Published online Jan 7, 2018. doi: 10.3748/wjg.v24.i1.46
Peer-review started: September 30, 2017
First decision: October 17, 2017
Revised: November 8, 2017
Accepted: November 21, 2017
Article in press: November 21, 2017
Published online: January 7, 2018
To measure the leptin levels in patients with diarrhea-predominant irritable bowel syndrome (IBS-D) and analyze the relationship of leptin with clinical features, visceral sensitivity, mast cells, and nerve fibers.
Forty-two patients with IBS-D fulfilling the Rome III criteria and 20 age- and sex-matched healthy controls underwent clinical and psychological evaluations using validated questionnaires (including IBS Symptom Severity Scale, IBS-specific Quality of Life, Hamilton Anxiety Scale, and Hamilton Depression Scale), along with colonoscopy, colonic mucosal biopsy, and visceral sensitivity testing. Serum leptin levels were assayed using enzyme-linked immunosorbent assay. Mucosal leptin expression and localization were evaluated using immunohistochemistry and immunofluorescence. Mucosal leptin mRNA levels were quantified using quantitative real-time reverse transcription polymerase chain reaction. Mast cell counts and activation rates were investigated by toluidine blue staining. Correlation analyses between these parameters were performed.
There were no statistically significant differences in age, gender, or body mass index between the IBS-D group and the control group. The median IBS Symptom Severity Scale score in the IBS-D group was 225.0 (range, 100-475). IBS-D patients had significantly increased anxiety [IBS-D: median, 6.5; interquartile range (IQR), 3.3; control: median, 2.0; IQR, 2.0; P < 0.001] and depression (IBS-D: median, 7.0; IQR, 3.0; control: median, 3.0; IQR, 2.0; P < 0.001) scores. IBS-D patients had significantly lower first sensation threshold (IBS-D: median, 50.6; IQR, 25.9; control: median, 80.5; IQR, 18.6; P < 0.001), defecation sensation threshold (IBS-D: median, 91.5; IQR, 29.3; control: median, 155.0; IQR, 21.1; P < 0.001) and maximum tolerable threshold (IBS-D: median, 163.2; IQR, 71.2; control: median, 226.2; IQR, 39.3; P < 0.001). Mucosal leptin expression, as reflected by integrated optical density (IBS-D: median, 4424.71; IQR, 4533.63; control: median, 933.65; IQR, 888.10; P < 0.001), leptin mRNA expression (IBS-D: median, 1.1226; IQR, 1.6351; control: median, 0.8947; IQR, 0.4595; P = 0.009), and mast cell activation rate (IBS-D: median, 71.2%; IQR, 12.9%; control group: median, 59.4%; IQR, 18.88%; P < 0.001) were significantly increased in IBS-D patients. The colocalization of leptin and leptin receptors was observed on mast cells and PGP9.5-positive nerve fibers in the intestinal mucosa. Also, leptin expression was positively correlated with anxiety, depression, and the mast cell activation rate, but negatively correlated with the defecation sensation threshold and the maximum tolerance threshold during visceral sensitivity testing (adjusted P < 0.0038).
Increased levels of mucosal leptin may interact with mast cells and the nervous system to contribute to the pathogenesis of IBS-D.
Core tip: Leptin is an important cytokine that exerts significant biological effects on gastrointestinal function and immune system modulation. We found that diarrhea-predominant irritable bowel syndrome (IBS-D) patients had significantly increased psychological symptoms, visceral hypersensitivity, mucosal leptin expression, leptin mRNA expression, and mast cell activation rate. Additionally, leptin expression was positively correlated with anxiety, depression, and the mast cell activation rate, but negatively correlated with the defecation sensation threshold and maximum tolerance threshold during visceral sensitivity testing. Increased levels of mucosal leptin may interact with mast cells and the nervous system to contribute to the pathogenesis of IBS-D.
- Citation: Liu DR, Xu XJ, Yao SK. Increased intestinal mucosal leptin levels in patients with diarrhea-predominant irritable bowel syndrome. World J Gastroenterol 2018; 24(1): 46-57
- URL: https://www.wjgnet.com/1007-9327/full/v24/i1/46.htm
- DOI: https://dx.doi.org/10.3748/wjg.v24.i1.46
Irritable bowel syndrome (IBS) is a commonly diagnosed functional gastrointestinal disease, with a prevalence rate of 5%-10% in most European countries, the United States, and China[1]. IBS is a typically recurrent disease characterized by abdominal pain or discomfort, stool irregularities, and bloating[2]. Symptoms may worsen over time and significantly impact patients’ quality of life and work productivity[3]. A major subtype of IBS is IBS with diarrhea (IBS-D), which accounts for 46% of all IBS cases[4].
The pathogenesis of IBS-D is complex and poorly understood. Recent studies of the pathophysiology of IBS-D have focused on molecular mechanisms and have suggested that the levels of luminal and mucosal cytokines originating from the gastrointestinal tract may be altered in the gut of IBS-D patients. These alterations could result in dysregulation of gastrointestinal secretion and motility and an increase in visceral hypersensitivity. These cytokines can also affect the peripheral and central nervous systems and disrupt communication between the brain and gut[5].
Leptin is a 16 kDa non-glycosylated peptide hormone belonging to the type I cytokine superfamily[6]. It is produced predominantly by mature adipocytes, but also by gastric and colonic epithelial cells, immune cells, placental trophoblasts, amniotic cells, chondrocytes, and synoviocytes[7]. When bound to receptor sites, leptin not only exerts significant biological effects, such as appetite control, by signaling satiety and increasing energy expenditure, but also modulates the immune system[8] and gastrointestinal function[9,10].
The number of mucosal mast cells has been reported to be increased in IBS-D patients[11], and mast cell number often correlates with symptoms including abdominal pain[12] and bloating[13]. Mast cells are key to the induction and maintenance of low-grade immune activation in IBS-D patients[14]. Gastrointestinal mast cells can express leptin and leptin receptors, indicating the modulatory effects of leptin on mast cells[15].
The enteric nervous system (ENS) is important in regulating gut motility, secretion, and nutrient absorption. Leptin has been shown to affect the ENS[16,17] and sensory afferent neurons[18]. Taken together, leptin may be involved in the regulation of both local gastrointestinal functions and the brain-gut axis.
Few studies have specifically addressed the role of leptin in the pathogenesis of IBS[19-21]. Therefore, the present study measured leptin expression in both the serum and intestinal mucosa of patients with IBS-D, and then analyzed the relationship of leptin with the clinical features, visceral sensitivity, the number and activation rate of mast cells, and nerve fibers in these patients
We recruited 42 IBS-D patients (15 women and 27 men; mean age, 29.4 years; age range, 22-40 years) and 20 healthy controls (8 women and 12 men; mean age, 28.9 years; age range, 20-38 years). All patients were treated at the gastroenterology department of the China-Japan Friendship Hospital from January 2016 to July 2016. IBS-D was diagnosed according to the Rome III criteria. Controls were recruited through public advertisement or from asymptomatic patients undergoing colonoscopy for colorectal cancer screening or polyposis follow-up. Exclusion criteria included the use of anti-spasmodics, analgesics, antibiotics, nonsteroidal anti-inflammatory drugs, corticosteroids, mast cell stabilizers, and anti-depressants or a history of organic diseases (documented by patient history, appropriate consultation, and laboratory testing), celiac disease (documented by celiac serology), allergic disease, major abdominal surgery, and severe psychiatric disorders.
