Published online Dec 14, 2017. doi: 10.3748/wjg.v23.i46.8152
Peer-review started: March 29, 2017
First decision: May 3, 2017
Revised: May 29, 2017
Accepted: August 9, 2017
Article in press: August 9, 2017
Published online: December 14, 2017
To investigate the hepatic differentiation potential of human umbilical cord-derived mesenchymal stem cells (hUC-MSCs) and to evaluate their therapeutic effect on liver fibrosis/cirrhosis.
A CCl4-induced liver fibrotic/cirrhotic rat model was used to assess the effect of hUC-MSCs. Histopathology was assessed by hematoxylin and eosin (H&E), Masson trichrome and Sirius red staining. The liver biochemical profile was measured using a Beckman Coulter analyzer. Expression analysis was performed using immunofluorescent staining, immunohistochemistry, Western blot, and real-time PCR.
We demonstrated that the infused hUC-MSCs could differentiate into hepatocytes in vivo. Functionally, the transplantation of hUC-MSCs to CCl4-treated rats improved liver transaminases and synthetic function, reduced liver histopathology and reversed hepatobiliary fibrosis. The reversal of hepatobiliary fibrosis was likely due to the reduced activation state of hepatic stellate cells, decreased collagen deposition, and enhanced extracellular matrix remodeling via the up-regulation of MMP-13 and down-regulation of TIMP-1.
Transplanted hUC-MSCs could differentiate into functional hepatocytes that improved both the biochemical and histopathologic changes in a CCl4-induced rat liver fibrosis model. hUC-MSCs may offer therapeutic opportunities for treating hepatobiliary diseases, including cirrhosis.
Core tip: Transplanted human umbilical cord-derived mesenchymal stem cells (hUC-MSCs) could differentiate into hepatocytes in vivo, thereby reducing the activation state of hepatic stellate cells, decreasing collagen deposition, and enhancing extracellular matrix remodeling in liver cirrhosis. hUC-MSCs play an important role in treating liver fibrosis and cirrhosis.
- Citation: Zhang GZ, Sun HC, Zheng LB, Guo JB, Zhang XL. In vivo hepatic differentiation potential of human umbilical cord-derived mesenchymal stem cells: Therapeutic effect on liver fibrosis/cirrhosis. World J Gastroenterol 2017; 23(46): 8152-8168
- URL: https://www.wjgnet.com/1007-9327/full/v23/i46/8152.htm
- DOI: https://dx.doi.org/10.3748/wjg.v23.i46.8152
Chronic liver inflammation is a major driving force for extracellular matrix (ECM) accumulation and can lead to liver cirrhosis with resultant complications such as portal hypertension and hepatocellular carcinoma[1,2]. The economic burden of cirrhosis is significant with the cost for treatment in 2008 ranging from $14 million to $2 billion in the United States, depending on the disease etiology[3]. This burden is expected to rise over the next 20 years. Given that cirrhosis is a worldwide problem that is associated with substantial socioeconomic burden, effective therapeutic strategies would be impactful.
Liver transplantation is an effective method for treating end-stage liver disease, but donor scarcity and immunological rejection limit its clinical application[4]. This has led to the search for alternative sources of hepatocytes, including cell-based therapy using mesenchymal stem cells (MSCs). MSCs are multipotent stromal cells that can differentiate into a variety of cell types. MSCs, including bone marrow-derived MSCs (BM-MSCs), have been demonstrated to reduce the generation of ECM and collagen deposition, thereby ameliorating liver fibrosis and cirrhosis[5,6]. In addition, BM-MSCs can differentiate into functional hepatocytes to repair damaged liver tissue, improving liver fibrosis and cirrhosis[7].
Among various MSCs types, human umbilical cord-derived MSCs (hUC-MSCs) may be best as an alternative for hepatocyte transplantation, because of their ease of extraction, low risk of viral transmission, low immunogenicity and immunosuppressive effects. To better understand the mechanism and therapeutic potential of hUC-MSCs, we developed a CCL4-induced rat liver fibrosis/cirrhosis model. We described the dynamic differentiation process of hUC-MSCs into functional hepatocytes in vivo and showed that transplanted hUC-MSCs can improve biochemical and histological abnormalities in this model. Our data suggest that hUC-MSCs may be an attractive, alternative therapeutic for treating liver disease, including cirrhosis.
The umbilical cord was cut into 1-mm3 pieces and filtered through a 1.5-mm mesh. Filtered umbilical cord pieces were seeded in DMEM/F12 complete medium. Fresh medium was replaced every 3 to 4 d, and non-adherent cells were discarded. When the observed cells reached 80% confluency, hUC-MSCs were separated by trypsin digestion, cultured, and identified by Alliancells Bioscience Co., Ltd.
One hundred and twenty adult male Wistar rats, weighing 350-450 g, were obtained from the Experimental Animal Center of Hebei Medical University. The research was conducted in accordance with the internationally accepted principles for laboratory animal use and care, as found in the US guidelines (NIH publication #85-23, revised in 1985). The experiment was performed in compliance with the national ethical guidelines for the care and use of laboratory animals (Certificate No. 911102).
Rat models of liver fibrosis and cirrhosis were established by hypodermic injection of CCl4 mixed with olive oil at a concentration of 40% (2 mL/kg) every Monday and Thursday. Rats injected with saline served as a control group. In the fibrosis group, after the sixth injection of CCl4, 5 × 106 hUC-MSCs were injected into the rats via the tail vein. Rats continued to be treated with CCl4. After 1, 2, or 4 wk of hUC-MSCs infusion, rats were sacrificed to assess the related index of liver fibrosis. In the cirrhosis group, rats were treated with CCl4 for up to 6 wk, and 5 × 106 hUC-MSCs were injected into the rats via the tail vein. Rats continued to be treated with CCl4. After 2, 4, or 8 wk, rats were sacrificed to determine the related indicators. In the control group, the same volumes of saline were injected into the rats via the tail vein.
Alanine aminotransferase (ALT), aspartate aminotransferase (AST), albumin (ALB), total bilirubin (TBIL) and direct bilirubin (DBIL) were evaluated in the samples of serum obtained at the end of the experiment. All tests were completed using the BECKMAN COULTER CX9 automatic biochemical analyzer.
Liver specimens were fixed for 12-24 h in 4% phosphate-buffered paraformaldehyde (Huarui Scientific and Technological Co.) and then embedded in paraffin for light microscopy examination. Tissue sections (3 μm thick) were stained with hematoxylin and eosin (H&E) for morphological evaluation and Masson trichrome (MT) to assess the degree of fibrosis.
Immunofluorescent studies were performed on 3-μm paraffin-embedded liver sections. Briefly, the sections were fixed with 4% phosphate-buffered paraformaldehyde and washed with 0.1% TritonX-100 TBS (TBSTx). Five percent bovine serum albumin (BSA) in TBSTx was used as a sealed liquid and then the specimens were incubated overnight at 4 °C with mouse monoclonal anti-human specific albumin (ALB) antibody (1:100; BETHYL) and mouse monoclonal anti-human specific α-fetoprotein (AFP) antibody (1:100; Thermo Scientific, Inc., United States). After the sections were washed, the Cy3-labeled goat anti-mouse IgG secondary antibody or FITC-labeled goat anti-rabbit (1:400 dilution) (Beyotime Institute of Biotechnology, Shanghai, China) was added and the sections were incubated at 37 °C for 1 h. DAPI (4’, 6-diamidino-2-phenylindole) was used for nuclear staining. The negative control samples were processed under the same conditions, except that 5% BSA in TBSTx was used in place of the primary antibody. The ALB and AFP positive expression levels were measured using a Motic Med 6.0 digital video image analysis system (Motic China Group Co., Ltd., Xiamen, China) and expressed as optical density values.
