Published online May 14, 2017. doi: 10.3748/wjg.v23.i18.3240
Peer-review started: December 17, 2016
First decision: December 29, 2016
Revised: January 27, 2017
Accepted: March 15, 2017
Article in press: March 15, 2017
Published online: May 14, 2017
To identify which blood and mucosal lymphocyte populations are specifically depleted by thiopurine use in vivo.
The thiopurines azathioprine and 6-mercaptopurine have been a mainstay of inflammatory bowel disease (IBD) therapy for decades, but their mechanism of action in vivo remains obscure. Although thiopurines are lymphotoxic at high doses, and have been reported to cause T cell apoptosis in vitro, their ability to control IBD at lower doses suggests that they may selectively deplete particular lymphocyte populations. Blood cells from 19 IBD patients on a thiopurine, 19 IBD patients not on a thiopurine, and 38 matched healthy control subjects were analyzed by multiple multi-color flow cytometry panels to quantify the immune cell subsets contained therein, both as a percent of cells, and as an absolute cell count. Similar analyses were performed on colon biopsies from 17 IBD patients on a thiopurine, 17 IBD patients not on a thiopurine, and 49 healthy screening colonoscopy recipients.
Complete blood counts revealed lower lymphocyte, but not monocyte or granulocyte, counts in IBD patients who were taking thiopurines at the time of sampling. This reduction was restricted to CD3-negative lymphocytes, wherein both natural killer (NK) and B cells were significantly reduced among thiopurine recipients. Among CD19+ B cells, the transitional B cells were particularly depleted, being nearly absent in both blood and colon biopsies of thiopurine recipients. No differences were associated with thiopurine use in CD8+ T cells, mucosa-associated invariant T (MAIT) cells, invariant natural killer T (iNKT) cells, gamma/delta T cells, Th1, Th17, regulatory T cells (Tregs) or naïve CD4+ T cells. However, patients with IBD had significantly more circulating FOXP3+, Helios+ Tregs and fewer iNKT and MAIT cells than healthy controls.
Thiopurine use is associated with reduced B and NK cell, but not T cell, subpopulations in the blood of IBD patients.
Core tip: Thiopurine medications have been used to treat inflammatory bowel disease (IBD) for almost half a century. However, the effect of thiopurines on the human immune system in vitro remains unclear. The enclosed manuscript performed a thorough flow cytometric analysis of peripheral blood specimens from IBD patients with or without thiopurine therapy and found significant differences in B and NK cell populations associated with therapy. These suggest that thiopurines function to suppress inflammation in IBD not by causing T cell apoptosis, as had been suggested by in vitro data, but rather by depleting other lymphocytes.
- Citation: Lord JD, Shows DM. Thiopurine use associated with reduced B and natural killer cells in inflammatory bowel disease. World J Gastroenterol 2017; 23(18): 3240-3251
- URL: https://www.wjgnet.com/1007-9327/full/v23/i18/3240.htm
- DOI: https://dx.doi.org/10.3748/wjg.v23.i18.3240
The thiopurine medications azathioprine and 6-mercaptopurine (6-MP) have been used to treat IBD since the 1960’s[1] as an effective maintenance therapy for both Crohn’s disease[2] and ulcerative colitis (UC)[3]. In more recent years, these agents have demonstrated utility as cotherapy with biopharmaceuticals, reducing the incidence of anti-drug antibodies and increasing treatment success rates[4,5]. While appealing for their oral delivery and relatively low cost, thiopurines are challenging to use, with a narrow therapeutic dose window, slow onset of efficacy[6] and a number needed to treat in the 4-6 range[2,3]. This benefit is balanced against a number of potential risks, including infections, and certain neoplasms[7].
The mechanisms by which thiopurines maintain IBD remission and prevent anti-biopharmaceutical antibody formation remain obscure. 6-thioguanine nucleotides are thought to be the active metabolites of both azathioprine and 6-MP, and originally were believed to function by incorporating into cellular nucleic acids to damage their structure[8] and thus inhibit T cell proliferation[9]. In vitro studies also demonstrated that thiopurines mediate apoptosis[10], and specifically the 6-thioguanine triphosphate (6-thio-GTP) metabolite may stimulate T cell apoptosis through inhibition of Rac1 activation, thus preventing CD28 costimulation from inducing Bcl-xL expression in these cells upon activation[11]. Leukopenia is a known effect of azathioprine therapy[12], and has been associated with therapeutic efficacy[13]. However, this association appears to be due to decreased neutrophil counts seen during the early phase of thiopurine use, with lymphopenia demonstrating no correlation with therapeutic efficacy[14]. Thus, if azathioprine suppresses the inflammation of IBD through anti-proliferative or pro-apoptotic effects on lymphocytes, these effects must be subtle, affecting only specific minor lymphocyte subpopulations, clonotypes, or anatomically sequestered populations not evident in the peripheral blood.
Early studies of azathioprine in UC showed that it reduced total plasma cell counts in the rectal mucosa[15] to levels resembling healthy controls[16]. However, it is unclear whether this is a specific effect of azathioprine vs simply a reflection of reduced lymphocytic infiltration as a consequence of decreased inflammation. These studies also demonstrated less antibody-dependent cell mediated cytotoxicity in the blood of azathioprine recipients[15-17], a phenomenon that is classically attributed to natural killer (NK) cells. More recent research comparing the mRNA transcripts of peripheral blood from Crohn’s patients revealed reduced expression of genes commonly expressed by NK and other cytotoxic lymphocytes in thiopurine recipients[18], suggesting that thiopurines may function through selective depletion of NK cells. One small study of Crohn’s patients prospectively examined the effect of azathioprine on immune cell subsets over a year, and found it to reduce total lymphocyte counts, but with no significant effect upon the percent of these lymphocytes expressing the NK markers CD16 and CD56[19].
Curiously, this study also found azathioprine to significantly increase the percent of lymphocytes expressing CD25[19]. Among CD4+ T cells, CD25 is a marker of FOXP3+ regulatory T cells (Tregs), which are known to play a central role in preventing intestinal inflammation in mice[20] and humans[21,22]. Although CD25+, FOXP3+ Tregs are not deficient in IBD patients[23], their frequency in the blood has been reported to be reduced in active vs quiescent disease, and their frequency in the intestinal mucosa, while enriched in inflammation[24,25], may be relatively low compared to other causes of intestinal inflammation[26]. Thus, an alternative mechanism by which thiopurines could control IBD may be by selectively sparing, and thus enriching, Tregs in the intestinal lamina propria.