All participants underwent venous blood sampling and colonoscopy. Before colonoscopy, all subjects underwent a standard bowel preparation in the form of 3 L of dissolved polyethylene glycol electrolyte powder (Fortrans, Beaufour Ipsen Industrie, Dreux, France). Four biopsies were obtained in each subject from the rectosigmoid junction in order to standardize the site of sampling. Two biopsy specimens were used for routine hematoxylin and eosin (H and E) staining, mast cell staining, and immunohistochemistry. The other two biopsy specimens were used for quantitative real-time reverse transcription polymerase chain reaction (qRT-PCR).
IBS-D patients and control patients both gave informed consent in a written form before entry into the study. The study protocol was approved by the China-Japan Friendship Hospital Ethics Committee (No. 2015-33) and the study was conducted in accordance with the Declaration of Helsinki.
Questionnaires: Clinical status was evaluated with the use of validated questionnaires. The IBS Symptom Severity Scale[22,23], a 5-item self-reporting questionnaire designed to measure disease severity, was given to each participant to complete. Questionnaire items included information regarding abdominal pain, bloating, satisfaction with bowel habits, and overall interference with quality of life. The total score of the questionnaire ranges from 0 to 500.
The IBS-specific Quality of Life questionnaire[24,25] was used to evaluate changes in patients’ quality of life. This scale evaluates 34 broad well-being factors based on variables including feelings of dysphoria, social interactions, body image, and health worries. The Hamilton Anxiety Scale[26,27] and Hamilton Depression Scale[27,28] were used to measure anxiety and depression, respectively.
Visceral sensitivity testing: Participants were placed in the left lateral decubitus position. After a digital rectal examination, a lubricated catheter with a latex balloon at the tip (Medical Measurement Systems, Enschede, the Netherlands) was inserted into the rectum such that the balloon was 8 cm proximal to the anal verge. Then, the balloon was inflated at a speed of 10 mL/5 s. During balloon inflation, feelings of initial perception, defecation urge, and discomfort/pain were reported by the participants. The balloon volumes were recorded at the first sensation threshold, defecating sensation threshold, and maximum tolerable threshold. All the tests were performed by the same investigator between 1 and 3 PM in a blinded fashion.
Serum leptin level: All fasting blood samples from both IBS-D patients and controls were collected between 11 AM and 1 PM to avoid diurnal variation. Whole blood was centrifuged immediately at 1151 g for 10 min, and stored at -80 °C until assay. Serum leptin levels were measured in duplicate using commercially available enzyme-linked immunosorbent assay (ELISA) kits (Human Leptin Instant ELISA; Bender MedSystems GmbH, Vienna, Austria).
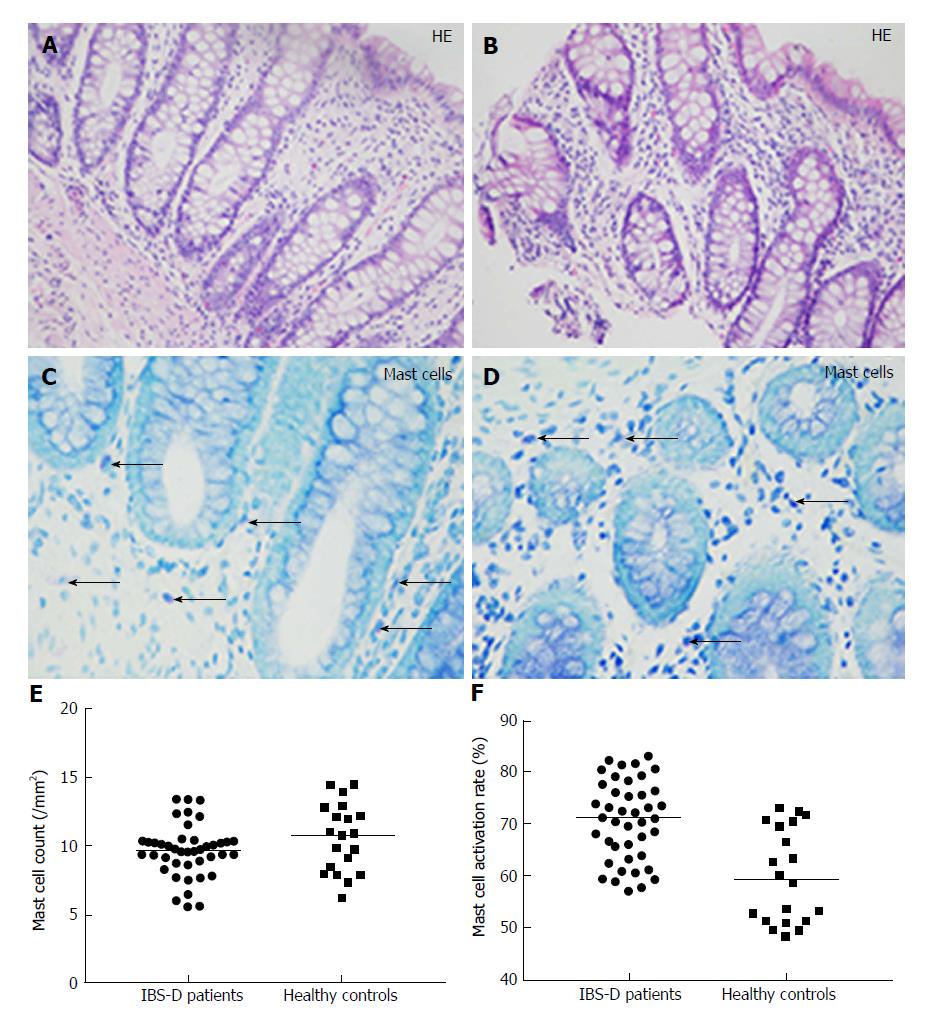
Histology, immunohistochemistry, and immunofluorescence: H and E sections from formalin-fixed and paraffin-embedded tissue samples were blindly assessed by independent observers. Mast cells were stained with toluidine blue. The slides were first soaked in 0.5% toluidine blue, and then differentiated with acetone. Afterwards, five 400 × magnification fields (field area, 0.237 mm2) were chosen randomly and scanned. Mast cells were identified using light microscopy by their metachromatic cytoplasmic granules. Finally, mast cell degranulation was assessed based on unclear or irregular cell membranes and the presence of extruded secretory granules. The mean mast cell number per millimetre square of the mucosal area (/mm2) was calculated. The percentage of degranulated mast cells (mast cell activation rate) was also calculated in each section (degranulated mast cells/the total number of mast cells × 100%).
Immunohistochemistry and immunofluorescence experiments were performed on paraffin-embedded, 4-mm-thick sections. The following primary antibodies were used: rabbit polyclonal anti-leptin antibody (1:100; Abcam, Cambridge, United Kingdom), rabbit polyclonal anti-leptin receptor antibody (1:50; Abcam, Cambridge, United Kingdom), mouse monoclonal anti-mast cell tryptase antibody (1:50; Abcam, Cambridge, United Kingdom), and mouse monoclonal anti-PGP9.5 antibody (1:50; Abcam, Cambridge, United Kingdom).
For immunohistochemistry studies, the sections were first incubated with the primary antibody overnight. Next, they were incubated at room temperature with a universal secondary antibody (EnVision Detection Systems, Dako, Denmark) for 60 min and then visualized using diaminobenzidine. Finally, the nuclei were labeled by counterstaining the sections with Mayer’s hematoxylin. The quantification of immunoreactivity was performed by two operators in a blinded fashion. Three randomly selected fields from each section were scanned under a Nikon Eclipse 80i microscope (Nikon Instruments Co., Ltd., Tokyo, Japan). Photographs were taken with a Nikon DS-Ri1 camera (Nikon Instruments Co., Ltd., Tokyo, Japan). The images were analyzed with Image-Pro Plus 6.0 software (Media Cybernetics, Bethesda, MD, United States). During the analysis, the average integrated option density (IOD) of positive staining substances of three non-overlapping fields chosen randomly was measured for the final analysis.
