spastic colitis”[34]. Increases in the number of mucosal MCs have been observed in IBS patients in the rectum[35,36], rectosigmoid colon[37,38], descending colon[39-44], ascending colon[36], cecum[45], terminal ileum[25,36,46], jejunum[47-49], and duodenum[29,50] (Figure 2).
However, discrepancies in data obtained from these studies could be due to sex-specific differences, bowel preparation artifacts, fixation protocols, tissue orientation, sample size, or IBS-related recruitment criteria[32,43,44,51]. Furthermore, clinical studies have also yielded conflicting evidence correlating MC numbers with the onset of abdominal pain[32].
The number of functionally active MCs (exhibiting changes in the release of tryptase and histamine), rather than the absolute number of MCs, plays a pivotal role in IBS[43,50]. Consequently, the role of MCs in IBS may be affected by cells that are functionally active and form close connections with enteric and extrinsic nerve terminals, thus determining visceral hypersensitivity and altered gut function[47].
The aforementioned inflammatory changes described in the mucosa of IBS patients show that immune activation may play a role in IBS pathophysiology[34]. Mucosal T- and B-type lymphocytes are also part of the gut adaptive immune response to pathogens[32].
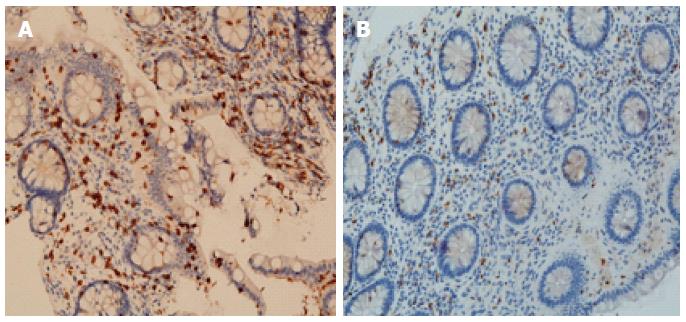
An increased density of T lymphocytes in the mucosa of IBS patients has been widely demonstrated. Specifically, the T-cell density is higher in the rectum[35,36,52], rectosigmoid colon[53], colon[38,48,54], cecum[26], jejunum[55], and duodenum[56]. Like for MCs, several studies showed that IBS patients exhibit a normal lymphocyte density in different intestinal tissue segments[19,36,38,53,57-61]. These discrepancies may be due to differences in the immunostaining techniques, quantification methods, and IBS-related recruitment criteria (Figure 3)[32].
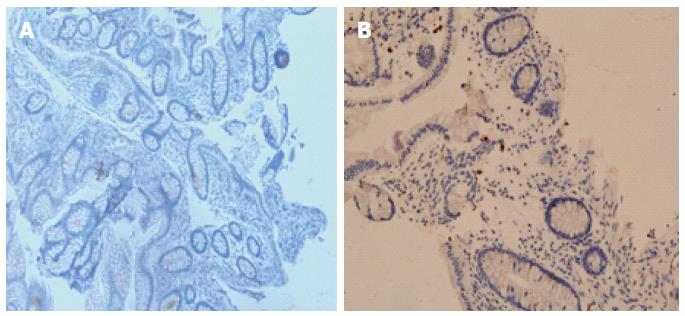
Conversely, the density of B-cells in the rectum[19,36], colon[19,43,53,62], cecum[19,20], ileum[36], or jejunum[60] did not differ in IBS patients. However, Forshammar and coworkers[62] and others[32] found a decrease in secretory B cells in the colon (Figure 4).
With regard to intraepithelial lymphocytes, several discrepancies have been reported in studies assessing the density of these cells in IBS patients. An increase in density has been demonstrated in the rectum[35,36,57], colon[57], jejunum[55,49], and duodenum[56], but these increases were not confirmed by other groups[20,32,45,49,59,61].
Dendritic cells are antigen-presenting cells that are usually located at the surveillance interfaces of the human body, such as the skin or mucosa, and play a pivotal role in the generation and regulation of immune responses[63]. In fact, they represent the link between allergen uptake and the clinical manifestations of intestinal inflammation[64,65]. Furthermore, the gut also harbors abundant macrophages. These cells do not function as typical antigen-presenting cells and lack the cellular machinery for the production of pro-inflammatory cytokines and induction of potent adaptive immune responses. However, they show very potent phagocytic activity[65].
In a Trichinella spiralis mouse model of PI-IBS, Long and coworkers[66] reported numerical and phenotypic alterations in the lamina propria dendritic cells following acute T. spiralis infection. In their study, the lamina propria dendritic cells expressed increased levels of costimulatory molecules and exhibited a greater ability to migrate and induce CD4+ T-cell proliferation[66]. Consequently, these changes favored increased levels of pro-inflammatory interferon-γ, interleukin-23, and tumor necrosis factor-α production in the so-called “PI-IBS stage”[66,67].
With regard to macrophages, the numbers of resident CD68+ macrophages are reduced in PI-IBS cases following Campylobacter jejuni infection, probably due to the cytotoxic nature of the pathogen inside host cells[50]. Similarly, Shigella spp.[68,69] and Salmonella infections have also been shown to be involved in PI-IBS, and both of these organisms are intracellular pathogens that induce phagocytosis by macrophages[70,71]. Furthermore, Salmonella seems to be less cytotoxic to macrophages[72] and also causes a marked interleukin-18 response[72] with important implications in exerting paracrine effects on surrounding immune cells (inducing interferon-γ expression). These changes result in increased levels of activated T cells in the infected intestine[47,52,54,55,57].
Enteroendocrine cells (residing among the epithelial cells of the mucosa in all gut segments, with the exception of the esophagus) secrete multiple regulatory molecules that control several functions, such as postprandial secretion and motility[12,73,74]. Animal experimental studies have demonstrated abnormalities in the function of enteroendocrine cells in the setting of gastrointestinal infection[73]. Enteroendocrine cells seem to be involved in visceral hypersensitivity, disturbed gastrointestinal motility, and abnormal gut secretion[12] that patients with IBS usually present[75-77].
In fact, visceral hypersensitivity has been shown in the colon of IBS patients[78-85], but the correlation of this disturbance with the severity of abdominal pain is currently poorly understood[12]. Some authors hypothesize the involvement of a peripheral mechanism in visceral hypersensitivity in IBS[86-88]. Because the gut mucosa can produce high levels of serotonin[88], a reduction in serotonin impairs intracellular uptake and degradation in the gut epithelial cells and consequently increases serotonin availability of in the gut mucosa[89-92]. Therefore, the amount of serotonin available at its receptors is markedly increased[12,87,88]. Due to this mechanism, the development of visceral hypersensitivity in PI-IBS patients may be due to the increase in serotonin at the 5-hydroxytryptamine 3 receptors of the sensory neurons of the enteric nervous system.
Dysmotility has also been shown in the small and large bowel of IBS patients, as evidenced by the involvement of cholecystokinin, ghrelin, secretin, serotonin, and peptide YY[12]. Both esophageal motility abnormalities and abnormal gastric emptying have been observed in IBS patients with conflicting results[93-108]. Specifically, IBS-C patients exhibit delayed gastric emptying, whereas accelerated gastric emptying was observed in IBS-D patients[77,98]. In this setting, ghrelin was shown to stimulate gastric and small- and large-bowel motility[106-117]. Conversely, serotonin relaxes the stomach through a nitrergic pathway and consequently delays gastric emptying[118-120]. Moreover, cholecystokinin[121-123] and secretin[124,125] were shown to relax the proximal stomach, which inhibited gastric emptying in a manner similar to secretin; furthermore, small-bowel transit was also found to be delayed overall in IBS-C patients and accelerated in IBS-D patients[126-131], but conflicting results have been reported[132-139].
Ghrelin, which is involved in the stimulation of small-bowel motility, and peptide YY, a regulator of the ileal brakes[140-145], play pivotal roles in gastric emptying by stimulating the absorption of water and electrolytes and inhibiting prostaglandin E2 and vasoactive intestinal polypeptide[146-148]. Therefore, ghrelin cell density is reportedly low in the stomach, and that of peptide YY is reported to be high in the ileal mucosa of IBS-C patients, whereas the ghrelin cell density is reported to be high in the stomach, and that of secretin is reported to be low in the duodenum of IBS-D patients[12]. Furthermore, colorectal transit was found to be delayed in IBS-C patients and accelerated in IBS-D patients[80,125,126,149-154], but contradictory results have been reported[12,105,152,154-176].
Finally, abnormal gastrointestinal secretion is common in IBS patients. Among the abnormalities in the enteroendocrine cells in IBS patients, low levels of duodenal cholecystokinin (which stimulates the secretion of digestive enzymes from pancreatic exocrine glands) and secretin (which stimulates pancreatic bicarbonate and fluid secretions)[122,123], as well as high levels of ileal peptide YY (which stimulates the absorption of water and electrolytes), were reported in IBS-C patients[12].
The term “mucosal barrier” was adopted by Cummings et al[177] in 2004 to describe “the complex structure that separates the internal milieu from the luminal environment, consisting of the vascular endothelium, the epithelial cell lining, and the mucus layer, next to which digestive secretions, immune molecules, cell products such as cytokines, inflammatory mediators, and antimicrobial peptides, are found, mainly produced by Paneth cells in the crypts of the small intestine”[177,178].
Conversely, impaired intestinal permeability is defined as “an altered permeability being nontransiently changed compared to the normal permeability leading to a loss of intestinal homeostasis, functional impairments and disease”[178]. Increases in the numbers of MCs in the gut of IBS patients were found to be related to changes in gut permeability[32,179-181]. In IBS patients, the assessment of permeability via the urinary recovery of orally administered markers has demonstrated increases in the permeability of the small and in the large bowels[45,182-184], but results have been contradictory[185-187]. Furthermore, rectal permeability was also reportedly increased in IBS-D patients following exposure to MC tryptase[188]. Finally, recent studies of the permeability of the epithelial barrier have reported a decrease in the colonic expression of the tight junction proteins occludin, claudin-1, and zonula occludens-1 in IBS patients[42,189] (Figure 5).