Noting that only lymphocyte counts were reduced in thiopurine recipients, our aim was to determine if and how thiopurine use is associated with depletion of specific lymphocyte populations. We evaluated IBD patients on or off thiopurines to correlate the use of these medications with changes in B, T, and NK cell subpopulations, and compared them with the frequency of these lymphocyte subsets in matched healthy control subjects.
Clinical data, including complete blood cell (CBC) counts presented in Figure 1, and specimens detailed below, were archived from consenting participants in a biorepository program at the Benaroya Research Institute, as authorized by an IRB-approved protocol in accordance with the declaration of Helsinki.
Five hundred and fifty-seven healthy controls, 42 IBD patients (31 Crohn’s, 10 UC, 1 indeterminate colitis) on azathioprine (n = 34) or 6-mercaptopurine (n = 8) and 168 IBD patients (105 Crohn’s, 61 UC, 2 indeterminate colitis) on no thiopurines provided CBC data in Figure 1. Leukocyte subsets in the latter were defined and reported according to the International Council for Standardization in Hematology (ICSH) guidelines (http://icsh.org/guidelines/).
Live, frozen peripheral blood mononuclear cells (PBMC) were obtained from 19 of the above IBD patients (14 Crohn’s, 5 UC) on azathioprine (n = 16) or 6-mercaptopurine (n = 3), and 19 IBD patients (also 14 Crohn’s, 5 UC) on no thiopurine medications, but matched in terms of whether or not taking a 5’ aminosalicylate agent. Also, PBMC were obtained from 38 healthy controls, age and gender-matched to each of these IBD patients. None of these blood donors were on glucocorticoids, biopharmaceuticals, or other systemic immunosuppressive agents at the time of phlebotomy.
Live, frozen, colonoscopic biopsies were obtained from 17 IBD patients (13 Crohn’s, 4 UC) on azathioprine (n = 14) or 6-mercaptopurine (n = 3), and 17 IBD patients (also 13 Crohn’s, 4 UC) on no thiopurines at the time of colonoscopy. Seven patients in each group were on an anti-TNF biopharmaceutical, and 3 patients in each group were on a glucocorticoid. Six patients in the thiopurine group and 8 in the no thiopurine group were on a 5’ aminosalicylate agent. The colonic mucosa biopsied was deemed by the colonoscopist to be actively inflamed in 6 of the patients on thiopurines and 7 of the patients on no thiopurines. As a control, biopsies from 49 healthy screening colonoscopy recipients were also examined.
Samples were thawed and colon biopsies were digested in a vortex at 37 degrees centigrade for 30 min in media containing collagenase and DNAse to liberate single cells. Cells were then filtered, washed, and stained extracellularly with panels of fluorophor-conjugated antibodies. Monoclonal antibodies against CD3 (clone SK7), CD4 (RPA-T4), CD8 (RPA-T8), CD19 (HIB19), CD25 (M-A251), CD27 (L128), CD38 (HIT2), CD49d (9F10), CD56 (NCAM16.2), CTLA4 (BNI3), and Ki67 (B56) were obtained from BD Biosciences (San Jose, CA, United States). Monoclonal antibodies against CD3 (SK7), CD4 (RPA-T4), CD8 (RPA-T8), CD16 (3G10), CD19 (HIB19), CD20 (2H7), CD45RA (HI100), CD49d (9F10), CD56 (HCD56), CD161 (HP-3G10), NKG2D (1D11), IFN gamma (4S.B3), IgD (1A6-2), IgM (MHM-88), TCRva24-Ja18 (6B11), TCRva7.2 (3C10), FOXP3 (236A/E7), and Helios (22F6) were obtained from BioLegend (San Diego, CA, United States). Monoclonal antibodies against CD8 (RPA-T8), NKp46 (9E2), integrin beta 7 (FIB504), gamma delta TCR (B1.1), and IL17A (eBio64DEC17) were obtained from eBiosciences (San Diego, CA, USA). Intracellular staining was performed with a FOXP3 staining kit (eBiosciences). For intracellular cytokine staining (ICCS), cells were first incubated overnight with PMA, ionomycin and brefeldin A. Stained cells were evaluated on either a FACSCanto (BD Biosciences) or, for ICCS, a FACS Calibur (BD Biosciences) flow cytometer. Data was analyzed with FlowJo (FlowJo, LLC, Ashland, OR) Excel (Microsoft, Inc., Redmond, WA), and GraphPad Prism (Graph Pad Software, Inc., La Jolla, CA, United States) software. All two-way comparisons were performed with two-tailed Mann-Whitney nonparametric analyses. The statistical methods of this study were reviewed by biostatistician Elizabeth Whalen, PhD, of the Benaroya Research Institute.