For immunofluorescence studies, the sections were prepared sequentially as follows: incubated with primary antibody (double labeling) overnight, rinsed with phosphate-buffered saline, and incubated at room temperature with goat anti-mouse IgG H and L (Alexa Fluor 647) (1/300; Abcam, Cambridge, United Kingdom) or goat anti-rabbit IgG H and L (Alexa Fluor® 488) (1/400; Abcam, Cambridge, United Kingdom) for 2 h. Specimens were examined by two operators in a blinded fashion using a Nikon Eclipse 90i microscope (Nikon Instruments Co., Ltd., Tokyo, Japan). Representative photomicrographs were taken using a Nikon DS-Ri1 camera.
Leptin gene expression analysis using qRT-PCR: The gene expression of leptin and the leptin receptor in the colonic mucosa was analyzed using qRT-PCR. Each supernatant was assayed in duplicate, and altogether three independent experiments were conducted. TRIzol reagent (Invitrogen Life Technologies, Waltham, MA, United States) was used to isolate total RNA. Then, the RevertAid First Strand cDNA Synthesis Kit (Thermo Scientific, Waltham, MA, United States) was used to perform reverse transcription. Next, real-time PCR was carried out using the FastStart Universal SYBR Green Master Rox kit (Roche, Shanghai, China) in the StepOnePlus Real-Time PCR System (Applied Biosystems, Waltham, MA, United States). Finally, leptin and leptin receptor mRNA expression was normalized to glyceraldehyde 3-phosphate dehydrogenase (GAPDH) expression. The following primers were used for PCR amplification: forward, 5’-ACTTTGGTATCGTGGAAGGACTCAT-3’ and reverse, 5’-GTTTTTCTAGACGGCAGGTCAGG-3’ for GAPDH; forward, 5’-ACACGCAGTCAGTCTCCTCCAA-3’ and reverse, 5’-CTGGAAGGCATACTGGTGAGGA-3’ for leptin; and forward, 5’-GCCAACAGCCAAACTCAACG-3’ and reverse, 5’-GGTGGGCTGGACCAAGAAATC-3’ for leptin receptor. The results were first normalized to GAPDH expression using the 2-ΔΔCt method[29]. Then, the leptin and leptin receptor mRNA levels in each sample are expressed as the fold-change relative to the average level of healthy controls, in whom the mean value was used as the reference and considered as 1.
All statistical analyses were performed using SPSS for Windows software, version 24.0 (SPSS Inc, Chicago, IL). All data are reported as values (mean ± SD) or medians [interquartile range (IQR)]. Independent sample t-tests or nonparametric Mann-Whitney U-tests were used to analyze quantitative data. The χ2 test or Fisher’s exact test were used to analyze qualitative data. Correlations between two parameters were performed using Spearman’s correlation coefficient, followed by Bonferroni correction to adjust multiple comparisons, with a corrected significance level of 0.0038 (0.05/13). Two-tailed P-values < 0.05 were considered significant.
There were no statistically significant differences in age, gender, or body mass index between the IBS-D group and the control group. The median IBS Symptom Severity Scale score in the IBS-D group was 225.0 (range, 100-475). There was no significant difference between male and female IBS-D patients in terms of IBS Symptom Severity Scale score (P = 0.053). The median duration of disease was 3.5 years (range, 1-13 years) in the IBS-D patients. The IBS-specific Quality of Life score was significantly lower in IBS-D patients (P < 0.001), and the differences in the scores of both the Hamilton Anxiety Scale and the Hamilton Depression Scale were also statistically significant between the IBS-D group and the control group (P < 0.001) (Table 1).
| IBS-D | Controls | P value | |
| Demographic and clinical feature | |||
| N | 42 | 20 | NA |
| Age (yr) | 29.4 ± 4.3 | 28.9 ± 5.1 | 0.673 |
| Gender (male: female) | 9:5 | 3:2 | 0.744 |
| Body mass index (kg/m2) | 22.5 ± 2.5 | 22.1 ± 2.2 | 0.553 |
| Duration of disease (yr) | 3.5 (3.3) | NA | NA |
| Questionnaire | |||
| IBS Symptom Severity Scale | 225.0 (50.0) | NA | NA |
| IBS-specific Quality of Life | 76.5 (17.3) | 100.0 (1.1) | < 0.001 |
| Hamilton Anxiety Scale | 6.5 (3.3) | 2.0 (2.0) | < 0.001 |
| Hamilton Depression Scale | 7.0 (3.0) | 3.0 (2.0) | < 0.001 |
| Visceral sensitivity | |||
| First sensation threshold (mL) | 50.6 (25.9) | 80.5 (18.6) | < 0.001 |
| Defecating sensation threshold (mL) | 91.5 (29.3) | 155.0 (21.1) | < 0.001 |
| Maximum sensation threshold (mL) | 163.2 (71.2) | 226.2 (39.3) | < 0.001 |
The first sensation threshold, defecation sensation threshold, and maximum tolerable threshold were significantly lower in the IBS-D patients compared with those in the control group (P < 0.001) (Table 1).
Serum leptin levels in the IBS-D patients (median, 1.28 ng/mL; IQR, 1.63 ng/mL) and healthy controls (median, 1.27 ng/mL; IQR, 0.83 ng/mL) were not significantly different (P = 0.657).
On H and E histology, all colonic mucosal biopsies were reported as normal (Figure 1A and B). Toluidine blue staining revealed that mast cells were often scattered in the lamina propria and submucosa (Figures 1C and D). The mast cell count was not significantly different between the two groups (IBS group: median, 9.62/mm2; IQR, 1.61/mm2vs control group: median, 10.75/mm2; IQR, 4.52/mm2; P = 0.164) (Figure 1E). However, the percentage of degranulated mast cells was significantly increased in IBS-D patients (IBS group: median, 71.2%; IQR, 12.9% vs control group: median, 59.4%; IQR, 18.88%; P < 0.001) (Figure 1F).
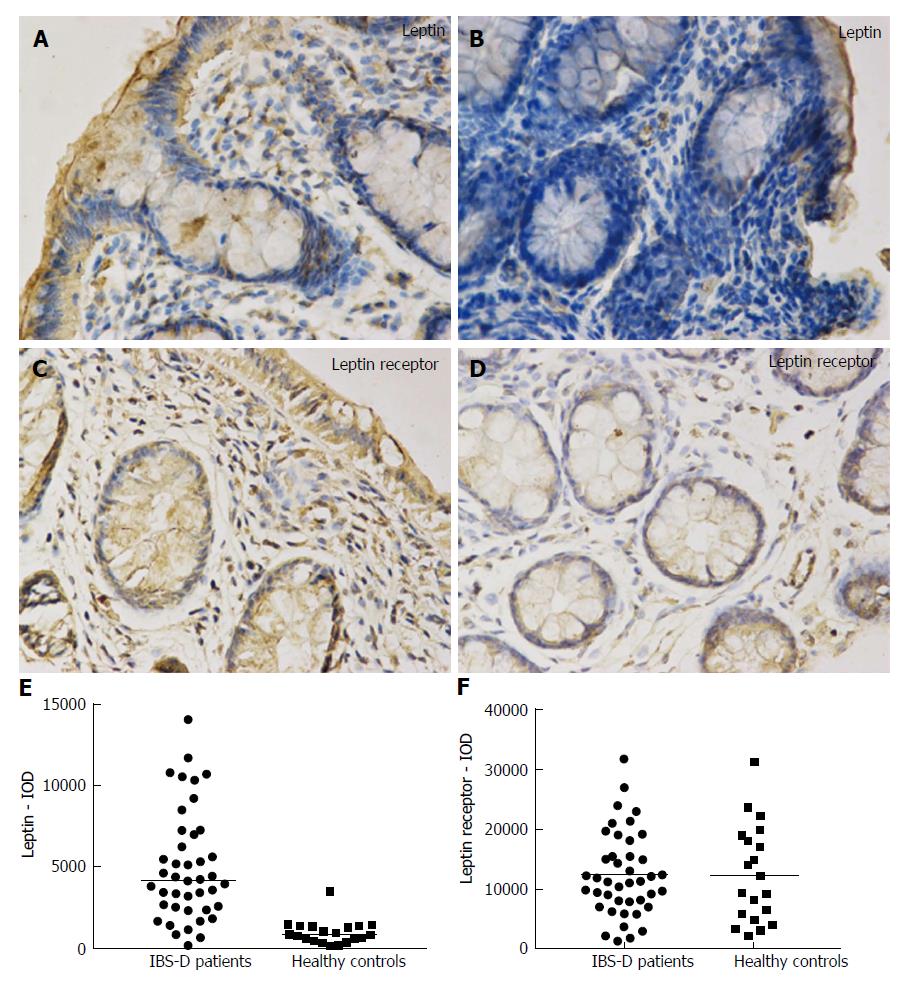
In the colonic mucosa, leptin and leptin receptor staining was mostly scattered in the epithelium and lamina propria (Figure 2A-D). The leptin protein level in mucosal biopsies was significantly increased in the IBS-D patients (IOD median, 4424.71; IQR, 4533.63) vs the control group (IOD median, 933.65; IQR, 888.10; P < 0.001) (Figure 2E). The leptin receptor expression was not significantly increased in the colonic mucosa obtained from IBS-D patients (12454.27 ± 6946.06) compared to controls (12508.07 ± 8088.46) (P = 0.979) (Figure 2F).
