Published online Mar 14, 2020. doi: 10.3748/wjg.v26.i10.1098
Peer-review started: October 4, 2019
First decision: November 4, 2019
Revised: January 12, 2020
Accepted: February 21, 2020
Article in press: February 21, 2020
Published online: March 14, 2020
There is conflincting evidence on the intravenous fluid (IVF) strategy for acute pancreatitis (AP). We perform a metaanalysis of the available evidence.
To investigate if aggressive IVF therapy in AP patients is beneficial to decrease mortality and improve outcomes.
Metaanalysis of available randomized controlled trials and cohort studies comparing aggressive IVF vs non-aggressive IVF resuscitation.
There was no significant difference in mortality between the aggressive (n = 1229) and non-aggressive IVF (n = 1397) patients. Patients receiving aggressive IVF therapy had higher risk for acute kidney injury and acute respiratory distress syndrome. There also was no significant difference in the overall incidence of systemic inflammatory response syndrome, persistent organ failure, pancreatic necrosis when comparing both study groups.
Early aggressive IVF therapy did not improve mortality. Moreover, aggressive IVF therapy could potentially increase the risk for acute kidney injury and pulmonary edema leading to respiratory failure and mechanical ventilation. Studies are needed to investigate which subset of AP patients could benefit from aggressive IVF therapy.
Core tip: Early aggressive intravenous fluid therapy did not improve mortality of acute pancreatitis patients and could potentially be harmful. The intravenous fluid therapy strategy in acute pancreatitis patients remains to be elucidated.
- Citation: Gad MM, Simons-Linares CR. Is aggressive intravenous fluid resuscitation beneficial in acute pancreatitis? A meta-analysis of randomized control trials and cohort studies. World J Gastroenterol 2020; 26(10): 1098-1106
- URL: https://www.wjgnet.com/1007-9327/full/v26/i10/1098.htm
- DOI: https://dx.doi.org/10.3748/wjg.v26.i10.1098
Acute pancreatitis (AP) is a common gastrointestinal disease that can lead to severe morbidity and mortality[1,2]. AP incidence has been increasing worldwide without an evident explanation[3]. AP is characterized by inflammation of the pancreas, and its natural disease can be categorized in two phases: The early phase that is accompanied with the systemic inflammatory response syndrome (SIRS) and usually last 1-2 wk; and the late phase refers for patients that suffer sequela of AP (fluid collections, infection). AP is classified in three subtypes, mild (usually interstitial), moderately–severe (transient organ failure) and severe (persistent organ failure). 80%-85% of cases are mild and typically interstitial, whereas 15%-20% are severe and/or necrotizing[4-6].
Intravenous fluid (IVF) resuscitation is one of the cornerstones for its management and is meant to counteract the third spacing and intravascular hypovolemia caused by the severe pancreatic inflammation. Early aggressive IVF resuscitation has been recommended by different guidelines[7-10], but most recently the AGA guidelines urged caution with this approach[8]. Vigorous IVF resuscitation has been traditionally given to prevent pancreatic hypoperfusion and necrosis. Although some studies have shown that is the persistent organ failure that puts the patient at higher mortality rather than necrosis alone. Other studies have raised concern on aggressive IVF been detrimental as it could increase the risk for pulmonary edema, respiratory failure, renal congestion, and acute kidney injury[11].
Despite the growing evidence, AP IVF resuscitation remains a controversial topic. The purpose of our study was to conduct a rigorous systematic review and meta-analysis of IVF resuscitation randomized trials and cohort studies.
Three electronic databases, Pubmed, Cochrane, and Embase, were searched from inception till 25 December 2018 for cohort studies as well as randomized controlled trials comparing aggressive fluid administration to non-aggressive fluid administration in patients with acute pancreatitis. Studies assessing IVF amount and timing of administration were included. Only articles published in the English language were screened. The references of the included studies were also evaluated for studies not incorporated by the initial search. This meta-analysis was performed in concurrence with the Preferred Reporting Items for Systematic Reviews and Meta-Analyses guidelines (Supplementary I) and was registered on PROSPERO international prospective register of systematic reviews(CRD42020146809).
A study was included if it satisfied all of the following: (1) A randomized trial, prospective cohort, or a retrospective cohort; and (2) Reporting outcomes of patients who received aggressive hydration versus patients who received non-aggressive hydration. Aggressive IVF amount administered had variation between studies (from 3 mL/kg/h to 5 mL/kg/h in first 24 h), but the definitions were still compliant with guideline definition of aggressive IVF hydration therapy. Studies that reported only outcomes of one group, studies that did not clearly define the rate of fluid administration, studies that were published as conference abstracts, case reports, narrative reviews, or studies that were designed as case-control studies were excluded from our current study.
Two authors (Mohamed M Gad, C Roberto Simons-Linares) independently screened the titles and abstracts of the search results after removing duplicated studies. Same two authors selected full-text studies for screening and performed the final data extraction of the baseline characteristics as well as outcomes of interest. Any conflicts were settled by consensus between the authors.
The primary outcome evaluated was in-hospital mortality. In-Hospital mortality was chosen due to the impactful consequential clinical management decision based on mortality outcomes as well as the uniform definition across all studies, corresponding with the least possible study heterogeneity. Secondary outcomes were SIRS, pancreatic necrosis, persistent organ failure, Acute Kidney Injury (AKI), and the need for mechanical ventilation. All outcomes were determined as per the study’s definition.
The Cochrane risk of bias tool was utilized in randomized controlled trials, and Newcastle-Ottawa Scale was used in observational studies as advised by the Cochrane handbook of systematic reviews and meta-analysis.
Conventional meta-analysis statistical analysis: Categorical variables were described using weighted frequencies, and weighted means/ SD were calculated for continuous variables. Weights were determined based on the sample size of each study. Fixed and Random effects risk ratios (RRs) were calculated for all outcomes using inverse variance method-DerSimonian-Laird estimator. I2 statistic was used to assess the heterogeneity between the included studies. A two-sided P value of < 0.05 and confidence interval (CI) of 95% were considered to be statistically significant, and all statistical analyses for the meta-analysis were performed with the use of RStudio® software package (meta) (RStudio, Boston, MA, United States).