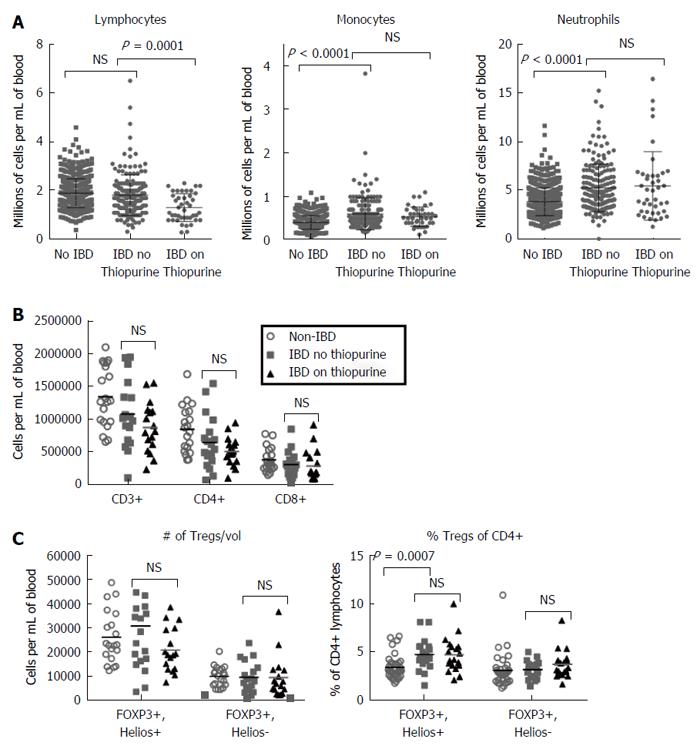
Comparing the CBC differentials of IBD patients revealed that patients chronically on thiopurines had significantly fewer circulating lymphocytes than patients not on thiopurines, although no differences in monocytes or granulocytes were evident (Figure 1A), as has been reported previously in IBD[16]. Thiopurine use was not associated with any significant reduction in total CD3+ T cells, or the CD4+ or CD8+ subsets thereof (Figure 1B). While this does not exclude the possibility that thiopurines selectively affect a minor T cell subpopulation, thiopurine use was not associated with any changes in the number of TCRγδ + T cells, TCRvα24/jα18+ invariant NKT cells, or CD161+, CD4-, TCRvα7.2+ mucosa-associated invariant T (MAIT) cells in circulation, although fewer circulating CD161+ iNKT (P = 0.00843) and MAIT cells (P = 0.00423) were seen in IBD patients than controls (Supplemental Figure 1).
To determine if a reported increase in CD25+ CD4+ T cells associated with thiopurine use[19] reflects an increase in FOXP3+ Treg populations, the above PBMC were also stained intracellularly for FOXP3 and the “natural” Treg (nTreg) marker Helios[27]. The Helios+ FOXP3+ nTreg fraction of circulating CD4+ T cells was significantly larger in untreated IBD patients than controls (P = 0.0007), but the Helios- FOXP3+ fraction was not, and there was no correlation between either Treg population and thiopurine use among IBD patients (Figure 1C). Both Treg populations and conventional FOXP3- T cells showed no difference in expression of the naïve T cell marker CD45RA, the inhibitory receptor CTLA4, the IL-2 receptor CD25, or the proliferation marker Ki67 between IBD patients on vs off thiopurines, and between total IBD patients and controls (Supplemental Figure 2A-D).
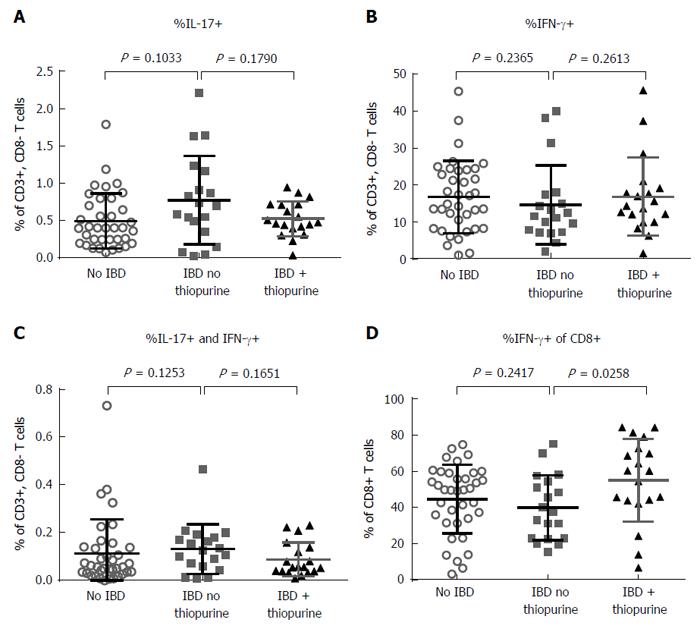
CD4+ effector T cells that make IFN-γ (Th1), IL-17 (Th17) or both (TH1/17) have been proposed to play a pathogenic role in IBD. Through intracellular cytokine staining, we found that thiopurine use had no significant correlation with Th17 (Figure 2A) Th1 (Figure 2B) or Th1/17 frequency (Figure 2C), which additionally were no different between IBD patients and controls. However, thiopurine use did correlate with an increased fraction of CD8+ T cells expressing IFN-γ (P = 0.0258) (Figure 2D).
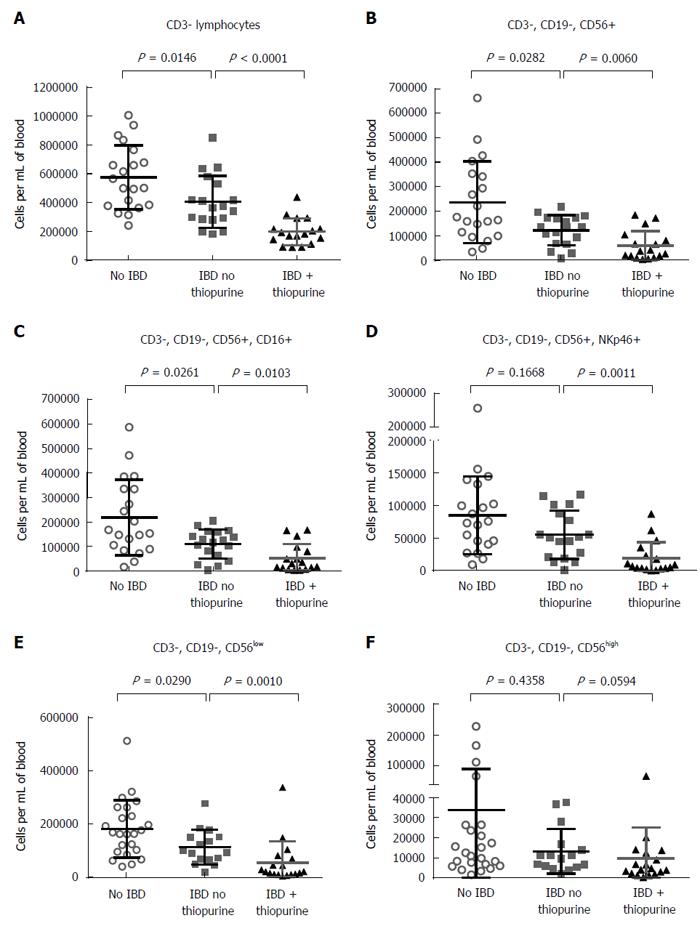
Finding no difference in T cells between patients on or off thiopurines, we next turned our attention to CD3- lymphocytes, which were found to be significantly reduced in the blood of IBD patients relative to controls (P = 0.0146), particularly if taking thiopurines (P < 0.0001) (Figure 3A). As this population contains both NK and B cells, we evaluated these populations independently. As with the total CD3- lymphocyte numbers, NK cells were significantly less numerous in IBD patients than controls (P = 0.0282), and less numerous in thiopurine recipients (P = 0.006), regardless of whether they were defined by the marker CD56 alone (Figure 3B), or more strictly with CD56 in combination with CD16 (Figure 3C) or NKp46 (Figure 3D). CD56 is expressed bimodally in NK cells, with a small but distinct CD56high population among CD16- cells discernable from the majority of NK cells, which are CD56low. Thiopurine use was associated with a decreased number of the latter cells (Figure 3E), but not significantly with any change in CD56high cells (Figure 3F). Although some of this reduction simply parallels the overall reduced lymphocyte counts associated with thiopurine use (Figure 1A), among IBD patients there were also significantly fewer CD56+ NK cells in thiopurine recipients when analyzed as a percentage of CD3-, CD19- lymphocytes (P = 0.0189).