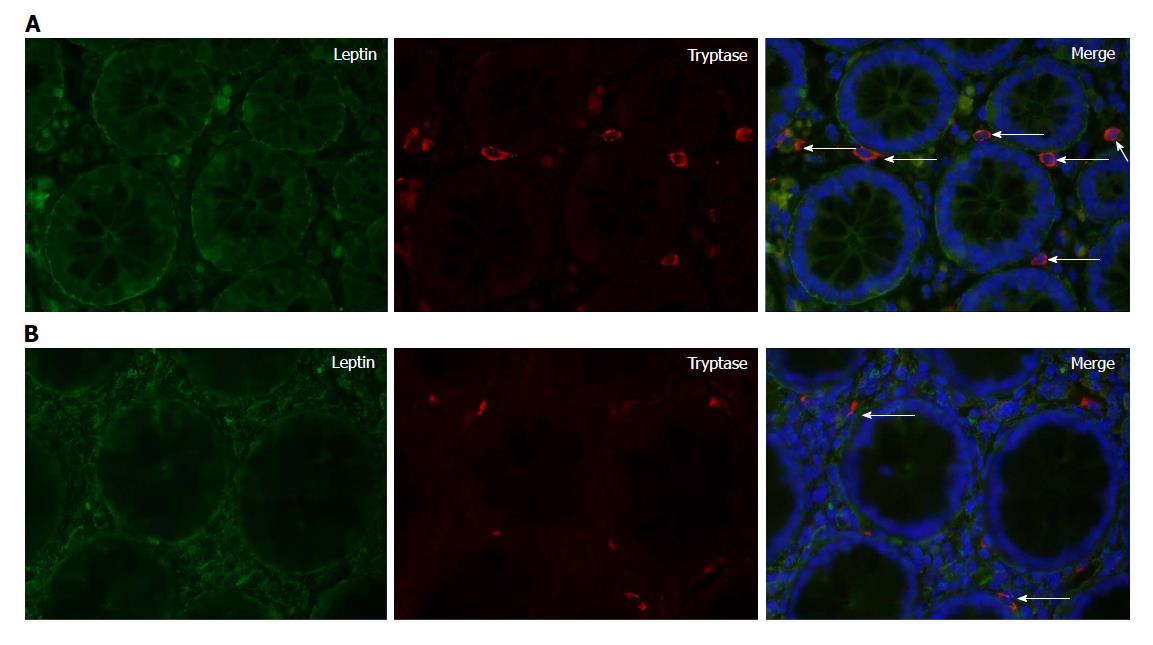
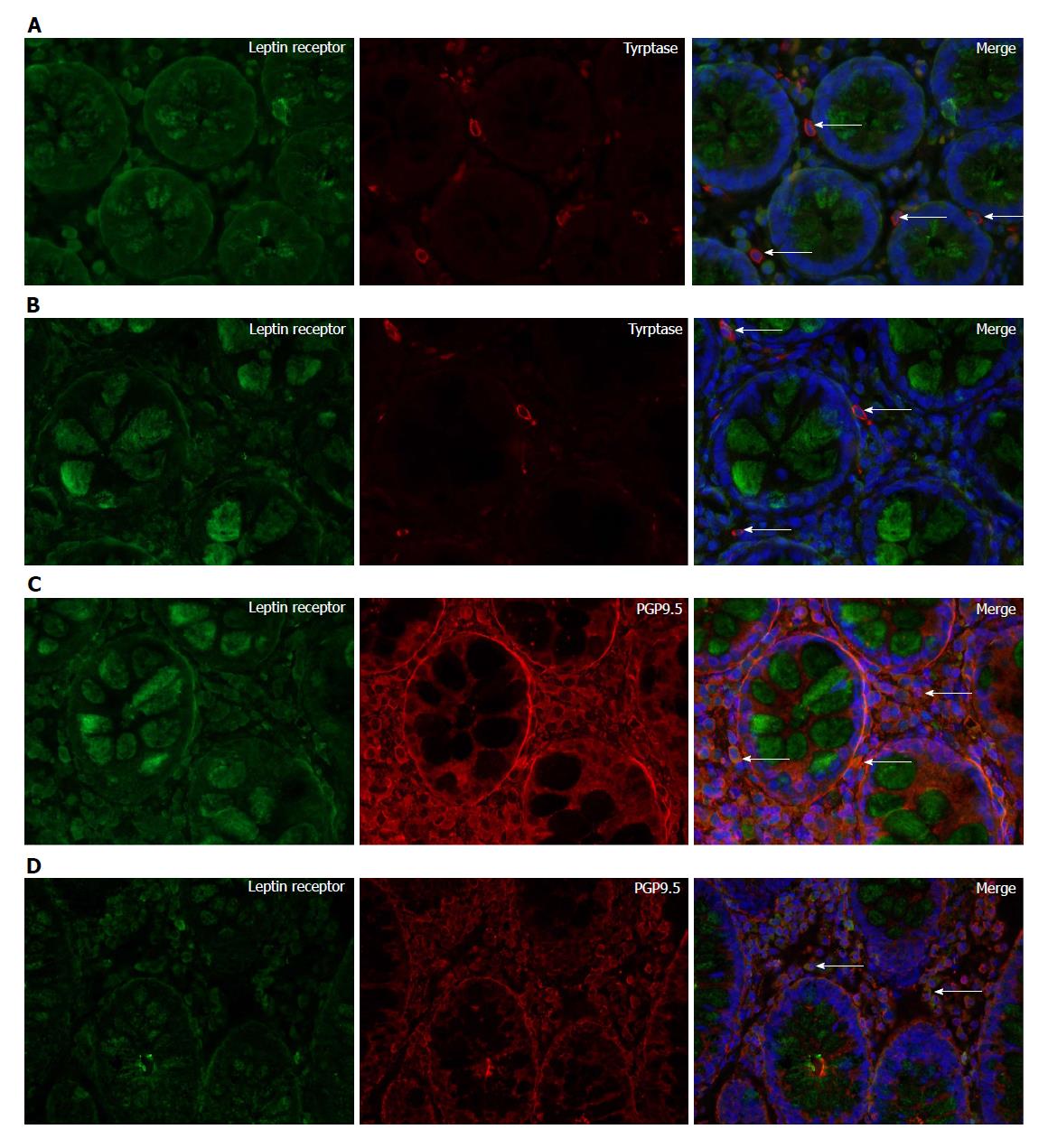
Leptin and tryptase double-labeling immunofluorescence experiments recorded colocalization of leptin and tryptase, indicating that mast cells were producers of leptin (Figure 3A and B). Moreover, leptin receptor and tryptase colocalization was found to exist in the colonic mucosa (Figure 4A and B). Double-staining experiments confirmed leptin receptor expression in PGP9.5-immunoreactive nerve fibers (Figure 4C and D).