Several aliments, as well as microbiota and bile acids, have been proposed to cause low-grade inflammation and altered permeability in IBS. In fact, in some patients, IBS was related to food allergy[190]. Furthermore, endogenous triggers, such as MC-derived histamine, proteases, and eicosanoids, could increase intestinal permeability, either directly or via the stimulation of neurons of the enteric nervous system[183]. Moreover, serotonin was also identified as an endogenous trigger of pain, inflammation, and increased permeability in IBS[40]; therefore, LX1031, an oral inhibitor of tryptophan hydroxylase, the principal enzyme needed for mucosal serotonin synthesis, has been successful for treatment of patients with non-constipating IBS[191].
Few studies have investigated the role of calcitonin gene related peptide and substance P. Wang et al[37] investigated the incidence of IBS in patients who had recovered from bacillary dysentery by focusing on neuroimmunologic changes, including changes in interleukins, MCs, neuropeptides, and the relationship between MCs and intestinal nerves[50]. The density of substance P-immunoreactive fibers was increased in both the ileal and the rectosigmoid samples of IBS patients[37], but the density of calcitonin gene related peptide-containing fibers remained unchanged. Palsson and coworkers[192] reported similar findings, and Kerkhoffs and coworkers[193] reported an increase of rectal substance P. However, these findings have not been universal[192,194], possibly reflecting region-specific discrepancies[51].
Neuronal plasticity in the enteric nervous system has also been investigated[195]. Akbar et al[38] investigated the capsaicin receptor transient receptor potential vanilloid 1-immunoreactive nerve fibers in colonic biopsies from patients with IBS. Specifically, they demonstrated that the number of nerve fibers exhibiting immunoreactivity for substance P and transient receptor potential vanilloid 1 was increased in IBS patients. Moreover, the number of these fibers did not differ by IBS subtype, but significantly correlated with patient pain scores[50,196].
Nerve growth factor has also been suggested to play a central role in promoting the growth and differentiation of primary afferent fibers[50]. Specifically, its expression was found to be markedly increased in rectal biopsies from pediatric[197] and adult IBS patients[198], suggesting both the sprouting of sensory afferent fibers expressing transient receptor potential vanilloid 1 and increases in receptor sensitivity in IBS, which consequently induced visceral hyperalgesia[50]. More recently, Dothel et al[199] also showed that nerve fiber density and sprouting, as well as the expression of nerve growth and neurotrophic tyrosine kinase receptor type 1, are significantly increased in the mucosal tissues of patients with IBS. Mucosal mediators participate in these neuroplastic changes.
Finally, the morphology of enteric glia, which are known to regulate intestinal barrier integrity and neuronal activity[200] has only been examined in one study of human intestinal biopsy samples from IBS patients and was found to be unchanged[50,201].
Low-grade intestinal inflammation plays a key role in the pathophysiology of IBS, and this role is likely multifactorial[202]. Several studies demonstrated microscopic and molecular abnormalities in IBS patients[202,203].
The above-reported evidence provides a rationale to test the efficacy of intestinal anti-inflammatory compounds in patients with IBS. Previously, treatment with corticosteroids was found to be ineffective in PI-IBS patients[204]; however, MC stabilizers have produced promising results, particularly in IBS-D, suggesting that immune mechanisms and MCs are involved in the generation of IBS symptoms[205,206]. Based on this approach, Clarke and coworkers[207] recently conducted a phase 3, multicenter, tertiary setting, randomized, double-blind, placebo-controlled trial in patients with Rome III-confirmed IBS to evaluate the efficacy and safety of mesalazine in patients with IBS. In this study, mesalazine treatment was not superior to placebo based on the study primary endpoint (68.6% vs 67.4%, 95%CI: 12.8-15.1, P = 0.870). However, the placebo response was high in this trial and this study enrolled both male and female subjects and patients with mild symptoms[207], which likely masked drug efficacy. Furthermore, a subgroup of patients with IBS showed a sustained therapy response and benefits from mesalazine therapy[207].
As mentioned above, abnormalities in the enteric nervous system of the gut may alter digestion, gastrointestinal motility, and visceral hypersensitivity, which contribute to symptom onset and play a pivotal role in the pathogenesis of IBS[12].
The enteric nervous system of the gut seems to be affected by genetic differences, diet, intestinal flora, and inflammation[12]. For example, the food content of FODMAPs and fibers, which interacts with the intestinal flora and drives subsequent fermentation, may increase intestinal osmotic pressure to induce hormonal and serotonin release[12]. Targeting these known factors may improve the control of IBS symptoms by acting on mechanisms that trigger these symptoms and regulate the pathophysiology of IBS. Finally, probiotics have also been found to be effective in select IBS patients, as suggested by several recent systematic reviews, guidelines and meta-analyses, by improving intestinal permeability[208-212].
In conclusion, a high proportion of IBS patients show low-grade inflammation, which is a multifactorial process, in the intestinal mucosa. Understanding the mechanisms underlying the low-grade inflammation in IBS may allow the design of clinical trials that test the efficacy and safety of drugs that target the pathophysiologic mechanism of this disease.
P- Reviewer: Jia HC S- Editor: Yu J L- Editor: Filipodia E- Editor: Wang CH
| 1. | Sinagra E, Romano C, Cottone M. Psychopharmacological treatment and psychological interventions in irritable bowel syndrome. Gastroenterol Res Pract. 2012;2012:486067. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 13] [Cited by in F6Publishing: 16] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 2. | Stamboldjiev T. Management of Irritable Bowel Syndrome in Primary Care. Mas-ter of Arts in Nursing Theses. 2011;10 Available from: http://sophia.stkate.edu/ma_nursing/10. [Cited in This Article: ] |
| 3. | Russo MW, Gaynes BN, Drossman DA. A national survey of practice patterns of gastroenterologists with comparison to the past two decades. J Clin Gastroenterol. 1999;29:339-343. [PubMed] [Cited in This Article: ] |
| 4. | Wald A, Rakel D. Behavioral and complementary approaches for the treatment of irritable bowel syndrome. Nutr Clin Pract. 1999;23:284-292. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 33] [Cited by in F6Publishing: 33] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 5. | Mulak A, Tachè Y. Sex difference in irritable bowel syndrome: do gonadal hormones play a role? Gastroenterol Pol. 2010;17:89-97. [PubMed] [Cited in This Article: ] |
| 6. | Heitkemper M, Jarrett M, Bond EF, Chang L. Impact of sex and gender on irritable bowel syndrome. Biol Res Nurs. 2003;5:56-65. [PubMed] [Cited in This Article: ] |
| 7. | Longstreth GF, Wolde-Tsadik G. Irritable bowel-type symptoms in HMO examinees. Prevalence, demographics, and clinical correlates. Dig Dis Sci. 1993;38:1581-1589. [PubMed] [Cited in This Article: ] |
| 8. | Toner BB, Akman D. Gender role and irritable bowel syndrome: literature review and hypothesis. Am J Gastroenterol. 2000;95:11-16. [PubMed] [Cited in This Article: ] |
| 9. | Longstreth GF, Thompson WG, Chey WD, Houghton LA, Mearin F, Spiller RC. Functional bowel disorders. Gastroenterology. 2006;130:1480-1491. [PubMed] [Cited in This Article: ] |
| 10. | Lewis SJ, Heaton KW. Stool form scale as a useful guide to intestinal transit time. Scand J Gastroenterol. 1997;32:920-924. [PubMed] [Cited in This Article: ] |
| 11. | El-Salhy M, Gundersen D, Hatlebakk JG, Hausken T. Irritable bowel syndrome: diagnosis, pathogenesis and treatment options. New York: Nova Science Publishers 2012; . [Cited in This Article: ] |
| 12. | El-Salhy M, Gundersen D, Gilja OH, Hatlebakk JG, Hausken T. Is irritable bowel syndrome an organic disorder? World J Gastroenterol. 2014;20:384-400. [PubMed] [DOI] [Cited in This Article: ] [Cited by in CrossRef: 71] [Cited by in F6Publishing: 68] [Article Influence: 6.8] [Reference Citation Analysis (0)] |
| 13. | Lee YJ, Park KS. Irritable bowel syndrome: emerging paradigm in pathophysiology. World J Gastroenterol. 2014;20:2456-2469. [PubMed] [DOI] [Cited in This Article: ] [Cited by in CrossRef: 102] [Cited by in F6Publishing: 99] [Article Influence: 9.9] [Reference Citation Analysis (2)] |
| 14. | Drossman DA, Camilleri M, Mayer EA, Whitehead WE. AGA technical review on irritable bowel syndrome. Gastroenterology. 2002;123:2108-2131. [PubMed] [Cited in This Article: ] |
| 15. | Spiller R, Aziz Q, Creed F, Emmanuel A, Houghton L, Hungin P, Jones R, Kumar D, Rubin G, Trudgill N. Guidelines on the irritable bowel syndrome: mechanisms and practical management. Gut. 2007;56:1770-1798. [PubMed] [Cited in This Article: ] |
| 16. | Fukudo S, Kaneko H, Akiho H, Inamori M, Endo Y, Okumura T, Kanazawa M, Kamiya T, Sato K, Chiba T. Evidence-based clinical practice guidelines for irritable bowel syndrome. J Gastroenterol. 2015;50:11-30. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 89] [Cited by in F6Publishing: 93] [Article Influence: 10.3] [Reference Citation Analysis (0)] |