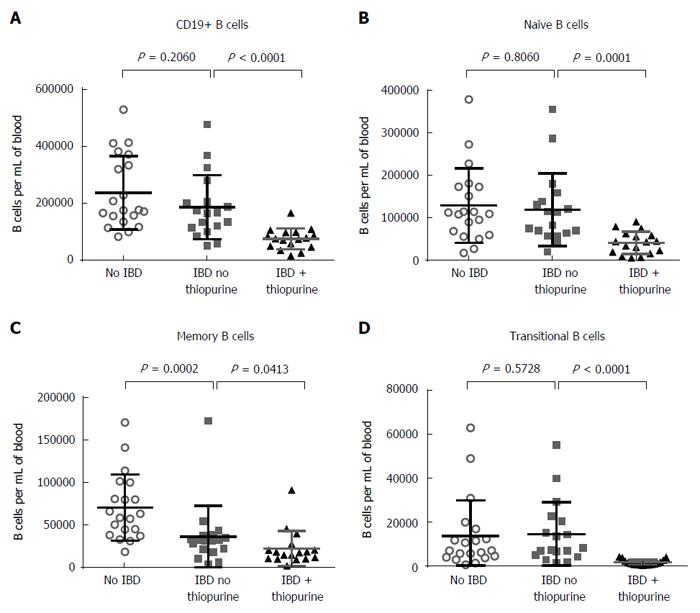
B cell counts were likewise reduced in thiopurine recipients (P < 0.0001; Figure 4A). This difference was more evident among the naïve (CD27-, CD38-, IgD+, P = 0.0001; Figure 4B), than memory B cells (CD27+, P = 0.0413; Figure 4C), although memory B cells were less numerous in the blood of IBD patients than controls (P = 0.0002). The latter difference was seen in both switched (IgD-) and unswitched (IgD+) memory B cells (data not shown). The most striking effect of thiopurines on B cells was in the transitional (CD38+, CD27-, IgD+) population, which was almost completely obliterated in all thiopurine recipients (P < 0.0001; Figure 4D). Restricting analyses to B cells bearing the gut-homing integrin α4β7 did not change whether or not these differences were significant (data not shown), suggesting that the effect of thiopurines on circulating B cells was not mediated by altering B cell trafficking to the gut. The number of plasmablasts (CD27+, CD38+, CD20-, IgD-) in circulation was low in all subjects, and did not correlate with either the diagnosis of IBD or the use of thiopurines (data not shown), indicating that the effect of thiopurines on transitional B cells was not due to a global suppression of CD38 expression.
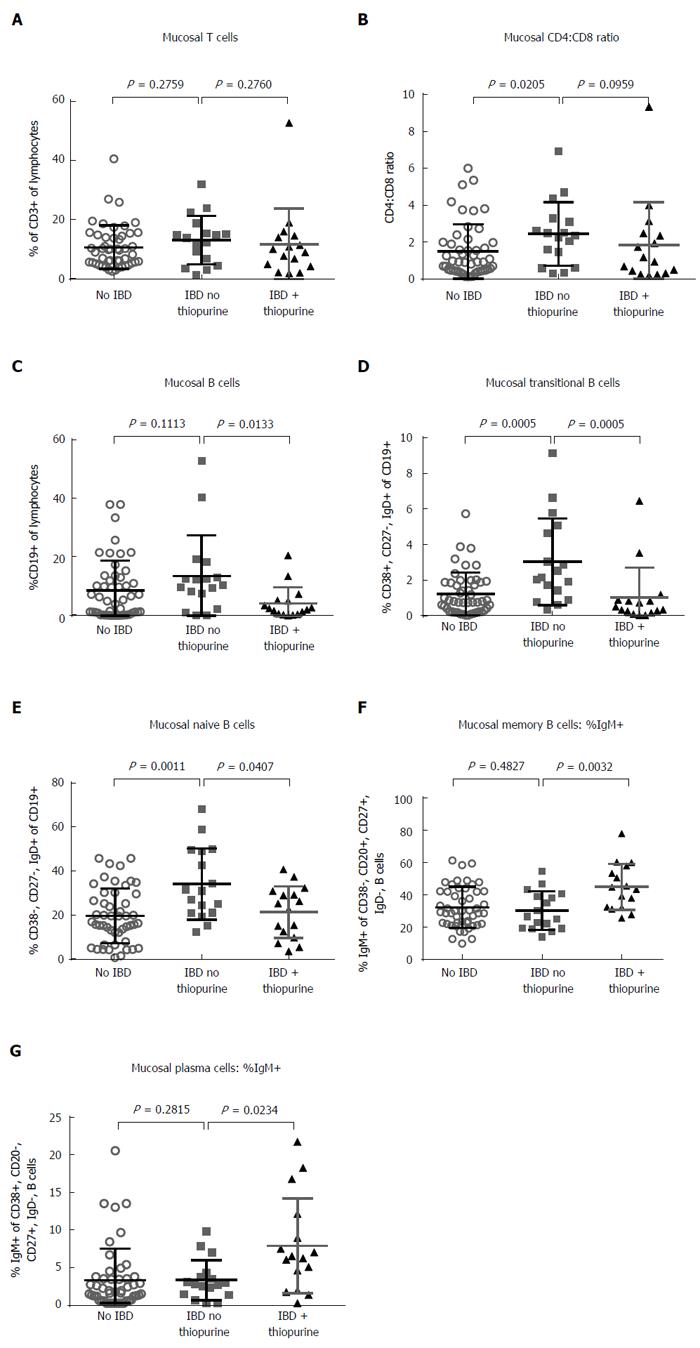
To determine if our findings in the peripheral blood were reflected at the site of IBD activity, we evaluated lymphocyte populations in the intestinal mucosa. Colon biopsies from IBD patients on as compared to those not on thiopurine, or from healthy screening colonoscopy recipients, were collagenase digested and evaluated by flow cytometry. In IBD patient biopsies, as in blood, thiopurine use had no effect on the mucosal CD3+ T cell fraction of live lymphocytes (P = 0.276; Figure 5A), nor on the CD4:CD8 ratio therein (P = 0.096; Figure 5B). The latter was lower (P = 0.02) and the percent of CD8+ T cells expressing CD103 was higher (P = 0.016) in healthy controls than in IBD patients off thiopurines, but these findings likely just reflect a generally lower ratio of epithelium to lamina propria in IBD, as the E-cadherin binding integrin CD103 is expressed by intraepithelial lymphocytes, most of which are CD8+.