The initial search revealed 2033 citations, but a total of 11 studies were included[11-21]; giving a total of 2686 patients. 1256 received aggressive resuscitation, and 1430 did not. Four randomized controlled trials (RCTs) addressed the issue of aggressive IVF resuscitation vs non-aggressive IVF. Seven cohort studies were also included. The end-points for outcomes varied among studies, and we tested for heterogeneity, which was found to be high between the studies.
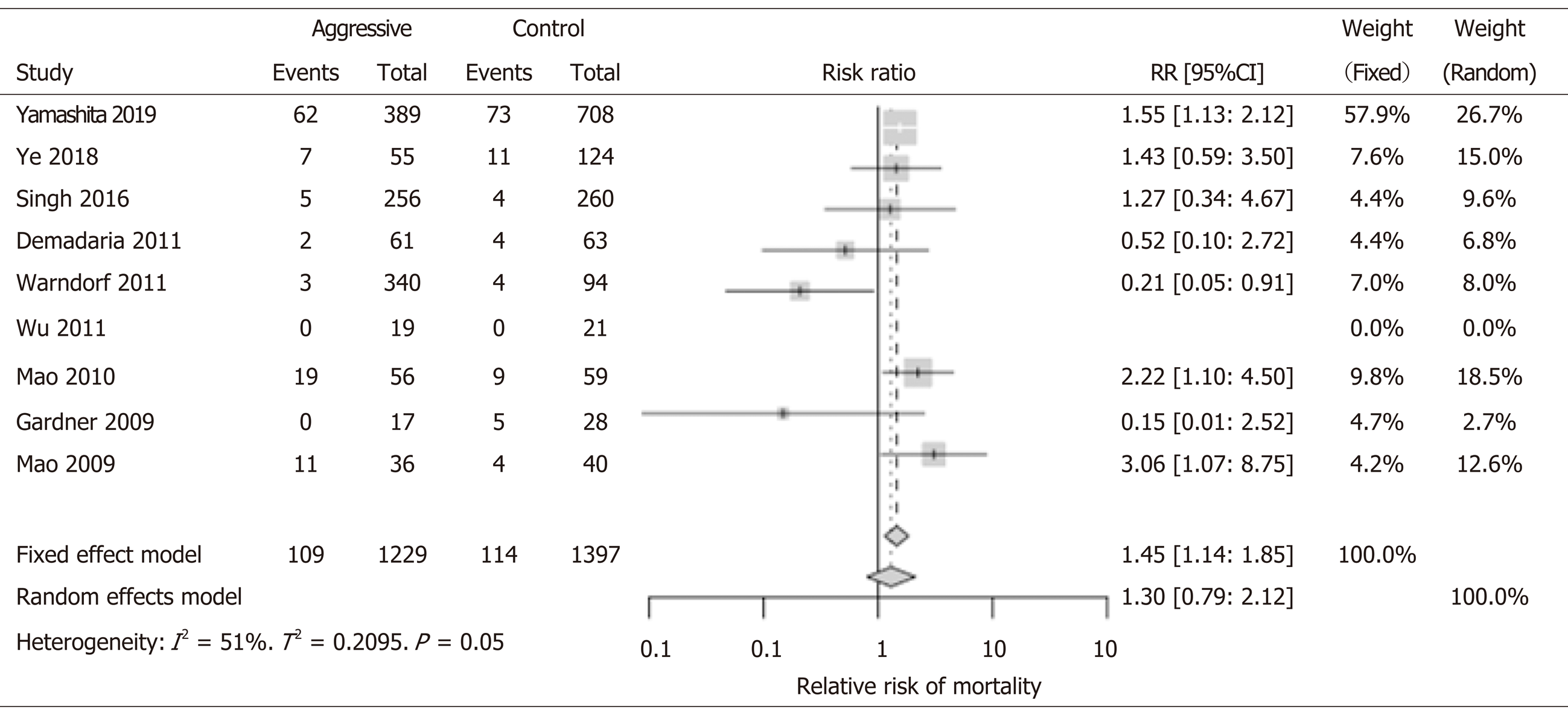
There was no significant difference between the aggressive (n = 1229) and non-aggressive IVF (n = 1397) patients in terms of mortality benefit (RR: 1.30; 95%CI: 0.79-2.12). In most studies, the mortality was not significantly different, and this could be due to the low mortality and small sample size. With the exception of the study by Yamashita et al[18] that studied over one thousand patients and had over 130 events (deaths); but the investigators did not find mortality difference between groups. However, in only one study by Warndorf et al[16], the aggressive IVF group showed to have a lower mortality (RR: 0.21, 95%CI: 0.05-0.91). In contrary, both RCT studies performed in China by Mao et al[12,22], revealed increased mortality when aggressive IVF strategy was used. These studies in China showed higher risk for increased mortality in the aggressive IVF group: 2009 study RR 3.06 (95%CI: 1.07-8.75) and 2010 study RR 2.22 (95%CI: 1.10-4.50) (Figure 1). Of note, most studies had low event rate (0-11 deaths in the study group) with the exception of two studies: Mao et al[22] 2010 had 19 deaths; and Yamashita et al[18] had 62 deaths.