Immunohistochemistry studies were performed on 3-μm paraffin-embedded liver sections. The sections were fixed with 4% phosphate-buffered paraformaldehyde and washed with PBS. Five percent BSA in TBSTx was used as a sealed liquid, and the specimens were then incubated overnight at 4 °C with the primary antibody mouse monoclonal anti-human specific CK18 antibody (1:200; Thermo Scientific, Inc.), mouse monoclonal anti-human specific CK19 antibody (1:200; Thermo Scientific, Inc.), mouse monoclonal anti-human specific vimentin antibody (1:200; Santa Cruz Biotechnology, Inc., Santa Cruz, CA, United States), mouse monoclonal anti-human specific E-cadherin antibody (1:200; Santa Cruz Biotechnology, Inc.), mouse monoclonal anti-human specific α-catenin antibody (1:200; Santa Cruz Biotechnology, Inc.), rabbit monoclonal anti-α-SMA antibody (1:200; Santa Cruz Biotechnology, Inc.), goat polyclonal anti-collagen I antibody (1:100; Santa Cruz Biotechnology, Inc.), mouse polyclonal anti-collagen III antibody (1:200; Santa Cruz Biotechnology, Inc.), rabbit polyclonal anti-MMP-13 antibody (1:200; Boaosen, Beijing, China), and rabbit polyclonal anti-TIMP-1 antibody (1:200; Santa Cruz Biotechnology, Inc.). After incubation with peroxidase-conjugated secondary antibody, signals were visualized with a diaminobenzidine peroxidase substrate kit (Vector Laboratories). The negative control samples were processed under the same conditions, except that 5% BSA in TBSTx was used in place of the primary antibody. The expression levels were measured using a Motic Med 6.0 digital video image analysis system (Motic China Group Co., Ltd.) and expressed as optical density values.
Primary antibodies included mouse anti-CK18, CK19, ALB, and AFP monoclonal antibodies (1:500); mouse anti-vimentin, E-cadherin, and α-catenin antibodies (1:200); goat anti-collagen I polyclonal antibody (1:100); mouse anti-collagen III polyclonal antibody (1:500); rabbit anti-MMP-13 and TIMP-1 polyclonal antibody (1:200) and rabbit anti-β-actin monoclonal antibody (1:200). Western blot was performed as previously described[8].
Total RNA was extracted with TRIzol reagent (Invitrogen, United States) according to the manufacturer’s instructions. cDNA was generated using 2 μg of total RNA as described[9].
Primer Express 5.0 was used to design the following primers: ALB forward primer, 5′-GCT TGA ATG TGC TGA TGA CAG G-3′ and reverse primer, 5′-TGG GAT TTT TCC AAC AGA GGT T-3′; AFP forward primer, 5′-AAG TGA AGA GGG AAG ACA TAA C-3′ and reverse primer, 5′-AGA AGA ATT GTA GGT GCA TAC A-3′; CK18 forward primer, 5′-TAA TCT TGG TGA TGC CTT GGA C-3′ and reverse primer, 5′-CCT CAG AAC TTT GGT GTC ATT G-3′; CK19 forward primer, 5′-GCC ACT ACT ACA CGA CCA TCC A-3′ and reverse primer, 5′-AGA GCC TGT TCC GTC TCA AAC T-3′; vimentin forward primer, 5′-ATG TTG ACA ATG CGT CTC TGG CTC CA-3′ and reverse primer, 5′-ATT TCC TCT TCG TGG AGT TTC T-3′; N-cadherin forward primer, 5′-CAG CCT CCA ACT GGT ATC TTC A-3′ and reverse primer, 5′-ATC TAC TGC ATG TGC CC TCA AA-3′; E-cadherin forward primer, 5′-CCA GGA GGT CTT TAA GGG GTC T-3′ and reverse primer, 5′-GCT GAGG ATG GTG TAA GCG ATG-3′; α-catenin forward primer, 5′-GCA AAG AAT GGA AAT GAG AAA G-3′ and reverse primer, 5′-AAT AAC CTG AGG ACA GAG GGC T-3′; collagen I forward primer, 5′-GTG CGA TGG GGT GCT ATG-3′ and reverse primer, 5′-TCT GCG TCT GGT GAT ACA TAT TC-3′; collagen III forward primer, 5′-ACC TGC TCC TGT CAT TCC-3′ and reverse primer, 5′- CCT CCG ACT CCA GAC TTG-3′; MMP-13 forward primer, 5′-CCA CCT TCT TCT TGT TGA GTT G-3′ and reverse primer, 5′-AAG AGT CAC AGG ATG GTA GTA TG-3′; TIMP-1 forward primer, 5′-CGC TGG TAT AAG GTG GTC TC-3′ and reverse primer, 5′-CGC TGG TAT AAG GTG GTC TC-3′; and GAPDH forward primer, 5′-GGC AAG TTC AAC GGC ACA G-3′ and reverse primer, 5′-CGC CAG TAG ACT CCA CGA CAT -3′. Real-time fluorescent quantitative PCR was performed on an ABI Prism 7700 real-time fluorescent quantitative PCR thermal cycler (Applied Biosystems, Foster City, CA, United States), and the expression of the above genes was normalized to that of GAPDH.
Data are presented as the mean ± SD and analyzed using SPSS18.0 software. The performed statistical analyses included one-way ANOVA, the LSD test and Pearson’s correlation analysis. P < 0.05 was considered statistically significant.
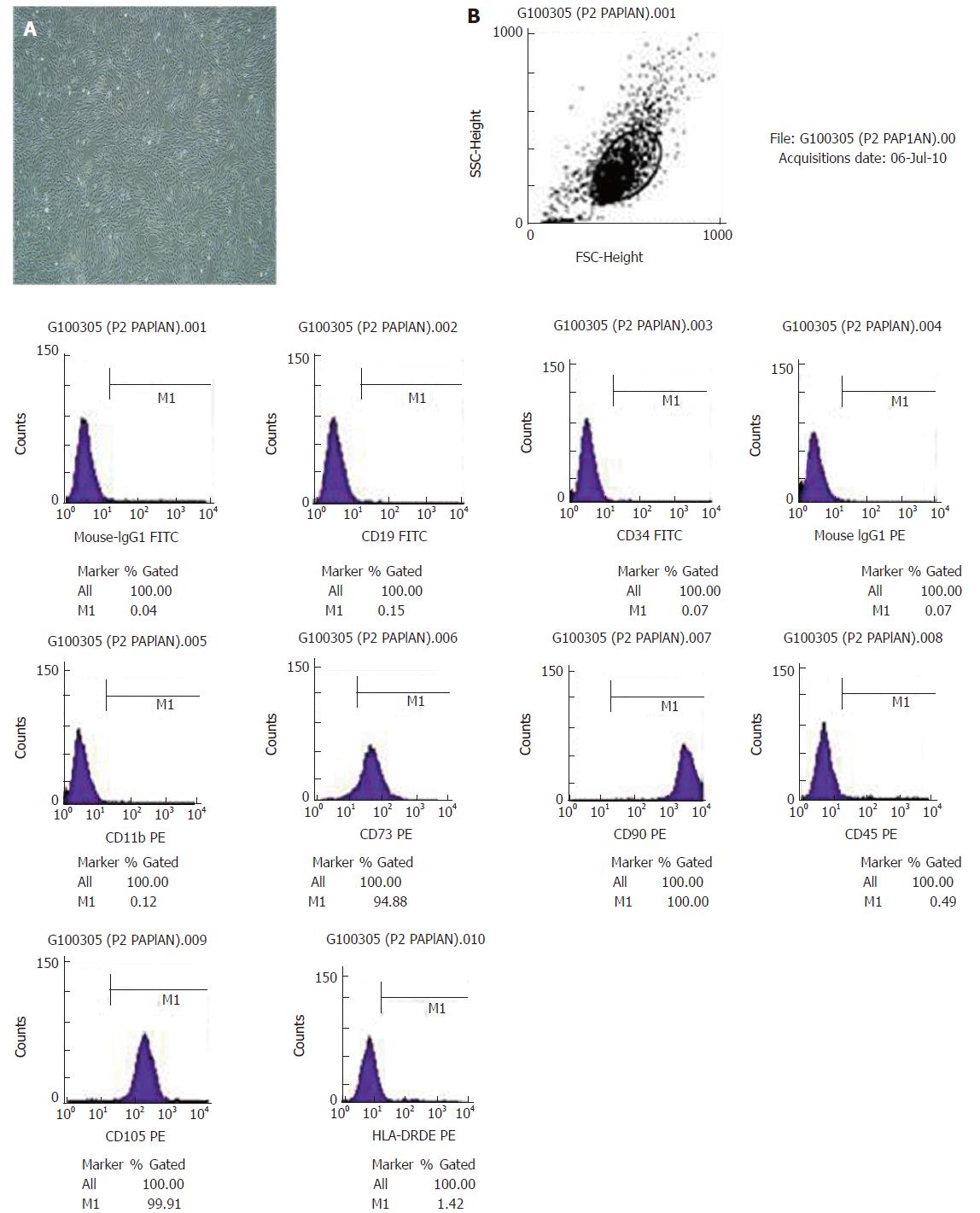
We isolated hUC-MSCs that exhibited fibroblast-like morphology from the umbilical cord (Figure 1A). Flow cytometry showed that hUC-MSCs expressed high levels of MSCs-specific markers CD90, CD105 and CD73 but no or low levels of CD34, CD19, CD11b, HLA-DR and CD45 (Figure 1B). We next transplanted hUC-MSCs into rats and assessed their potential to differentiate into hepatocytes. We detected ALB, AFP, CK18 and CK19 from human origin, but the rat-derived ALB, AFP, CK18 and CK19 were not detected. Therefore, the differentiated hepatocytes of rats had no effect on the results. Prior to transplantation, there was no positive expression of human ALB, AFP, CK18 or CK19. After transplantation, we detected the expression of the above markers.