| 17. | Ishihara S, Yashima K, Kushiyama Y, Izumi A, Kawashima K, Fujishiro H, Kojo H, Komazawa Y, Hamamoto T, Yamamoto T. Prevalence of organic colonic lesions in patients meeting Rome III criteria for diagnosis of IBS: a prospective multi-center study utilizing colonoscopy. J Gastroenterol. 2012;47:1084-1090. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 27] [Cited by in F6Publishing: 29] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 18. | Hsiao CW, Huang WY, Ke TW, Muo CH, Chen WT, Sung FC, Kao CH. Association between irritable bowel syndrome and colorectal cancer: a nationwide population-based study. Eur J Intern Med. 2014;25:82-86. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 28] [Cited by in F6Publishing: 21] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 19. | Ahn E, Son KY, Shin DW, Han MK, Lee H, An AR, Kim EH, Cho B. Perceived risk as a barrier to appropriate diagnosis of irritable bowel syndrome. World J Gastroenterol. 2014;20:18360-18366. [PubMed] [DOI] [Cited in This Article: ] [Cited by in CrossRef: 4] [Cited by in F6Publishing: 3] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 20. | Lieberman DA, Williams JL, Holub JL, Morris CD, Logan JR, Eisen GM, Carney P. Colonoscopy utilization and outcomes 2000 to 2011. Gastrointest Endosc. 2014;80:133-143. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 86] [Cited by in F6Publishing: 88] [Article Influence: 8.8] [Reference Citation Analysis (0)] |
| 21. | McKenna BJ. Is it really colitis? Dealing with the nearly normal colonic biopsy and variations of microscopic colitis. Pathol Case Rev. 2004;9:106-114. [DOI] [Cited in This Article: ] [Cited by in Crossref: 6] [Cited by in F6Publishing: 6] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 22. | Kirsch R, Riddell RH. Histopathological alterations in irritable bowel syndrome. Mod Pathol. 2006;19:1638-1645. [PubMed] [Cited in This Article: ] |
| 23. | Barbara G, De Giorgio R, Stanghellini V, Cremon C, Corinaldesi R. A role for inflammation in irritable bowel syndrome? Gut. 2002;51 Suppl 1:i41-i44. [PubMed] [Cited in This Article: ] |
| 24. | Lee E, Schiller LR, Fordtran JS. Quantification of colonic lamina propria cells by means of a morphometric point-counting method. Gastroenterology. 1988;94:409-418. [PubMed] [Cited in This Article: ] |
| 25. | Salzmann JL, Peltier-Koch F, Bloch F, Petite JP, Camilleri JP. Morphometric study of colonic biopsies: a new method of estimating inflammatory diseases. Lab Invest. 1989;60:847-851. [PubMed] [Cited in This Article: ] |
| 26. | O’Sullivan M, Clayton N, Breslin NP, Harman I, Bountra C, McLaren A, O’Morain CA. Increased mast cells in the irritable bowel syndrome. Neurogastroenterol Motil. 2000;12:449-457. [PubMed] [Cited in This Article: ] |
| 27. | Piche T, Saint-Paul MC, Dainese R, Marine-Barjoan E, Iannelli A, Montoya ML, Peyron JF, Czerucka D, Cherikh F, Filippi J. Mast cells and cellularity of the colonic mucosa correlated with fatigue and depression in irritable bowel syndrome. Gut. 2008;57:468-473. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 142] [Cited by in F6Publishing: 141] [Article Influence: 8.8] [Reference Citation Analysis (0)] |
| 28. | Ford AC, Talley NJ. Mucosal inflammation as a potential etiological factor in irritable bowel syndrome: a systematic review. J Gastroenterol. 2011;46:421-431. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 129] [Cited by in F6Publishing: 138] [Article Influence: 10.6] [Reference Citation Analysis (0)] |
| 29. | Walker MM, Talley NJ, Prabhakar M, Pennaneac’h CJ, Aro P, Ronkainen J, Storskrubb T, Harmsen WS, Zinsmeister AR, Agreus L. Duodenal mastocytosis, eosinophilia and intraepithelial lymphocytosis as possible disease markers in the irritable bowel syndrome and functional dyspepsia. Aliment Pharmacol Ther. 2009;29:765-773. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 199] [Cited by in F6Publishing: 195] [Article Influence: 13.0] [Reference Citation Analysis (0)] |
| 30. | Ford AC, Marwaha A, Lim A, Moayyedi P. Systematic review and meta-analysis of the prevalence of irritable bowel syndrome in individuals with dyspepsia. Clin Gastroenterol Hepatol. 2010;8:401-409. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 130] [Cited by in F6Publishing: 132] [Article Influence: 9.4] [Reference Citation Analysis (0)] |
| 31. | Lembo AJ, Neri B, Tolley J, Barken D, Carroll S, Pan H. Use of serum biomarkers in a diagnostic test for irritable bowel syndrome. Aliment Pharmacol Ther. 2009;29:834-842. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 74] [Cited by in F6Publishing: 63] [Article Influence: 4.2] [Reference Citation Analysis (0)] |
| 32. | Matricon J, Meleine M, Gelot A, Piche T, Dapoigny M, Muller E, Ardid D. Review article: Associations between immune activation, intestinal permeability and the irritable bowel syndrome. Aliment Pharmacol Ther. 2012;36:1009-1031. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 145] [Cited by in F6Publishing: 134] [Article Influence: 11.2] [Reference Citation Analysis (1)] |
| 33. | Hiatt RB, Katz L. Mast cells in inflammatory conditions of the gastrointestinal tract. Am J Gastroenterol. 1962;37:541-545. [PubMed] [Cited in This Article: ] |
| 34. | De Giorgio R, Barbara G. Is irritable bowel syndrome an inflammatory disorder? Curr Gastroenterol Rep. 2008;10:385-390. [PubMed] [Cited in This Article: ] |
| 35. | Dunlop SP, Jenkins D, Spiller RC. Distinctive clinical, psychological, and histological features of postinfective irritable bowel syndrome. Am J Gastroenterol. 2003;98:1578-1583. [PubMed] [Cited in This Article: ] |
| 36. | Park JH, Rhee PL, Kim HS, Lee JH, Kim YH, Kim JJ, Rhee JC. Mucosal mast cell counts correlate with visceral hypersensitivity in patients with diarrhea predominant irritable bowel syndrome. J Gastroenterol Hepatol. 2006;21:71-78. [PubMed] [Cited in This Article: ] |
| 37. | Wang LH, Fang XC, Pan GZ. Bacillary dysentery as a causative factor of irritable bowel syndrome and its pathogenesis. Gut. 2004;53:1096-1101. [PubMed] [Cited in This Article: ] |
| 38. | Akbar A, Yiangou Y, Facer P, Walters JR, Anand P, Ghosh S. Increased capsaicin receptor TRPV1-expressing sensory fibres in irritable bowel syndrome and their correlation with abdominal pain. Gut. 2008;57:923-929. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 359] [Cited by in F6Publishing: 362] [Article Influence: 22.6] [Reference Citation Analysis (0)] |
| 39. | Barbara G, Wang B, Stanghellini V, de Giorgio R, Cremon C, Di Nardo G, Trevisani M, Campi B, Geppetti P, Tonini M. Mast cell-dependent excitation of visceral-nociceptive sensory neurons in irritable bowel syndrome. Gastroenterology. 2007;132:26-37. [PubMed] [Cited in This Article: ] |
| 40. | Cremon C, Carini G, Wang B, Vasina V, Cogliandro RF, De Giorgio R, Stanghellini V, Grundy D, Tonini M, De Ponti F. Intestinal serotonin release, sensory neuron activation, and abdominal pain in irritable bowel syndrome. Am J Gastroenterol. 2011;106:1290-1298. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 143] [Cited by in F6Publishing: 156] [Article Influence: 12.0] [Reference Citation Analysis (0)] |
| 41. | Buhner S, Li Q, Vignali S, Barbara G, De Giorgio R, Stanghellini V, Cremon C, Zeller F, Langer R, Daniel H. Activation of human enteric neurons by supernatants of colonic biopsy specimens from patients with irritable bowel syndrome. Gastroenterology. 2009;137:1425-1434. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 254] [Cited by in F6Publishing: 262] [Article Influence: 17.5] [Reference Citation Analysis (0)] |
| 42. | Coëffier M, Gloro R, Boukhettala N, Aziz M, Lecleire S, Vandaele N, Antonietti M, Savoye G, Bôle-Feysot C, Déchelotte P. Increased proteasome-mediated degradation of occludin in irritable bowel syndrome. Am J Gastroenterol. 2010;105:1181-1188. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 125] [Cited by in F6Publishing: 132] [Article Influence: 9.4] [Reference Citation Analysis (0)] |
| 43. | Cremon C, Gargano L, Morselli-Labate AM, Santini D, Cogliandro RF, De Giorgio R, Stanghellini V, Corinaldesi R, Barbara G. Mucosal immune activation in irritable bowel syndrome: gender-dependence and association with digestive symptoms. Am J Gastroenterol. 2009;104:392-400. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 244] [Cited by in F6Publishing: 254] [Article Influence: 16.9] [Reference Citation Analysis (0)] |
| 44. | Barbara G, Stanghellini V, De Giorgio R, Cremon C, Cottrell GS, Santini D, Pasquinelli G, Morselli-Labate AM, Grady EF, Bunnett NW. Activated mast cells in proximity to colonic nerves correlate with abdominal pain in irritable bowel syndrome. Gastroenterology. 2004;126:693-702. [PubMed] [Cited in This Article: ] |
| 45. | Vivinus-Nébot M, Dainese R, Anty R, Saint-Paul MC, Nano JL, Gonthier N, Marjoux S, Frin-Mathy G, Bernard G, Hébuterne X. Combination of allergic factors can worsen diarrheic irritable bowel syndrome: role of barrier defects and mast cells. Am J Gastroenterol. 2012;107:75-81. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 99] [Cited by in F6Publishing: 100] [Article Influence: 8.3] [Reference Citation Analysis (0)] |
| 46. | Wang SH, Dong L, Luo JY, Gong J, Li L, Lu XL, Han SP. Decreased expression of serotonin in the jejunum and increased numbers of mast cells in the terminal ileum in patients with irritable bowel syndrome. World J Gastroenterol. 2007;13:6041-6047. [PubMed] [DOI] [Cited in This Article: ] [Cited by in CrossRef: 30] [Cited by in F6Publishing: 51] [Article Influence: 3.2] [Reference Citation Analysis (0)] |
