Published online Jul 28, 2017. doi: 10.3748/wjg.v23.i28.5086
Peer-review started: April 10, 2017
First decision: April 21,2017
Revised: May 2, 2017
Accepted: June 19, 2017
Article in press: June 19, 2017
Published online: July 28, 2017
Colorectal cancer (CRC) is a significant cause of morbidity and mortality worldwide. However, colon cancer incidence and mortality is declining over the past decade owing to adoption of effective screening programs. Nevertheless, in some parts of the world, CRC incidence and mortality remain on the rise, likely due to factors including “westernized” diet, lifestyle, and lack of health-care infrastructure and resources. Participation and adherence to different national screening programs remain obstacles limiting the achievement of screening goals. Different modalities are available ranging from stool based tests to radiology and endoscopy with varying sensitivity and specificity. However, the availability of these tests is limited to areas with high economic resources. Recently, FDA approved a blood-based test (Epi procolon®) for CRC screening. This blood based test may serve to increase the participation and adherence rates. Hence, leading to increase in colon cancer detection and prevention. This article will discuss various CRC screening tests with a particular focus on the data regarding the new approved blood test. Finally, we will propose an algorithm for a simple cost-effective CRC screening program.
Core tip: Multiple societies have published screening guidelines concerning colorectal cancer (CRC) screening. Despite that, global participation can be challenging due to wide variability in the availability of screening tools, especially the newer resources. Additionally, patient friendly approach, improving patient uptake, adherence, and compliance to attain national CRC screening goals are still lacking. Regardless of the screening approaches utilized, it is necessary to demonstrate high sensitivity for detection of advanced neoplasia and CRC, as well as high specificity for costeffectiveness. Furthermore, they must have broad acceptability to the general population, healthcare providers, and third-party payers. Hence, achieving most of the screening value that is derived from cancer prevention over cancer detection. Recently, a very appealing blood test was FDA approved for screening, this modality certainly carries significant appeal, but how does it fare when we compare to the rest of tests? This is the question we aim to answer through this review.
- Citation: Issa IA, Noureddine M. Colorectal cancer screening: An updated review of the available options. World J Gastroenterol 2017; 23(28): 5086-5096
- URL: https://www.wjgnet.com/1007-9327/full/v23/i28/5086.htm
- DOI: https://dx.doi.org/10.3748/wjg.v23.i28.5086
Colorectal cancer (CRC) is the third most common cancer in men and the second most common cancer in women worldwide[1] with 1.36 million people affected globally, accounting for nearly 10% of cancers[2]. It remains the second leading cause of cancer in the United States and worldwide[1,3]. Due to its slow progression from detectable precancerous lesions and to the much better prognosis of patients diagnosed at early stages, the potential for reducing the burden of the disease by early detection is significant. While screening has noticeably been shown to reduce the risk of CRC associated mortality[4], its effectiveness is jeopardized by a multitude of factors including the limitations of test performance, lack of accessibility, and suboptimal screening compliance. Consequently, resulting in a marked variation in CRC incidence and mortality globally[1,5]. The newly approved blood screening test overcomes most of the above mentioned factors, this may lead to better participation rates. This article will discuss various CRC screening tests particularly the new blood based test. Moreover, we will debate the different CRC risk assessment scores, and screening programs participation and adherence rates, as well as the issue of total cost. Furthermore, we will propose a potential screening algorithm that might attain a high rate of population participation and adherence, especially in lower income countries.
The ideal screening study should be efficient with high sensitivity and specificity, safe, available, convenient, and cheap. Current CRC screening methods are divided into invasive and non-invasive tests.
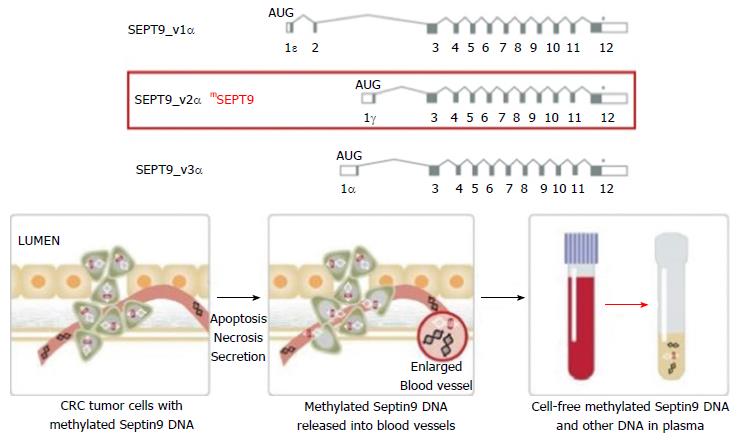
The non-invasive tests include stool and blood-based tests and radiologic tests. The stool-based tests currently available are the guaiac-based fecal occult blood test (gFOBT), fecal immunochemical test (FIT), and the newer fecal DNA testing (Multitarget stool DNA, MT-sDNA, Cologuard®). Those tests are based on the concept of detecting blood or shredded cell debris by vascularized polyps, adenomas and cancers[6]. The radiologic examinations include double contrast barium enema, capsule endoscopy and Computed tomographic colonography (CTC). Their role revolves around radiographic visualization and identification of an advanced colonic polyp or cancer in addition to the possibility of detection of extra-colonic findings (by CTC). The newly emerged blood test (Epi procolon®) is a qualitative in vitro diagnostic polymerase chain reaction (PCR) test for the detection of mutated methylated septin9 DNA in EDTA plasma derived from patient whole blood specimens. Methylated SEPT9 has been associated with the occurrence of CRC[7].
Invasive tests include the flexible sigmoidoscopy (FS) and colonoscopy which offer direct visualization and detection of a colonic polyp or advanced neoplasia with the advantage of getting a pathology specimen. In real life, colonoscopy has been introduced as a primary screening tool in a number of countries in recent years including United States, Germany, Poland, Austria, and parts of Italy. gFOBT is still being used in France, Finland, and the United Kingdom. Netherlands and many other European countries have shifted from gFOBT to FIT. Sigmoidoscopy remains a screening option in the United Kingdom. In United States all the available screening methods are still being offered.
In the United States, the two main principle guidelines for CRC screening are: (1) joint guidelines from the American Cancer Society, the United States MultiSociety Task Force on Colorectal Cancer, and the American College of Radiology[8]; and (2) the United States Preventive Services Task Force guidelines which have been updated recently[9]. Other organizations have issued their own guidelines as well, such as the American College of Gastroenterology[10], the American College of Physicians[11], and the National Comprehensive Cancer Network[12]. Table 1 summarizes the latest recommendations from these different sets of guidelines for average-risk individuals. It is noteworthy that almost all the guidelines are coherent in terms of their recommendations, with the exception of barium enema being dropped for reasons of low sensitivity of only 48%[13]. Additionally, the frequency of stool DNA analysis is controversial, as such a test is rather new. Nonetheless, all the remaining screening options are well endorsed by all the societies as potential screening methods.
| ACG (2009) | ACP (2015) | NCCN (2015) | USPSTF (2016) | ACS (2016) | |
| Sigmoidoscopy (yr) | Q 5-10 | Q 5 | Q 5 | Q 5 or Q 10 + stl | Q 5 |
| Colonoscopy (yr) | Q 10 | Q 10 | Q 10 | Q 10 | Q 10 |
| CT colonography (yr) | Q 5 | N/A | N/A | Q 5 | Q 5 |
| Ba enema (yr) | N/A | N/A | Q 5 | N/A | Q 5 |
| Stool eFOBT (yr) | Q 1 | Q 1 | Q 1 | Q 1 | Q 1 |
| Stool FIT (yr) | Q 1 | Q 1 | Q 1 | Q 1 | Q 1 |
| Stool MT-sDNA (yr) | N/A | N/A | N/A | Q 1-3 | Q 3 |
In the 1980’s and 1990’s, most screening methods put in use were FOBT and sigmoidoscopy. However, since the year 2000, most CRC screening in the United States shifted towards colonoscopy, even though its effects on reducing CRC incidence and mortality has never been proven in a solid randomized controlled trail. Therefore, the evident question would be: has it been effective?