NK cells were more difficult to define in intestinal biopsies than in blood, as only a small minority of CD56+, CD3- lymphocytes expressed CD16 or CD161 (data not shown). However, the fraction of CD3- lymphocytes expressing the NK marker CD56 did not correlate with thiopurine use in IBD patients (P = 0.986, data not shown).
In contrast, the use of thiopurines by IBD patients was associated with a smaller fraction of intramucosal lymphocytes being B cells (P = 0.0133; Figure 5C), as in blood. Transitional B cells were, again, significantly reduced as a fraction of total B cells in the setting of thiopurine use (P = 0.0005), although their frequency among thiopurine recipients resembled that of healthy controls, who had significantly fewer transitional B cells than untreated IBD patients (P = 0.0005; Figure 5D). Naïve cells were also a greater fraction of colonic B cells in IBD than controls (P = 0.0011), although the effect of thiopurines thereupon was less significant (P = 0.04) (Figure 5E). No significant associations with thiopurine use among IBD patients were observed in the fraction of B cells resembling memory (P = 0.557) or plasma cells (P = 0.154) (data not shown). However, thiopurine use was associated with significantly more IgM+ cells in the memory B cell (P = 0.0032; Figure 5F) and plasma cell (P = 0.0234, Figure 5G) compartments in IBD samples, suggesting that it may impair immunoglobin class-switching.
We performed a thorough characterization of lymphocyte subpopulations in well-matched cohorts of IBD patients on vs not on thiopurine immunosuppressants to determine if the lymphopenia associated with chronic use of these medications is attributable to selective depletion of a particular cell type. We found that NK and B cells, but not T cell populations, are reduced in the peripheral blood of IBD patients taking thiopurines, with the B cell depletion also being evident in intestinal samples. In particular, the transitional B cell compartment was depleted in the setting of thiopurine use.
Thiopurines have a relatively long history as monotherapy for IBD[1-3,12]. However, in recent years this role has been somewhat supplanted by their use concomitant with anti-TNF biopharmaceuticals, as a consequence of comparative efficacy trials demonstrating clear benefit of dual therapy with both a thiopurine and anti-TNF agent over monotherapy with either agent alone for IBD[4,5]. In this setting, a roughly 15% absolute increase in efficacy with dual therapy over anti-TNF monotherapy was paralleled by a roughly 15% absolute decrease in patients generating anti-drug antibodies (ADA’s) directed against the biopharmaceutical, as well as higher anti-TNF serum levels[4,5]. As ADA’s have clearly been associated with increased biopharmaceutical clearance, as well as hazardous infusion reactions[28], it is believed that the prevention of ADA’s is a primary mechanism by which thiopurines increase the safety and efficacy of anti-TNF therapy. Thus a potential clinical significance of our findings concerning B cells in thiopurine recipients is that they may help identify a peripheral biomarker for successful thiopurine-mediated ADA prophylaxis, or even identify drug-naïve IBD patients at low enough risk of ADA to obviate the need for thiopurine prophylaxis.
Our data suggests that thiopurines may shrink the pool of naïve and, particularly, transitional B cells to prevent these cells from ultimately becoming ADA-producing plasma cells upon de novo exposure to foreign biopharmaceutical proteins, while leaving intact pre-existing antigen-experienced memory B cells and plasma cells. Transitional B cells represent an early stage in B cell development, at which immature B cells that have undergone primary negative selection and receptor editing exit the bone marrow to become mature, but naïve, B cells in the spleen. As such, they retain a susceptibility to B cell receptor-mediated apoptosis common to immature B cells, making them vulnerable to negative selection[29]. As thiopurine metabolites have been shown to enhance T cell susceptibility to antigen receptor-mediated apoptosis in vitro[11], they may likewise lower the threshold for negative selection in apoptosis-prone transitional B cells in vivo, perhaps to such a level that nearly all nascent transitional B cells entering the periphery are spontaneously deleted, consistent with our findings (Figure 3F). Over time, this would result in a similar depletion of the naïve B cells derived from transitional B cells, which we observed (Figure 3D).
We also observed a decrease in circulating NK cells associated with thiopurine use. NK cells play an important role in providing an antiviral immune response and immune surveillance against tumors. Consequently, our findings may explain why thiopurine recipients are at increased risk of certain types of cancer, particularly lymphoma mediated by Epstein-Barr virus[30], or cervical cancer mediated by the human papilloma virus[31]. However, our study was not powered to capture such rare events and thus determine if NK cell frequency could be a biomarker for cancer susceptibility in thiopurine recipients.