| 47. | Martínez C, Vicario M, Ramos L, Lobo B, Mosquera JL, Alonso C, Sánchez A, Guilarte M, Antolín M, de Torres I. The jejunum of diarrhea-predominant irritable bowel syndrome shows molecular alterations in the tight junction signaling pathway that are associated with mucosal pathobiology and clinical manifestations. Am J Gastroenterol. 2012;107:736-746. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 150] [Cited by in F6Publishing: 157] [Article Influence: 13.1] [Reference Citation Analysis (0)] |
| 48. | Martínez C, Lobo B, Pigrau M, Ramos L, González-Castro AM, Alonso C, Guilarte M, Guilá M, de Torres I, Azpiroz F. Diarrhoea-predominant irritable bowel syndrome: an organic disorder with structural abnormalities in the jejunal epithelial barrier. Gut. 2013;62:1160-1168. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 193] [Cited by in F6Publishing: 189] [Article Influence: 17.2] [Reference Citation Analysis (0)] |
| 49. | Guilarte M, Santos J, de Torres I, Alonso C, Vicario M, Ramos L, Martínez C, Casellas F, Saperas E, Malagelada JR. Diarrhoea-predominant IBS patients show mast cell activation and hyperplasia in the jejunum. Gut. 2007;56:203-209. [PubMed] [Cited in This Article: ] |
| 50. | Nasser Y, Boeckxstaens GE, Wouters MM, Schemann M, Vanner S. Using human intestinal biopsies to study the pathogenesis of irritable bowel syndrome. Neurogastroenterol Motil. 2014;26:455-469. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 39] [Cited by in F6Publishing: 35] [Article Influence: 3.5] [Reference Citation Analysis (0)] |
| 51. | Schemann M, Camilleri M. Functions and imaging of mast cell and neural axis of the gut. Gastroenterology. 2013;144:698-704.e4. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 58] [Cited by in F6Publishing: 64] [Article Influence: 5.8] [Reference Citation Analysis (0)] |
| 52. | Spiller RC, Jenkins D, Thornley JP, Hebden JM, Wright T, Skinner M, Neal KR. Increased rectal mucosal enteroendocrine cells, T lymphocytes, and increased gut permeability following acute Campylobacter enteritis and in post-dysenteric irritable bowel syndrome. Gut. 2000;47:804-811. [PubMed] [Cited in This Article: ] |
| 53. | Kim HS, Lim JH, Park H, Lee SI. Increased immunoendocrine cells in intestinal mucosa of postinfectious irritable bowel syndrome patients 3 years after acute Shigella infection--an observation in a small case control study. Yonsei Med J. 2010;51:45-51. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 57] [Cited by in F6Publishing: 66] [Article Influence: 4.7] [Reference Citation Analysis (0)] |
| 54. | Ohman L, Isaksson S, Lindmark AC, Posserud I, Stotzer PO, Strid H, Sjövall H, Simrén M. T-cell activation in patients with irritable bowel syndrome. Am J Gastroenterol. 2009;104:1205-1212. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 108] [Cited by in F6Publishing: 116] [Article Influence: 7.7] [Reference Citation Analysis (0)] |
| 55. | Törnblom H, Lindberg G, Nyberg B, Veress B. Full-thickness biopsy of the jejunum reveals inflammation and enteric neuropathy in irritable bowel syndrome. Gastroenterology. 2002;123:1972-1979. [PubMed] [Cited in This Article: ] |
| 56. | Foley S, Garsed K, Singh G, Duroudier NP, Swan C, Hall IP, Zaitoun A, Bennett A, Marsden C, Holmes G. Impaired uptake of serotonin by platelets from patients with irritable bowel syndrome correlates with duodenal immune activation. Gastroenterology. 2011;140:1434-1443.e1. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 96] [Cited by in F6Publishing: 99] [Article Influence: 7.6] [Reference Citation Analysis (0)] |
| 57. | Chadwick VS, Chen W, Shu D, Paulus B, Bethwaite P, Tie A, Wilson I. Activation of the mucosal immune system in irritable bowel syndrome. Gastroenterology. 2002;122:1778-1783. [PubMed] [Cited in This Article: ] |
| 58. | Lee KJ, Kim YB, Kim JH, Kwon HC, Kim DK, Cho SW. The alteration of enterochromaffin cell, mast cell, and lamina propria T lymphocyte numbers in irritable bowel syndrome and its relationship with psychological factors. J Gastroenterol Hepatol. 2008;23:1689-1694. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 118] [Cited by in F6Publishing: 119] [Article Influence: 7.4] [Reference Citation Analysis (0)] |
| 59. | Faure C, Patey N, Gauthier C, Brooks EM, Mawe GM. Serotonin signaling is altered in irritable bowel syndrome with diarrhea but not in functional dyspepsia in pediatric age patients. Gastroenterology. 2010;139:249-258. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 113] [Cited by in F6Publishing: 122] [Article Influence: 8.7] [Reference Citation Analysis (0)] |
| 60. | Park JH, Rhee PL, Kim G, Lee JH, Kim YH, Kim JJ, Rhee JC, Song SY. Enteroendocrine cell counts correlate with visceral hypersensitivity in patients with diarrhoea-predominant irritable bowel syndrome. Neurogastroenterol Motil. 2006;18:539-546. [PubMed] [Cited in This Article: ] |
| 61. | Park H. [The pathophysiology of irritable bowel syndrome: inflammation and motor disorder]. Korean J Gastroenterol. 2006;47:101-110. [PubMed] [Cited in This Article: ] |
| 62. | Forshammar J, Isaksson S, Strid H, Stotzer PO, Sjövall H, Simrén M, Ohman L. A pilot study of colonic B cell pattern in irritable bowel syndrome. Scand J Gastroenterol. 2008;43:1461-1466. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 16] [Cited by in F6Publishing: 17] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 63. | Steinman RM. The dendritic cell system and its role in immunogenicity. Annu Rev Immunol. 1991;9:271-296. [PubMed] [Cited in This Article: ] |
| 64. | Adams S, O’Neill DW, Bhardwaj N. Recent advances in dendritic cell biology. J Clin Immunol. 2005;25:177-188. [PubMed] [Cited in This Article: ] |
| 65. | Koido S, Ohkusa T, Kan S, Takakura K, Saito K, Komita H, Ito Z, Kobayashi H, Takami S, Uchiyama K. Production of corticotropin-releasing factor and urocortin from human monocyte-derived dendritic cells is stimulated by commensal bacteria in intestine. World J Gastroenterol. 2014;20:14420-14429. [PubMed] [DOI] [Cited in This Article: ] [Cited by in CrossRef: 16] [Cited by in F6Publishing: 17] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 66. | Long Y, Wang W, Wang H, Hao L, Qian W, Hou X. Characteristics of intestinal lamina propria dendritic cells in a mouse model of postinfectious irritable bowel syndrome. J Gastroenterol Hepatol. 2012;27:935-944. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 22] [Cited by in F6Publishing: 27] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 67. | Beatty JK, Bhargava A, Buret AG. Post-infectious irritable bowel syndrome: mechanistic insights into chronic disturbances following enteric infection. World J Gastroenterol. 2014;20:3976-3985. [PubMed] [DOI] [Cited in This Article: ] [Cited by in CrossRef: 69] [Cited by in F6Publishing: 64] [Article Influence: 6.4] [Reference Citation Analysis (0)] |
| 68. | Ji S, Park H, Lee D, Song YK, Choi JP, Lee SI. Post-infectious irritable bowel syndrome in patients with Shigella infection. J Gastroenterol Hepatol. 2005;20:381-386. [PubMed] [Cited in This Article: ] |
| 69. | Kim HS, Kim MS, Ji SW, Park H. [The development of irritable bowel syndrome after Shigella infection: 3 year follow-up study]. Korean J Gastroenterol. 2006;47:300-305. [PubMed] [Cited in This Article: ] |
| 70. | Kindt S, Van Oudenhove L, Broekaert D, Kasran A, Ceuppens JL, Bossuyt X, Fischler B, Tack J. Immune dysfunction in patients with functional gastrointestinal disorders. Neurogastroenterol Motil. 2009;21:389-398. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 105] [Cited by in F6Publishing: 111] [Article Influence: 7.4] [Reference Citation Analysis (0)] |
| 71. | Liebregts T, Adam B, Bredack C, Röth A, Heinzel S, Lester S, Downie-Doyle S, Smith E, Drew P, Talley NJ. Immune activation in patients with irritable bowel syndrome. Gastroenterology. 2007;132:913-920. [PubMed] [Cited in This Article: ] |
| 72. | Miao EA, Rajan JV. Salmonella and Caspase-1: A complex Interplay of Detection and Evasion. Front Microbiol. 2011;2:85. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 38] [Cited by in F6Publishing: 42] [Article Influence: 3.2] [Reference Citation Analysis (0)] |
| 73. | Moran GW, Leslie FC, Levison SE, Worthington J, McLaughlin JT. Enteroendocrine cells: neglected players in gastrointestinal disorders? Therap Adv Gastroenterol. 2008;1:51-60. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 61] [Cited by in F6Publishing: 64] [Article Influence: 4.9] [Reference Citation Analysis (0)] |
| 74. | Rindi G, Inzani F, Solcia E. Pathology of gastrointestinal disorders. Endocrinol Metab Clin North Am. 2010;39:713-727. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 25] [Cited by in F6Publishing: 25] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 75. | Gunnarsson J, Simrén M. Peripheral factors in the pathophysiology of irritable bowel syndrome. Dig Liver Dis. 2009;41:788-793. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 34] [Cited by in F6Publishing: 36] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 76. | Delgado-Aros S, Camilleri M. Visceral hypersensitivity. J Clin Gastroenterol. 2005;39:S194-S203; discussion S210. [PubMed] [Cited in This Article: ] |
| 77. | Lee OY. Asian motility studies in irritable bowel syndrome. J Neurogastroenterol Motil. 2010;16:120-130. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 33] [Cited by in F6Publishing: 36] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 78. | Posserud I, Syrous A, Lindström L, Tack J, Abrahamsson H, Simrén M. Altered rectal perception in irritable bowel syndrome is associated with symptom severity. Gastroenterology. 2007;133:1113-1123. [PubMed] [Cited in This Article: ] |