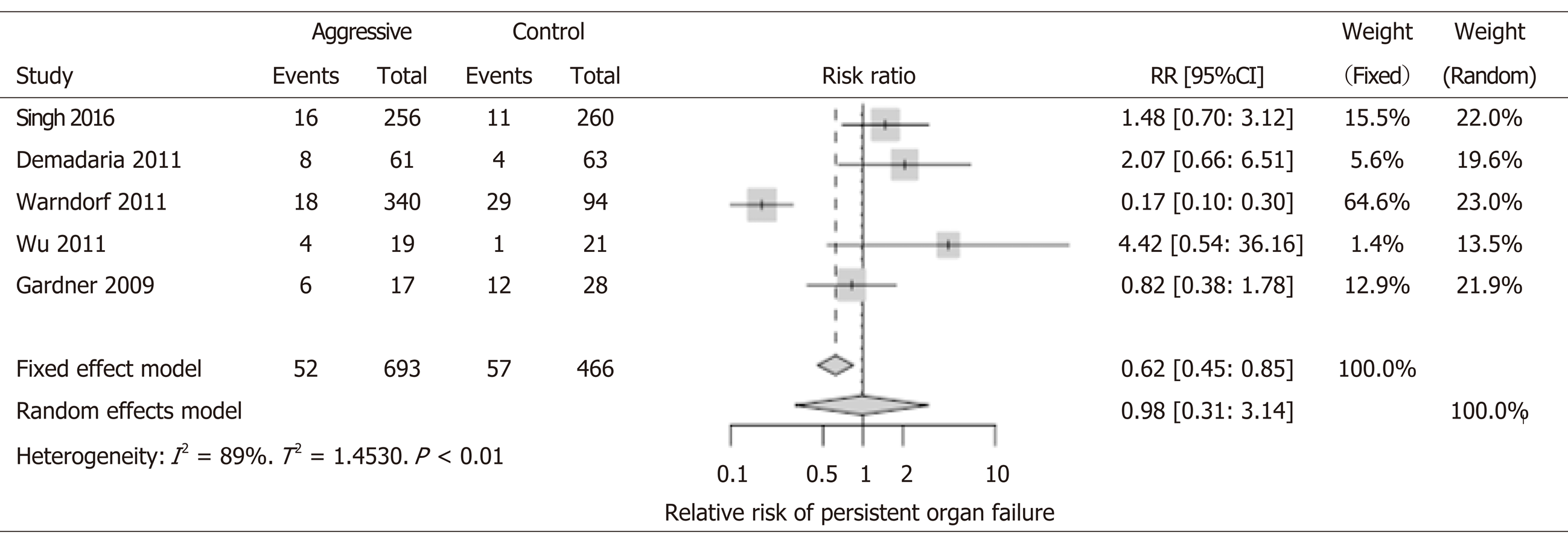
Not distinguishing between transient and persistent organ failures was one of the most important limitations of RCTs. Including cohort studies allowed us to compare between them, but with a lower level of evidence. We were able to assess this outcome in 5 studies (n = 1159), of which only one study had statistically significant findings in favor of aggressive IVF (Warndorf et al[16], RR: 0.17, 95%CI: 0.10-0.30) but this benefit was not observed in the overall calculations of the metaanalysis (RR: 0.98, 95%CI: 0.31-3.14) (Figure 2).
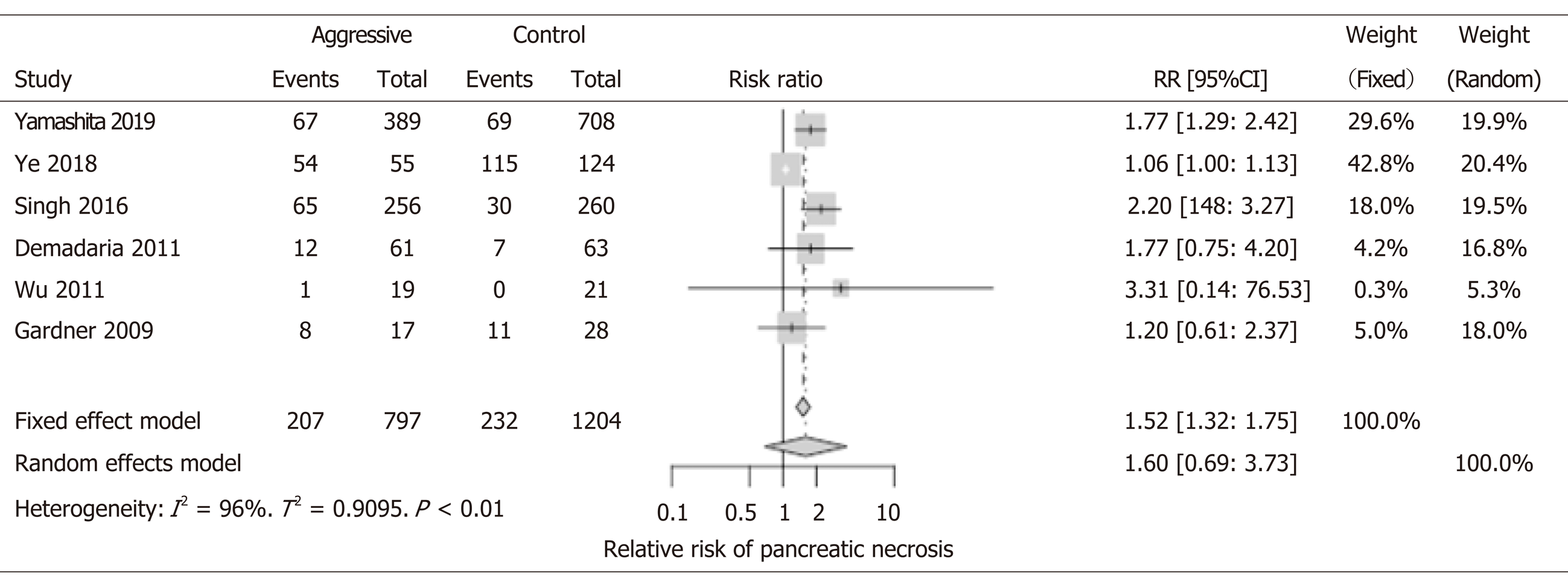
Eight studies with 2001 patients were included in the analysis. In the fixed model of out metaanalysis, patients receiving aggressive IVF therapy (n = 848) seemed to have higher risk for pancreatic necrosis (RR: 1.52; 95%CI: 1.32-1.75); this could be explained by assuming that probably patients who had necrosis were more likely to receive aggressive IVF therapy and more prolong IVF therapy that could be detrimental (Figure 3). However, due to the significant differences between the included studies (differences in terms of end points, patients enrollment method, and overall studies designs), we interpreted the results of our metaanalysis by using the random effect models for all our results (rather than the fixed model). In the random effect model, there was no difference in pancreatic necrosis rates between groups (RR: 1.60, 95%CI: 0.69-3.73). Location, extension, and degree of necrosis were not provided by studies. Additionally, the incidence of infection of the necrosis was not studied.