Leptin mRNA expression was significantly increased in IBS-D patients (median, 1.1226; IQR, 1.6351) vs that seen in the control group (median, 0.8947; IQR, 0.4595; P = 0.009). Similarly, leptin receptor gene expression was also significantly increased in IBS-D patients (median, 1.5491; IQR, 2.0721) vs that seen in the control group (median, 0.9062; IQR, 0.3850; P = 0.001).
In patients with IBS-D, the leptin protein expression level showed a significantly positive correlation with anxiety, depression, and mast cell activation rate, and a negative correlation with the defecation sensation threshold and maximum tolerable threshold (adjusted P < 0.0038). The leptin mRNA level was significantly positively correlated with IBS symptom severity, anxiety, and depression, and negatively correlated with the visceral sensation threshold (adjusted P < 0.0038) (Table 2).
| Leptin expression | Leptin mRNA | |
| Questionnaire | ||
| IBS-SSS | 0.417 (0.006)1 | 0.678 (< 0.001) |
| HAMA | 0.540 (< 0.001) | 0.675 (< 0.001) |
| HAMD | 0.488 (0.001) | 0.589 (< 0.001) |
| Visceral sensitivity | ||
| First sensation | -0.438 (0.004)1 | -0.604 (< 0.001) |
| Defecating sensation | -0.654 (< 0.001) | -0.480 (0.001) |
| Maximum tolerance | -0.576 (< 0.001) | -0.706 (< 0.001) |
| Mucosal parameter | ||
| Mast cell activation rate (%) | 0.510 (0.001) | 0.430 (0.005)1 |
| Leptin mRNA | 0.666 (< 0.001) | NA |
| Leptin expression | NA | 0.666 (< 0.001) |
The present study investigated the possible role of leptin in the pathogenesis of IBS-D. Biopsies from IBS-D patients revealed significant upregulation of both leptin protein and mRNA levels. The increased leptin protein or mRNA expression closely correlated with IBS symptom severity, anxiety, depression, and visceral sensation thresholds, which may provide a putative basis for the development of IBS symptoms. To our knowledge, this was the first study to preliminarily confirm that leptin may play a certain role in the pathophysiology of IBS-D.
Visceral hypersensitivity has been identified in 20%-90% of IBS patients and is defined as a low threshold for the perception of stimuli arising from the gut[30,31]. Mechanisms of visceral hypersensitivity are complex, including the sensitization of gut wall sensory endings, enhanced nociceptive information, reduced antinociceptive effects at the level of the spinal cord, and hypervigilance of the central nervous system[31]. In line with these mechanisms, the present study found that visceral sensation thresholds were significantly lower in IBS-D patients than those of healthy controls. Limitations regarding the method of rectal distention used were discussed in our previous study[32] and include: (1) the sensory threshold, reported by the subject, may be susceptible to a perceptual response bias[33]; (2) progressive rectal distention may lead to hypervigilance and alter the response of the participants; and (3) volume measurements, rather than balloon pressures recorded with a barostat, may not accurately reflect the biological properties of the rectum, leading to measurement errors[34]. In spite of these limitations, this method is convenient to perform and well-tolerated by patients. Furthermore, some studies have shown that volume thresholds are a valid measure of rectal sensory function[35-38].
Mast cell proliferation has been recognized as a common feature of IBS patients[39-41]. Some studies have suggested that the number of mast cells is increased in IBS patients[42,43], while other studies have shown that the density of mast cells is not significantly different in these patients[44-46]. Additional studies have shown that the degranulation or activation rate of mast cells, rather than the number of mast cells, best reflects the severity of IBS in patients[47]. In IBS patients, activated mast cells release mediators such as histamine, serotonin, and bradykinin[41]. These mediators contribute to the development of major IBS-D symptoms through the modulation of visceral sensation, gastrointestinal motility, intestinal immune function, and epithelial permeability and secretion[41]. In the present study, the mast cell activation rate was found to be significantly higher in IBS-D patients, while the mast cell count was not significantly different from that seen in the control group, indicating that activated mast cells are important in the pathophysiology of IBS-D.
Few studies have specifically analyzed serum leptin levels in IBS patients[19-21], and the results of these studies have been inconsistent. In the present study, serum leptin levels in patients with IBS-D were not significantly different from those seen in healthy controls. However, both leptin and leptin receptor mRNA levels were significantly increased in IBS-D patients compared to the control group. Complex factors influencing serum leptin levels and the relatively small sample size may have contributed to the lack of a difference in serum leptin levels.
The potential role of immune activation in the pathogenesis and symptom formation in IBS patients has been reported in previous studies[14,48,49]. Mast cells are a key component in the induction and maintenance of low-grade immune activation in IBS patients[48]. A recent report has demonstrated that leptin, a regulatory hormone that has multiple modulatory effects on immune cells, including T cells, macrophages, mast cells, dendritic cells, neutrophils, eosinophils, basophils, and NK cells, was able to promote the generation of an inflammatory phenotype of mast cells through the induction of interferon-γ and the suppression of interleukin-4[50]. In animal experiments, leptin has been proven to be effective in activating the colonic submucosal and myenteric neurons of guinea pigs[16] and in modulating the activity of enteric inhibitory and excitatory neurons in the proximal colon of rats[17]. It has also been reported that leptin can stimulate the activity of sensory afferent neurons[18]. In this study, the expression and location of leptin, leptin receptors, tryptase, and PGP9.5 were analyzed by immunohistochemistry and immunofluorescence. We found that the expression of leptin, rather than the leptin receptor, increased significantly. In addition, the colocalization of leptin and leptin receptors was observed on mast cells and PGP9.5-positive nerve fibers in the intestinal mucosa. These results indicate that leptin may be involved in the pathogenesis of IBS-D via mechanisms similar to those noted in the above studies.
Correlation analyses were performed between intestinal mucosa leptin levels and other parameters. Leptin expression was positively correlated with anxiety, depression, and the mast cell activation rate, and was negatively correlated with the defecation sensation threshold and the maximum tolerable threshold during visceral sensitivity testing. Leptin mRNA levels were positively correlated with disease severity, anxiety, and depression, and negatively correlated with the visceral sensation threshold. The correlation analyses indicate that leptin might be an important factor involved in the pathogenesis and symptom formation of IBS-D patients. However, correlations cannot be interpreted as causal associations, and further studies are needed to confirm this finding.
Based on the results of our study, we speculate that psychosocial and other stimulations cause disruption in the brain-gut axis[51]. Corticotrophin-releasing hormone (CRH) and cortisol are released with activation of the hypothalamic-pituitary axis[51]. CRH and cortisol are also increased with the production of leptin[51,52]. Also, intestinal mucosal mast cells produce histamine, serotonin, and bradykinin when activated. As a key component of low-grade mucosal inflammation, mast cells also cause increased epithelial permeability, visceral hypersensitivity, and a disruption of gastrointestinal secretory and motility functions. Leptin-stimulated mast cells modulate low-grade mucosal inflammation, activate nervous system, and increase epithelial tight junction permeability[53], all of which contribute to the development of IBS-D symptoms.