Data from a recent study examining the incidence of CRC in males and females established a drop in both incidence and mortality from CRC in both genders concomitant with the beginning of the screening programs[3]. In addition to that, over a 20-year period (SEER data 1991-2011), the United States colorectal cancer incidence (all races and genders confounded) has declined from 59.5 cases to 39.3 cases per 100000 (35% reduction) with a corresponding mortality reduction over the same time period from 24.0 to 15.1 deaths per 100000 (37% reduction)[14]. Moreover, in the National Polyp Study (NPS), CRC was prevented by colonoscopic removal of adenomatous polyps. They evaluated the long-term effect of polypectomy in a study on mortality from CRC. Among 2602 patients who had adenomas removed during participation, after a median of 15.8 years, 1246 patients had died from any cause and 12 had died from CRC. Given an estimated 25.4 expected deaths from CRC in the general population (SEER cohort), the standardized incidence-based mortality ratio was 0.47 (95%CI: 0.26-0.80) with colonoscopic polypectomy, suggesting a 53% reduction in mortality[15]. Although the NPS does not address the effectiveness of screening colonoscopy in the general population, their findings provide an indirect estimate of the effect of removing adenomas, which is the primary interventional measure in screening colonoscopy. These findings support the hypothesis that colonoscopic removal of adenomatous polyps prevents death from CRC.
Colonoscopy is considered to be the gold standard tool of screening with a high sensitivity and specificity. This test affords the opportunity to detect and resect neoplasia and precancerous lesions across the entire large bowel and is the definitive examination when other screening tests are positive. It is relatively safe with recent data suggesting a less than 1/1000 perforation rate most often due to a polypectomy rather than the act itself. On the other hand, colonoscopy requires full bowel preparation and sedation[16-20]. Moreover, despite it being readily available, it is not considered cheap or easily affordable to the general population, hence rendering its application difficult on mass screening basis[21-24]. Multiple case-control and prospective cohort studies have estimated cancer mortality to be 68% to 88% lower among persons who undergo screening colonoscopy than among those who do not[18,24-26]. A meta-analysis of observational studies showed that despite a 68% lower overall mortality, limited benefit from colonoscopy was seen with respect to cancer in the proximal colon[17]. Another study showed that there was a 29% reduction in overall CRC mortality, a 47% reduction in mortality from distal CRC, and no reduction in mortality from proximal CRC. This study concluded that colonoscopy significantly reduces mortality from CRC, but the benefit is not uniform across different areas of the colon[22]. This discrepancy may be due to several factors affecting the quality of the act itself[27-31] (i.e., incomplete colonoscopy, training level and experience of the gastroenterologist, inadequate bowel preparation, or technical difficulties with polyp removal in the proximal colon) or possibly differences in the biologic characteristics of proximal and distal colorectal cancers[32]. To address these issues thoroughly, data from large controlled randomized trails are still lacking but are currently under way. The Colonoscopy vs Fecal Immunochemical Test in Reducing Mortality from Colorectal Cancer (CONFIRM) trial (ClinicalTrials.gov number, NCT01239082) is a randomized comparison of one-time colonoscopy with annual FIT plus colonoscopy as follow-up to a positive test, to examine CRC incidence and mortality over 10 years. A similar trial comparing colonoscopy with FIT is being conducted in Spain (COLONPREV) trail (ClinicalTrials.gov number, NCT00906997)[33]. Two additional European studies are comparing screening colonoscopy with no screening [the Nordic-European Initiative on Colorectal Cancer (NordICC)] trial (ClinicalTrials.gov number, NCT00883792)[34,35] or with FIT or no screening [Screening of Swedish Colons (SCREESCO), NCT02078804] with respect to mortality from CRC.
Sigmoidoscopy offers limited bowel preparation compared to colonoscopy. In addition, several randomized controlled trials have shown that screening with FS, followed by colonoscopy if precancerous polyps are detected, reduces CRC mortality[36,37]. Analysis from several large, randomized, controlled trials have confirmed the efficacy of one-time and periodic (every 3 to 5 years) sigmoidoscopy, with a 26% to 31% lower mortality from CRC among patients who underwent FS screening than among those who underwent no screening[17,38-41]. However, the benefit of sigmoidoscopy is limited to cancer in the distal colon (rectum, sigmoid, and descending colon), for which the reduction in mortality was reported to be 46%[17]. Many programs have abandoned this strategy in favor of colonoscopy for better prevention results.
gFOBT detects the presence of blood in feces through a chemical reaction dependent upon the peroxidase activity of heme. It is an inexpensive, simple, and widely available test. A landmark study that evaluated the fecal occultblood test randomized 46551 participants 50 to 80 years of age to screening for CRC on once a year basis, every two years, or to a control group. They concluded that annual fecal occult-blood testing with rehydration of the samples decreased the 13-year cumulative mortality from CRC by 33%[42]. Another randomized study compared mortality rates after FOB tests every 2 years during a 10-year period with those of unscreened similar controls. They found that after 10 years of follow-up, screening by FOB every 2 years (Hemoccult-II without rehydration) led to a reduction of 18% in CRC mortality. This was independent of sex and age, in individuals aged 45-75 years[43,44]. In the Minnesota Colon Cancer Control Study a 30-year follow-up of patients randomly assigned to annual/or biennial gFOBT vs usual care showed a 32% decrease in CRC mortality. Incidentally mortality reduction was more pronounced in men compared to women[45]. Several other randomized, controlled trials have shown lower mortality from CRC with this strategy compared to no screening[46-49]. However, this test requires a moderate quantity of heme to effect a visible change in color and thus is not analytically very sensitive to the presence of blood[6]. Once-only test sensitivity for cancer may approximate 50%[50] although many other studies indicate it is lower[51]. The method relies on simple oxidation, and therefore, any dietary peroxidases, such as heme from myoglobin in red meat, peroxidase in plants, etc., or any antioxidant, such as vitamin C, have the potential to confound the result. The gFOBT is therefore an inherently non-specific test with a very low PPV of 3%-10%[8,52]. Consequently, a test showing higher statistical results was urgently needed.
FIT is considered to be a newer version of the guaiac based FOBT. It is an antibody to human globin that does not cross react with dietary meats. Therefore, no need to avoid foods with peroxidase activity. FIT carefully measures the colonic blood since upper gastrointestinal globin is degraded readily by digestive proteolytic enzymes. The FIT sampling technique is simple and easy to collect with fewer fecal samples required compared to FOBT. Trials have shown that FIT has a greater sensitivity for detecting advanced adenomas and CRC than both standard and sensitive gFOBT[53-57]. More recent systematic review and meta-analysis including 19 qualified studies showed an overall accuracy of FIT for detection of CRC of 95% with a cumulative respective 79% sensitivity and 94% specificity[58]. Moreover, data for its protective effect can already be extrapolated from several screening programs. For instance, an organized biennial single FIT screening program in Florence where 6961 were screened with an average follow-up period of 11 years have shown a 22% reduction in CRC incidence[59]. One of the disadvantages of FIT is its low sensitivity for detecting colon polyps[57]. Additionally, many types of tests are available, the measures of accuracy vary greatly between tests within a technology as well as between technologies and according to how the test is applied (e.g., sample number) and the various confusing cutoff levels[60-62].
CTC or virtual colonoscopy is a rapid radiographic non-invasive imaging test that requires no sedation with lower procedural risks compared to colonoscopy[63-65]. In addition to that, it carries the advantage of extra colonic evaluation[66-69]. In a recent comparative meta-analysis the estimated pooled sensitivity and specificity per patient for polyps detection in asymptomatic screened patients were 66.8% and 80.3% respectively for CT colonography, and 92.5% and 73.2% respectively for colonoscopy. Analysis according to size showed that both studies have similar sensitivity for large polyps but evident lower CTC sensitivity for polyps < 8 mm. Regarding overall detection of CRC, the pooled sensitivity of CT colonography (96%) was not statistically significant from that of colonoscopy (91%)[70]. However, CTC is not a very pleasant study since the patient must take the same preparation as for colonoscopy in addition to the same discomfort during procedure insufflation. Moreover, contrast allergy, radiation exposure, and the need for colonoscopy if positive findings are considered additional disadvantages of CTC[71]. Perforation risk is an existing drawback, although to a lesser degree than colonoscopy[72]. To note that, published data from randomized studies evaluating the impact of CT colonography on CRC incidence and mortality are lacking.
In August 2014, Cologuard® became the first multi-target stool DNA approved by the FDA for general CRC screening[73]. Stool DNA test targets molecular debris in stool including abnormal DNA present in malignancies such as mutant KRA, actin, FIT, aberrantly methylated BMP3, and NDRG4 promoter regions. One multicenter study on nearly 10000 patients comparing Cologuard® to FIT using colonoscopy as the gold standard showed that the fecal DNA test had a higher sensitivity than FIT for detecting CRC (92% vs 74%). Unfortunately, Cologuard® detected fewer than half of all large advanced adenomas (42%), limiting its preventive role. Fecal DNA test had lower specificity at 87%-90% compared to FIT (95%-96%)[74]. Moreover, a new elegant study from Stanford university used a Markov model of average-risk CRC screening to compare the effectiveness and cost effectiveness of screening with the MT-sDNA test vs FIT or colonoscopy. They found FIT and colonoscopy to be more effective and less costly than the MT-sDNA test when participation rates were equal for all strategies. For the MT-sDNA test to be cost effective, the patient support program included in its cost would need to achieve substantially higher participation rates than those for FIT, whether in organized programs or under the opportunistic screening setting that is more common in the United States[75]. Additionally, the screening interval differs between FIT and MT-sDNA, which makes a comparison of the effectiveness of any programed screening difficult.