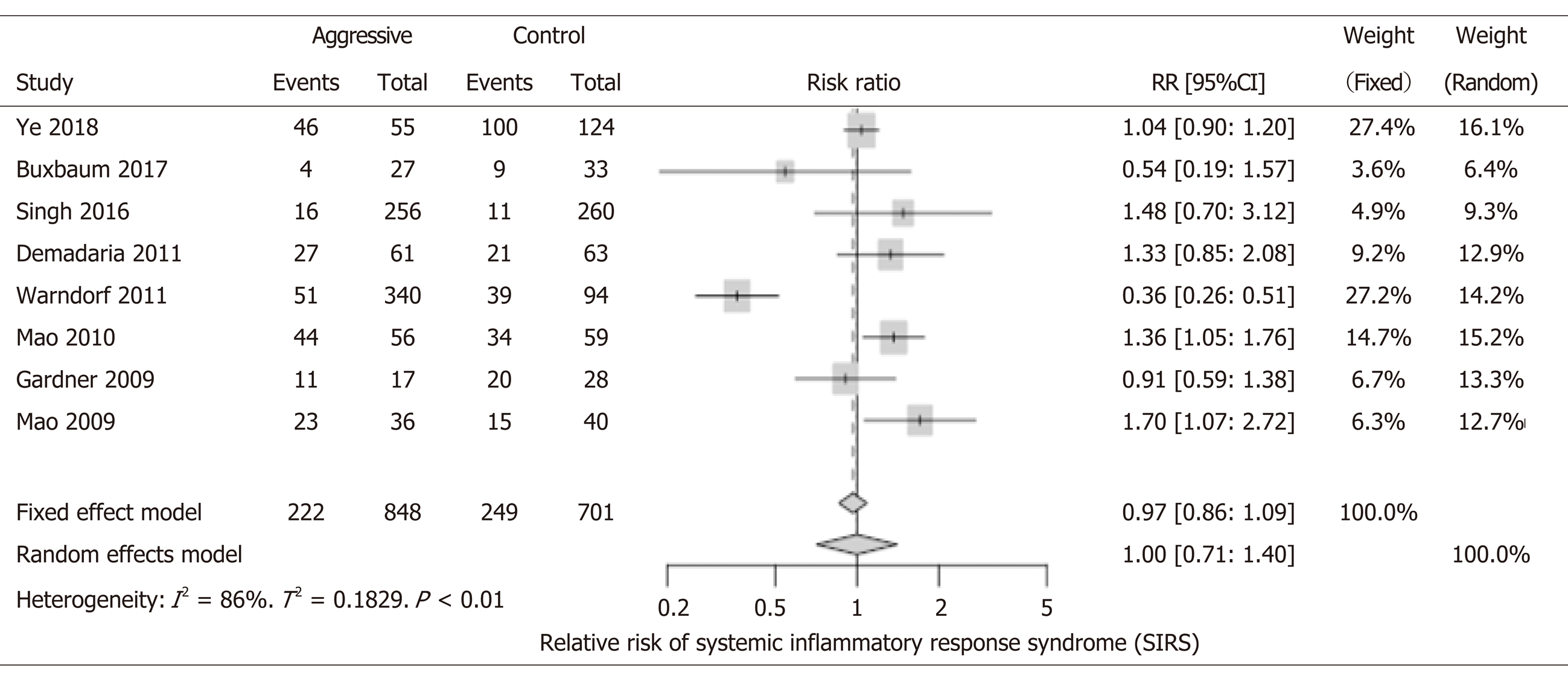
1549 patients were included, and there was no significant difference in the overall incidence of SIRS when comparing both study groups (RR: 1.0; 95%CI: 0.71-1.40) (Figure 4). There were two cohort studies and one RCT that reported a decreased incidence of SIRS in the aggressive IVF group[14,16,20]. Contrary, there were two RCTs and three cohort studies that showed that the aggressive IVF group had higher rates of SIRS[9,11,15,18,22]. However, the only statistically significant results for this outcome were reported in three studies: Mao et al[22] 2010 study and 2009 study, both reported increased incidence of SIRS with aggressive IVF: RR: 1.36, 95%CI: 1.05-1.76 and RR: 1.70, 95%CI: 1.07-2.72, respectively. Warndorf et al[16] reported a significant benefit of decreasing SIRS with aggressive IVF (RR: 0.36, 95%CI: 0.26-0.51).