| 79. | Ritchie J. Pain from distension of the pelvic colon by inflating a balloon in the irritable colon syndrome. Gut. 1973;14:125-132. [PubMed] [Cited in This Article: ] |
| 80. | Mertz H, Naliboff B, Munakata J, Niazi N, Mayer EA. Altered rectal perception is a biological marker of patients with irritable bowel syndrome. Gastroenterology. 1995;109:40-52. [PubMed] [Cited in This Article: ] |
| 81. | Whitehead WE, Palsson OS. Is rectal pain sensitivity a biological marker for irritable bowel syndrome: psychological influences on pain perception. Gastroenterology. 1998;115:1263-1271. [PubMed] [Cited in This Article: ] |
| 82. | Whitehead WE, Holtkotter B, Enck P, Hoelzl R, Holmes KD, Anthony J, Shabsin HS, Schuster MM. Tolerance for rectosigmoid distention in irritable bowel syndrome. Gastroenterology. 1990;98:1187-1192. [PubMed] [Cited in This Article: ] |
| 83. | Bouin M, Plourde V, Boivin M, Riberdy M, Lupien F, Laganière M, Verrier P, Poitras P. Rectal distention testing in patients with irritable bowel syndrome: sensitivity, specificity, and predictive values of pain sensory thresholds. Gastroenterology. 2002;122:1771-1777. [PubMed] [Cited in This Article: ] |
| 84. | Bradette M, Delvaux M, Staumont G, Fioramonti J, Bueno L, Frexinos J. Evaluation of colonic sensory thresholds in IBS patients using a barostat. Definition of optimal conditions and comparison with healthy subjects. Dig Dis Sci. 1994;39:449-457. [PubMed] [Cited in This Article: ] |
| 85. | Camilleri M, McKinzie S, Busciglio I, Low PA, Sweetser S, Burton D, Baxter K, Ryks M, Zinsmeister AR. Prospective study of motor, sensory, psychologic, and autonomic functions in patients with irritable bowel syndrome. Clin Gastroenterol Hepatol. 2008;6:772-781. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 182] [Cited by in F6Publishing: 193] [Article Influence: 12.1] [Reference Citation Analysis (0)] |
| 86. | Wendelbo I, Mazzawi T, El-Salhy M. Increased serotonin transporter immunoreactivity intensity in the ileum of patients with irritable bowel disease. Mol Med Rep. 2014;9:180-184. [PubMed] [Cited in This Article: ] |
| 87. | Coates MD, Mahoney CR, Linden DR, Sampson JE, Chen J, Blaszyk H, Crowell MD, Sharkey KA, Gershon MD, Mawe GM. Molecular defects in mucosal serotonin content and decreased serotonin reuptake transporter in ulcerative colitis and irritable bowel syndrome. Gastroenterology. 2004;126:1657-1664. [PubMed] [Cited in This Article: ] |
| 88. | El-Salhy M, Wendelbo IH, Gundersen D. Reduced chromogranin A cell density in the ileum of patients with irritable bowel syndrome. Mol Med Rep. 2013;7:1241-1244. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 24] [Cited by in F6Publishing: 28] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 89. | Chen JX, Pan H, Rothman TP, Wade PR, Gershon MD. Guinea pig 5-HT transporter: cloning, expression, distribution, and function in intestinal sensory reception. Am J Physiol. 1998;275:G433-G448. [PubMed] [Cited in This Article: ] |
| 90. | Keating C, Beyak M, Foley S, Singh G, Marsden C, Spiller R, Grundy D. Afferent hypersensitivity in a mouse model of post-inflammatory gut dysfunction: role of altered serotonin metabolism. J Physiol. 2008;586:4517-4530. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 66] [Cited by in F6Publishing: 71] [Article Influence: 4.4] [Reference Citation Analysis (0)] |
| 91. | Coleman NS, Foley S, Dunlop SP, Wheatcroft J, Blackshaw E, Perkins AC, Singh G, Marsden CA, Holmes GK, Spiller RC. Abnormalities of serotonin metabolism and their relation to symptoms in untreated celiac disease. Clin Gastroenterol Hepatol. 2006;4:874-881. [PubMed] [Cited in This Article: ] |
| 92. | Gershon MD, Tack J. The serotonin signaling system: from basic understanding to drug development for functional GI disorders. Gastroenterology. 2007;132:397-414. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 988] [Cited by in F6Publishing: 1025] [Article Influence: 60.3] [Reference Citation Analysis (0)] |
| 93. | Whorwell PJ, Clouter C, Smith CL. Oesophageal motility in the irritable bowel syndrome. Br Med J (Clin Res Ed). 1981;282:1101-1102. [PubMed] [Cited in This Article: ] |
| 94. | Clouse RE, Eckert TC. Gastrointestinal symptoms of patients with esophageal contraction abnormalities. Dig Dis Sci. 1986;31:236-240. [PubMed] [Cited in This Article: ] |
| 95. | Soffer EE, Scalabrini P, Pope CE, Wingate DL. Effect of stress on oesophageal motor function in normal subjects and in patients with the irritable bowel syndrome. Gut. 1988;29:1591-1594. [PubMed] [Cited in This Article: ] |
| 96. | Lind CD. Motility disorders in the irritable bowel syndrome. Gastroenterol Clin North Am. 1991;20:279-295. [PubMed] [Cited in This Article: ] |
| 97. | van Wijk HJ, Smout AJ, Akkermans LM, Roelofs JM, ten Thije OJ. Gastric emptying and dyspeptic symptoms in the irritable bowel syndrome. Scand J Gastroenterol. 1992;27:99-102. [PubMed] [Cited in This Article: ] |
| 98. | Charles F, Phillips SF, Camilleri M, Thomforde GM. Rapid gastric emptying in patients with functional diarrhea. Mayo Clin Proc. 1997;72:323-328. [PubMed] [DOI] [Cited in This Article: ] [Cited by in F6Publishing: 2] [Reference Citation Analysis (0)] |
| 99. | Caballero-Plasencia AM, Valenzuela-Barranco M, Herrerías-Gutiérrez JM, Esteban-Carretero JM. Altered gastric emptying in patients with irritable bowel syndrome. Eur J Nucl Med. 1999;26:404-409. [PubMed] [Cited in This Article: ] |
| 100. | Morin DR. The patient’s records and the defense of dental malpractice claims. Am J Orthod Dentofacial Orthop. 1992;102:569-570. [PubMed] [Cited in This Article: ] |
| 101. | Stanghellini V, Tosetti C, Barbara G, De Giorgio R, Cogliandro L, Cogliandro R, Corinaldesi R. Dyspeptic symptoms and gastric emptying in the irritable bowel syndrome. Am J Gastroenterol. 2002;97:2738-2743. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 66] [Cited by in F6Publishing: 67] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 102. | Leahy A, Besherdas K, Clayman C, Mason I, Epstein O. Abnormalities of the electrogastrogram in functional gastrointestinal disorders. Am J Gastroenterol. 1999;94:1023-1028. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 80] [Cited by in F6Publishing: 89] [Article Influence: 3.6] [Reference Citation Analysis (0)] |
| 103. | Evans PR, Bak YT, Shuter B, Hoschl R, Kellow JE. Gastroparesis and small bowel dysmotility in irritable bowel syndrome. Dig Dis Sci. 1997;42:2087-2093. [PubMed] [Cited in This Article: ] |
| 104. | Nielsen OH, Gjørup T, Christensen FN. Gastric emptying rate and small bowel transit time in patients with irritable bowel syndrome determined with 99mTc-labeled pellets and scintigraphy. Dig Dis Sci. 1986;31:1287-1291. [PubMed] [Cited in This Article: ] |
| 105. | Acharya U, Waite N, Howlett P, Tanner AR, Smith CL. Failure to demonstrate altered gastric emptying in irritable bowel syndrome. Dig Dis Sci. 1983;28:889-892. [PubMed] [Cited in This Article: ] |
| 106. | El-Salhy M. Ghrelin in gastrointestinal diseases and disorders: a possible role in the pathophysiology and clinical implications (review). Int J Mol Med. 2009;24:727-732. [PubMed] [Cited in This Article: ] |
| 107. | Asakawa A, Ataka K, Fujino K, Chen CY, Kato I, Fujimiya M, Inui A. Ghrelin family of peptides and gut motility. J Gastroenterol Hepatol. 2011;26 Suppl 3:73-74. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 13] [Cited by in F6Publishing: 14] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 108. | Dornonville de la Cour C, Lindström E, Norlén P, Håkanson R. Ghrelin stimulates gastric emptying but is without effect on acid secretion and gastric endocrine cells. Regul Pept. 2004;120:23-32. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 114] [Cited by in F6Publishing: 126] [Article Influence: 6.3] [Reference Citation Analysis (0)] |
| 109. | Fukuda H, Mizuta Y, Isomoto H, Takeshima F, Ohnita K, Ohba K, Omagari K, Taniyama K, Kohno S. Ghrelin enhances gastric motility through direct stimulation of intrinsic neural pathways and capsaicin-sensitive afferent neurones in rats. Scand J Gastroenterol. 2004;39:1209-1214. [PubMed] [Cited in This Article: ] |
| 110. | Levin F, Edholm T, Schmidt PT, Grybäck P, Jacobsson H, Degerblad M, Höybye C, Holst JJ, Rehfeld JF, Hellström PM. Ghrelin stimulates gastric emptying and hunger in normal-weight humans. J Clin Endocrinol Metab. 2006;91:3296-3302. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 213] [Cited by in F6Publishing: 223] [Article Influence: 12.4] [Reference Citation Analysis (0)] |
| 111. | Edholm T, Levin F, Hellström PM, Schmidt PT. Ghrelin stimulates motility in the small intestine of rats through intrinsic cholinergic neurons. Regul Pept. 2004;121:25-30. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 99] [Cited by in F6Publishing: 108] [Article Influence: 5.4] [Reference Citation Analysis (0)] |
| 112. | Tack J, Depoortere I, Bisschops R, Delporte C, Coulie B, Meulemans A, Janssens J, Peeters T. Influence of ghrelin on interdigestive gastrointestinal motility in humans. Gut. 2006;55:327-333. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 259] [Cited by in F6Publishing: 276] [Article Influence: 15.3] [Reference Citation Analysis (0)] |
| 113. | Ariga H, Tsukamoto K, Chen C, Mantyh C, Pappas TN, Takahashi T. Endogenous acyl ghrelin is involved in mediating spontaneous phase III-like contractions of the rat stomach. Neurogastroenterol Motil. 2007;19:675-680. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 80] [Cited by in F6Publishing: 82] [Article Influence: 4.8] [Reference Citation Analysis (0)] |