Septins are a group of scaffolding proteins that provide structural support during cell division[76]. Individual septins exist in stable six-to eight-subunit core heteromers, and the octamer contains two molecules of each of SEPT2, SEPT6, SEPT7, and SEPT9 subunits[77]. It was suggested that SEPT9 occupies a terminal position in the complex and plays a key role in subunit polymerization and the whole octamer stabilization[78]. It is also critical for the final separation of daughter cells during cytokinesis[79]. Therefore, cytokinesis may be seriously affected if abnormal SEPT9 or no SEPT9 is expressed, and this could be a key factor in CRC carcinogenesis when the promoter region of the SEPT9 gene is hypermethylated and the transcription is compromised[80]. Hypermethylated Septin9 DNA can be found in the tumor DNA that has been shed into the bloodstream from all intestinal anatomical sites[81]. Epi proColon® (also referred to as the mSEPT9 assay) became FDA approved for CRC screening in April 2016, it is the first blood test used for this goal. The mSEPT9 assay relies on qualitative detection by Real-Time PCR of the methylated Septin 9 gene that is present in increased levels in patients with colon cancer[82,83] (Figure 1). In initial retrospective case-control studies, the mSEPT9 test showed a great promise, with a sensitivity of about 70% and specificity of 90% for CRC detection[81,82]. A subsequent prospective trial in an asymptomatic screening cohort reported lower rates of sensitivity (48%) and specificity (92%) for CRC. However, this sensitivity decreased to 35% for stage I CRC and 11% for advanced adenomas almost totally eliminating its preventive role[84]. In a prospective multi-center study, compared with FIT, Sept9 testing showed similar sensitivity (68% vs 73%) but markedly decreased specificity (97% vs 81%). While the overall sensitivity for CRC detection of Septin9 may be superior to gFOBT, it is non-inferior to that of FIT[85]. However, relative to Cologuard®, the Epi proColon® test appears to be less sensitive for both CRC and advanced adenomas in actual practice, but with a higher specificity for cancer[86].
Nonetheless, evidence suggests that some patients who are reluctant to undergo the usual screening would be receptive to a blood test. An observational study showed that 97% of subjects refusing colonoscopy accepted a non-invasive screening test, of these 83% chose a blood test. This demonstrates that offering non-invasive test options might significantly increase compliance and screening participation[87]. A cost-utility analysis comparing SEPT9, FOBT, FIT, sigmoidoscopy, and colonoscopy suggested that while the use of SEPT9 appeared cost-effective for screening compared to no screening, to be cost-effective relative to other established methods SEPT9 or any other blood-based biomarker with similar test performance characteristics would need to achieve substantially higher uptake and adherence rates than the alternatives[88].
Moreover, SEPT9 blood testing raises concerns for potential abuse leading to inadequate screening, a similar drawback is seen with the prostate specific antigen for prostate cancer. Lastly, much like the other non-invasive tests a second intervention is needed if the test was positive[89].
Stratifying the population by colon cancer risk offers the potential to improve the efficiency of screening and helps establishing a screening program. A quick review of the literature reveals more than 50 proposed risk scores for colon cancer that have the potential to identify individuals at high risk. A recent systematic review that examined all the available risk scores, showed that the discrimination of the models, compare favorably with risk models used for other cancers, and several include only variables recorded in routine medical records. Grouping risk models according to type and number of variables included also showed that there is no clear improvement in discrimination as increasing numbers of variables are added from self-completed questionnaires to routine data. A small number of risk models developed from case-control studies of genetic biomarkers showed serious promise but still require further external validation in population-based samples. This review also showed that risk models exist, with the potential to stratify the general population into risk categories, and allow screening and preventive strategies to be targeted at those most likely to benefit, while leaving those at low risk of disease unexposed to direct and indirect side effects of screening programs. This might improve the cost-effectiveness of CRC screening and would address concerns about demand and capacity for colonoscopy. The use of risk prediction models would also potentially increase acceptance of screening and provide an opportunity to give information to encourage lifestyle modification[90]. The two most commonly used and validated scores are the Cleveland Clinic test and the NCI test; which are both self-completed questionnaire and can provide a suggested 10-year risk assessment. A prospective examination of the relationship between predicted 10-year CRC risk and the prevalence of advanced neoplasia (AN), defined as advanced or multiple (≥ 3 adenomatous, ≥ 5 serrated) adenomas or sessile serrated polyps, in individuals undergoing screening colonoscopy was studied in 509 screeners. AN was found in 11%. The prevalence of AN increased progressively from 6% in the lowest risk-score quintile to 17% in the highest risk-score quintile (P = 0.002). The discriminatory accuracy of the tool was modest, with AUC of 0.61 overall (95%CI: 0.54-0.69)[91].
Despite the various modalities offered for CRC screening, it is still underused by populations. Screening rates have not increased appreciably since 2010 and remain at approximately 60%[92]. Several complex factors play a role in affecting the patients participation and sustained adherence. Barriers to screening include elevated cost, lack of proper education regarding CRC, under appreciation of the benefit of screening, a sense of fatalism, or simply fear of the screening tests[93,94]. Several interventions used in randomized, controlled trials have been shown to increase patient participation rates; such interventions include sending patients invitations from their primary care provider, sending reminder letters, making telephone calls, and mailing fecal occult blood test kits to patients’ homes.
Ladabaum et al[88] suggested through an elaborate markov model reproduction that at comparable screening participation rates among different strategies, FIT or screening colonoscopy are likely to be more effective and less costly than MT-sDNA. They concluded that in comparison to an organized screening program of yearly FIT, a program of MT-sDNA testing every 3 years could be cost-effective if this program could accomplish a steady participation rate of approximately 66%[82]. Several National screening programs that examined patterns of CRC screening using gFOBT and FIT concluded that there is 20%-29% rate of non-responders and after 3 or 4 cycles there is an additional drop out of up to 30%[95-98]. For instance in an annual FIT screening program in California there was a 48% initial participation with a 75% adherence after 4 cycles[99]. Analysis from those programs also indicate a strong sex and socioeconomic inequalities in CRC screening uptake. Repeated invitations to screening successfully engage previous non-responders. Many respond to at least one screening invitation over multiple rounds with a considerably smaller number of persons responding consistently to all invitations. Therefore, efforts to increase (continued) engagement among these “at-risk” groups are essential to optimize the long-term benefit of organized screening programs[95].
Total patient support, which can include navigation, has emerged as an important component of many CRC screening efforts. The most successful programs use patient navigators to reduce logistic barriers, address cultural issues, and encourage participants to undergo screening. Moreover, patient support costs in the CDC and Prevention’s Colorectal Cancer Screening Demonstration Program averaged $153 per person over approximately 1 year[100]. The use of patient navigators is especially important in underserved populations[101,102]. It is estimated to be likely cost-effective and increase people’s adherence[103-106].
The National Colorectal Cancer Round table has established a goal of 80% adherence to CRC screening programs by the year 2018. Kaiser Permanente has implemented a comprehensive strategy focused on FIT screening, with colonoscopy performed as follow-up to a positive test. They have reached and maintained the goal of 80% adherence rate through four rounds of screening[99]. Adherence to screening tests varies among strategies, and preference of strategy varies by race and ethnic group; data have shown that white participants more commonly prefer colonoscopy, and non-white participants tend to prefer fecal testing[93,107]. To achieve the highest level of adherence to CRC screening, it may be best to provide participants a choice, because the “best” strategy is the one that they will adhere to consistently.
Maximizing the benefit of CRC screening requires a programmatic approach to implementing screening strategies. The quality of a screening program should be measured by its ability to identify patients who are due for screening, provide access to screening, assess adherence to the screening test and to a follow-up colonoscopy if a non-invasive screening test is positive. It also needs to document test outcomes and disseminate accurate follow-up recommendations, identify patients with a negative test to follow them up for repeat screening at the appropriate intervals, and provide timely surgery for cancers. The rate of adenoma detection (the percentage of patients in whom precancerous polyps are detected during screening colonoscopy) differs substantially among endoscopists and may be used as a measure of the ability of screening to prevent CRC[108]. A retrospective study showed that for every 1% increase in the rate of adenoma detection, there is a 3% decrease in the rate of cancer developing after a colonoscopy[109]. One important factor to increase the adenoma detection rate is the adequate bowel cleansing. Therefore, bowel preparation is considered a crucial step in any CRC screening program. Patient compliance with various bowel preparation procedures remains one of the most difficult tasks to achieve before a colonoscopy. Failure to complete the preparation has several drawbacks in terms of decreased cecal intubation rates, increased rates of missing important lesions, and prolonged colonoscopy duration further increasing patient discomfort[27-30]. Tepeš et al[110] evaluated the effectiveness of bowel cleansing with magnesium sulphate and low-volume polyethylene glycol with electrolytes in addition to the effect of timing of the colonoscopy. They found excellent bowel cleansing in 82.61% participants and in more participants with young age with colonoscopy performed in the afternoon[110].