There were several limitations in this study. First, because of the nature of our research foundation[32,54], the present study focused on patients with the IBS-D subtype. Therefore, the findings may not be generalizable to constipation-predominant IBS, mixed-type IBS, and unsubtyped IBS patients. Second, leptin has been shown to increase during the process of inflammation[55]. However, we cannot be sure that all postinfective IBS patients were excluded from the study. Because these two subgroups may be different in pathogenesis, future experiments should study these two types separately. Third, due to the lack of previous data related to our study, we did not estimate sample size and power, which may reduce the reliability of our conclusion. Fourth, in qRT-PCR, we used only one reference gene (GAPDH), which may influence the accuracy of the results. Finally, we cannot make a cause and effect inference on the basis of our findings; additional studies of the effects of leptin on mast cells and the nervous system as well as larger clinical studies are needed.
Irritable bowel syndrome (IBS) is a commonly diagnosed functional gastrointestinal disease. Symptoms may worsen over time and significantly impact patient quality of life and work productivity. A major subtype of IBS is IBS with diarrhea (IBS-D). The pathogenesis of IBS-D is complex and poorly understood. Leptin not only exerts significant biological effects, such as appetite control, by signaling satiety and increasing energy expenditure, but also modulates the immune system and gastrointestinal function. Few studies have specifically addressed the role of leptin in the pathogenesis of IBS. To our knowledge, this was the first study to preliminarily investigate the possible role of leptin in the pathophysiology of IBS-D.
The main topics of the present study include evaluating clinical symptoms, psychological characteristics, and visceral sensitivity of IBS-D patients and healthy controls; examining leptin expression, mast cells, and PGP9.5 nerve fibres in IBS-D and healthy controls; and performing correlation analyses between these parameters. Our study proposed a mechanism by which leptin may contribute to the pathophysiology of IBS.
This study aimed to measure leptin expression in both the serum and intestinal mucosa of patients with IBS-D subtype disease and to analyze the relationship of leptin with the clinical features, visceral sensitivity, and number of mast cells and nerve fibers in these patients.
Participants underwent clinical and psychological evaluations using validated questionnaires (including IBS Symptom Severity Scale, IBS-specific Quality of Life, Hamilton Anxiety Scale and Hamilton Depression Scale), along with colonoscopy, colonic mucosal biopsy, and visceral sensitivity testing. Serum leptin levels were assayed using enzyme-linked immunosorbent assay. Mucosal leptin expression and localization were evaluated using immunohistochemistry and immunofluorescence. Mucosal leptin mRNA levels were quantified using quantitative real-time reverse transcription polymerase chain reaction. Mast cell counts and activation rates were investigated by toluidine blue staining. Correlation analyses between these parameters were performed. All statistical analyses were performed using SPSS for Windows software, version 24.0 (SPSS Inc, Chicago, IL).
The authors found that IBS-D patients had significantly increased psychological symptoms and visceral hypersensitivity, and their mucosal leptin expression, leptin mRNA levels, and mast cell activation rates were significantly increased. Also, leptin expression was positively correlated with anxiety, depression, and the mast cell activation rate, but negatively correlated with the defecation sensation threshold and the maximum tolerance threshold during visceral sensitivity testing. Increased levels of mucosal leptin may interact with mast cells and the nervous system to contribute to the pathogenesis of IBS-D.
This study presents evidence that leptin levels are increased in the intestinal mucosa of IBS-D patients, proposes a mechanism by which leptin may contribute to the pathophysiology of IBS, and provides some potential avenues for more specific and effective treatments in these patients. The authors believe that this study makes a significant contribution to the literature because it is an original research that provides information that not only contributes to the understanding of the pathogenesis and pathophysiology of IBS, but also suggests area for future research and therapeutic intervention.
This study preliminarily investigated the possible role of leptin involved in the pathogenesis of IBS-D. Future studies should focus on the following aspects. First, the present study focused on patients with the IBS-D subtype. Therefore, the findings may not be generalizable to constipation-predominant IBS, mixed-type IBS, and unsubtyped IBS patients. Future research should investigate the possible role of leptin in the pathophysiology of the other three subtypes. Second, leptin has been shown to increase during the process of inflammation. However, we cannot be sure that all postinfective IBS patients were excluded from the study. Because these two subgroups may be different in pathogenesis, future experiments should study these two types separately. Finally, we cannot make a cause and effect inference on the basis of our findings, therefore, additional studies of the effects of leptin on mast cells and the nervous system as well as larger clinical studies are needed.
We thank Dr. Li Y and Dr. Wang H for enrollment of participants, and Dr. Du S, Dr. Zhang M, Dr. Qin G and Dr. Liu F for assistance of colonoscopy and biopsy.
Manuscript source: Unsolicited manuscript
Specialty type: Gastroenterology and hepatology
Country of origin: China
Peer-review report classification
Grade A (Excellent): 0
Grade B (Very good): 0
Grade C (Good): C, C
Grade D (Fair): 0
Grade E (Poor): 0
P- Reviewer: De Winter BY, Silva LD S- Editor: Ma YJ L- Editor: Wang TQ E- Editor: Li D
| 1. | Lovell RM, Ford AC. Global prevalence of and risk factors for irritable bowel syndrome: a meta-analysis. Clin Gastroenterol Hepatol. 2012;10:712-721.e4. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 1251] [Cited by in F6Publishing: 1292] [Article Influence: 107.7] [Reference Citation Analysis (1)] |
| 2. | Enck P, Aziz Q, Barbara G, Farmer AD, Fukudo S, Mayer EA, Niesler B, Quigley EM, Rajilić-Stojanović M, Schemann M. Irritable bowel syndrome. Nat Rev Dis Primers. 2016;2:16014. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 601] [Cited by in F6Publishing: 565] [Article Influence: 70.6] [Reference Citation Analysis (0)] |
| 3. | Spiegel BM. The burden of IBS: looking at metrics. Curr Gastroenterol Rep. 2009;11:265-269. [PubMed] [Cited in This Article: ] |
| 4. | Kibune Nagasako C, Garcia Montes C, Silva Lorena SL, Mesquita MA. Irritable bowel syndrome subtypes: Clinical and psychological features, body mass index and comorbidities. Rev Esp Enferm Dig. 2016;108:59-64. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 15] [Cited by in F6Publishing: 23] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 5. | O’Malley D. Neuroimmune Cross Talk in the Gut. Neuroendocrine and neuroimmune pathways contribute to the pathophysiology of irritable bowel syndrome. Am J Physiol Gastrointest Liver Physiol. 2016;311:G934-G941. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 17] [Cited by in F6Publishing: 17] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 6. | Barbier M, Vidal H, Desreumaux P, Dubuquoy L, Bourreille A, Colombel JF, Cherbut C, Galmiche JP. Overexpression of leptin mRNA in mesenteric adipose tissue in inflammatory bowel diseases. Gastroenterol Clin Biol. 2003;27:987-991. [PubMed] [Cited in This Article: ] |