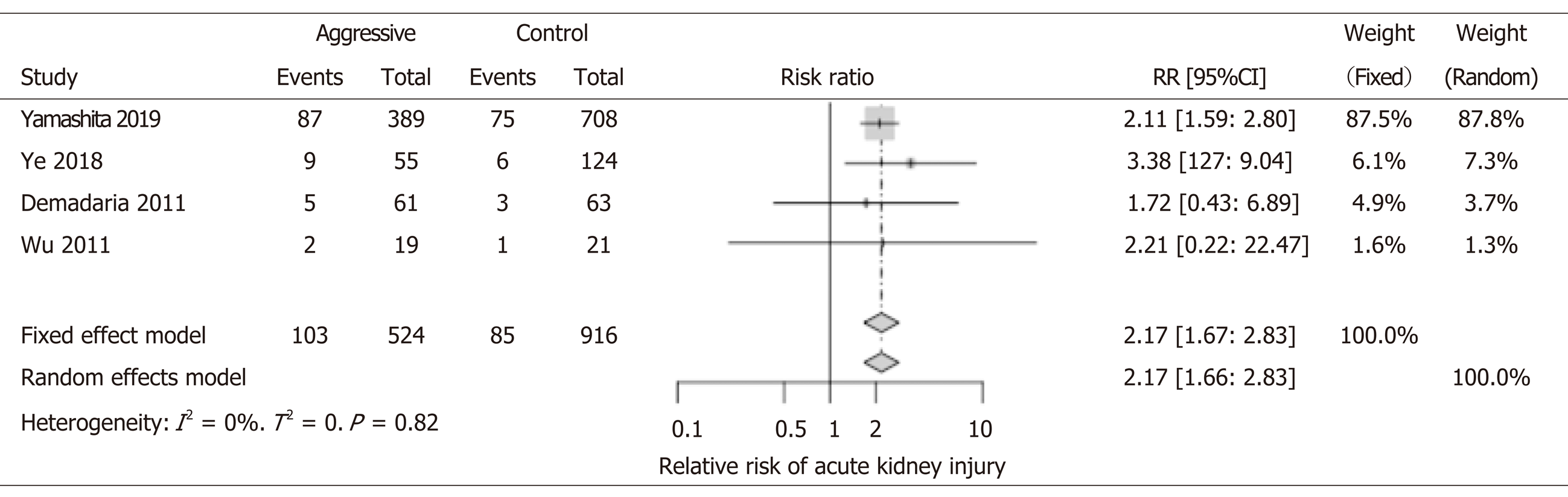
We included four studies involving 1440 patients for the analysis of AKI. All four studies showed an increased risk for AKI with aggressive IVF. Although, only two studies reached statistical significance (Yamashita et al[18], Ye et al[11]). Interestingly, our metaanalysis shows that patients that received aggressive IVF therapy were more than two times more likely to develop AKI (RR: 2.17; 95%CI: 1.66-2.83). Renal vascular congestion, similar pathophysiology of the cardiorenal syndromes–has been demonstrated that could cause injury to the glomeruli. The latter mechanism could be implicated and was reported in some of the papers included in our analysis (Figure 5).
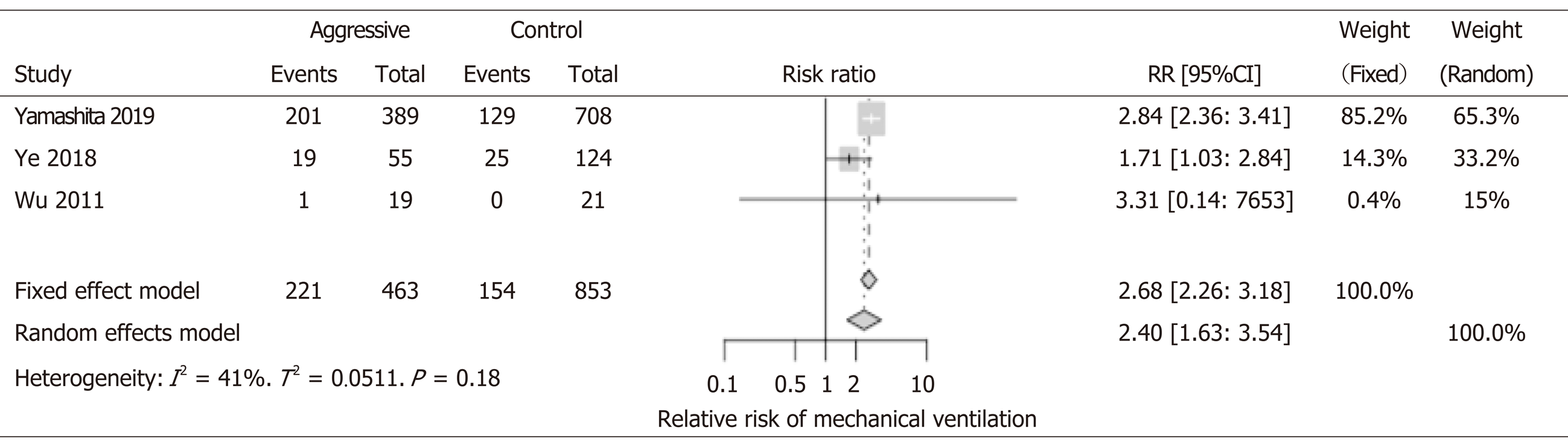
1316 patients from 3 studies were included in the analysis. All three showed worse outcomes with aggressive IVF; Two studies (Yamashita et al[18], Ye et al[11]) reached statistical significance. Not surprising, patients who received aggressive IVF therapy were also more likely to develop pulmonary edema, fluid overload that leads to respiratory failure, and mechanical ventilation support. Overall, aggressive IVF patients were two times more at risk to develop respiratory failure (RR: 2.40, 95%CI: 1.63-3.54) (Figure 6).
One study (Buxbaum et al[19]) was not designed to have mortality as an outcome and hence not reported. There were significant variations on the definitions of the outcomes (persistent organ failure, respiratory failure, AKI). Another concern we have is how AKI was defined; unfortunately there are no details about this in the vast majority of the studies. Finally, one of the biggest concerns that the authors of this paper have is in regards to persistent organ failure–there was significant heterogeneity of this definition, and none of the RCTs reported this. We have evidence that is persistent organ failure that drives mortality in AP rather than isolated pancreatic necrosis without persistent organ failure. The mentioned concerns are limitations of the study, and our findings should be interpreted with caution (Supplemental I Table 1).
We explored studying our outcomes in subgroups according to AP etiology and also according to age groups (older > 55 years old vs younger < 55 years old). However, very few studies had data for us to include in the subgroup analysis and most studies did not report appropriate data for this analysis. From the available data, we did not find any significant differences in the subgroups analysis (Supplemental II). Subgroup analysis by AP severity could not be studied due to lack of data.
The capillary leak from pancreatic inflammation behaves similar to other diseases such as sepsis and burn injuries[23-26]. The intravascular depletion, hypovolemia, and third-spacing of fluid causes pancreatic tissue hypoperfusion and necrosis. It is also known that this hypoperfusion state damages other sensitive organs such as the kidneys, lungs, and heart; leading to multi-organ failure with or without hypovolemic shock. Apart from the hypovolemia and hypoperfusion, pancreatitis itself causes severe inflammation in its early phase that can also lead to other organ failures such as acute respiratory distress syndrome, hypercoagulable state, and venous thromboembolisms[6].