One of the National CRC screening programs was conducted in Slovenia with promising results. They used the FIT test with a positive result being followed by colonoscopy. Assessment of the first round showed an adherence rate of 56.9%. The overall adenoma detection rate was 51.3%. CRC was found in 6.2% of participants who underwent colonoscopy. A localized clinical stage was found in 70.2% of cases. Cancer was cured in 22.8% of CRC patients with endoscopic resection only[111].
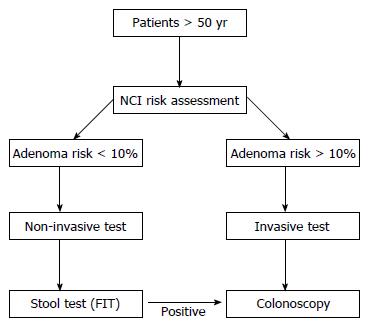
Figure 2 illustrates a proposed cost-effective algorithm for a screening program for low-resources countries using the NCT CRC screening risk score.
A multitude of options currently exist for CRC screening. A quick review reveals wide variability between programs all over the world. Additionally, one should not forget that most screening, especially in low income countries, is still performed on opportunistic basis with no solid structure. CRC screening must be optimized to reach the golden target of reducing incidence of the disease and eventually mortality. Most importantly we need to achieve high rates of participation and adherence in different screening programs by seeking correction of all the confounding factors. Benefiting from all the available screening tools in the correct settings of each population will increase the compliance of different populations. Consistent with this goal, adoption of cost-effective non-invasive methodologies designed to reduce complications, reduce anxiety over CRC screening, and improve overall acceptance of the screening process would be highly desirable.
Despite the current limitations and caveats, blood based markers may have a solid future. Screening with a relatively inexpensive serum or plasma marker (or marker panel) could increase screening compliance and be cost-saving if participation and performance were both elevated. Such a marker has the potential to replace more cumbersome stool based test in programs that employ a 2-stage paradigm. All the initial enthusiasm raised by the new blood marker have now faded as the rather disappointing results from the prospective trials were published. At this point due to the significant variability in sensitivity and specificity between various trials, it is very difficult to recommend it for mass screening. Other potential options include the noninvasive stool tests and in that regard the FIT seems to still be the most appropriate due to the present prohibitive cost of the multi-strain stool DNA test and the lack of significant difference in their performance. Colonoscopy is still the most appropriate test in high risk individuals or as second procedure following a positive first test.
Future studies should not only focus on the statistical performance of different tests but also on the characterization of complete screening programs, from the invitation to screening to the completion of colonoscopy for patients with a positive test.
Manuscript source: Invited manuscript
Specialty type: Gastroenterology and hepatology
Country of origin: Lebanon
Peer-review report classification
Grade A (Excellent): 0
Grade B (Very good): B, B, B
Grade C (Good): C
Grade D (Fair): 0
Grade E (Poor): 0
P- Reviewer: Barreto S, Gassler N, Lakatos PL, Tepes B S- Editor: Ma YJ L- Editor: A E- Editor: Zhang FF
| 1. | Torre LA, Bray F, Siegel RL, Ferlay J, Lortet-Tieulent J, Jemal A. Global cancer statistics, 2012. CA Cancer J Clin. 2015;65:87-108. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 18694] [Cited by in F6Publishing: 20827] [Article Influence: 2314.1] [Reference Citation Analysis (2)] |
| 2. | Ferlay J, Soerjomataram I, Dikshit R, Eser S, Mathers C, Rebelo M, Parkin DM, Forman D, Bray F. Cancer incidence and mortality worldwide: sources, methods and major patterns in GLOBOCAN 2012. Int J Cancer. 2015;136:E359-E386. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 20108] [Cited by in F6Publishing: 19772] [Article Influence: 2196.9] [Reference Citation Analysis (17)] |
| 3. | Siegel R, Desantis C, Jemal A. Colorectal cancer statistics, 2014. CA Cancer J Clin. 2014;64:104-117. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 1848] [Cited by in F6Publishing: 2025] [Article Influence: 202.5] [Reference Citation Analysis (0)] |
| 4. | Bond JH. Fecal occult blood test screening for colorectal cancer. Gastrointest Endosc Clin N Am. 2002;12:11-21. [PubMed] [Cited in This Article: ] |
| 5. | Jemal A, Bray F, Center MM, Ferlay J, Ward E, Forman D. Global cancer statistics. CA Cancer J Clin. 2011;61:69-90. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 23762] [Cited by in F6Publishing: 25175] [Article Influence: 1936.5] [Reference Citation Analysis (3)] |
| 6. | Carroll MR, Seaman HE, Halloran SP. Tests and investigations for colorectal cancer screening. Clin Biochem. 2014;47:921-939. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 60] [Cited by in F6Publishing: 64] [Article Influence: 6.4] [Reference Citation Analysis (0)] |
| 7. | Centers for Disease Control and Prevention (CDC). Vital signs: Colorectal cancer screening, incidence, and mortality--United States, 2002-2010. MMWR Morb Mortal Wkly Rep. 2011;60:884-889. [PubMed] [Cited in This Article: ] |
| 8. | Levin B, Lieberman DA, McFarland B, Smith RA, Brooks D, Andrews KS, Dash C, Giardiello FM, Glick S, Levin TR. Screening and surveillance for the early detection of colorectal cancer and adenomatous polyps, 2008: a joint guideline from the American Cancer Society, the US Multi-Society Task Force on Colorectal Cancer, and the American College of Radiology. CA Cancer J Clin. 2008;58:130-160. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 1169] [Cited by in F6Publishing: 1163] [Article Influence: 72.7] [Reference Citation Analysis (0)] |
| 9. | US Preventive Services Task Force, Bibbins-Domingo K, Grossman DC, Curry SJ, Davidson KW, Epling JW Jr, García FAR, Gillman MW, Harper DM, Kemper AR, Krist AH, Kurth AE, Landefeld CS, Mangione CM, Owens DK, Phillips WR, Phipps MG, Pignone MP, Siu AL. Screening for Colorectal Cancer: US Preventive Services Task Force Recommendation Statement. JAMA. 2016;315:2564-2575. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 1249] [Cited by in F6Publishing: 1279] [Article Influence: 159.9] [Reference Citation Analysis (1)] |
| 10. | Rex DK, Johnson DA, Anderson JC, Schoenfeld PS, Burke CA, Inadomi JM; American College of Gastroenterology. American College of Gastroenterology guidelines for colorectal cancer screening 2009 [corrected]. Am J Gastroenterol. 2009;104:739-750. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 981] [Cited by in F6Publishing: 1035] [Article Influence: 69.0] [Reference Citation Analysis (0)] |
| 11. | Qaseem A, Denberg TD, Hopkins RH Jr, Humphrey LL, Levine J, Sweet DE, Shekelle P; Clinical Guidelines Committee of the American College of Physicians. Screening for colorectal cancer: a guidance statement from the American College of Physicians. Ann Intern Med. 2012;156:378-386. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 225] [Cited by in F6Publishing: 243] [Article Influence: 20.3] [Reference Citation Analysis (1)] |
| 12. | Provenzale D, Jasperson K, Ahnen DJ, Aslanian H, Bray T, Cannon JA, David DS, Early DS, Erwin D, Ford JM. Colorectal Cancer Screening, Version 1.2015. J Natl Compr Canc Netw. 2015;13:959-968; quiz 968. [PubMed] [Cited in This Article: ] |
| 13. | Rockey DC, Paulson E, Niedzwiecki D, Davis W, Bosworth HB, Sanders L, Yee J, Henderson J, Hatten P, Burdick S. Analysis of air contrast barium enema, computed tomographic colonography, and colonoscopy: prospective comparison. Lancet. 2005;365:305-311. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 231] [Cited by in F6Publishing: 217] [Article Influence: 11.4] [Reference Citation Analysis (1)] |
| 14. | Surveillance, Epidemiology, and End Results Program. SEER Stat Fact Sheets: Colon and Rectum Cancer: Centers for Disease Control; 2014. . [Cited in This Article: ] |
| 15. | Zauber AG, Winawer SJ, O’Brien MJ, Lansdorp-Vogelaar I, van Ballegooijen M, Hankey BF, Shi W, Bond JH, Schapiro M, Panish JF. Colonoscopic polypectomy and long-term prevention of colorectal-cancer deaths. N Engl J Med. 2012;366:687-696. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 1952] [Cited by in F6Publishing: 2084] [Article Influence: 173.7] [Reference Citation Analysis (1)] |