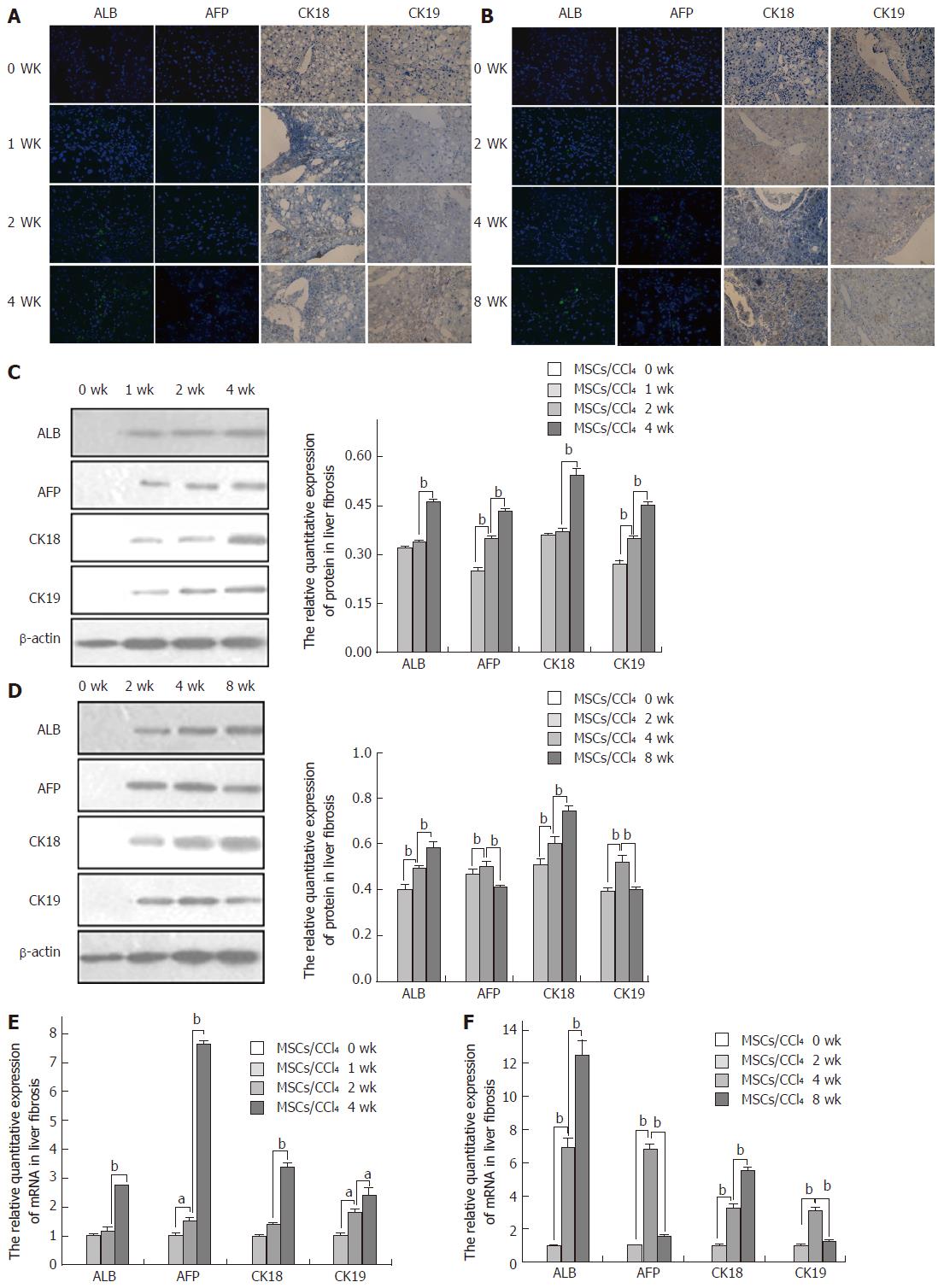
Immunofluorescence and immunohistochemistry showed that compared to the saline/CCl4 groups, the positive expression of the above markers showed different patterns in the MSCs/CCl4 fibrotic and cirrhotic groups. The expression of ALB, AFP, CK18 and CK19 was weakly positive and faint in spots at 1 or 2 wk, respectively, in the MSCs/CCl4 fibrotic and cirrhotic groups. However, at 2, 4, or 8 wk after hUC-MSC infusion, with an extension of the transplantation time, the positive expression of ALB, AFP, CK18 and CK19 was gradually increased, in addition to the decreased expression of the AFP and CK19 in the MSCs/CCl4 cirrhotic group at 8 wk after hUC-MSC infusion.
The mRNA levels of ALB and CK18 were not significantly different between 2 wk and 1 wk in the MSCs/CCl4 fibrotic groups, while the expression of ALB and CK18 mRNAs was significantly increased at 4 wk compared to 2 wk (P < 0.01). With the extension of the transplantation time, the expression levels of AFP and CK19 mRNAs were significantly elevated (P < 0.01). In the cirrhotic MSCs/CCl4 groups, the expression levels of AFP and CK19 mRNAs were significantly elevated at 4 wks compared to 2 wk (P < 0.01). However, the expression levels of AFP and CK19 mRNAs were significantly decreased at 8 wk compared to 4 wk (P < 0.01); as the time of hUC-MSC transplantation was extended, the expression levels of ALB and hCK18 increased, and the relative protein quantitation was consistent with the above results (Figure 2).