| 114. | Ariga H, Nakade Y, Tsukamoto K, Imai K, Chen C, Mantyh C, Pappas TN, Takahashi T. Ghrelin accelerates gastric emptying via early manifestation of antro-pyloric coordination in conscious rats. Regul Pept. 2008;146:112-116. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 38] [Cited by in F6Publishing: 44] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 115. | Tümer C, Oflazoğlu HD, Obay BD, Kelle M, Taşdemir E. Effect of ghrelin on gastric myoelectric activity and gastric emptying in rats. Regul Pept. 2008;146:26-32. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 33] [Cited by in F6Publishing: 36] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 116. | Tack JF, Janssens J, Vantrappen G, Wood JD. Actions of 5-hydroxytryptamine on myenteric neurons in guinea pig gastric antrum. Am J Physiol. 1992;263:G838-G846. [PubMed] [Cited in This Article: ] |
| 117. | Michel K, Sann H, Schaaf C, Schemann M. Subpopulations of gastric myenteric neurons are differentially activated via distinct serotonin receptors: projection, neurochemical coding, and functional implications. J Neurosci. 1997;17:8009-8017. [PubMed] [Cited in This Article: ] |
| 118. | Camilleri M. Integrated upper gastrointestinal response to food intake. Gastroenterology. 2006;131:640-658. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 181] [Cited by in F6Publishing: 183] [Article Influence: 10.2] [Reference Citation Analysis (0)] |
| 119. | Lal S, McLaughlin J, Barlow J, D’Amato M, Giacovelli G, Varro A, Dockray GJ, Thompson DG. Cholecystokinin pathways modulate sensations induced by gastric distension in humans. Am J Physiol Gastrointest Liver Physiol. 2004;287:G72-G79. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 54] [Cited by in F6Publishing: 62] [Article Influence: 3.1] [Reference Citation Analysis (0)] |
| 120. | Chey WY, Chang TM. Secretin, 100 years later. J Gastroenterol. 2003;38:1025-1035. [PubMed] [Cited in This Article: ] |
| 121. | Cann PA, Read NW, Brown C, Hobson N, Holdsworth CD. Irritable bowel syndrome: relationship of disorders in the transit of a single solid meal to symptom patterns. Gut. 1983;24:405-411. [PubMed] [Cited in This Article: ] |
| 122. | Sadik R, Stotzer PO, Simrén M, Abrahamsson H. Gastrointestinal transit abnormalities are frequently detected in patients with unexplained GI symptoms at a tertiary centre. Neurogastroenterol Motil. 2008;20:197-205. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 60] [Cited by in F6Publishing: 61] [Article Influence: 3.8] [Reference Citation Analysis (0)] |
| 123. | Jian R, Najean Y, Bernier JJ. Measurement of intestinal progression of a meal and its residues in normal subjects and patients with functional diarrhoea by a dual isotope technique. Gut. 1984;25:728-731. [PubMed] [Cited in This Article: ] |
| 124. | Corbett CL, Thomas S, Read NW, Hobson N, Bergman I, Holdsworth CD. Electrochemical detector for breath hydrogen determination: measurement of small bowel transit time in normal subjects and patients with the irritable bowel syndrome. Gut. 1981;22:836-840. [PubMed] [Cited in This Article: ] |
| 125. | Kellow JE, Phillips SF, Miller LJ, Zinsmeister AR. Dysmotility of the small intestine in irritable bowel syndrome. Gut. 1988;29:1236-1243. [PubMed] [Cited in This Article: ] |
| 126. | Kellow JE, Phillips SF. Altered small bowel motility in irritable bowel syndrome is correlated with symptoms. Gastroenterology. 1987;92:1885-1893. [PubMed] [Cited in This Article: ] |
| 127. | Kellow JE, Gill RC, Wingate DL. Prolonged ambulant recordings of small bowel motility demonstrate abnormalities in the irritable bowel syndrome. Gastroenterology. 1990;98:1208-1218. [PubMed] [Cited in This Article: ] |
| 128. | Quigley EM, Donovan JP, Lane MJ, Gallagher TF. Antroduodenal manometry. Usefulness and limitations as an outpatient study. Dig Dis Sci. 1992;37:20-28. [PubMed] [Cited in This Article: ] |
| 129. | Quigley EM. Intestinal manometry--technical advances, clinical limitations. Dig Dis Sci. 1992;37:10-13. [PubMed] [Cited in This Article: ] |
| 130. | Gorard DA, Libby GW, Farthing MJ. Ambulatory small intestinal motility in ‘diarrhoea’ predominant irritable bowel syndrome. Gut. 1994;35:203-210. [PubMed] [Cited in This Article: ] |
| 131. | Zhao JH, Dong L, Hao XQ. [Small intestine motility and gastrointestinal hormone levels in irritable bowel syndrome]. Nanfang Yike Daxue Xuebao. 2007;27:1492-1495. [PubMed] [Cited in This Article: ] |
| 132. | Kellow JE, Eckersley CM, Jones MP. Enhanced perception of physiological intestinal motility in the irritable bowel syndrome. Gastroenterology. 1991;101:1621-1627. [PubMed] [Cited in This Article: ] |
| 133. | Thompson DG, Laidlow JM, Wingate DL. Abnormal small-bowel motility demonstrated by radiotelemetry in a patient with irritable colon. Lancet. 1979;2:1321-1323. [PubMed] [Cited in This Article: ] |
| 134. | Kingham JG, Bown R, Colson R, Clark ML. Jejunal motility in patients with functional abdominal pain. Gut. 1984;25:375-380. [PubMed] [Cited in This Article: ] |
| 135. | Schmidt T, Pfeiffer A, Kaess H. Abnormal intestinal motility in irritable bowel syndrome. Gastroenterology. 1996;111:1400-1401. [PubMed] [Cited in This Article: ] |
| 136. | Schmidt T, Hackelsberger N, Widmer R, Meisel C, Pfeiffer A, Kaess H. Ambulatory 24-hour jejunal motility in diarrhea-predominant irritable bowel syndrome. Scand J Gastroenterol. 1996;31:581-589. [PubMed] [Cited in This Article: ] |
| 137. | Hellström PM, Näslund E, Edholm T, Schmidt PT, Kristensen J, Theodorsson E, Holst JJ, Efendic S. GLP-1 suppresses gastrointestinal motility and inhibits the migrating motor complex in healthy subjects and patients with irritable bowel syndrome. Neurogastroenterol Motil. 2008;20:649-659. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 88] [Cited by in F6Publishing: 91] [Article Influence: 5.7] [Reference Citation Analysis (0)] |
| 138. | Simrén M, Castedal M, Svedlund J, Abrahamsson H, Björnsson E. Abnormal propagation pattern of duodenal pressure waves in the irritable bowel syndrome (IBS) [correction of (IBD)]. Dig Dis Sci. 2000;45:2151-2161. [PubMed] [Cited in This Article: ] |
| 139. | Stanghellini V, Ghidini C, Maccarini MR, Paparo GF, Corinaldesi R, Barbara L. Fasting and postprandial gastrointestinal motility in ulcer and non-ulcer dyspepsia. Gut. 1992;33:184-190. [PubMed] [Cited in This Article: ] |
| 140. | Björnsson ES, Abrahamsson H. Interdigestive gastroduodenal manometry in humans. Indication of duodenal phase III as a retroperistaltic pump. Acta Physiol Scand. 1995;153:221-230. [PubMed] [DOI] [Cited in This Article: ] [Cited by in F6Publishing: 1] [Reference Citation Analysis (0)] |
| 141. | Maljaars PW, Keszthelyi D, Masclee AA. An ileal brake-through? Am J Clin Nutr. 2010;92:467-468. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 20] [Cited by in F6Publishing: 21] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 142. | Van Citters GW, Lin HC. Ileal brake: neuropeptidergic control of intestinal transit. Curr Gastroenterol Rep. 2006;8:367-373. [PubMed] [Cited in This Article: ] |
| 143. | Lin HC, Zhao XT, Wang L, Wong H. Fat-induced ileal brake in the dog depends on peptide YY. Gastroenterology. 1996;110:1491-1495. [PubMed] [Cited in This Article: ] |
| 144. | Pironi L, Stanghellini V, Miglioli M, Corinaldesi R, De Giorgio R, Ruggeri E, Tosetti C, Poggioli G, Morselli Labate AM, Monetti N. Fat-induced ileal brake in humans: a dose-dependent phenomenon correlated to the plasma levels of peptide YY. Gastroenterology. 1993;105:733-739. [PubMed] [Cited in This Article: ] |
| 145. | Spiller RC, Trotman IF, Adrian TE, Bloom SR, Misiewicz JJ, Silk DB. Further characterisation of the ‘ileal brake’ reflex in man--effect of ileal infusion of partial digests of fat, protein, and starch on jejunal motility and release of neurotensin, enteroglucagon, and peptide YY. Gut. 1988;29:1042-1051. [PubMed] [Cited in This Article: ] |
| 146. | Spiller RC, Trotman IF, Higgins BE, Ghatei MA, Grimble GK, Lee YC, Bloom SR, Misiewicz JJ, Silk DB. The ileal brake--inhibition of jejunal motility after ileal fat perfusion in man. Gut. 1984;25:365-374. [PubMed] [Cited in This Article: ] |
| 147. | Goumain M, Voisin T, Lorinet AM, Ducroc R, Tsocas A, Rozé C, Rouet-Benzineb P, Herzog H, Balasubramaniam A, Laburthe M. The peptide YY-preferring receptor mediating inhibition of small intestinal secretion is a peripheral Y(2) receptor: pharmacological evidence and molecular cloning. Mol Pharmacol. 2001;60:124-134. [PubMed] [Cited in This Article: ] |
| 148. | Souli A, Chariot J, Voisin T, Presset O, Tsocas A, Balasubramaniam A, Laburthe M, Rozé C. Several receptors mediate the antisecretory effect of peptide YY, neuropeptide Y, and pancreatic polypeptide on VIP-induced fluid secretion in the rat jejunum in vivo. Peptides. 1997;18:551-557. [PubMed] [Cited in This Article: ] |
| 149. | Whang EE, Hines OJ, Reeve JR, Grandt D, Moser JA, Bilchik AJ, Zinner MJ, McFadden DW, Ashley SW. Antisecretory mechanisms of peptide YY in rat distal colon. Dig Dis Sci. 1997;42:1121-1127. [PubMed] [Cited in This Article: ] |
| 150. | Konturek SJ, Konturek PC, Brzozowska I, Pawlik M, Sliwowski Z, Cześnikiewicz-Guzik M, Kwiecień S, Brzozowski T, Bubenik GA, Pawlik WW. Localization and biological activities of melatonin in intact and diseased gastrointestinal tract (GIT). J Physiol Pharmacol. 2007;58:381-405. [PubMed] [Cited in This Article: ] |