| 16. | Nishihara R, Wu K, Lochhead P, Morikawa T, Liao X, Qian ZR, Inamura K, Kim SA, Kuchiba A, Yamauchi M. Long-term colorectal-cancer incidence and mortality after lower endoscopy. N Engl J Med. 2013;369:1095-1105. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 968] [Cited by in F6Publishing: 1047] [Article Influence: 95.2] [Reference Citation Analysis (0)] |
| 17. | Brenner H, Stock C, Hoffmeister M. Effect of screening sigmoidoscopy and screening colonoscopy on colorectal cancer incidence and mortality: systematic review and meta-analysis of randomised controlled trials and observational studies. BMJ. 2014;348:g2467. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 501] [Cited by in F6Publishing: 537] [Article Influence: 53.7] [Reference Citation Analysis (2)] |
| 18. | Kahi CJ, Imperiale TF, Juliar BE, Rex DK. Effect of screening colonoscopy on colorectal cancer incidence and mortality. Clin Gastroenterol Hepatol. 2009;7:770-775; quiz 711. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 271] [Cited by in F6Publishing: 280] [Article Influence: 18.7] [Reference Citation Analysis (0)] |
| 19. | Manser CN, Bachmann LM, Brunner J, Hunold F, Bauerfeind P, Marbet UA. Colonoscopy screening markedly reduces the occurrence of colon carcinomas and carcinoma-related death: a closed cohort study. Gastrointest Endosc. 2012;76:110-117. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 95] [Cited by in F6Publishing: 97] [Article Influence: 8.1] [Reference Citation Analysis (0)] |
| 20. | Baxter NN, Goldwasser MA, Paszat LF, Saskin R, Urbach DR, Rabeneck L. Association of colonoscopy and death from colorectal cancer. Ann Intern Med. 2009;150:1-8. [PubMed] [Cited in This Article: ] |
| 21. | Ransohoff DF. How much does colonoscopy reduce colon cancer mortality? Ann Intern Med. 2009;150:50-52. [PubMed] [Cited in This Article: ] |
| 22. | Singh H, Nugent Z, Demers AA, Kliewer EV, Mahmud SM, Bernstein CN. The reduction in colorectal cancer mortality after colonoscopy varies by site of the cancer. Gastroenterology. 2010;139:1128-1137. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 341] [Cited by in F6Publishing: 363] [Article Influence: 25.9] [Reference Citation Analysis (0)] |
| 23. | Sandler RS. Editorial: colonoscopy and colorectal cancer mortality: strong beliefs or strong facts? Am J Gastroenterol. 2010;105:1633-1635. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 7] [Cited by in F6Publishing: 8] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 24. | Neugut AI, Lebwohl B. Colonoscopy vs sigmoidoscopy screening: getting it right. JAMA. 2010;304:461-462. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 43] [Cited by in F6Publishing: 46] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 25. | Jørgensen OD, Kronborg O, Fenger C, Rasmussen M. Influence of long-term colonoscopic surveillance on incidence of colorectal cancer and death from the disease in patients with precursors (adenomas). Acta Oncol. 2007;46:355-360. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 19] [Cited by in F6Publishing: 19] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 26. | Brenner H, Chang-Claude J, Seiler CM, Rickert A, Hoffmeister M. Protection from colorectal cancer after colonoscopy: a population-based, case-control study. Ann Intern Med. 2011;154:22-30. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 571] [Cited by in F6Publishing: 575] [Article Influence: 44.2] [Reference Citation Analysis (0)] |
| 27. | Rex DK, Bond JH, Winawer S, Levin TR, Burt RW, Johnson DA, Kirk LM, Litlin S, Lieberman DA, Waye JD. S. Multi-Society Task Force on Colorectal Cancer. Quality in the technical performance of colonoscopy and the continuous quality improvement process for colonoscopy: recommendations of the U.S. Multi-Society Task Force on Colorectal Cancer. Am J Gastroenterol. 2002;97:1296-1308. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 691] [Cited by in F6Publishing: 700] [Article Influence: 31.8] [Reference Citation Analysis (0)] |
| 28. | Rex DK, Petrini JL, Baron TH, Chak A, Cohen J, Deal SE, Hoffman B, Jacobson BC, Mergener K, Petersen BT. Quality indicators for colonoscopy. Am J Gastroenterol. 2006;101:873-885. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 532] [Cited by in F6Publishing: 548] [Article Influence: 30.4] [Reference Citation Analysis (0)] |
| 29. | Lieberman D. A call to action--measuring the quality of colonoscopy. N Engl J Med. 2006;355:2588-2589. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 46] [Cited by in F6Publishing: 54] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 30. | Kaminski MF, Regula J, Kraszewska E, Polkowski M, Wojciechowska U, Didkowska J, Zwierko M, Rupinski M, Nowacki MP, Butruk E. Quality indicators for colonoscopy and the risk of interval cancer. N Engl J Med. 2010;362:1795-1803. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 1287] [Cited by in F6Publishing: 1351] [Article Influence: 96.5] [Reference Citation Analysis (0)] |
| 31. | Atkin W, Rogers P, Cardwell C, Cook C, Cuzick J, Wardle J, Edwards R. Wide variation in adenoma detection rates at screening flexible sigmoidoscopy. Gastroenterology. 2004;126:1247-1256. [PubMed] [Cited in This Article: ] |
| 32. | Rabeneck L, Paszat LF, Saskin R. Endoscopist specialty is associated with incident colorectal cancer after a negative colonoscopy. Clin Gastroenterol Hepatol. 2010;8:275-279. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 125] [Cited by in F6Publishing: 133] [Article Influence: 9.5] [Reference Citation Analysis (0)] |
| 33. | Castells A, Quintero E. Programmatic screening for colorectal cancer: the COLONPREV study. Dig Dis Sci. 2015;60:672-680. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 19] [Cited by in F6Publishing: 18] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 34. | Kaminski MF, Bretthauer M, Zauber AG, Kuipers EJ, Adami HO, van Ballegooijen M, Regula J, van Leerdam M, Stefansson T, Påhlman L. The NordICC Study: rationale and design of a randomized trial on colonoscopy screening for colorectal cancer. Endoscopy. 2012;44:695-702. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 119] [Cited by in F6Publishing: 126] [Article Influence: 10.5] [Reference Citation Analysis (0)] |
| 35. | Bretthauer M, Kaminski MF, Løberg M, Zauber AG, Regula J, Kuipers EJ, Hernán MA, McFadden E, Sunde A, Kalager M, Dekker E, Lansdorp-Vogelaar I, Garborg K, Rupinski M, Spaander MC, Bugajski M, Høie O, Stefansson T, Hoff G, Adami HO; Nordic-European Initiative on Colorectal Cancer (NordICC) Study Group. Population-Based Colonoscopy Screening for Colorectal Cancer: A Randomized Clinical Trial. JAMA Intern Med. 2016;176:894-902. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 226] [Cited by in F6Publishing: 206] [Article Influence: 25.8] [Reference Citation Analysis (0)] |
| 36. | Hoff G, Grotmol T, Skovlund E, Bretthauer M; Norwegian Colorectal Cancer Prevention Study Group. Risk of colorectal cancer seven years after flexible sigmoidoscopy screening: randomised controlled trial. BMJ. 2009;338:b1846. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 218] [Cited by in F6Publishing: 242] [Article Influence: 16.1] [Reference Citation Analysis (0)] |
| 37. | Segnan N, Armaroli P, Bonelli L, Risio M, Sciallero S, Zappa M, Andreoni B, Arrigoni A, Bisanti L, Casella C. Once-only sigmoidoscopy in colorectal cancer screening: follow-up findings of the Italian Randomized Controlled Trial--SCORE. J Natl Cancer Inst. 2011;103:1310-1322. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 428] [Cited by in F6Publishing: 427] [Article Influence: 32.8] [Reference Citation Analysis (0)] |
| 38. | Atkin WS, Edwards R, Kralj-Hans I, Wooldrage K, Hart AR, Northover JM, Parkin DM, Wardle J, Duffy SW, Cuzick J; UK Flexible Sigmoidoscopy Trial Investigators. Once-only flexible sigmoidoscopy screening in prevention of colorectal cancer: a multicentre randomised controlled trial. Lancet. 2010;375:1624-1633. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 1121] [Cited by in F6Publishing: 1083] [Article Influence: 77.4] [Reference Citation Analysis (0)] |