Given in vitro mechanistic data suggesting that T cells are the primary target of thiopurines[11], we focused particularly upon T cell populations, including rare T cell subsets to exclude the possibility that a subtle effect of thiopurines could be selectively hidden therein. However, we found no selective depletion of any T cell population associated with thiopurine use in IBD patients. We did find that, regardless of thiopurine use, IBD patients have paradoxically more Helios+ FOXP3+ CD4+ T cells, resembling thymically-derived, “natural” Tregs[27], in their blood than matched healthy controls. While this could suggest that these immunoregulatory cells are being excluded from the bowel to facilitate intestinal inflammation, we have previously shown that, in UC, there is likewise a paradoxically higher frequency of Helios+ nTregs in the inflamed colonic mucosa than in controls[32]. Thus, we hypothesize that Tregs are ineffective at controlling the inflammation of IBD, rather than being numerically deficient, as they are in the enteropathy of the IPEX syndrome[21,33]. However, no significant differences were found between IBD patients and controls in the phenotype of these Tregs (See supplemental digital content 2).
In contrast to nTregs, we found a lower frequency of circulating iNKT and MAIT cells in IBD patient relative to controls. Decreased circulating MAIT cells in IBD have been previously reported[34]. Although these MAIT cells are known to express high levels of the multi-drug resistance gene ABCB1[35], which facilitates resistance to chemotherapeutic drugs[36] such as thiopurines, MAIT cells were not selectively enriched by thiopurine use in either the peripheral blood (See supplemental digital content 1) or intestine (data not shown) of IBD patients.
In summary, we have demonstrated that a relative lymphopenia associated with thiopurine use is attributable to decreased NK and B cells, rather than T cell depletion. Future studies associating them with clinical outcomes, such as ADA formation, may provide useful biomarkers to guide therapy for IBD patients. Furthermore, exploring the mechanism by which such changes occur may elucidate more selective means with which to tailor therapy while reducing the risk of off-target toxicities currently associated with thiopurine medications.
In conclusion, thiopurine use is associated with reduced B and NK cell, but not T cell, subpopulations in the blood of IBD patients, and reduced B cells in the colonic mucosa. These findings suggest a mechanism by which thiopurines reduce anti-drug antibodies and increase the risk of neoplasia, respectively.
We would like to thank the Digestive Disease Institute and its associated doctors, nurses and staff at Virginia Mason Hospital for funding these studies and allowing patient interactions. We thank Kassidy Benoscek and Melissa Peda for recruiting and consenting subjects to the biorepository used for this work. We thank Thien-Son Nguyen for biorepository management. We thank “Aru” K. Arumuganathan and Katharine Schwedhelm for assistance with flow cytometry. We thank biostatistician Elizabeth Whalen, PhD for manuscript review. We thank Brenda Norris for assistance with manuscript preparation.
The thiopurines azathioprine and 6-mercaptopurine have been a mainstay of inflammatory bowel disease (IBD) therapy for decades, but their mechanism of action in vivo remains obscure. Although thiopurines are lymphotoxic at high doses, and have been reported to cause T cell apoptosis in vitro, their ability to control IBD at lower doses suggests that they may selectively deplete particular lymphocyte populations.
Although long used for clinical benefit in IBD, the mechanism by which thiopurines suppress the immune system remains obscure. Understanding how such medications affect the human immune system in vivo is critical for improving upon them and identifying meaningful biomarkers with which to tailor therapy.
Historical in vitro mechanistic studies have suggested thiopurines control inflammation through T cell apoptosis, thus causing lymphopenia. The authors tested this hypothesis in vivo by correlating the frequency of major and minor lymphocyte populations with thiopurine use in IBD patients, and found that it was not T cell, but rather B and NK cell populations, that were decreased in thiopurine recipients. In particular, transitional B cells were all but eliminated in the blood of thiopurine recipients, a finding which was mirrored in the colon.
In addition to their role as a primary therapy for IBD, thiopurines have become widely used to suppress the development of antibodies to biopharmaceuticals, which are made by B cell progeny. Additionally, thiopurine use has been associated with an increased incidence of neoplasia, particularly from virally mediated tumors such as HSV-associated cervical cancer and EBV-associated lymphoma, against which NK cells perform immune surveillance. Thus, by finding selective decreases in B and NK cells associated with thiopurine use, they provide potential mechanistic evidence of how each of these clinical events occur.
In this study authors aimed to determine if and how thiopurine use is associated with depletion of specific lymphocyte populations. Authors demonstrated that a relative lymphopenia associated with thiopurine use is attributable to decreased NK and B cells, rather than T cell depletion. This is a good study and it gives us a well information in terms of thiopurine mechanism.