| 7. | Fantuzzi G, Faggioni R. Leptin in the regulation of immunity, inflammation, and hematopoiesis. J Leukoc Biol. 2000;68:437-446. [PubMed] [Cited in This Article: ] |
| 8. | La Cava A, Matarese G. The weight of leptin in immunity. Nat Rev Immunol. 2004;4:371-379. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 598] [Cited by in F6Publishing: 599] [Article Influence: 30.0] [Reference Citation Analysis (0)] |
| 9. | Gallagher TK, Geoghegan JG, Baird AW, Winter DC. Implications of altered gastrointestinal motility in obesity. Obes Surg. 2007;17:1399-1407. [PubMed] [Cited in This Article: ] |
| 10. | Kiely JM, Noh JH, Graewin SJ, Pitt HA, Swartz-Basile DA. Altered intestinal motility in leptin-deficient obese mice. J Surg Res. 2005;124:98-103. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 24] [Cited by in F6Publishing: 26] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 11. | Guilarte M, Santos J, de Torres I, Alonso C, Vicario M, Ramos L, Martínez C, Casellas F, Saperas E, Malagelada JR. Diarrhoea-predominant IBS patients show mast cell activation and hyperplasia in the jejunum. Gut. 2007;56:203-209. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 275] [Cited by in F6Publishing: 301] [Article Influence: 17.7] [Reference Citation Analysis (0)] |
| 12. | Barbara G, Stanghellini V, De Giorgio R, Cremon C, Cottrell GS, Santini D, Pasquinelli G, Morselli-Labate AM, Grady EF, Bunnett NW. Activated mast cells in proximity to colonic nerves correlate with abdominal pain in irritable bowel syndrome. Gastroenterology. 2004;126:693-702. [PubMed] [Cited in This Article: ] |
| 13. | Cremon C, Gargano L, Morselli-Labate AM, Santini D, Cogliandro RF, De Giorgio R, Stanghellini V, Corinaldesi R, Barbara G. Mucosal immune activation in irritable bowel syndrome: gender-dependence and association with digestive symptoms. Am J Gastroenterol. 2009;104:392-400. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 244] [Cited by in F6Publishing: 254] [Article Influence: 16.9] [Reference Citation Analysis (0)] |
| 14. | Barbara G, Cremon C, Carini G, Bellacosa L, Zecchi L, De Giorgio R, Corinaldesi R, Stanghellini V. The immune system in irritable bowel syndrome. J Neurogastroenterol Motil. 2011;17:349-359. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 137] [Cited by in F6Publishing: 135] [Article Influence: 10.4] [Reference Citation Analysis (0)] |
| 15. | Taildeman J, Pérez-Novo CA, Rottiers I, Ferdinande L, Waeytens A, De Colvenaer V, Bachert C, Demetter P, Waelput W, Braet K. Human mast cells express leptin and leptin receptors. Histochem Cell Biol. 2009;131:703-711. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 36] [Cited by in F6Publishing: 36] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 16. | Reichardt F, Krueger D, Schemann M. Leptin excites enteric neurons of guinea-pig submucous and myenteric plexus. Neurogastroenterol Motil. 2011;23:e165-e170. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 15] [Cited by in F6Publishing: 14] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 17. | Florian V, Caroline F, Francis C, Camille S, Fabielle A. Leptin modulates enteric neurotransmission in the rat proximal colon: an in vitro study. Regul Pept. 2013;185:73-78. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 12] [Cited by in F6Publishing: 12] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 18. | Dunn TN, Adams SH. Relations between metabolic homeostasis, diet, and peripheral afferent neuron biology. Adv Nutr. 2014;5:386-393. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 12] [Cited by in F6Publishing: 13] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 19. | Russo F, Chimienti G, Clemente C, D’Attoma B, Linsalata M, Orlando A, De Carne M, Cariola F, Semeraro FP, Pepe G. Adipokine profile in celiac patients: differences in comparison with patients suffering from diarrhea-predominant IBS and healthy subjects. Scand J Gastroenterol. 2013;48:1377-1385. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 24] [Cited by in F6Publishing: 23] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 20. | Semnani S, Roshandel G, Keshtkar A, Najafi L, Amiriani T, Farajollahi M, Moradi A, Joshaghani H. Serum leptin levels and irritable bowel syndrome: a new hypothesis. J Clin Gastroenterol. 2009;43:826-830. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 13] [Cited by in F6Publishing: 15] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 21. | Piche T, Huet PM, Gelsi E, Barjoan EM, Cherick F, Caroli-Bosc FX, Hébuterne X, Tran A. Fatigue in irritable bowel syndrome: characterization and putative role of leptin. Eur J Gastroenterol Hepatol. 2007;19:237-243. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 24] [Cited by in F6Publishing: 31] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 22. | Francis CY, Morris J, Whorwell PJ. The irritable bowel severity scoring system: a simple method of monitoring irritable bowel syndrome and its progress. Aliment Pharmacol Ther. 1997;11:395-402. [PubMed] [Cited in This Article: ] |
| 23. | Bao C, Zhang J, Liu J, Liu H, Wu L, Shi Y, Li J, Hu Z, Dong Y, Wang S. Moxibustion treatment for diarrhea-predominant irritable bowel syndrome: study protocol for a randomized controlled trial. BMC Complement Altern Med. 2016;16:408. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 16] [Cited by in F6Publishing: 15] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 24. | Schmulson M, Ortiz O, Mejia-Arangure JM, Hu YB, Morris C, Arcila D, Gutierrez-Reyes G, Bangdiwala S, Drossman DA. Further validation of the IBS-QOL: female Mexican IBS patients have poorer quality of life than females from North Carolina. Dig Dis Sci. 2007;52:2950-2955. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 24] [Cited by in F6Publishing: 24] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 25. | Tang YR, Yang WW, Liang ML, Xu XY, Wang MF, Lin L. Age-related symptom and life quality changes in women with irritable bowel syndrome. World J Gastroenterol. 2012;18:7175-7183. [PubMed] [DOI] [Cited in This Article: ] [Cited by in CrossRef: 40] [Cited by in F6Publishing: 35] [Article Influence: 2.9] [Reference Citation Analysis (0)] |
| 26. | HAMILTON M. The assessment of anxiety states by rating. Br J Med Psychol. 1959;32:50-55. [PubMed] [Cited in This Article: ] |
| 27. | Zhao JM, Wu LY, Liu HR, Hu HY, Wang JY, Huang RJ, Shi Y, Tao SP, Gao Q, Zhou CL. Factorial study of moxibustion in treatment of diarrhea-predominant irritable bowel syndrome. World J Gastroenterol. 2014;20:13563-13572. [PubMed] [DOI] [Cited in This Article: ] [Cited by in CrossRef: 15] [Cited by in F6Publishing: 16] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 28. | HAMILTON M. A rating scale for depression. J Neurol Neurosurg Psychiatry. 1960;23:56-62. [PubMed] [Cited in This Article: ] |
| 29. | Schmittgen TD, Livak KJ. Analyzing real-time PCR data by the comparative C(T) method. Nat Protoc. 2008;3:1101-1108. [PubMed] [Cited in This Article: ] |
| 30. | Azpiroz F, Bouin M, Camilleri M, Mayer EA, Poitras P, Serra J, Spiller RC. Mechanisms of hypersensitivity in IBS and functional disorders. Neurogastroenterol Motil. 2007;19:62-88. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 260] [Cited by in F6Publishing: 258] [Article Influence: 15.2] [Reference Citation Analysis (0)] |
| 31. | Barbara G, Cremon C, De Giorgio R, Dothel G, Zecchi L, Bellacosa L, Carini G, Stanghellini V, Corinaldesi R. Mechanisms underlying visceral hypersensitivity in irritable bowel syndrome. Curr Gastroenterol Rep. 2011;13:308-315. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 83] [Cited by in F6Publishing: 73] [Article Influence: 5.6] [Reference Citation Analysis (0)] |