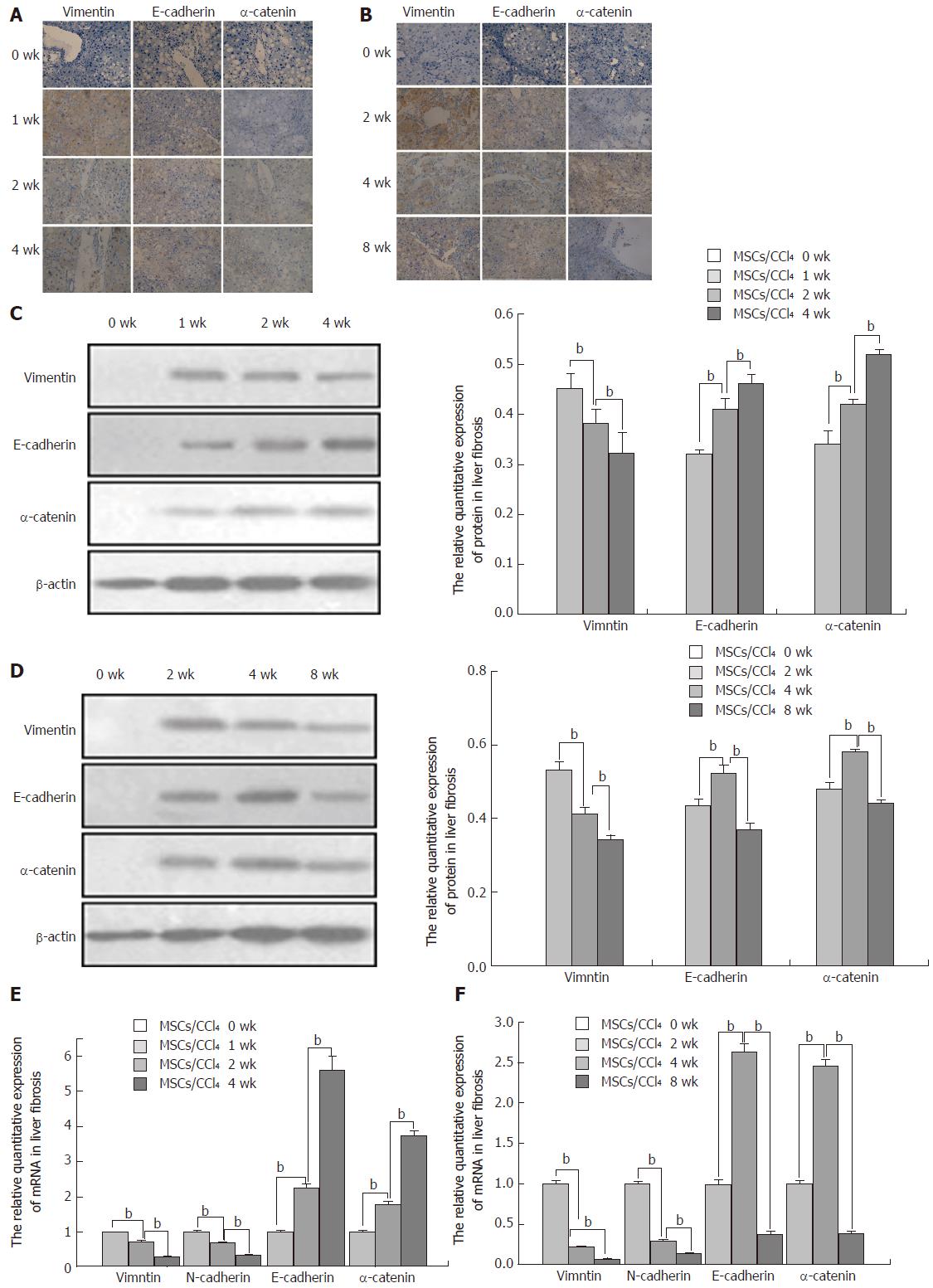
Next, we analyzed the levels of human vimentin (a mesenchymal marker) and human E-cadherin and α-catenin (epithelial markers) by immunohistochemistry, Western blot and real-time PCR. To determine whether hUC-MSCs experienced the mesenchymal-to-epithelial transition (MET) during hepatocyte differentiation in vivo, the expression levels of vimentin, E-cadherin and α-catenin were detected. After 1 or 2 wk of hUC-MSC transplantation, in the fibrotic and cirrhotic MSCs/CCl4 groups, many positive cells for vimentin were expressed in the portal vein and portal area. As the time of hUC-MSC transplantation extended, the expression of vimentin was significantly decreased in both the fibrotic and cirrhotic MSCs/CCl4 groups. At 8 wk after hUC-MSC transplantation, a low level of vimentin was expressed. There was no expression before hUC-MSC transplantation. The relative expression levels of protein and mRNA were consistent with the above results (P < 0.01).
In the fibrotic MSCs/CCl4 groups, at 1 wk after hUC-MSC transplantation, low levels of E-cadherin and α-catenin expression were detected by immunohistochemistry, which gradually increased with the extension of hUC-MSC transplantation. In the cirrhotic MSCs/CCl4 groups, at 2 wk after hUC-MSC transplantation, low E-cadherin and α-catenin levels were seen. The E-cadherin and α-catenin expressions levels were significantly increased at 4 wk after hUC-MSCs transplantation compared to 2 wk in the fibrotic and cirrhotic MSCs/CCl4 groups. By contrast, the levels of the above indicators decreased significantly at 8 wk compared to 4 wk after hUC-MSC transplantation. The relative protein and mRNA expression levels were consistent with these results (P < 0.01; Figure 3).
CCl4-induced hepatic injury in rats has been used as a model system to study liver damage and fibrosis, and we used this model to assess the therapeutic effect of hUC-MSCs. The hUC-MSCs were transplanted into a CCl4-induced liver fibrotic/cirrhotic rat model, and we observed a significant reduction in the serum levels of ALT and AST at 2 and 4 wk in the fibrotic MSCs/CCl4 groups and at 2, 4, and 8 wk in the cirrhotic MSCs/CCl4 groups (P < 0.05). In addition, the serum level of ALB was gradually reduced with further CCl4 induction in the fibrotic and cirrhotic saline/CCl4 groups, whereas after the transplantation of hUC-MSCs, the serum levels of ALB were markedly increased at 4 wk in the fibrotic MSCs/CCl4 groups and at 4 and 8 wk in the cirrhotic MSCs/CCl4 groups (P < 0.05). Compared with the saline/CCl4 groups, the serum levels of TBIL and DBIL greatly dropped at 4 wk in the fibrotic MSCs/CCl4 groups and at 2, 4, and 8 wk in the cirrhotic MSCs/CCl4 groups (P < 0.05; Table 1). The inflammation score in rat liver fibrosis and cirrhosis induced by CCl4 in each group are listed in Table 2.