Manuscript source: Unsolicited manuscript
Specialty type: Gastroenterology and hepatology
Country of origin: United States
Peer-review report classification
Grade A (Excellent): 0
Grade B (Very good): B, B
Grade C (Good): C
Grade D (Fair): 0
Grade E (Poor): 0
P- Reviewer: Capasso R, Leclercq G, Yuksel I S- Editor: Gong ZM L- Editor: A E- Editor: Wang CH
| 1. | Brooke BN, Hoffmann DC, Swarbrick ET. Azathioprine for Crohn’s disease. Lancet. 1969;2:612-614. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 121] [Cited by in F6Publishing: 118] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 2. | Prefontaine E, Sutherland LR, Macdonald JK, Cepoiu M. Azathioprine or 6-mercaptopurine for maintenance of remission in Crohn’s disease. Cochrane Database Syst Rev. 2009;CD000067. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 37] [Cited by in F6Publishing: 67] [Article Influence: 4.5] [Reference Citation Analysis (0)] |
| 3. | Timmer A, McDonald JW, Tsoulis DJ, Macdonald JK. Azathioprine and 6-mercaptopurine for maintenance of remission in ulcerative colitis. Cochrane Database Syst Rev. 2012;CD000478. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 41] [Cited by in F6Publishing: 69] [Article Influence: 5.8] [Reference Citation Analysis (0)] |
| 4. | Colombel JF, Sandborn WJ, Reinisch W, Mantzaris GJ, Kornbluth A, Rachmilewitz D, Lichtiger S, D’Haens G, Diamond RH, Broussard DL. Infliximab, azathioprine, or combination therapy for Crohn’s disease. N Engl J Med. 2010;362:1383-1395. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 2221] [Cited by in F6Publishing: 2210] [Article Influence: 157.9] [Reference Citation Analysis (0)] |
| 5. | Panaccione R, Ghosh S, Middleton S, Márquez JR, Scott BB, Flint L, van Hoogstraten HJ, Chen AC, Zheng H, Danese S. Combination therapy with infliximab and azathioprine is superior to monotherapy with either agent in ulcerative colitis. Gastroenterology. 2014;146:392-400.e3. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 614] [Cited by in F6Publishing: 621] [Article Influence: 62.1] [Reference Citation Analysis (0)] |
| 6. | Pearson DC, May GR, Fick GH, Sutherland LR. Azathioprine and 6-mercaptopurine in Crohn disease. A meta-analysis. Ann Intern Med. 1995;123:132-142. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 721] [Cited by in F6Publishing: 702] [Article Influence: 24.2] [Reference Citation Analysis (0)] |
| 7. | de Boer NK, van Bodegraven AA, Jharap B, de Graaf P, Mulder CJ. Drug Insight: pharmacology and toxicity of thiopurine therapy in patients with IBD. Nat Clin Pract Gastroenterol Hepatol. 2007;4:686-694. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 90] [Cited by in F6Publishing: 93] [Article Influence: 5.5] [Reference Citation Analysis (0)] |
| 8. | Marathias VM, Sawicki MJ, Bolton PH. 6-Thioguanine alters the structure and stability of duplex DNA and inhibits quadruplex DNA formation. Nucleic Acids Res. 1999;27:2860-2867. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 54] [Cited by in F6Publishing: 58] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 9. | Smith SR, Terminelli C, Kipilman CT, Smith Y. Comparative effects of azathioprine, cyclophosphamide and frentizole on cellular immunity in mice. J Immunopharmacol. 1981;3:133-170. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 10] [Cited by in F6Publishing: 10] [Article Influence: 0.2] [Reference Citation Analysis (0)] |
| 10. | Quéméneur L, Gerland LM, Flacher M, Ffrench M, Revillard JP, Genestier L. Differential control of cell cycle, proliferation, and survival of primary T lymphocytes by purine and pyrimidine nucleotides. J Immunol. 2003;170:4986-4995. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 161] [Cited by in F6Publishing: 160] [Article Influence: 7.6] [Reference Citation Analysis (0)] |
| 11. | Tiede I, Fritz G, Strand S, Poppe D, Dvorsky R, Strand D, Lehr HA, Wirtz S, Becker C, Atreya R. CD28-dependent Rac1 activation is the molecular target of azathioprine in primary human CD4+ T lymphocytes. J Clin Invest. 2003;111:1133-1145. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 547] [Cited by in F6Publishing: 552] [Article Influence: 26.3] [Reference Citation Analysis (0)] |
| 12. | Candy S, Wright J, Gerber M, Adams G, Gerig M, Goodman R. A controlled double blind study of azathioprine in the management of Crohn’s disease. Gut. 1995;37:674-678. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 435] [Cited by in F6Publishing: 455] [Article Influence: 15.7] [Reference Citation Analysis (0)] |
| 13. | Colonna T, Korelitz BI. The role of leukopenia in the 6-mercaptopurine-induced remission of refractory Crohn’s disease. Am J Gastroenterol. 1994;89:362-366. [PubMed] [Cited in This Article: ] |
| 14. | Fraser AG, Orchard TR, Jewell DP. The efficacy of azathioprine for the treatment of inflammatory bowel disease: a 30 year review. Gut. 2002;50:485-489. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 452] [Cited by in F6Publishing: 449] [Article Influence: 20.4] [Reference Citation Analysis (0)] |
| 15. | Campbell AC, Skinner JM, Hersey P, Roberts-Thomson P, MacLennan IC, Truelove SC. Immunosuppression in the treatment of inflammatory bowel disease. I. Changes in lymphoid sub-populations in the blood and rectal mucosa following cessation of treatment with azathioprine. Clin Exp Immunol. 1974;16:521-533. [PubMed] [Cited in This Article: ] |
| 16. | Campbell AC, Skinner JM, Maclennan IC, Hersey P, Waller CA, Wood J, Jewell DP, Truelove SC. Immunosuppression in the treatment of inflammatory bowel disease. II. The effects of azathioprine on lymphoid cell populations in a double blind trial in ulcerative colitis. Clin Exp Immunol. 1976;24:249-258. [PubMed] [Cited in This Article: ] |
| 17. | Eckhardt R, Kloos P, Dierich MP, Meyer zum Büschenfelde KH. K-lymphocytes (killer-cells) in Crohn’s disease and acute virus B-hepatitis. Gut. 1977;18:1010-1016. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 21] [Cited by in F6Publishing: 19] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 18. | Bouma G, Baggen JM, van Bodegraven AA, Mulder CJ, Kraal G, Zwiers A, Horrevoets AJ, van der Pouw Kraan CT. Thiopurine treatment in patients with Crohn’s disease leads to a selective reduction of an effector cytotoxic gene expression signature revealed by whole-genome expression profiling. Mol Immunol. 2013;54:472-481. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 8] [Cited by in F6Publishing: 9] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 19. | Cattan S, Lémann M, Thuillier F, Bengoufa D, Rabian C, Ngo Y, Bouhnik Y, Messing B, Rambaud JC, Modigliani R. 6-mercaptopurine levels and study of blood lymphocyte subsets during azathioprine treatment of Crohn’s disease. Gastroenterol Clin Biol. 1998;22:160-167. [PubMed] [Cited in This Article: ] |