| 32. | Xu XJ, Zhang YL, Liu L, Pan L, Yao SK. Increased expression of nerve growth factor correlates with visceral hypersensitivity and impaired gut barrier function in diarrhoea-predominant irritable bowel syndrome: a preliminary explorative study. Aliment Pharmacol Ther. 2017;45:100-114. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 49] [Cited by in F6Publishing: 48] [Article Influence: 6.9] [Reference Citation Analysis (0)] |
| 33. | Whitehead WE, Palsson OS. Is rectal pain sensitivity a biological marker for irritable bowel syndrome: psychological influences on pain perception. Gastroenterology. 1998;115:1263-1271. [PubMed] [Cited in This Article: ] |
| 34. | Whitehead WE, Delvaux M. Standardization of barostat procedures for testing smooth muscle tone and sensory thresholds in the gastrointestinal tract. The Working Team of Glaxo-Wellcome Research, UK. Dig Dis Sci. 1997;42:223-241. [PubMed] [Cited in This Article: ] |
| 35. | Sabate JM, Veyrac M, Mion F, Siproudhis L, Ducrotte P, Zerbib F, Grimaud JC, Dapoigny M, Dyard F, Coffin B. Relationship between rectal sensitivity, symptoms intensity and quality of life in patients with irritable bowel syndrome. Aliment Pharmacol Ther. 2008;28:484-490. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 39] [Cited by in F6Publishing: 34] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 36. | Klooker TK, Kuiken SD, Lei A, Boeckxstaens GE. Effect of long-term treatment with octreotide on rectal sensitivity in patients with non-constipated irritable bowel syndrome. Aliment Pharmacol Ther. 2007;26:605-615. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 22] [Cited by in F6Publishing: 22] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 37. | Hammer HF, Phillips SF, Camilleri M, Hanson RB. Rectal tone, distensibility, and perception: reproducibility and response to different distensions. Am J Physiol. 1998;274:G584-G590. [PubMed] [Cited in This Article: ] |
| 38. | Zhu Y, Zheng X, Cong Y, Chu H, Fried M, Dai N, Fox M. Bloating and distention in irritable bowel syndrome: the role of gas production and visceral sensation after lactose ingestion in a population with lactase deficiency. Am J Gastroenterol. 2013;108:1516-1525. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 88] [Cited by in F6Publishing: 90] [Article Influence: 8.2] [Reference Citation Analysis (0)] |
| 39. | Santos J, Guilarte M, Alonso C, Malagelada JR. Pathogenesis of irritable bowel syndrome: the mast cell connection. Scand J Gastroenterol. 2005;40:129-140. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 52] [Cited by in F6Publishing: 51] [Article Influence: 2.7] [Reference Citation Analysis (0)] |
| 40. | Wouters MM, Vicario M, Santos J. The role of mast cells in functional GI disorders. Gut. 2016;65:155-168. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 208] [Cited by in F6Publishing: 211] [Article Influence: 26.4] [Reference Citation Analysis (0)] |
| 41. | Zhang L, Song J, Hou X. Mast Cells and Irritable Bowel Syndrome: From the Bench to the Bedside. J Neurogastroenterol Motil. 2016;22:181-192. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 81] [Cited by in F6Publishing: 85] [Article Influence: 10.6] [Reference Citation Analysis (0)] |
| 42. | Philpott H, Gibson P, Thien F. Irritable bowel syndrome - An inflammatory disease involving mast cells. Asia Pac Allergy. 2011;1:36-42. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 42] [Cited by in F6Publishing: 51] [Article Influence: 3.9] [Reference Citation Analysis (0)] |
| 43. | Goral V, Kucukoner M, Buyukbayram H. Mast cells count and serum cytokine levels in patients with irritable bowel syndrome. Hepatogastroenterology. 2010;57:751-754. [PubMed] [Cited in This Article: ] |
| 44. | La JH, Kim TW, Sung TS, Kim HJ, Kim JY, Yang IS. Role of mucosal mast cells in visceral hypersensitivity in a rat model of irritable bowel syndrome. J Vet Sci. 2004;5:319-324. [PubMed] [Cited in This Article: ] |
| 45. | Celik AF, Demirkesen C, Pamuk ON, Pamuk GE, Uzunismail H. Mast cells: do they really have a role in disturbed bowel habits of IBS patients? Am J Gastroenterol. 2001;96:927-929. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 4] [Cited by in F6Publishing: 4] [Article Influence: 0.2] [Reference Citation Analysis (0)] |
| 46. | Theoharides TC, Asadi S, Chen J, Huizinga JD. Irritable bowel syndrome and the elusive mast cells. Am J Gastroenterol. 2012;107:727-729. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 13] [Cited by in F6Publishing: 13] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 47. | Theoharides TC. Mast cells in irritable bowel syndrome and ulcerative colitis: function not numbers is what makes all the difference. Dig Dis Sci. 2014;59:897-898. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 19] [Cited by in F6Publishing: 18] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 48. | Barbara G, Cremon C, Annese V, Basilisco G, Bazzoli F, Bellini M, Benedetti A, Benini L, Bossa F, Buldrini P. Randomised controlled trial of mesalazine in IBS. Gut. 2016;65:82-90. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 70] [Cited by in F6Publishing: 81] [Article Influence: 10.1] [Reference Citation Analysis (0)] |
| 49. | Lam C, Tan W, Leighton M, Hastings M, Lingaya M, Falcone Y, Zhou X, Xu L, Whorwell P, Walls AF. A mechanistic multicentre, parallel group, randomised placebo-controlled trial of mesalazine for the treatment of IBS with diarrhoea (IBS-D). Gut. 2016;65:91-99. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 61] [Cited by in F6Publishing: 69] [Article Influence: 8.6] [Reference Citation Analysis (0)] |
| 50. | Zhou Y, Yu X, Chen H, Sjöberg S, Roux J, Zhang L, Ivoulsou AH, Bensaid F, Liu CL, Liu J. Leptin Deficiency Shifts Mast Cells toward Anti-Inflammatory Actions and Protects Mice from Obesity and Diabetes by Polarizing M2 Macrophages. Cell Metab. 2015;22:1045-1058. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 99] [Cited by in F6Publishing: 97] [Article Influence: 10.8] [Reference Citation Analysis (0)] |
| 51. | Elsenbruch S. Abdominal pain in Irritable Bowel Syndrome: a review of putative psychological, neural and neuro-immune mechanisms. Brain Behav Immun. 2011;25:386-394. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 202] [Cited by in F6Publishing: 209] [Article Influence: 16.1] [Reference Citation Analysis (0)] |
| 52. | Hernández C, Simó R, Chacón P, Sabin P, Baena JA, Castellanos JM, Planas M. Influence of surgical stress and parenteral nutrition on serum leptin concentration. Clin Nutr. 2000;19:61-64. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 17] [Cited by in F6Publishing: 19] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 53. | Le Dréan G, Haure-Mirande V, Ferrier L, Bonnet C, Hulin P, de Coppet P, Segain JP. Visceral adipose tissue and leptin increase colonic epithelial tight junction permeability via a RhoA-ROCK-dependent pathway. FASEB J. 2014;28:1059-1070. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 41] [Cited by in F6Publishing: 45] [Article Influence: 4.1] [Reference Citation Analysis (0)] |
| 54. | Liu L, Liu BN, Chen S, Wang M, Liu Y, Zhang YL, Yao SK. Visceral and somatic hypersensitivity, autonomic cardiovascular dysfunction and low-grade inflammation in a subset of irritable bowel syndrome patients. J Zhejiang Univ Sci B. 2014;15:907-914. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 10] [Cited by in F6Publishing: 13] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 55. | Abella V, Scotece M, Conde J, Pino J, Gonzalez-Gay MA, Gómez-Reino JJ, Mera A, Lago F, Gómez R, Gualillo O. Leptin in the interplay of inflammation, metabolism and immune system disorders. Nat Rev Rheumatol. 2017;13:100-109. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 284] [Cited by in F6Publishing: 322] [Article Influence: 46.0] [Reference Citation Analysis (0)] |