| 151. | Thor PJ, Krolczyk G, Gil K, Zurowski D, Nowak L. Melatonin and serotonin effects on gastrointestinal motility. J Physiol Pharmacol. 2007;58 Suppl 6:97-103. [PubMed] [Cited in This Article: ] |
| 152. | Lu WZ, Song GH, Gwee KA, Ho KY. The effects of melatonin on colonic transit time in normal controls and IBS patients. Dig Dis Sci. 2009;54:1087-1093. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 34] [Cited by in F6Publishing: 36] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 153. | Charles F, Camilleri M, Phillips SF, Thomforde GM, Forstrom LA. Scintigraphy of the whole gut: clinical evaluation of transit disorders. Mayo Clin Proc. 1995;70:113-118. [PubMed] [DOI] [Cited in This Article: ] [Cited by in F6Publishing: 2] [Reference Citation Analysis (0)] |
| 154. | van der Sijp JR, Kamm MA, Nightingale JM, Britton KE, Mather SJ, Morris GP, Akkermans LM, Lennard-Jones JE. Radioisotope determination of regional colonic transit in severe constipation: comparison with radio opaque markers. Gut. 1993;34:402-408. [PubMed] [Cited in This Article: ] |
| 155. | Vassallo M, Camilleri M, Phillips SF, Brown ML, Chapman NJ, Thomforde GM. Transit through the proximal colon influences stool weight in the irritable bowel syndrome. Gastroenterology. 1992;102:102-108. [PubMed] [Cited in This Article: ] |
| 156. | Snape WJ, Carlson GM, Cohen S. Colonic myoelectric activity in the irritable bowel syndrome. Gastroenterology. 1976;70:326-330. [PubMed] [Cited in This Article: ] |
| 157. | Snape WJ. Myoelectric and motor activity of the colon in normal and abnormal states. Scand J Gastroenterol Suppl. 1984;96:55-60. [PubMed] [Cited in This Article: ] |
| 158. | Sarna S, Latimer P, Campbell D, Waterfall WE. Effect of stress, meal and neostigmine on rectosigmoid electrical control activity (ECA) in normals and in irritable bowel syndrome patients. Dig Dis Sci. 1982;27:582-591. [PubMed] [Cited in This Article: ] |
| 159. | Latimer P, Sarna S, Campbell D, Latimer M, Waterfall W, Daniel EE. Colonic motor and myoelectrical activity: a comparative study of normal subjects, psychoneurotic patients, and patients with irritable bowel syndrome. Gastroenterology. 1981;80:893-901. [PubMed] [Cited in This Article: ] |
| 160. | Welgan P, Meshkinpour H, Ma L. Role of anger in antral motor activity in irritable bowel syndrome. Dig Dis Sci. 2000;45:248-251. [PubMed] [Cited in This Article: ] |
| 161. | Welgan P, Meshkinpour H, Hoehler F. The effect of stress on colon motor and electrical activity in irritable bowel syndrome. Psychosom Med. 1985;47:139-149. [PubMed] [Cited in This Article: ] |
| 162. | Katschinski M, Lederer P, Ellermann A, Ganzleben R, Lux G, Arnold R. Myoelectric and manometric patterns of human rectosigmoid colon in irritable bowel syndrome and diverticulosis. Scand J Gastroenterol. 1990;25:761-768. [PubMed] [Cited in This Article: ] |
| 163. | Chey WY, Jin HO, Lee MH, Sun SW, Lee KY. Colonic motility abnormality in patients with irritable bowel syndrome exhibiting abdominal pain and diarrhea. Am J Gastroenterol. 2001;96:1499-1506. [PubMed] [Cited in This Article: ] |
| 164. | Vassallo MJ, Camilleri M, Phillips SF, Steadman CJ, Talley NJ, Hanson RB, Haddad AC. Colonic tone and motility in patients with irritable bowel syndrome. Mayo Clin Proc. 1992;67:725-731. [PubMed] [Cited in This Article: ] |
| 165. | Corsetti M, Ogliari C, Marino B, Basilisco G. Perceptual sensitivity and response bias during rectal distension in patients with irritable bowel syndrome. Neurogastroenterol Motil. 2005;17:541-547. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 12] [Cited by in F6Publishing: 14] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 166. | Corsetti M, Cesana B, Bhoori S, Basilisco G. Rectal hyperreactivity to distention in patients with irritable bowel syndrome: role of distention rate. Clin Gastroenterol Hepatol. 2004;2:49-56. [PubMed] [Cited in This Article: ] |
| 167. | Bassotti G, de Roberto G, Chistolini F, Sietchiping-Nzepa F, Morelli O, Morelli A. Twenty-four-hour manometric study of colonic propulsive activity in patients with diarrhea due to inflammatory (ulcerative colitis) and non-inflammatory (irritable bowel syndrome) conditions. Int J Colorectal Dis. 2004;19:493-497. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 37] [Cited by in F6Publishing: 38] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 168. | Bassotti G, Sietchiping-Nzepa F, De Roberto G, Chistolini F, Morelli A. Colonic regular contractile frequency patterns in irritable bowel syndrome: the ‘spastic colon’ revisited. Eur J Gastroenterol Hepatol. 2004;16:613-617. [PubMed] [Cited in This Article: ] |
| 169. | Clemens CH, Samsom M, Van Berge Henegouwen GP, Smout AJ. Abnormalities of left colonic motility in ambulant nonconstipated patients with irritable bowel syndrome. Dig Dis Sci. 2003;48:74-82. [PubMed] [Cited in This Article: ] |
| 170. | Clemens CH, Samsom M, Roelofs JM, van Berge Henegouwen GP, Smout AJ. Association between pain episodes and high amplitude propagated pressure waves in patients with irritable bowel syndrome. Am J Gastroenterol. 2003;98:1838-1843. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 26] [Cited by in F6Publishing: 28] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 171. | Rogers J, Henry MM, Misiewicz JJ. Increased segmental activity and intraluminal pressures in the sigmoid colon of patients with the irritable bowel syndrome. Gut. 1989;30:634-641. [PubMed] [Cited in This Article: ] |
| 172. | Simrén M, Abrahamsson H, Björnsson ES. An exaggerated sensory component of the gastrocolonic response in patients with irritable bowel syndrome. Gut. 2001;48:20-27. [PubMed] [Cited in This Article: ] |
| 173. | Di Stefano M, Miceli E, Missanelli A, Mazzocchi S, Corazza GR. Meal induced rectosigmoid tone modification: a low caloric meal accurately separates functional and organic gastrointestinal disease patients. Gut. 2006;55:1409-1414. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 31] [Cited by in F6Publishing: 36] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 174. | Park JH, Baek YH, Park DI, Kim HJ, Cho YK, Sohn CI, Jeon WK, Kim BI, Rhee PL. Analysis of rectal dynamic and static compliances in patients with irritable bowel syndrome. Int J Colorectal Dis. 2008;23:659-664. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 11] [Cited by in F6Publishing: 13] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 175. | Steens J, Van Der Schaar PJ, Penning C, Brussee J, Masclee AA. Compliance, tone and sensitivity of the rectum in different subtypes of irritable bowel syndrome. Neurogastroenterol Motil. 2002;14:241-247. [PubMed] [Cited in This Article: ] |
| 176. | Kwan CL, Davis KD, Mikula K, Diamant NE. Abnormal rectal motor physiology in patients with irritable bowel syndrome. Neurogastroenterol Motil. 2004;16:251-263. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 26] [Cited by in F6Publishing: 24] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 177. | Cummings JH, Antoine JM, Azpiroz F, Bourdet-Sicard R, Brandtzaeg P, Calder PC, Gibson GR, Guarner F, Isolauri E, Pannemans D. PASSCLAIM--gut health and immunity. Eur J Nutr. 2004;43 Suppl 2:II118-II173. [PubMed] [Cited in This Article: ] |
| 178. | Bischoff SC, Barbara G, Buurman W, Ockhuizen T, Schulzke JD, Serino M, Tilg H, Watson A, Wells JM. Intestinal permeability--a new target for disease prevention and therapy. BMC Gastroenterol. 2014;14:189. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 893] [Cited by in F6Publishing: 1021] [Article Influence: 102.1] [Reference Citation Analysis (0)] |
| 179. | Santos J, Yang PC, Söderholm JD, Benjamin M, Perdue MH. Role of mast cells in chronic stress induced colonic epithelial barrier dysfunction in the rat. Gut. 2001;48:630-636. [PubMed] [Cited in This Article: ] |
| 180. | Söderholm JD, Yang PC, Ceponis P, Vohra A, Riddell R, Sherman PM, Perdue MH. Chronic stress induces mast cell-dependent bacterial adherence and initiates mucosal inflammation in rat intestine. Gastroenterology. 2002;123:1099-1108. [PubMed] [Cited in This Article: ] |
| 181. | McDermott JR, Bartram RE, Knight PA, Miller HR, Garrod DR, Grencis RK. Mast cells disrupt epithelial barrier function during enteric nematode infection. Proc Natl Acad Sci USA. 2003;100:7761-7766. [PubMed] [Cited in This Article: ] |
| 182. | Zhou Q, Souba WW, Croce CM, Verne GN. MicroRNA-29a regulates intestinal membrane permeability in patients with irritable bowel syndrome. Gut. 2010;59:775-784. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 188] [Cited by in F6Publishing: 202] [Article Influence: 14.4] [Reference Citation Analysis (0)] |
| 183. | Piche T, Barbara G, Aubert P, Bruley des Varannes S, Dainese R, Nano JL, Cremon C, Stanghellini V, De Giorgio R, Galmiche JP. Impaired intestinal barrier integrity in the colon of patients with irritable bowel syndrome: involvement of soluble mediators. Gut. 2009;58:196-201. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 365] [Cited by in F6Publishing: 381] [Article Influence: 25.4] [Reference Citation Analysis (0)] |
| 184. | Zhou Q, Zhang B, Verne GN. Intestinal membrane permeability and hypersensitivity in the irritable bowel syndrome. Pain. 2009;146:41-46. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 246] [Cited by in F6Publishing: 262] [Article Influence: 17.5] [Reference Citation Analysis (0)] |
| 185. | Shulman RJ, Eakin MN, Czyzewski DI, Jarrett M, Ou CN. Increased gastrointestinal permeability and gut inflammation in children with functional abdominal pain and irritable bowel syndrome. J Pediatr. 2008;153:646-650. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 134] [Cited by in F6Publishing: 140] [Article Influence: 8.8] [Reference Citation Analysis (0)] |