| 39. | Schoen RE, Pinsky PF, Weissfeld JL, Yokochi LA, Church T, Laiyemo AO, Bresalier R, Andriole GL, Buys SS, Crawford ED. Colorectal-cancer incidence and mortality with screening flexible sigmoidoscopy. N Engl J Med. 2012;366:2345-2357. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 672] [Cited by in F6Publishing: 697] [Article Influence: 58.1] [Reference Citation Analysis (1)] |
| 40. | Holme Ø, Løberg M, Kalager M, Bretthauer M, Hernán MA, Aas E, Eide TJ, Skovlund E, Schneede J, Tveit KM, Hoff G. Effect of flexible sigmoidoscopy screening on colorectal cancer incidence and mortality: a randomized clinical trial. JAMA. 2014;312:606-615. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 279] [Cited by in F6Publishing: 283] [Article Influence: 28.3] [Reference Citation Analysis (0)] |
| 41. | Lin JS, Piper MA, Perdue LA, Rutter CM, Webber EM, O’Connor E, Smith N, Whitlock EP. Screening for Colorectal Cancer: Updated Evidence Report and Systematic Review for the US Preventive Services Task Force. JAMA. 2016;315:2576-2594. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 506] [Cited by in F6Publishing: 516] [Article Influence: 64.5] [Reference Citation Analysis (0)] |
| 42. | Mandel JS, Bond JH, Church TR, Snover DC, Bradley GM, Schuman LM, Ederer F. Reducing mortality from colorectal cancer by screening for fecal occult blood. Minnesota Colon Cancer Control Study. N Engl J Med. 1993;328:1365-1371. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 2183] [Cited by in F6Publishing: 2118] [Article Influence: 68.3] [Reference Citation Analysis (1)] |
| 43. | Hardcastle JD, Chamberlain JO, Robinson MH, Moss SM, Amar SS, Balfour TW, James PD, Mangham CM. Randomised controlled trial of faecal-occult-blood screening for colorectal cancer. Lancet. 1996;348:1472-1477. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 1858] [Cited by in F6Publishing: 1800] [Article Influence: 64.3] [Reference Citation Analysis (0)] |
| 44. | Kronborg O, Fenger C, Olsen J, Jørgensen OD, Søndergaard O. Randomised study of screening for colorectal cancer with faecal-occult-blood test. Lancet. 1996;348:1467-1471. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 1640] [Cited by in F6Publishing: 1573] [Article Influence: 56.2] [Reference Citation Analysis (0)] |
| 45. | Shaukat A, Mongin SJ, Geisser MS, Lederle FA, Bond JH, Mandel JS, Church TR. Long-term mortality after screening for colorectal cancer. N Engl J Med. 2013;369:1106-1114. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 552] [Cited by in F6Publishing: 587] [Article Influence: 53.4] [Reference Citation Analysis (0)] |
| 46. | Faivre J, Dancourt V, Lejeune C, Tazi MA, Lamour J, Gerard D, Dassonville F, Bonithon-Kopp C. Reduction in colorectal cancer mortality by fecal occult blood screening in a French controlled study. Gastroenterology. 2004;126:1674-1680. [PubMed] [Cited in This Article: ] |
| 47. | Scholefield JH, Moss SM, Mangham CM, Whynes DK, Hardcastle JD. Nottingham trial of faecal occult blood testing for colorectal cancer: a 20-year follow-up. Gut. 2012;61:1036-1040. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 205] [Cited by in F6Publishing: 216] [Article Influence: 18.0] [Reference Citation Analysis (0)] |
| 48. | Lindholm E, Brevinge H, Haglind E. Survival benefit in a randomized clinical trial of faecal occult blood screening for colorectal cancer. Br J Surg. 2008;95:1029-1036. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 200] [Cited by in F6Publishing: 216] [Article Influence: 13.5] [Reference Citation Analysis (0)] |
| 49. | Kronborg O, Jørgensen OD, Fenger C, Rasmussen M. Randomized study of biennial screening with a faecal occult blood test: results after nine screening rounds. Scand J Gastroenterol. 2004;39:846-851. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 169] [Cited by in F6Publishing: 174] [Article Influence: 8.7] [Reference Citation Analysis (0)] |
| 50. | Lansdorp-Vogelaar I, van Ballegooijen M, Boer R, Zauber A, Habbema JD. A novel hypothesis on the sensitivity of the fecal occult blood test: Results of a joint analysis of 3 randomized controlled trials. Cancer. 2009;115:2410-2419. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 63] [Cited by in F6Publishing: 69] [Article Influence: 4.6] [Reference Citation Analysis (0)] |
| 51. | Allison JE, Tekawa IS, Ransom LJ, Adrain AL. A comparison of fecal occult-blood tests for colorectal-cancer screening. N Engl J Med. 1996;334:155-159. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 430] [Cited by in F6Publishing: 387] [Article Influence: 13.8] [Reference Citation Analysis (0)] |
| 52. | Young GP, Symonds EL, Allison JE, Cole SR, Fraser CG, Halloran SP, Kuipers EJ, Seaman HE. Advances in Fecal Occult Blood Tests: the FIT revolution. Dig Dis Sci. 2015;60:609-622. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 108] [Cited by in F6Publishing: 121] [Article Influence: 13.4] [Reference Citation Analysis (0)] |
| 53. | Allison JE, Fraser CG, Halloran SP, Young GP. Population screening for colorectal cancer means getting FIT: the past, present, and future of colorectal cancer screening using the fecal immunochemical test for hemoglobin (FIT). Gut Liver. 2014;8:117-130. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 108] [Cited by in F6Publishing: 121] [Article Influence: 12.1] [Reference Citation Analysis (0)] |
| 54. | Allison JE, Sakoda LC, Levin TR, Tucker JP, Tekawa IS, Cuff T, Pauly MP, Shlager L, Palitz AM, Zhao WK. Screening for colorectal neoplasms with new fecal occult blood tests: update on performance characteristics. J Natl Cancer Inst. 2007;99:1462-1470. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 249] [Cited by in F6Publishing: 246] [Article Influence: 14.5] [Reference Citation Analysis (0)] |
| 55. | Allison JE, Lawson M. Screening tests for colorectal cancer: a menu of options remains relevant. Curr Oncol Rep. 2006;8:492-498. [PubMed] [Cited in This Article: ] |
| 56. | Whitlock EP, Lin JS, Liles E, Beil TL, Fu R. Screening for colorectal cancer: a targeted, updated systematic review for the U.S. Preventive Services Task Force. Ann Intern Med. 2008;149:638-658. [PubMed] [Cited in This Article: ] |
| 57. | Lieberman DA. Clinical practice. Screening for colorectal cancer. N Engl J Med. 2009;361:1179-1187. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 194] [Cited by in F6Publishing: 213] [Article Influence: 14.2] [Reference Citation Analysis (0)] |
| 58. | Lee JK, Liles EG, Bent S, Levin TR, Corley DA. Accuracy of fecal immunochemical tests for colorectal cancer: systematic review and meta-analysis. Ann Intern Med. 2014;160:171. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 389] [Cited by in F6Publishing: 426] [Article Influence: 42.6] [Reference Citation Analysis (0)] |
| 59. | Ventura L, Mantellini P, Grazzini G. The effect of introduction of immunochemical faecal occult blood testing on colorectal cancer incidence. International Cancer Screening Network Biennial Meeting, Sydney Australia. October 23-25, 2012. . [Cited in This Article: ] |
| 60. | van Rossum LG, van Rijn AF, Laheij RJ, van Oijen MG, Fockens P, Jansen JB, Verbeek AL, Dekker E. Cutoff value determines the performance of a semi-quantitative immunochemical faecal occult blood test in a colorectal cancer screening programme. Br J Cancer. 2009;101:1274-1281. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 73] [Cited by in F6Publishing: 82] [Article Influence: 5.5] [Reference Citation Analysis (0)] |
| 61. | Fraser CG, Halloran SP, Allison JE, Young GP. Making colorectal cancer screening FITTER for purpose with quantitative faecal immunochemical tests for haemoglobin (FIT). Clin Chem Lab Med. 2013;51:2065-2067. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 11] [Cited by in F6Publishing: 14] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 62. | McDonald PJ, Strachan JA, Digby J, Steele RJ, Fraser CG. Faecal haemoglobin concentrations by gender and age: implications for population-based screening for colorectal cancer. Clin Chem Lab Med. 2011;50:935-940. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 39] [Cited by in F6Publishing: 50] [Article Influence: 3.8] [Reference Citation Analysis (0)] |
| 63. | Kim DH, Pickhardt PJ, Taylor AJ, Leung WK, Winter TC, Hinshaw JL, Gopal DV, Reichelderfer M, Hsu RH, Pfau PR. CT colonography versus colonoscopy for the detection of advanced neoplasia. N Engl J Med. 2007;357:1403-1412. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 536] [Cited by in F6Publishing: 448] [Article Influence: 26.4] [Reference Citation Analysis (0)] |