The rationale of intravenous fluid resuscitation is to provide hemodynamic support and expand the severely depleted intravascular space to aid the perfusion of vital organs. Unfortunately, there is no medication approved as of yet to help counteract the capillary leak from AP systemic inflammation. Rapid hemodilution was studied in multiple retrospective studies and data showed that rapidly administering IVF therapy and using the hematocrit and blood urea nitrogen as markers to achieve rapid hemodilution were effective[19,27-29].
In recent years, some studies have raised concerns about aggressive IVF resuscitation causing serious side effects such as AKI and pulmonary edema leading to respiratory failure[11-13,19]. Our meta-analysis found that individuals in the aggressive IVF group were two times more likely to develop AKI (Figure 5). AKI could worsen with aggressive IVF therapy through multiple possible mechanisms. First renal congestion with excessive intravascular fluid, a similar mechanism to cardiorenal syndrome. Second, visceral edema and congestion of the renal vasculature bed can also affect the kidney’s perfusion and lead to AKI. Third, the type of IV fluid used may also impact; for example, excessive chloride is a risk for kidney injury. Fourth, if patients are fluid overload, the use of diuretics to treat the fluid overload can impact the kidneys and contribute to the multifactorial causes of AKI during AP. Less common, intra-abdominal compartment syndrome could cause constriction of the renal vasculature and lead to AKI as well[30,31].
Our study also found that patients in the aggressive IVF group were two times more at risk to develop respiratory failure and require mechanical ventilation. Although direct conclusion cannot be drawn from this finding; for example, these patients had more pulmonary edema from excessive IV fluids, but it could also be that these patients were just sicker and developed acute respiratory distress syndrome from pancreatitis itself. There is undoubtedly a concern for this possible and detrimental side effect of aggressive IVF to the lungs, that could lead to higher risk for mechanical ventilation in the aggressive IVF group. However, we are unable to conclude that the higher rates of respiratory failure in our analysis was due to a more aggressive IVF strategy.
In our metaanalysis we were also able to assess for mortality, persistent organ failure, pancreatic necrosis and SIRS; but we did not find an statistical difference between groups. The available studies in the literature and the ones included in this metaanalysis have significant heterogenicity in terms of design, populations studied (variations in AP severity, races), IVF types, IVF amount/definitions of aggressive IVF; hence we do not believe that the outcomes of this metanalysis can be used to draw strong or definitive conclusions. However, the present study contributes to the current literature with a summary of the available studies and it also shows the signifcant heterogenicty among the published studies and the need for a well design multicenter randomized control trial to answer the question if aggressive IVF is beneficial and in what type of patient it would be beneficial.
In conclusion, there is very limited evidence to support aggressive over goal-directed IVF resuscitation. RCTs are needed to first address the baseline accurate fluid status of the patient with a non-invasive hemodynamic assessment. Moreover, the fluid responsiveness of the patient also needs to be studied, as all patients may not be responsive to IV fluid resuscitation, and additional therapies remained to be elucidated.
The background, present status, and significance of the study should be described in detail. Intravenous fluid (IVF) resuscitation is the cornerstone for Acute pancreatitis (AP) management and Early Aggressive IVF therapy has been traditionally recommended. Recent evidence has raised concern for detrimental effect of aggressive IVF therapy, hence we analyzed the evidence of randomized controlled trials (RCTs) and cohort studies comparing aggressive IVF vs non-aggressive IVF therapy.
There is growing controversial evidence on AP IVF resuscitation and the IVF strategy remains a controversial topic. The purpose of our study was to conduct a rigorous systematic review and meta-analysis of IVF therapy for AP reported in randomized trials and cohort studies.
To investigate if aggressive IVF therapy in AP patients is beneficial to decrease mortality and improve outcomes.
We perform a metaanalysis of RCTs and cohort studies. Three electronic databases (Pubmed, Cochrane, and Embase) were searched from inception till 25 December 2018 for studies comparing aggressive IVF to non-aggressive IVF therapy in patients with AP.
A total of 11 studies were included; giving a total of 2686 patients. Our study found that early aggressive IVF therapy did not improve mortality and it could potentially increase the risk for AKI, pulmonary edema leading to respiratory failure and mechanical ventilation requirement. This controversial topic remains to be studied and more studies are needed to investigate which subset of AP patients could benefit from aggressive IVF therapy.
Early Aggressive IV fluid therapy did not improve mortality. RCTs are needed to first address the baseline accurate fluid status of the patient with a non-invasive hemodynamic assessment. There is very limited data comparing aggressive IVF to non-aggressive IVF therapy, and the published studies are very heterogenous; which difficults the proper assessment to draw conclusions. It seems that aggressive IVF therapy in AP patients is not for everyone and the look to identify the subset of AP patients who may benefit from is still ongoing. We first need to address a baseline and accurate fluid status of AP patient and we could use non-invasive hemodynamic assessment technology such as the one that has been extensively used in the critical care, trauma, burns and cardiology settings. Additionally, the fluid responsiveness of patients also needs to be studied, as all patients may not be responsive to aggressive IVF resuscitation, and hence additional therapies may be needed and remained to be elucidated.
As there is very limited and heterogenous evidence to support aggressive IVF over goal-directed IVF therapy, further studies are needed to assess the baseline fluid status of the patient before, during, and after IVF resuscitation. Non-invasive identification of the fluid responder patients would be beneficial to help optimize the management of AP patients and avoid pancreatic necrosis, multiorgan failure and mortality.