| 20. | Ziegler SF. FOXP3: of mice and men. Annu Rev Immunol. 2006;24:209-226. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 719] [Cited by in F6Publishing: 765] [Article Influence: 42.5] [Reference Citation Analysis (0)] |
| 21. | Bennett CL, Christie J, Ramsdell F, Brunkow ME, Ferguson PJ, Whitesell L, Kelly TE, Saulsbury FT, Chance PF, Ochs HD. The immune dysregulation, polyendocrinopathy, enteropathy, X-linked syndrome (IPEX) is caused by mutations of FOXP3. Nat Genet. 2001;27:20-21. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 2432] [Cited by in F6Publishing: 2398] [Article Influence: 104.3] [Reference Citation Analysis (0)] |
| 22. | Wildin RS, Ramsdell F, Peake J, Faravelli F, Casanova JL, Buist N, Levy-Lahad E, Mazzella M, Goulet O, Perroni L. X-linked neonatal diabetes mellitus, enteropathy and endocrinopathy syndrome is the human equivalent of mouse scurfy. Nat Genet. 2001;27:18-20. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 1371] [Cited by in F6Publishing: 1329] [Article Influence: 57.8] [Reference Citation Analysis (0)] |
| 23. | Makita S, Kanai T, Oshima S, Uraushihara K, Totsuka T, Sawada T, Nakamura T, Koganei K, Fukushima T, Watanabe M. CD4+CD25bright T cells in human intestinal lamina propria as regulatory cells. J Immunol. 2004;173:3119-3130. [PubMed] [DOI] [Cited in This Article: ] [Cited by in F6Publishing: 1] [Reference Citation Analysis (0)] |
| 24. | Saruta M, Yu QT, Fleshner PR, Mantel PY, Schmidt-Weber CB, Banham AH, Papadakis KA. Characterization of FOXP3+CD4+ regulatory T cells in Crohn’s disease. Clin Immunol. 2007;125:281-290. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 142] [Cited by in F6Publishing: 150] [Article Influence: 8.8] [Reference Citation Analysis (0)] |
| 25. | Yu QT, Saruta M, Avanesyan A, Fleshner PR, Banham AH, Papadakis KA. Expression and functional characterization of FOXP3+ CD4+ regulatory T cells in ulcerative colitis. Inflamm Bowel Dis. 2007;13:191-199. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 131] [Cited by in F6Publishing: 127] [Article Influence: 7.5] [Reference Citation Analysis (0)] |
| 26. | Maul J, Loddenkemper C, Mundt P, Berg E, Giese T, Stallmach A, Zeitz M, Duchmann R. Peripheral and intestinal regulatory CD4+ CD25(high) T cells in inflammatory bowel disease. Gastroenterology. 2005;128:1868-1878. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 470] [Cited by in F6Publishing: 467] [Article Influence: 24.6] [Reference Citation Analysis (0)] |
| 27. | Thornton AM, Korty PE, Tran DQ, Wohlfert EA, Murray PE, Belkaid Y, Shevach EM. Expression of Helios, an Ikaros transcription factor family member, differentiates thymic-derived from peripherally induced Foxp3+ T regulatory cells. J Immunol. 2010;184:3433-3441. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 936] [Cited by in F6Publishing: 1016] [Article Influence: 72.6] [Reference Citation Analysis (0)] |
| 28. | Baert F, Noman M, Vermeire S, Van Assche G, D’ Haens G, Carbonez A, Rutgeerts P. Influence of immunogenicity on the long-term efficacy of infliximab in Crohn’s disease. N Engl J Med. 2003;348:601-608. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 1523] [Cited by in F6Publishing: 1453] [Article Influence: 69.2] [Reference Citation Analysis (0)] |
| 29. | Chung JB, Silverman M, Monroe JG. Transitional B cells: step by step towards immune competence. Trends Immunol. 2003;24:343-349. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 172] [Cited by in F6Publishing: 162] [Article Influence: 7.7] [Reference Citation Analysis (0)] |
| 30. | Vos AC, Bakkal N, Minnee RC, Casparie MK, de Jong DJ, Dijkstra G, Stokkers P, van Bodegraven AA, Pierik M, van der Woude CJ, Oldenburg B, Hommes DW; Initiative on Crohn’s and Colitis (ICC). Risk of malignant lymphoma in patients with inflammatory bowel diseases: a Dutch nationwide study. Inflamm Bowel Dis. 2011;17:1837-1845. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 72] [Cited by in F6Publishing: 60] [Article Influence: 4.6] [Reference Citation Analysis (0)] |
| 31. | Dugué PA, Rebolj M, Hallas J, Garred P, Lynge E. Risk of cervical cancer in women with autoimmune diseases, in relation with their use of immunosuppressants and screening: population-based cohort study. Int J Cancer. 2015;136:E711-E719. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 32] [Cited by in F6Publishing: 33] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 32. | Lord J, Chen J, Thirlby RC, Sherwood AM, Carlson CS. T-cell receptor sequencing reveals the clonal diversity and overlap of colonic effector and FOXP3+ T cells in ulcerative colitis. Inflamm Bowel Dis. 2015;21:19-30. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 22] [Cited by in F6Publishing: 20] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 33. | Gambineri E, Torgerson TR, Ochs HD. Immune dysregulation, polyendocrinopathy, enteropathy, and X-linked inheritance (IPEX), a syndrome of systemic autoimmunity caused by mutations of FOXP3, a critical regulator of T-cell homeostasis. Curr Opin Rheumatol. 2003;15:430-435. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 425] [Cited by in F6Publishing: 414] [Article Influence: 19.7] [Reference Citation Analysis (0)] |
| 34. | Serriari NE, Eoche M, Lamotte L, Lion J, Fumery M, Marcelo P, Chatelain D, Barre A, Nguyen-Khac E, Lantz O. Innate mucosal-associated invariant T (MAIT) cells are activated in inflammatory bowel diseases. Clin Exp Immunol. 2014;176:266-274. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 254] [Cited by in F6Publishing: 244] [Article Influence: 24.4] [Reference Citation Analysis (0)] |
| 35. | Dusseaux M, Martin E, Serriari N, Péguillet I, Premel V, Louis D, Milder M, Le Bourhis L, Soudais C, Treiner E. Human MAIT cells are xenobiotic-resistant, tissue-targeted, CD161hi IL-17-secreting T cells. Blood. 2011;117:1250-1259. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 674] [Cited by in F6Publishing: 720] [Article Influence: 51.4] [Reference Citation Analysis (0)] |
| 36. | Nooter K, Herweijer H. Multidrug resistance (mdr) genes in human cancer. Br J Cancer. 1991;63:663-669. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 150] [Cited by in F6Publishing: 152] [Article Influence: 4.6] [Reference Citation Analysis (0)] |