| 64. | Ranasinghe I, Parzynski CS, Searfoss R, Montague J, Lin Z, Allen J, Vender R, Bhat K, Ross JS, Bernheim S. Differences in Colonoscopy Quality Among Facilities: Development of a Post-Colonoscopy Risk-Standardized Rate of Unplanned Hospital Visits. Gastroenterology. 2016;150:103-113. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 40] [Cited by in F6Publishing: 41] [Article Influence: 5.1] [Reference Citation Analysis (0)] |
| 65. | Pickhardt PJ. Incidence of colonic perforation at CT colonography: review of existing data and implications for screening of asymptomatic adults. Radiology. 2006;239:313-316. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 169] [Cited by in F6Publishing: 180] [Article Influence: 10.0] [Reference Citation Analysis (0)] |
| 66. | Hassan C, Pickhardt PJ, Laghi A, Kim DH, Zullo A, Iafrate F, Di Giulio L, Morini S. Computed tomographic colonography to screen for colorectal cancer, extracolonic cancer, and aortic aneurysm: model simulation with cost-effectiveness analysis. Arch Intern Med. 2008;168:696-705. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 126] [Cited by in F6Publishing: 115] [Article Influence: 7.2] [Reference Citation Analysis (0)] |
| 67. | Pickhardt PJ, Hassan C, Laghi A, Kim DH. CT colonography to screen for colorectal cancer and aortic aneurysm in the Medicare population: cost-effectiveness analysis. AJR Am J Roentgenol. 2009;192:1332-1340. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 55] [Cited by in F6Publishing: 57] [Article Influence: 3.8] [Reference Citation Analysis (0)] |
| 68. | Pickhardt PJ, Lee LJ, del Rio AM, Lauder T, Bruce RJ, Summers RM, Pooler BD, Binkley N. Simultaneous screening for osteoporosis at CT colonography: bone mineral density assessment using MDCT attenuation techniques compared with the DXA reference standard. J Bone Miner Res. 2011;26:2194-2203. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 186] [Cited by in F6Publishing: 200] [Article Influence: 15.4] [Reference Citation Analysis (0)] |
| 69. | Ziemlewicz TJ, Binkley N, Pickhardt PJ. Opportunistic Osteoporosis Screening: Addition of Quantitative CT Bone Mineral Density Evaluation to CT Colonography. J Am Coll Radiol. 2015;12:1036-1041. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 33] [Cited by in F6Publishing: 35] [Article Influence: 4.4] [Reference Citation Analysis (0)] |
| 70. | Martín-López JE, Beltrán-Calvo C, Rodríguez-López R, Molina-López T. Comparison of the accuracy of CT colonography and colonoscopy in the diagnosis of colorectal cancer. Colorectal Dis. 2014;16:O82-O89. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 17] [Cited by in F6Publishing: 17] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 71. | Lin OS. Computed tomographic colonography: hope or hype? World J Gastroenterol. 2010;16:915-920. [PubMed] [DOI] [Cited in This Article: ] [Cited by in CrossRef: 10] [Cited by in F6Publishing: 10] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 72. | Burling D, Halligan S, Slater A, Noakes MJ, Taylor SA. Potentially serious adverse events at CT colonography in symptomatic patients: national survey of the United Kingdom. Radiology. 2006;239:464-471. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 151] [Cited by in F6Publishing: 161] [Article Influence: 8.9] [Reference Citation Analysis (0)] |
| 73. | Ahlquist DA. Multi-target stool DNA test: a new high bar for noninvasive screening. Dig Dis Sci. 2015;60:623-633. [PubMed] [Cited in This Article: ] |
| 74. | Imperiale TF, Ransohoff DF, Itzkowitz SH. Multitarget stool DNA testing for colorectal-cancer screening. N Engl J Med. 2014;371:187-188. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 44] [Cited by in F6Publishing: 97] [Article Influence: 9.7] [Reference Citation Analysis (0)] |
| 75. | Ladabaum U, Mannalithara A. Comparative Effectiveness and Cost Effectiveness of a Multitarget Stool DNA Test to Screen for Colorectal Neoplasia. Gastroenterology. 2016;151:427-439.e6. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 89] [Cited by in F6Publishing: 92] [Article Influence: 11.5] [Reference Citation Analysis (0)] |
| 76. | Longtine MS, DeMarini DJ, Valencik ML, Al-Awar OS, Fares H, De Virgilio C, Pringle JR. The septins: roles in cytokinesis and other processes. Curr Opin Cell Biol. 1996;8:106-119. [PubMed] [Cited in This Article: ] |
| 77. | Sellin ME, Sandblad L, Stenmark S, Gullberg M. Deciphering the rules governing assembly order of mammalian septin complexes. Mol Biol Cell. 2011;22:3152-3164. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 135] [Cited by in F6Publishing: 137] [Article Influence: 10.5] [Reference Citation Analysis (0)] |
| 78. | Kim MS, Froese CD, Estey MP, Trimble WS. SEPT9 occupies the terminal positions in septin octamers and mediates polymerization-dependent functions in abscission. J Cell Biol. 2011;195:815-826. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 129] [Cited by in F6Publishing: 124] [Article Influence: 10.3] [Reference Citation Analysis (0)] |
| 79. | Estey MP, Di Ciano-Oliveira C, Froese CD, Bejide MT, Trimble WS. Distinct roles of septins in cytokinesis: SEPT9 mediates midbody abscission. J Cell Biol. 2010;191:741-749. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 171] [Cited by in F6Publishing: 169] [Article Influence: 12.1] [Reference Citation Analysis (0)] |
| 80. | Song L, Li Y. Methylated Sept9 Gene is a Sensitive Biomarker for all Stages of Colorectal Cancer. Colorec Cancer. 2015;1:1-7. [DOI] [Cited in This Article: ] |
| 81. | deVos T, Tetzner R, Model F, Weiss G, Schuster M, Distler J, Steiger KV, Grützmann R, Pilarsky C, Habermann JK. Circulating methylated SEPT9 DNA in plasma is a biomarker for colorectal cancer. Clin Chem. 2009;55:1337-1346. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 361] [Cited by in F6Publishing: 379] [Article Influence: 25.3] [Reference Citation Analysis (0)] |
| 82. | Grützmann R, Molnar B, Pilarsky C, Habermann JK, Schlag PM, Saeger HD, Miehlke S, Stolz T, Model F, Roblick UJ. Sensitive detection of colorectal cancer in peripheral blood by septin 9 DNA methylation assay. PLoS One. 2008;3:e3759. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 281] [Cited by in F6Publishing: 291] [Article Influence: 18.2] [Reference Citation Analysis (0)] |
| 83. | Lofton-Day C, Model F, Devos T, Tetzner R, Distler J, Schuster M, Song X, Lesche R, Liebenberg V, Ebert M. DNA methylation biomarkers for blood-based colorectal cancer screening. Clin Chem. 2008;54:414-423. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 340] [Cited by in F6Publishing: 367] [Article Influence: 21.6] [Reference Citation Analysis (0)] |
| 84. | Church TR, Wandell M, Lofton-Day C, Mongin SJ, Burger M, Payne SR, Castaños-Vélez E, Blumenstein BA, Rösch T, Osborn N. Prospective evaluation of methylated SEPT9 in plasma for detection of asymptomatic colorectal cancer. Gut. 2014;63:317-325. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 486] [Cited by in F6Publishing: 509] [Article Influence: 50.9] [Reference Citation Analysis (0)] |
| 85. | Johnson DA, Barclay RL, Mergener K, Weiss G, König T, Beck J, Potter NT. Plasma Septin9 versus fecal immunochemical testing for colorectal cancer screening: a prospective multicenter study. PLoS One. 2014;9:e98238. [PubMed] [Cited in This Article: ] |
| 86. | Pickhardt PJ. Emerging stool-based and blood-based non-invasive DNA tests for colorectal cancer screening: the importance of cancer prevention in addition to cancer detection. Abdom Radiol (NY). 2016;41:1441-1444. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 30] [Cited by in F6Publishing: 33] [Article Influence: 4.1] [Reference Citation Analysis (0)] |
| 87. | Adler A, Geiger S, Keil A, Bias H, Schatz P, deVos T, Dhein J, Zimmermann M, Tauber R, Wiedenmann B. Improving compliance to colorectal cancer screening using blood and stool based tests in patients refusing screening colonoscopy in Germany. BMC Gastroenterol. 2014;14:183. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 111] [Cited by in F6Publishing: 129] [Article Influence: 12.9] [Reference Citation Analysis (0)] |