Manuscript source: Unsolicited manuscript
Specialty type: Gastroenterology and hepatology
Country of origin: United States
Peer-review report classification
Grade A (Excellent): 0
Grade B (Very good): B, B, B
Grade C (Good): C
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Bramhall SR, Dambrauskas Z, Liu WH, Schietroma M S-Editor: Zhang L L-Editor: A E-Editor: Zhang YL
| 1. | Peery AF, Dellon ES, Lund J, Crockett SD, McGowan CE, Bulsiewicz WJ, Gangarosa LM, Thiny MT, Stizenberg K, Morgan DR, Ringel Y, Kim HP, DiBonaventura MD, Carroll CF, Allen JK, Cook SF, Sandler RS, Kappelman MD, Shaheen NJ. Burden of gastrointestinal disease in the United States: 2012 update. Gastroenterology. 2012;143:1179-1187.e3. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 1355] [Cited by in F6Publishing: 1373] [Article Influence: 114.4] [Reference Citation Analysis (1)] |
| 2. | Banks PA, Bollen TL, Dervenis C, Gooszen HG, Johnson CD, Sarr MG, Tsiotos GG, Vege SS; Acute Pancreatitis Classification Working Group. Classification of acute pancreatitis--2012: revision of the Atlanta classification and definitions by international consensus. Gut. 2013;62:102-111. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 4134] [Cited by in F6Publishing: 3692] [Article Influence: 335.6] [Reference Citation Analysis (38)] |
| 3. | Krishna SG, Kamboj AK, Hart PA, Hinton A, Conwell DL. The Changing Epidemiology of Acute Pancreatitis Hospitalizations: A Decade of Trends and the Impact of Chronic Pancreatitis. Pancreas. 2017;46:482-488. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 138] [Cited by in F6Publishing: 124] [Article Influence: 17.7] [Reference Citation Analysis (0)] |
| 4. | Working Group IAP/APA Acute Pancreatitis Guidelines. IAP/APA evidence-based guidelines for the management of acute pancreatitis. Pancreatology. 2013;13:e1-15. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 1080] [Cited by in F6Publishing: 921] [Article Influence: 83.7] [Reference Citation Analysis (3)] |
| 5. | Chua TY, Walsh RM, Baker ME, Stevens T. Necrotizing pancreatitis: Diagnose, treat, consult. Cleve Clin J Med. 2017;84:639-648. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 11] [Cited by in F6Publishing: 11] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 6. | Garber A, Frakes C, Arora Z, Chahal P. Mechanisms and Management of Acute Pancreatitis. Gastroenterol Res Pract. 2018;2018:6218798. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 37] [Cited by in F6Publishing: 38] [Article Influence: 6.3] [Reference Citation Analysis (0)] |
| 7. | Crockett S, Falck-Ytter Y, Wani S, Gardner TB. Acute Pancreatitis Guideline. Gastroenterology. 2018;154:1102. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 14] [Cited by in F6Publishing: 14] [Article Influence: 2.3] [Reference Citation Analysis (2)] |
| 8. | Crockett SD, Wani S, Gardner TB, Falck-Ytter Y, Barkun AN; American Gastroenterological Association Institute Clinical Guidelines Committee. American Gastroenterological Association Institute Guideline on Initial Management of Acute Pancreatitis. Gastroenterology. 2018;154:1096-1101. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 405] [Cited by in F6Publishing: 437] [Article Influence: 72.8] [Reference Citation Analysis (0)] |
| 9. | de-Madaria E, Soler-Sala G, Sánchez-Payá J, Lopez-Font I, Martínez J, Gómez-Escolar L, Sempere L, Sánchez-Fortún C, Pérez-Mateo M. Influence of fluid therapy on the prognosis of acute pancreatitis: a prospective cohort study. Am J Gastroenterol. 2011;106:1843-1850. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 139] [Cited by in F6Publishing: 129] [Article Influence: 9.9] [Reference Citation Analysis (0)] |
| 10. | Tenner S, Baillie J, DeWitt J, Vege SS; American College of Gastroenterology. American College of Gastroenterology guideline: management of acute pancreatitis. Am J Gastroenterol. 2013;108:1400-15; 1416. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 1232] [Cited by in F6Publishing: 1254] [Article Influence: 114.0] [Reference Citation Analysis (3)] |
| 11. | Ye B, Mao W, Chen Y, Tong Z, Li G, Zhou J, Ke L, Li W. Aggressive Resuscitation Is Associated with the Development of Acute Kidney Injury in Acute Pancreatitis. Dig Dis Sci. 2019;64:544-552. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 15] [Cited by in F6Publishing: 20] [Article Influence: 4.0] [Reference Citation Analysis (0)] |
| 12. | Mao EQ, Tang YQ, Fei J, Qin S, Wu J, Li L, Min D, Zhang SD. Fluid therapy for severe acute pancreatitis in acute response stage. Chin Med J (Engl). 2009;122:169-173. [PubMed] [Cited in This Article: ] |
| 13. | Mao EQ, Tang YQ, Li L, Qin S, Wu J, Liu W, Lei RQ, Zhang SD. [Strategy of controlling fluid resuscitation for severe acute pancreatitis in acute phase]. Zhonghua Wai Ke Za Zhi. 2007;45:1331-1334. [PubMed] [Cited in This Article: ] |
| 14. | Gardner TB, Vege SS, Chari ST, Petersen BT, Topazian MD, Clain JE, Pearson RK, Levy MJ, Sarr MG. Faster rate of initial fluid resuscitation in severe acute pancreatitis diminishes in-hospital mortality. Pancreatology. 2009;9:770-776. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 142] [Cited by in F6Publishing: 130] [Article Influence: 9.3] [Reference Citation Analysis (0)] |
| 15. | Singh VK, Gardner TB, Papachristou GI, Rey-Riveiro M, Faghih M, Koutroumpakis E, Afghani E, Acevedo-Piedra NG, Seth N, Sinha A, Quesada-Vázquez N, Moya-Hoyo N, Sánchez-Marin C, Martínez J, Lluís F, Whitcomb DC, Zapater P, de-Madaria E. An international multicenter study of early intravenous fluid administration and outcome in acute pancreatitis. United European Gastroenterol J. 2017;5:491-498. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 25] [Cited by in F6Publishing: 27] [Article Influence: 3.4] [Reference Citation Analysis (0)] |