| Group | ALT(U/L) | AST(U/L) | ALB(g/L) | TBIL (µmol/L) | DBIL (µmol/L) | |
| Liver fibrosis groups | Saline/CCl4 1 wk | 141.00 ± 16.33 | 150.76 ± 15.13 | 29.99 ± 1.53 | 0.97 ± 0.15 | 0.70 ± 0.02 |
| MSCs/CCl4 1 wk | 130.32 ± 15.35 | 142.32 ± 14.24 | 32.17 ± 2.25 | 0.93 ± 0.08 | 0.60 ± 0.04 | |
| Saline/CCl4 2 wk | 152.35 ± 14.56 | 172.22 ± 19.34 | 24.54 ± 1.21 | 1.51 ± 0.21 | 0.90 ± 0.07 | |
| MSCs/CCl4 2 wk | 132.88 ± 13.23a | 146.23 ± 13.23a | 26.60 ± 2.34 | 1.33 ± 0.25 | 0.70 ± 0.05 | |
| Saline/CCl4 4 wk | 239.24 ± 26.32 | 262.34 ± 28.12 | 28.12 ± 2.13 | 2.10 ± 0.23 | 1.10 ± 0.17 | |
| MSCs/CCl4 4 wk | 192.32 ± 21.24b | 204.52 ± 23.24b | 21.12 ± 1.94b | 1.57 ± 0.15b | 0.50 ± 0.03b | |
| Liver cirrhosis groups | Saline/CCl4 2 wk | 334.36 ± 37.48 | 375.24 ± 41.26 | 26.55 ± 1.51 | 2.27 ± 0.15 | 1.32 ± 0.12 |
| MSCs/CCl4 2 wk | 204.31 ± 22.52c | 289.13 ± 32.22c | 19.62 ± 1.54 | 1.90 ± 0.14c | 0.80 ± 0.09c | |
| Saline/CCl4 4 wk | 353.35 ± 38.76 | 406.24 ± 44.31 | 22.69 ± 2.72 | 3.34 ± 0.76 | 2.07 ± 0.15 | |
| MSCs/CCl4 4 wk | 214.11 ± 23.24d | 292.24 ± 32.32d | 20.36 ± 1.53d | 2.06 ± 0.14d | 1.10 ± 0.13d | |
| Saline/CCl4 8 wk | 391.22 ± 43.57 | 418.63 ± 45.71 | 19.31 ± 1.52 | 4.82 ± 0.52 | 3.74 ± 0.25 | |
| MSCs/CCl4 8 wk | 247.31 ± 27.36e | 307.22 ± 34.36e | 23.70 ± 7.72e | 3.32 ± 0.31e | 2.13 ± 0.15e | |
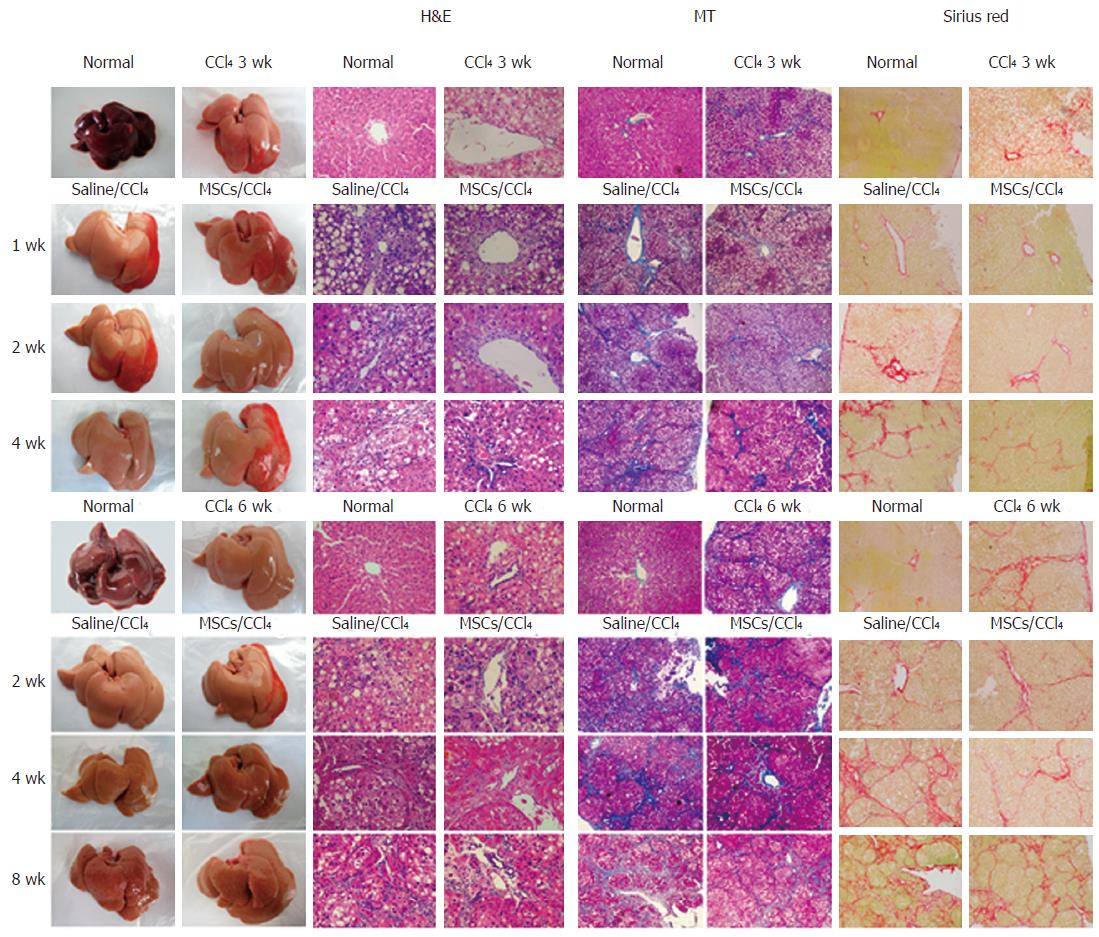
Transplantation of hUC-MSCs improved the gross liver morphology by reducing the surface coarseness, reducing the liver turgor, and improving the liver perfusion (Figure 4, right panels).
Histologic examination of the CCl4-treated liver revealed swelling of the hepatocytes, fatty degeneration, necrosis and regeneration, particularly with continued CCl4 administration (Figure 4). These alterations were reduced after the infusion of hUC-MSCs.
To determine the extent of the collagen deposition, MT and Sirius red staining were performed. By 3 wk after CCl4 injection, liver fibrosis was observed (Figure 4), as evidenced by fiber extension, large fibrous septa formation, pseudo lobe separation and collagen accumulation in the periportal region. These alterations were remarkably reduced after infusion of hUC-MSCs (Figure 4). Together, our data show that transplanted hUC-MSCs can reverse established liver histopathology induced by CCl4.