| 186. | Dunlop SP, Hebden J, Campbell E, Naesdal J, Olbe L, Perkins AC, Spiller RC. Abnormal intestinal permeability in subgroups of diarrhea-predominant irritable bowel syndromes. Am J Gastroenterol. 2006;101:1288-1294. [PubMed] [Cited in This Article: ] |
| 187. | Kerckhoffs AP, Akkermans LM, de Smet MB, Besselink MG, Hietbrink F, Bartelink IH, Busschers WB, Samsom M, Renooij W. Intestinal permeability in irritable bowel syndrome patients: effects of NSAIDs. Dig Dis Sci. 2010;55:716-723. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 45] [Cited by in F6Publishing: 47] [Article Influence: 3.4] [Reference Citation Analysis (0)] |
| 188. | Lee JW, Park JH, Park DI, Park JH, Kim HJ, Cho YK, Sohn CI, Jeon WK, Kim BI. Subjects with diarrhea-predominant IBS have increased rectal permeability responsive to tryptase. Dig Dis Sci. 2010;55:2922-2928. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 42] [Cited by in F6Publishing: 39] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 189. | Bertiaux-Vandaële N, Youmba SB, Belmonte L, Lecleire S, Antonietti M, Gourcerol G, Leroi AM, Déchelotte P, Ménard JF, Ducrotté P. The expression and the cellular distribution of the tight junction proteins are altered in irritable bowel syndrome patients with differences according to the disease subtype. Am J Gastroenterol. 2011;106:2165-2173. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 195] [Cited by in F6Publishing: 200] [Article Influence: 15.4] [Reference Citation Analysis (1)] |
| 190. | Bischoff SC. Food allergy and eosinophilic gastroenteritis and colitis. Curr Opin Allergy Clin Immunol. 2010;10:238-245. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 37] [Cited by in F6Publishing: 40] [Article Influence: 2.9] [Reference Citation Analysis (0)] |
| 191. | Brown PM, Drossman DA, Wood AJ, Cline GA, Frazier KS, Jackson JI, Bronner J, Freiman J, Zambrowicz B, Sands A. The tryptophan hydroxylase inhibitor LX1031 shows clinical benefit in patients with nonconstipating irritable bowel syndrome. Gastroenterology. 2011;141:507-516. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 83] [Cited by in F6Publishing: 85] [Article Influence: 6.5] [Reference Citation Analysis (0)] |
| 192. | Palsson OS, Morteau O, Bozymski EM, Woosley JT, Sartor RB, Davies MJ, Johnson DA, Turner MJ, Whitehead WE. Elevated vasoactive intestinal peptide concentrations in patients with irritable bowel syndrome. Dig Dis Sci. 2004;49:1236-1243. [PubMed] [Cited in This Article: ] |
| 193. | Kerckhoffs AP, ter Linde JJ, Akkermans LM, Samsom M. SERT and TPH-1 mRNA expression are reduced in irritable bowel syndrome patients regardless of visceral sensitivity state in large intestine. Am J Physiol Gastrointest Liver Physiol. 2012;302:G1053-G1060. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 62] [Cited by in F6Publishing: 71] [Article Influence: 5.9] [Reference Citation Analysis (0)] |
| 194. | Simrén M, Stotzer PO, Sjövall H, Abrahamsson H, Björnsson ES. Abnormal levels of neuropeptide Y and peptide YY in the colon in irritable bowel syndrome. Eur J Gastroenterol Hepatol. 2003;15:55-62. [PubMed] [Cited in This Article: ] |
| 195. | Barbara G, Cremon C, De Giorgio R, Dothel G, Zecchi L, Bellacosa L, Carini G, Stanghellini V, Corinaldesi R. Mechanisms underlying visceral hypersensitivity in irritable bowel syndrome. Curr Gastroenterol Rep. 2011;13:308-315. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 83] [Cited by in F6Publishing: 73] [Article Influence: 5.6] [Reference Citation Analysis (0)] |
| 196. | van Wanrooij SJ, Wouters MM, Van Oudenhove L, Vanbrabant W, Mondelaers S, Kollmann P, Kreutz F, Schemann M, Boeckxstaens GE. Sensitivity testing in irritable bowel syndrome with rectal capsaicin stimulations: role of TRPV1 upregulation and sensitization in visceral hypersensitivity? Am J Gastroenterol. 2014;109:99-109. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 71] [Cited by in F6Publishing: 67] [Article Influence: 6.7] [Reference Citation Analysis (0)] |
| 197. | Willot S, Gauthier C, Patey N, Faure C. Nerve growth factor content is increased in the rectal mucosa of children with diarrhea-predominant irritable bowel syndrome. Neurogastroenterol Motil. 2012;24:734-739, e347. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 42] [Cited by in F6Publishing: 43] [Article Influence: 3.6] [Reference Citation Analysis (0)] |
| 198. | Barbara G, Gargano L, Cremon C, Vasina V, Dothel G, Carini G, De Giorgio R, Stanghellini V, Cogliandro R, Tonini M. Nerve growth and plasticity in the colonic mucosa of patients with irritable bowel syndrome. Gastroenterology. 2010;138:S65. [DOI] [Cited in This Article: ] [Cited by in Crossref: 4] [Cited by in F6Publishing: 3] [Article Influence: 0.2] [Reference Citation Analysis (0)] |
| 199. | Dothel G, Barbaro MR, Boudin H, Vasina V, Cremon C, Gargano L, Bellacosa L, De Giorgio R, Le Berre-Scoul C, Aubert P. Nerve fiber outgrowth is increased in the intestinal mucosa of patients with irritable bowel syndrome. Gastroenterology. 2015;148:1002-1011.e4. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 113] [Cited by in F6Publishing: 117] [Article Influence: 13.0] [Reference Citation Analysis (0)] |
| 200. | Gulbransen BD, Sharkey KA. Novel functional roles for enteric glia in the gastrointestinal tract. Nat Rev Gastroenterol Hepatol. 2012;9:625-632. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 239] [Cited by in F6Publishing: 265] [Article Influence: 22.1] [Reference Citation Analysis (0)] |
| 201. | Andrews CN, Shaffer E, Ho W, Nasser Y, O’Hara JR, Sharkey KA. Enteric glia and enteroen-docrine cells in irritable bowel syndrome: controlled pilot study. Gastroenterology. 2005;128:A270. [Cited in This Article: ] |
| 202. | Barbara G, Cremon C, Annese V, Basilisco G, Bazzoli F, Bellini M, Benedetti A, Benini L, Bossa F, Buldrini P. Randomised controlled trial of mesalazine in IBS. Gut. 2016;65:82-90. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 70] [Cited by in F6Publishing: 81] [Article Influence: 10.1] [Reference Citation Analysis (0)] |
| 203. | Barbara G, Cremon C, Carini G, Bellacosa L, Zecchi L, De Giorgio R, Corinaldesi R, Stanghellini V. The immune system in irritable bowel syndrome. J Neurogastroenterol Motil. 2011;17:349-359. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 137] [Cited by in F6Publishing: 135] [Article Influence: 10.4] [Reference Citation Analysis (0)] |
| 204. | Dunlop SP, Jenkins D, Neal KR, Naesdal J, Borgaonker M, Collins SM, Spiller RC. Randomized, double-blind, placebo-controlled trial of prednisolone in post-infectious irritable bowel syndrome. Aliment Pharmacol Ther. 2003;18:77-84. [PubMed] [Cited in This Article: ] |
| 205. | Barbara G, Stanghellini V, Cremon C, De Giorgio R, Fronzoni L, Serra M, Corinaldesi R. Aminosalicylates and other anti-inflammatory compounds for irritable bowel syndrome. Dig Dis. 2009;27 Suppl 1:115-121. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 20] [Cited by in F6Publishing: 24] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 206. | Klooker TK, Braak B, Koopman KE, Welting O, Wouters MM, van der Heide S, Schemann M, Bischoff SC, van den Wijngaard RM, Boeckxstaens GE. The mast cell stabiliser ketotifen decreases visceral hypersensitivity and improves intestinal symptoms in patients with irritable bowel syndrome. Gut. 2010;59:1213-1221. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 268] [Cited by in F6Publishing: 288] [Article Influence: 20.6] [Reference Citation Analysis (0)] |
| 207. | Clarke G, Cryan JF, Dinan TG, Quigley EM. Review article: probiotics for the treatment of irritable bowel syndrome--focus on lactic acid bacteria. Aliment Pharmacol Ther. 2012;35:403-413. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 148] [Cited by in F6Publishing: 149] [Article Influence: 12.4] [Reference Citation Analysis (0)] |
| 208. | Whelan K. Probiotics and prebiotics in the management of irritable bowel syndrome: a review of recent clinical trials and systematic reviews. Curr Opin Clin Nutr Metab Care. 2011;14:581-587. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 89] [Cited by in F6Publishing: 92] [Article Influence: 7.1] [Reference Citation Analysis (0)] |
| 209. | DuPont AW, DuPont HL. The intestinal microbiota and chronic disorders of the gut. Nat Rev Gastroenterol Hepatol. 2011;8:523-531. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 217] [Cited by in F6Publishing: 215] [Article Influence: 16.5] [Reference Citation Analysis (0)] |
| 210. | Brenner DM, Moeller MJ, Chey WD, Schoenfeld PS. The utility of probiotics in the treatment of irritable bowel syndrome: a systematic review. Am J Gastroenterol. 2009;104:1033-1049; quiz 1050. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 236] [Cited by in F6Publishing: 252] [Article Influence: 16.8] [Reference Citation Analysis (0)] |
| 211. | Hoveyda N, Heneghan C, Mahtani KR, Perera R, Roberts N, Glasziou P. A systematic review and meta-analysis: probiotics in the treatment of irritable bowel syndrome. BMC Gastroenterol. 2009;9:15. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 182] [Cited by in F6Publishing: 181] [Article Influence: 12.1] [Reference Citation Analysis (0)] |
| 212. | Schmulson M, Chang L. Review article: the treatment of functional abdominal bloating and distension. Aliment Pharmacol Ther. 2011;33:1071-1086. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 47] [Cited by in F6Publishing: 41] [Article Influence: 3.2] [Reference Citation Analysis (0)] |