| 16. | Warndorf MG, Kurtzman JT, Bartel MJ, Cox M, Mackenzie T, Robinson S, Burchard PR, Gordon SR, Gardner TB. Early fluid resuscitation reduces morbidity among patients with acute pancreatitis. Clin Gastroenterol Hepatol. 2011;9:705-709. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 151] [Cited by in F6Publishing: 138] [Article Influence: 10.6] [Reference Citation Analysis (0)] |
| 17. | Wu BU, Hwang JQ, Gardner TH, Repas K, Delee R, Yu S, Smith B, Banks PA, Conwell DL. Lactated Ringer's solution reduces systemic inflammation compared with saline in patients with acute pancreatitis. Clin Gastroenterol Hepatol. 2011;9:710-717.e1. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 328] [Cited by in F6Publishing: 297] [Article Influence: 22.8] [Reference Citation Analysis (0)] |
| 18. | Yamashita T, Horibe M, Sanui M, Sasaki M, Sawano H, Goto T, Ikeura T, Hamada T, Oda T, Yasuda H, Ogura Y, Miyazaki D, Hirose K, Kitamura K, Chiba N, Ozaki T, Koinuma T, Oshima T, Yamamoto T, Hirota M, Masuda Y, Tokuhira N, Kobayashi M, Saito S, Izai J, Lefor AK, Iwasaki E, Kanai T, Mayumi T. Large Volume Fluid Resuscitation for Severe Acute Pancreatitis is Associated With Reduced Mortality: A Multicenter Retrospective Study. J Clin Gastroenterol. 2019;53:385-391. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 16] [Cited by in F6Publishing: 17] [Article Influence: 3.4] [Reference Citation Analysis (0)] |
| 19. | Buxbaum JL, Quezada M, Da B, Jani N, Lane C, Mwengela D, Kelly T, Jhun P, Dhanireddy K, Laine L. Early Aggressive Hydration Hastens Clinical Improvement in Mild Acute Pancreatitis. Am J Gastroenterol. 2017;112:797-803. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 70] [Cited by in F6Publishing: 78] [Article Influence: 11.1] [Reference Citation Analysis (0)] |
| 20. | Buxbaum J, Mwengela D, Jani N, Kelly T, Dhanireddy K, Nneji J. Randomized trial of moderate versus aggressive fluid therapy in patients with mild to moderate acute pancreatitis. Pancreas. 2014;43:1346-1346. [Cited in This Article: ] |
| 21. | Weitz G, Woitalla J, Wellhöner P, Schmidt K, Büning J, Fellermann K. Detrimental effect of high volume fluid administration in acute pancreatitis - a retrospective analysis of 391 patients. Pancreatology. 2014;14:478-483. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 17] [Cited by in F6Publishing: 15] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 22. | Mao EQ, Fei J, Peng YB, Huang J, Tang YQ, Zhang SD. Rapid hemodilution is associated with increased sepsis and mortality among patients with severe acute pancreatitis. Chin Med J (Engl). 2010;123:1639-1644. [PubMed] [Cited in This Article: ] |
| 23. | Evers LH, Bhavsar D, Mailänder P. The biology of burn injury. Exp Dermatol. 2010;19:777-783. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 169] [Cited by in F6Publishing: 202] [Article Influence: 14.4] [Reference Citation Analysis (0)] |
| 24. | Kottke MA, Walters TJ. Where's the Leak in Vascular Barriers? A Review. Shock. 2016;46:20-36. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 29] [Cited by in F6Publishing: 29] [Article Influence: 4.8] [Reference Citation Analysis (0)] |
| 25. | Schaefer TJ, Nunez Lopez O. Burn Resuscitation And Management 2020. [PubMed] [Cited in This Article: ] |
| 26. | Siddall E, Khatri M, Radhakrishnan J. Capillary leak syndrome: etiologies, pathophysiology, and management. Kidney Int. 2017;92:37-46. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 130] [Cited by in F6Publishing: 165] [Article Influence: 23.6] [Reference Citation Analysis (0)] |
| 27. | Koutroumpakis E, Wu BU, Bakker OJ, Dudekula A, Singh VK, Besselink MG, Yadav D, Mounzer R, van Santvoort HC, Whitcomb DC, Gooszen HG, Banks PA, Papachristou GI. Admission Hematocrit and Rise in Blood Urea Nitrogen at 24 h Outperform other Laboratory Markers in Predicting Persistent Organ Failure and Pancreatic Necrosis in Acute Pancreatitis: A Post Hoc Analysis of Three Large Prospective Databases. Am J Gastroenterol. 2015;110:1707-1716. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 74] [Cited by in F6Publishing: 91] [Article Influence: 10.1] [Reference Citation Analysis (0)] |
| 28. | Lin S, Hong W, Basharat Z, Wang Q, Pan J, Zhou M. Blood Urea Nitrogen as a Predictor of Severe Acute Pancreatitis Based on the Revised Atlanta Criteria: Timing of Measurement and Cutoff Points. Can J Gastroenterol Hepatol. 2017;2017:9592831. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 22] [Cited by in F6Publishing: 27] [Article Influence: 3.9] [Reference Citation Analysis (0)] |
| 29. | Valverde-López F, Matas-Cobos AM, Alegría-Motte C, Jiménez-Rosales R, Úbeda-Muñoz M, Redondo-Cerezo E. BISAP, RANSON, lactate and others biomarkers in prediction of severe acute pancreatitis in a European cohort. J Gastroenterol Hepatol. 2017;32:1649-1656. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 41] [Cited by in F6Publishing: 43] [Article Influence: 6.1] [Reference Citation Analysis (0)] |
| 30. | De Waele JJ, De Laet I, Kirkpatrick AW, Hoste E. Intra-abdominal Hypertension and Abdominal Compartment Syndrome. Am J Kidney Dis. 2011;57:159-169. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 110] [Cited by in F6Publishing: 93] [Article Influence: 7.2] [Reference Citation Analysis (0)] |
| 31. | Mohmand H, Goldfarb S. Renal dysfunction associated with intra-abdominal hypertension and the abdominal compartment syndrome. J Am Soc Nephrol. 2011;22:615-621. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 133] [Cited by in F6Publishing: 123] [Article Influence: 9.5] [Reference Citation Analysis (1)] |