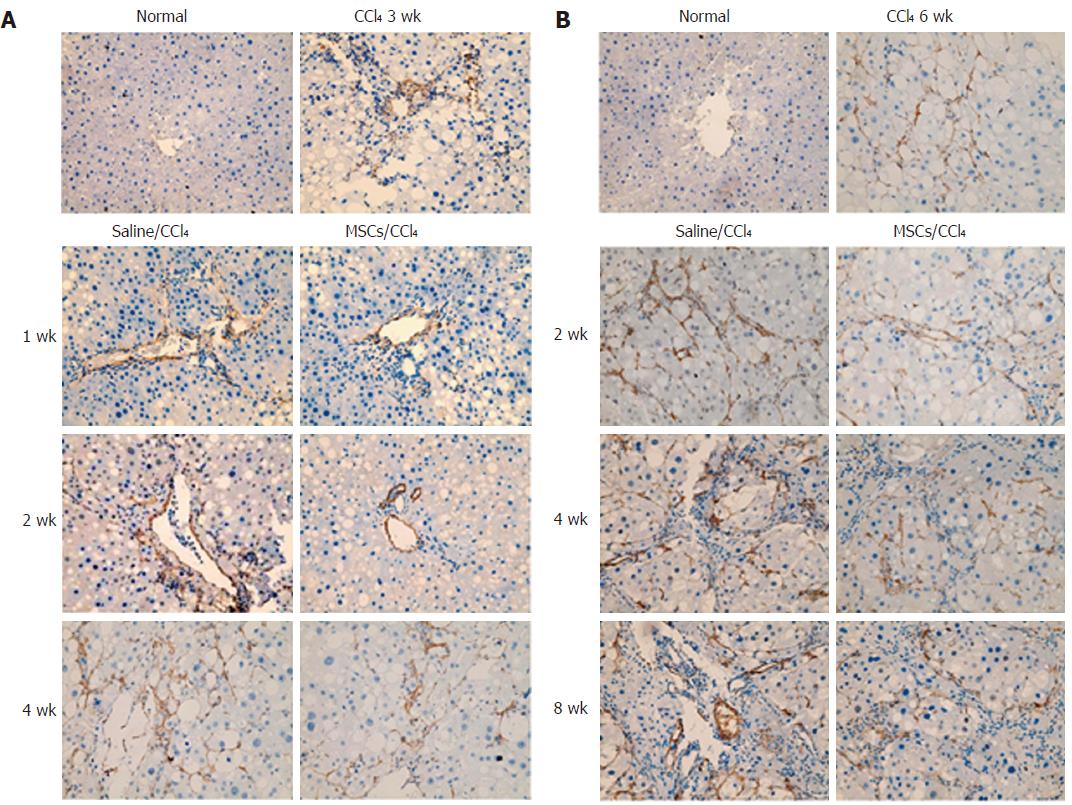
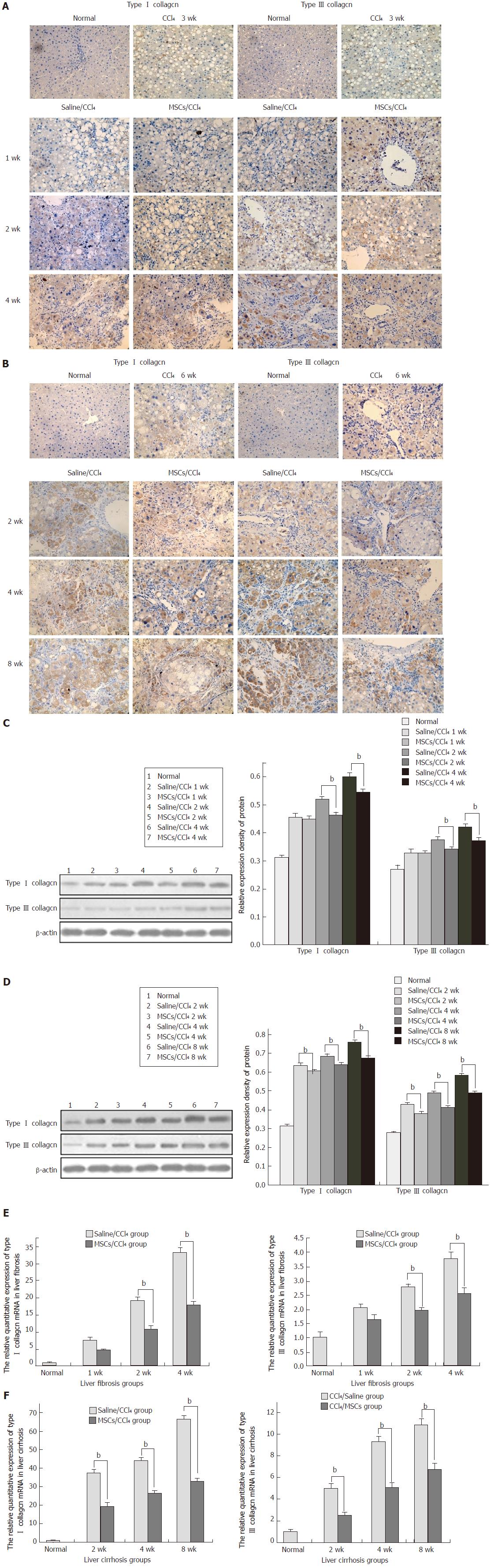
To study the possible impact of hUC-MSCs on HSC activation and collagen deposition, immunohistochemistry was performed to detect the α-SMA and collagen I and III expression. With the progression of liver fibrosis, positive α-SMA, collagen I and collagen III cells in the rat liver tissue gradually increased, mainly in the portal area, fibrous septa and hyperplasia of bile duct cells. Compared with the saline/CCl4 group, the positive expression of α-SMA and collagen I and III in the MSCs/CCl4 group was reduced, although they gradually increased as the time of transplantation extended. In addition, hUC-MSCs significantly reduced the protein and mRNA expression of collagen I and III in the fibrotic and cirrhotic groups (P < 0.05), except in the first week after hUC-MSC transplantation in these groups (Figures 5 and 6).
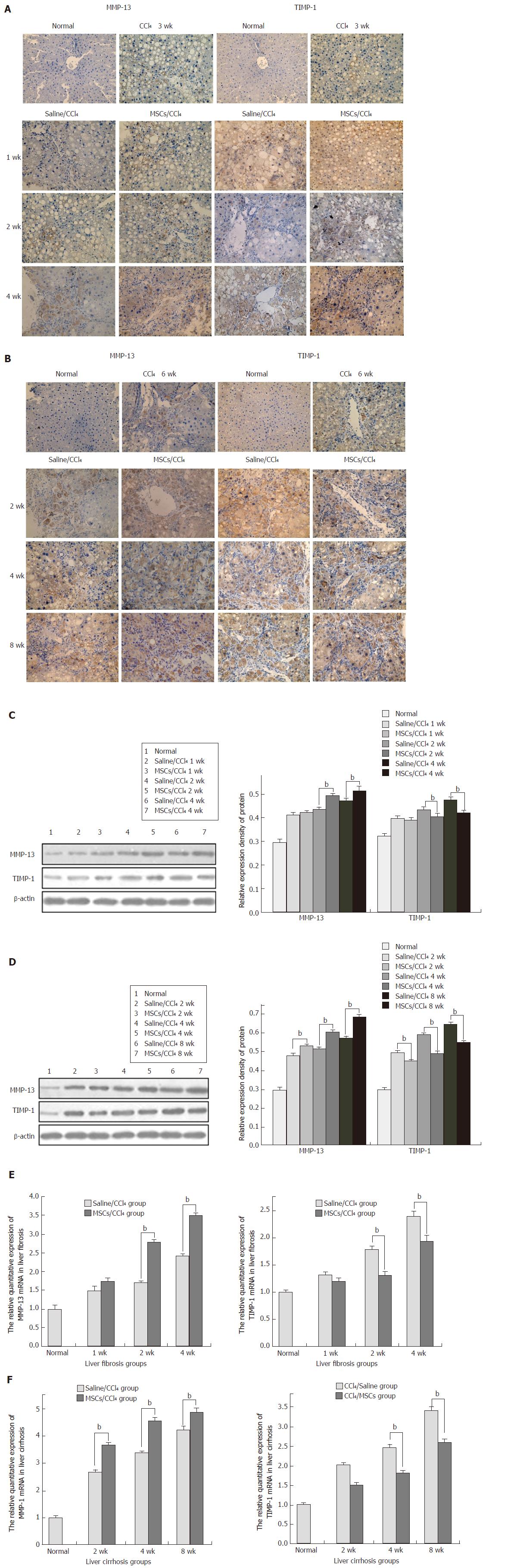
To detect the mechanism by which hUC-MSCs reduce collagen deposition, immunohistochemical analysis for MMP-13 and TIMP-1, which are mainly expressed in the cells of portal area and the central vein area, was performed in the liver tissues of fibrotic and cirrhotic rats. With the extension of the CCl4 induction time, MMP-13 and TIMP-1 gradually increased in both the saline/CCl4 groups and MSCs/CCl4 groups. After the same CCl4 injection, the MMP-13 in the MSCs/CCl4 groups significantly increased compared with the saline/CCl4 groups. However, in the MSCs/CCl4 groups, the expression of TIMP-1 decreased compared with the saline/CCl4 groups. Western blot and real-time PCR were further applied, which showed that hUC-MSCs significantly lowered the collagen deposition by increasing MMP-13 expression (P < 0.05) and decreasing TIMP-1 (P < 0.05) expression, except in the first week after the transplantation of hUC-MSCs in the fibrotic MSCs/CCl4 groups (Figure 7).