| 88. | Ladabaum U, Allen J, Wandell M, Ramsey S. Colorectal cancer screening with blood-based biomarkers: cost-effectiveness of methylated septin 9 DNA versus current strategies. Cancer Epidemiol Biomarkers Prev. 2013;22:1567-1576. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 54] [Cited by in F6Publishing: 60] [Article Influence: 5.5] [Reference Citation Analysis (0)] |
| 89. | Parikh RB, Prasad V. Blood-Based Screening for Colon Cancer: A Disruptive Innovation or Simply a Disruption? JAMA. 2016;315:2519-2520. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 23] [Cited by in F6Publishing: 24] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 90. | Usher-Smith JA, Walter FM, Emery JD, Win AK, Griffin SJ. Risk Prediction Models for Colorectal Cancer: A Systematic Review. Cancer Prev Res (Phila). 2016;9:13-26. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 98] [Cited by in F6Publishing: 103] [Article Influence: 11.4] [Reference Citation Analysis (1)] |
| 91. | Ladabaum U, Patel A, Mannalithara A, Sundaram V, Mitani A, Desai M. Predicting advanced neoplasia at colonoscopy in a diverse population with the National Cancer Institute colorectal cancer risk-assessment tool. Cancer. 2016;122:2663-2670. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 22] [Cited by in F6Publishing: 22] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 92. | Sabatino SA, White MC, Thompson TD, Klabunde CN; Centers for Disease Control and Prevention (CDC). Cancer screening test use - United States, 2013. MMWR Morb Mortal Wkly Rep. 2015;64:464-468. [PubMed] [Cited in This Article: ] |
| 93. | Brenner AT, Ko LK, Janz N, Gupta S, Inadomi J. Race/Ethnicity and Primary Language: Health Beliefs about Colorectal Cancer Screening in a Diverse, Low-Income Population. J Health Care Poor Underserved. 2015;26:824-838. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 10] [Cited by in F6Publishing: 12] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 94. | Doubeni CA, Corley DA, Zauber AG. Colorectal Cancer Health Disparities and the Role of US Law and Health Policy. Gastroenterology. 2016;150:1052-1055. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 20] [Cited by in F6Publishing: 23] [Article Influence: 2.9] [Reference Citation Analysis (0)] |
| 95. | Lo SH, Halloran S, Snowball J, Seaman H, Wardle J, von Wagner C. Colorectal cancer screening uptake over three biennial invitation rounds in the English bowel cancer screening programme. Gut. 2015;64:282-291. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 93] [Cited by in F6Publishing: 99] [Article Influence: 11.0] [Reference Citation Analysis (0)] |
| 96. | Crotta S, Segnan N, Paganin S, Dagnes B, Rosset R, Senore C. High rate of advanced adenoma detection in 4 rounds of colorectal cancer screening with the fecal immunochemical test. Clin Gastroenterol Hepatol. 2012;10:633-638. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 80] [Cited by in F6Publishing: 94] [Article Influence: 7.8] [Reference Citation Analysis (0)] |
| 97. | Duncan A, Turnbull D, Wilson C, Osborne JM, Cole SR, Flight I, Young GP. Behavioural and demographic predictors of adherence to three consecutive faecal occult blood test screening opportunities: a population study. BMC Public Health. 2014;14:238. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 40] [Cited by in F6Publishing: 42] [Article Influence: 4.2] [Reference Citation Analysis (0)] |
| 98. | Kapidzic A, Grobbee EJ, Hol L, van Roon AH, van Vuuren AJ, Spijker W, Izelaar K, van Ballegooijen M, Kuipers EJ, van Leerdam ME. Attendance and yield over three rounds of population-based fecal immunochemical test screening. Am J Gastroenterol. 2014;109:1257-1264. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 82] [Cited by in F6Publishing: 89] [Article Influence: 8.9] [Reference Citation Analysis (0)] |
| 99. | Jensen CD, Corley DA, Quinn VP, Doubeni CA, Zauber AG, Lee JK, Zhao WK, Marks AR, Schottinger JE, Ghai NR. Fecal Immunochemical Test Program Performance Over 4 Rounds of Annual Screening: A Retrospective Cohort Study. Ann Intern Med. 2016;164:456-463. [PubMed] [Cited in This Article: ] |
| 100. | Subramanian S, Tangka FK, Hoover S, Degroff A, Royalty J, Seeff LC. Clinical and programmatic costs of implementing colorectal cancer screening: evaluation of five programs. Eval Program Plann. 2011;34:147-153. [PubMed] [Cited in This Article: ] |
| 101. | Myers RE, Bittner-Fagan H, Daskalakis C, Sifri R, Vernon SW, Cocroft J, Dicarlo M, Katurakes N, Andrel J. A randomized controlled trial of a tailored navigation and a standard intervention in colorectal cancer screening. Cancer Epidemiol Biomarkers Prev. 2013;22:109-117. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 56] [Cited by in F6Publishing: 58] [Article Influence: 4.8] [Reference Citation Analysis (0)] |
| 102. | Ritvo PG, Myers RE, Paszat LF, Tinmouth JM, McColeman J, Mitchell B, Serenity M, Rabeneck L. Personal navigation increases colorectal cancer screening uptake. Cancer Epidemiol Biomarkers Prev. 2015;24:506-511. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 42] [Cited by in F6Publishing: 45] [Article Influence: 4.5] [Reference Citation Analysis (0)] |
| 103. | Ladabaum U, Mannalithara A, Jandorf L, Itzkowitz SH. Cost-effectiveness of patient navigation to increase adherence with screening colonoscopy among minority individuals. Cancer. 2015;121:1088-1097. [PubMed] [Cited in This Article: ] |
| 104. | Chen LA, Santos S, Jandorf L, Christie J, Castillo A, Winkel G, Itzkowitz S. A program to enhance completion of screening colonoscopy among urban minorities. Clin Gastroenterol Hepatol. 2008;6:443-450. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 129] [Cited by in F6Publishing: 131] [Article Influence: 8.2] [Reference Citation Analysis (0)] |
| 105. | Jandorf L, Braschi C, Ernstoff E, Wong CR, Thelemaque L, Winkel G, Thompson HS, Redd WH, Itzkowitz SH. Culturally targeted patient navigation for increasing african americans’ adherence to screening colonoscopy: a randomized clinical trial. Cancer Epidemiol Biomarkers Prev. 2013;22:1577-1587. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 70] [Cited by in F6Publishing: 78] [Article Influence: 7.1] [Reference Citation Analysis (0)] |
| 106. | Elkin EB, Shapiro E, Snow JG, Zauber AG, Krauskopf MS. The economic impact of a patient navigator program to increase screening colonoscopy. Cancer. 2012;118:5982-5988. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 41] [Cited by in F6Publishing: 42] [Article Influence: 3.5] [Reference Citation Analysis (0)] |
| 107. | Inadomi JM, Vijan S, Janz NK, Fagerlin A, Thomas JP, Lin YV, Muñoz R, Lau C, Somsouk M, El-Nachef N. Adherence to colorectal cancer screening: a randomized clinical trial of competing strategies. Arch Intern Med. 2012;172:575-582. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 339] [Cited by in F6Publishing: 409] [Article Influence: 34.1] [Reference Citation Analysis (0)] |
| 108. | Barclay RL, Vicari JJ, Doughty AS, Johanson JF, Greenlaw RL. Colonoscopic withdrawal times and adenoma detection during screening colonoscopy. N Engl J Med. 2006;355:2533-2541. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 911] [Cited by in F6Publishing: 901] [Article Influence: 50.1] [Reference Citation Analysis (0)] |
| 109. | Corley DA, Jensen CD, Marks AR, Zhao WK, Lee JK, Doubeni CA, Zauber AG, de Boer J, Fireman BH, Schottinger JE. Adenoma detection rate and risk of colorectal cancer and death. N Engl J Med. 2014;370:1298-1306. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 1251] [Cited by in F6Publishing: 1371] [Article Influence: 137.1] [Reference Citation Analysis (0)] |
| 110. | Tepeš B, Mlakar DN, Metličar T. Bowel preparation for colonoscopy with magnesium sulphate and low-volume polyethylene glycol. Eur J Gastroenterol Hepatol. 2014;26:616-620. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 7] [Cited by in F6Publishing: 8] [Article Influence: 0.8] [Reference Citation Analysis (5)] |
| 111. | Tepeš B, Bracko M, Novak Mlakar D, Stefanovic M, Stabuc B, Frkovic Grazio S, Maucec Zakotnik J. Results of the FIT-based National Colorectal Cancer Screening Program in Slovenia. J Clin Gastroenterol. 2017;51:e52-e59. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 16] [Cited by in F6Publishing: 17] [Article Influence: 2.4] [Reference Citation Analysis (0)] |