At present, liver cirrhosis and other chronic liver diseases seriously affect human health, and liver transplantation is an effective treatment. However, many of its side effects limit its clinical applications. Stem cell-based therapy is of potential value in tissue and organ replacement and regeneration. Among various types of MSCs, hUC-MSCs are optimal with many advantages, such as easy extraction, low risk of viral transmission, low immunogenicity and immunosuppressive effects[10,11].
Hepatocytes undergo damage in case of liver cirrhosis. In addition, under the given conditions, MSCs can differentiate into bone, nerve cells, hepatocytes and many other cell types[12-16]. Human MSCs differentiated into hepatocyte-like cells in vitro[17]. Can MSCs differentiate into hepatocyte-like cells to replace damaged hepatocytes that play a role in vivo? Piryaei et al[18] found that BM-MSCs could differentiate into hepatocyte-like cells in the liver fibrotic mice in vivo. Another study also confirmed that MSCs infused into the CCl4-induced liver fibrotic rats could differentiate into hepatocyte-like cells[19]. Human MSCs labelled with CM-Di I well integrated into the injured liver tissue in CCl4-induced cirrhotic rats[20]. Our study showed that the expression of human ALB, AFP, CK18 and CK19 was detected in liver tissue of the fibrotic and cirrhotic rats after hUC-MSC transplantation. Human ALB and CK18 were weakly detected after 1 wk of hUC-MSC transplantation, and they were increased in a time-dependent manner. Interestingly, the human AFP and CK19 expression levels first increased and then decreased with the extension of the transplantation time. These results suggest that transplanted hUC-MSCs could migrate into the injured liver in CCl4-induced fibrosis and cirrhosis, where they could differentiate into hepatocyte-like cells. Few hUC-MSCs differentiated into hepatocyte-like cells within 1 wk after hUC-MSC transplantation. hUC-MSCs first differentiated into immature hepatocytes and later became mature, which was a dynamic differentiation process.
Yamamoto et al[21] induced adipose tissue-derived MSCs (AT-MSCs) to differentiate into AT-MSC-derived hepatocytes (AT-MSC-Hepas) in vitro. AT-MSC-Hepas had epithelial cell-like morphology. However, undifferentiated AT-MSCs had a fibroblast-like shape, indicating that during hepatic differentiation, the cell morphology changed markedly from fibroblast cell-like to epithelial cell-like. Meanwhile, microarray analysis showed that Twist and Snail, which induced the epithelial-to-mesenchymal transition, were down-regulated in the hepatic differentiation process. In addition, the expression levels of E-cadherin and α-catenin, genes expressed in epithelial cells, were up-regulated in MSC-derived hepatocytes. In contrast, the expression of vimentin and N-cadherin, genes expressed in interstitial cells, was down-regulated after differentiation. Tsai et al[22] reported that the phosphorylation of a factor of liver mesenchymal epithelial conversion was up-regulated in rats with HMSC transplantation. In summary, these data support the hypothesis that MET occurs during the hepatic differentiation of MSCs.
We previously confirmed that hUC-MSCs could differentiate into functional hepatocytes in liver fibrosis and cirrhosis in rats. Do hUC-MSCs undergo the MET during this differentiation? A recent study speculated that MET might be one of the mechanisms for lung hMSCs to treat chronic lung diseases[23]. Our study confirmed that vimentin and N-cadherin expression was gradually reduced in both fibrotic and cirrhotic rats with the extension of hUC-MSC transplantation. However, E-cadherin and α-catenin expression first increased and then decreased, indicating that hUC-MSCs did not directly differentiate into functional hepatocytes; instead, they first differentiated into epithelial cell-like cells and then differentiated into hepatocyte-like cells. During this differentiation process, epithelial cell-like cells were in the intermediate stage.
BM-MSCs and hepatocytes transplanted into CCl4-induced liver fibrotic rats were evaluated after co-culture for 14 d; then, after 1, 3, or 4 wk, rats were sacrificed. The results showed that hepatic fibrosis was markedly improved, indicating the therapeutic effect of BM-MSCs on liver fibrosis[24]. In liver cirrhosis, activated HSCs are the main source of the ECM, with collagen I and III as the main ingredients. A recent study has demonstrated that human MSCs implanted into CCl4-induced liver cirrhotic rats inhibited the expression of collagen I and α-SMA, improving liver cirrhosis[25]. Our results showed that hUC-MSC infusion significantly reduced the deposition of collagen I and III, except in the first week after hUC-MSCs transplantation, indicating that hUC-MSCs had a therapeutic effect at a late stage rather than immediately at onset.
Under normal conditions, the collagenase mainly maintains collagen balance. MMP-13, a rodent interstitial metal collagenase, plays a major role in the balance of the contents of collagen I and III, which can be inhibited by TIMP-1. How do the MSCs participate in collagen metabolism? In this study, hUC-MSC transplantation degraded collagen deposition and clearly improved liver fibrosis with the up-regulation of MMP-13 expression and reduction of TIMP-1 expression. As indicated by immunohistochemical staining, Western blot and real-time PCR, MSCs reduced the collagen deposition by up-regulating MMP expression and down-regulating TIMP expression.
These data support that hUC-MSCs have the potential for hepatic differentiation in vivo and hUC-MSC transplantation could exert a protective effect against liver fibrosis/cirrhosis, suggesting that these cells may provide a new approach for cell therapy in liver diseases. The exact mechanisms by which hUC-MSCs repair liver injury and undergo hepatic differentiation remain to be elucidated in future studies.
Chronic liver inflammation is a major driving force for extracellular matrix accumulation, leading to liver cirrhosis with complications. Liver transplantation is an effective method for treating end-stage liver disease, but donor scarcity and immunological rejection limit its clinical application. This has led to the search for alternative sources of hepatocytes, including cell-based therapy using mesenchymal stem cells (MSCs). MSCs have been demonstrated to improve liver disease.
Among the various MSCs types, human umbilical cord-derived mesenchymal stem cells (hUC-MSCs) may be optimal as an alternative for hepatocyte transplantation, because of ease of extraction, low risk of viral transmission, low immunogenicity and immunosuppressive effects. The authors used a CCl4-induced rat liver fibrosis/cirrhosis model to better understand the mechanism and therapeutic potential of hUC-MSCs.
This is the first study to evaluate the in vivo hepatic differentiation potential of human umbilical cord-derived MSCs and their therapeutic effect on liver fibrosis.
Transplanted hUC-MSCs could differentiate into functional hepatocytes that improved both biochemical and histopathologic changes in a CCl4-induced rat liver fibrosis model. hUC-MSCs may offer therapeutic opportunities in the treatment of hepatobiliary diseases, including cirrhosis.
The mesenchymal-to-epithelial transition is a crucial physiologic event that converts motile mesenchymal cells to polarized epithelial cells, favoring the epithelial regeneration. hUC-MSCs differentiated into hepatocyte-like cells via the mensenchymal-to-epithelial transition in vivo.
Good research on cord stem cells in the treatment of liver disease. Change all typos saying “collagen I and III TO collagen I and III”.
We thank Alliancells Bioscience Co., Ltd. for providing hUC-MSCs and the ENT laboratory, The First Hospital of Hebei Medical University for providing laboratory equipment on immunofluorescence and real-time PCR.
Manuscript source: Unsolicited manuscript
Specialty type: Gastroenterology and hepatology
Country of origin: China
Peer-review report classification
Grade A (Excellent): 0
Grade B (Very good): B
Grade C (Good): 0
Grade D (Fair): D
Grade E (Poor): 0
P- Reviewer: Julie NL, Sun BC S- Editor: Qi Y L- Editor: Wang TQ E- Editor: Ma YJ
| 1. | Afdhal NH, Manning D. Diagnosis of liver fibrosis in 2008 and beyond. Gastroenterol Clin Biol. 2008;32:88-90. [PubMed] [DOI] [Cited in This Article: ] |
| 2. | Mormone E, Lu Y, Ge X, Fiel MI, Nieto N. Fibromodulin, an oxidative stress-sensitive proteoglycan, regulates the fibrogenic response to liver injury in mice. Gastroenterology. 2012;142:612-621.e5. [PubMed] [DOI] [Cited in This Article: ] |
| 3. | Neff GW, Duncan CW, Schiff ER. The current economic burden of cirrhosis. Gastroenterol Hepatol (N Y). 2011;7:661-671. [PubMed] [Cited in This Article: ] |
| 4. | Baertschiger RM, Serre-Beinier V, Morel P, Bosco D, Peyrou M, Clément S, Sgroi A, Kaelin A, Buhler LH, Gonelle-Gispert C. Fibrogenic potential of human multipotent mesenchymal stromal cells in injured liver. PLoS One. 2009;4:e6657. [PubMed] [DOI] [Cited in This Article: ] |
| 5. | Oyagi S, Hirose M, Kojima M, Okuyama M, Kawase M, Nakamura T, Ohgushi H, Yagi K. Therapeutic effect of transplanting HGF-treated bone marrow mesenchymal cells into CCl4-injured rats. J Hepatol. 2006;44:742-748. [PubMed] [DOI] [Cited in This Article: ] |
| 6. | Zhao DC, Lei JX, Chen R, Yu WH, Zhang XM, Li SN, Xiang P. Bone marrow-derived mesenchymal stem cells protect against experimental liver fibrosis in rats. World J Gastroenterol. 2005;11:3431-3440. [PubMed] [DOI] [Cited in This Article: ] |
| 7. | Ju S, Teng GJ, Lu H, Jin J, Zhang Y, Zhang A, Ni Y. In vivo differentiation of magnetically labeled mesenchymal stem cells into hepatocytes for cell therapy to repair damaged liver. Invest Radiol. 2010;45:625-633. [PubMed] [DOI] [Cited in This Article: ] |
| 8. | Hao LS, Zhang XL, An JY, Karlin J, Tian XP, Dun ZN, Xie SR, Chen S. PTEN expression is down-regulated in liver tissues of rats with hepatic fibrosis induced by biliary stenosis. APMIS. 2009;117:681-691. [PubMed] [DOI] [Cited in This Article: ] |
| 9. | Zheng L, Chen X, Guo J, Sun H, Liu L, Shih DQ, Zhang X. Differential expression of PTEN in hepatic tissue and hepatic stellate cells during rat liver fibrosis and its reversal. Int J Mol Med. 2012;30:1424-1430. [PubMed] [DOI] [Cited in This Article: ] |
| 10. | Chen MY, Lie PC, Li ZL, Wei X. Endothelial differentiation of Wharton’s jelly-derived mesenchymal stem cells in comparison with bone marrow-derived mesenchymal stem cells. Exp Hematol. 2009;37:629-640. [PubMed] [DOI] [Cited in This Article: ] |
| 11. | Zhao Q, Ren H, Li X, Chen Z, Zhang X, Gong W, Liu Y, Pang T, Han ZC. Differentiation of human umbilical cord mesenchymal stromal cells into low immunogenic hepatocyte-like cells. Cytotherapy. 2009;11:414-426. [PubMed] [DOI] [Cited in This Article: ] |
| 12. | Gregory CA, Prockop DJ, Spees JL. Non-hematopoietic bone marrow stem cells: molecular control of expansion and differentiation. Exp Cell Res. 2005;306:330-335. [PubMed] [DOI] [Cited in This Article: ] |
| 13. | La Rocca G, Anzalone R, Corrao S, Magno F, Loria T, Lo Iacono M, Di Stefano A, Giannuzzi P, Marasà L, Cappello F. Isolation and characterization of Oct-4+/HLA-G+ mesenchymal stem cells from human umbilical cord matrix: differentiation potential and detection of new markers. Histochem Cell Biol. 2009;131:267-282. [PubMed] [DOI] [Cited in This Article: ] |
| 14. | Yang XF, He X, He J, Zhang LH, Su XJ, Dong ZY, Xu YJ, Li Y, Li YL. High efficient isolation and systematic identification of human adipose-derived mesenchymal stem cells. J Biomed Sci. 2011;18:59. [PubMed] [DOI] [Cited in This Article: ] |
| 15. | Thanabalasundaram G, Arumalla N, Tailor HD, Khan WS. Regulation of differentiation of mesenchymal stem cells into musculoskeletal cells. Curr Stem Cell Res Ther. 2012;7:95-102. [PubMed] [DOI] [Cited in This Article: ] |
| 16. | Satija NK, Singh VK, Verma YK, Gupta P, Sharma S, Afrin F, Sharma M, Sharma P, Tripathi RP, Gurudutta GU. Mesenchymal stem cell-based therapy: a new paradigm in regenerative medicine. J Cell Mol Med. 2009;13:4385-4402. [PubMed] [DOI] [Cited in This Article: ] |
| 17. | Hong SH, Gang EJ, Jeong JA, Ahn C, Hwang SH, Yang IH, Park HK, Han H, Kim H. In vitro differentiation of human umbilical cord blood-derived mesenchymal stem cells into hepatocyte-like cells. Biochem Biophys Res Commun. 2005;330:1153-1161. [PubMed] [DOI] [Cited in This Article: ] |
| 18. | Piryaei A, Valojerdi MR, Shahsavani M, Baharvand H. Differentiation of bone marrow-derived mesenchymal stem cells into hepatocyte-like cells on nanofibers and their transplantation into a carbon tetrachloride-induced liver fibrosis model. Stem Cell Rev. 2011;7:103-118. [PubMed] [DOI] [Cited in This Article: ] |
| 19. | Pan Q, Fouraschen SM, Kaya FS, Verstegen MM, Pescatori M, Stubbs AP, van Ijcken W, van der Sloot A, Smits R, Kwekkeboom J. Mobilization of hepatic mesenchymal stem cells from human liver grafts. Liver Transpl. 2011;17:596-609. [PubMed] [DOI] [Cited in This Article: ] |
| 20. | Jung KH, Shin HP, Lee S, Lim YJ, Hwang SH, Han H, Park HK, Chung JH, Yim SV. Effect of human umbilical cord blood-derived mesenchymal stem cells in a cirrhotic rat model. Liver Int. 2009;29:898-909. [PubMed] [DOI] [Cited in This Article: ] |
| 21. | Yamamoto Y, Banas A, Murata S, Ishikawa M, Lim CR, Teratani T, Hatada I, Matsubara K, Kato T, Ochiya T. A comparative analysis of the transcriptome and signal pathways in hepatic differentiation of human adipose mesenchymal stem cells. FEBS J. 2008;275:1260-1273. [PubMed] [DOI] [Cited in This Article: ] |
| 22. | Tsai PC, Fu TW, Chen YM, Ko TL, Chen TH, Shih YH, Hung SC, Fu YS. The therapeutic potential of human umbilical mesenchymal stem cells from Wharton’s jelly in the treatment of rat liver fibrosis. Liver Transpl. 2009;15:484-495. [PubMed] [DOI] [Cited in This Article: ] |
| 23. | Ricciardi M, Malpeli G, Bifari F, Bassi G, Pacelli L, Nwabo Kamdje AH, Chilosi M, Krampera M. Comparison of epithelial differentiation and immune regulatory properties of mesenchymal stromal cells derived from human lung and bone marrow. PLoS One. 2012;7:e35639. [PubMed] [DOI] [Cited in This Article: ] |
| 24. | Li TZ, Kim JH, Cho HH, Lee HS, Kim KS, Lee SW, Suh H. Therapeutic potential of bone-marrow-derived mesenchymal stem cells differentiated with growth-factor-free coculture method in liver-injured rats. Tissue Eng Part A. 2010;16:2649-2659. [PubMed] [DOI] [Cited in This Article: ] |