Published online Jun 15, 2001. doi: 10.3748/wjg.v7.i3.317
Revised: April 3, 2001
Accepted: April 15, 2001
Published online: June 15, 2001
- Citation: Lovat LB, Bown SG. Lasers in gastroenterology. World J Gastroenterol 2001; 7(3): 317-323
- URL: https://www.wjgnet.com/1007-9327/full/v7/i3/317.htm
- DOI: https://dx.doi.org/10.3748/wjg.v7.i3.317
Endoscopy has revolutionised our management of many gastrointestinal disorders over the past 30 years. We are increasingly able to diagnose gastrointestinal (GI) tumors at an early stage, and endoscopic therapy has made a difference to the outcome of GI haemorrhage. We still rely on surgery for cure of cancer but as diagnostic techniques improve the goal of minimally invasive diagnosis and therapy appears ever more attainable. As populations get older, it is also increasingly desirable. Laser light can be used for both diagnosis and therapy in the gut. This article reviews the value of lasers in these areas.
Lasers are sophisticated sources of monochromatic light in the visible and near infrared part of the optical spectrum. The ones of most interest to gastroenterologists are those where the beam penetrates living tissue well and which can be transmitted via thin, flexible fibers, so they can be used with flexible endoscopes. These can be used to deliver light as heat to cause thermal contraction of soft tissue. The most important laser in this group has been the Neodymium yttrium aluminium garnet (NdYAG) laser with a near infrared beam at 1064 nm. Short, sharp shots from this laser cause thermal contraction in soft tissues, which provides good haemostasis. Longer shots at high power can vaporise tissue and coagulate the underlying layers, which is effective for debulking advanced cancers. At much lower powers, it is possible to coagulate a larger volume of tissue without vaporisation.
The other main group of effects is photodynamic where there is no increase in tissue temperature, but laser light is used to activate a previously administered photosensitising drug. This causes the release of highly reactive singlet oxygen which causes cell death by necrosis and apoptosis over a prolonged period. This can be used to completely eradicate small tumours.
A minor application is to use pulsed lasers endoscopically to fragment gall stones. These effects are summarised in Table 1.
| Laser effect | Clinical use |
| High power thermal: | Haemostasis |
| Cutting or debulking of tissue by vaporisation and coagulation | |
| Low power thermal: (Interstitial laser photocoagulation, ILP) | Gentle coagulation of lesions within solid organs |
| Photochemical: (Photodynamic therapy, PDT) | Non-thermal destruction of tissue by activation of a previously administered photosensitising drug |
| Pulsed shock wave | Fragmentation of gall stones |
All living tissue display a number of interactions with light, which are altered in areas of dysplasia. Intrinsic fluorescence may be detected at endoscopy by exciting the tissue with blue laser light and using special detector cameras on the endoscope. Elastic scattering depends on the different way light is scattered depending on the density of cellular and nuclear packing. These approaches may allow us to detect premalignant lesions of the GI tract that would otherwise beinvisible at conventional endoscopy.
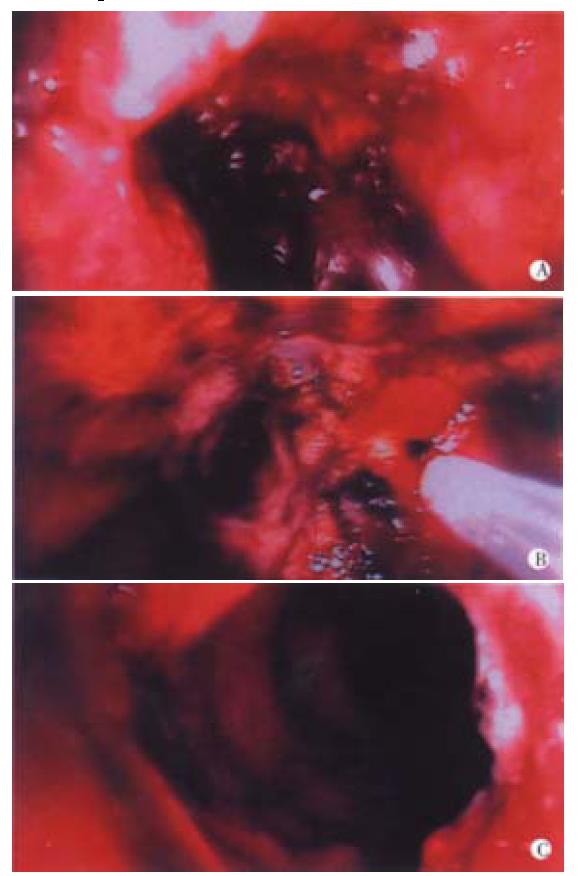
The main role of high power, thermal lasers like the NdYAG in current practice is for palliation of advanced, inoperable cancers of the upper and lower gastrointestinal tract. Under direct vision, nodules of exophytic tumour can be vaporised and underlying tumour coagulated either to relieve obstruction or to reduce blood loss (Figure 1). The incidence of complications is low, although it often takes several treatments to achieve optimum recanalisation. These laser beams are dangerous if viewed directly, so safety filters must be fitted to fibreoptic scopes. There is no risk to operators with video scopes, although filters are required to protect the chips in the camera.
Most patients with cancers of the oesophagus or gastric cardia present when the disease is too advanced for there to be any prospect of cure and the main aim of treatment is to relieve dysphagia as simply and rapidly as possible[1]. The main endoscopic options are stent insertion and laser therapy. Tumor dilatation and insertion of a silicone rubber stent was standard practice in Europe until 5 years ago, although these stents were never popular in North America. Using this approach most patients were unable to eat any more than pureed food. Since their introduction in the early 1990s, expanding metal stents have become popular as they require less dilatation (hence less risk of perforation) and are easier to insert. It is becoming clear with experience that they are far from ideal, particularly as the relief of dysphagia has never been shown to be better than silicone stents. New designs are appearing at regular intervals and the current generation of metal stent are covered to prevent tumour ingrowth and conical in shape to prevent stent slippage. Nevertheless, intractable pain occurs in 10% of patients after stent insertion and up to 40% will need further endoscopic interventions[2,3].
Laser therapy has been shown to improve dysphagia to a similar degree as stents, and does not cause pain. The disadvantage is that laser therapy alone has to be repeated on average every 5-6 weeks. The addition of a palliative dose of external beam radiotherapy can increase this to 9 weeks and a single fraction of brachytherapy (intraluminal radiotherapy) will bring relief of dysphagia for a median of 5 months. Since the median survival time for advanced disease is less than this, a single course of laser and brachytherapy is an attractive option. The relative merits of lasers and stents are summarised in Table 2. Common sense dictates that the two are complimentary rather than competitive. An eccentric, exophytic tumour is best debulked with the laser whereas a circumferential tumour with little exophytic component is best stented. A fistula must be stented, whereas high cervical tumours can seldom be stented. What little data there is on comparative costs suggests that the lifetime treatment costs are similar for each of these approaches[4].
| Laser | Conventional stent | Self expanding metal stent | |
| Technique | Basically safe (risk of perforation if dilatation also needed) | 10% risk of perforation on insertion | Usually safe and easy to insert |
| Cost | High setup cost Low patient costs | Low cost | High cost |
| Contra | Fistula | High lesion | High lesion |
| indications | No endoscopic target | Tracheal compression | Tracheal compression Care with lesions crossing cardia |
| Dysphagia post | Variable, can be | Semi-solids | Variable, can be |
| therapy | close to normal | some solids | close to normal |
| Repeat Therapy | Possible. Usually required after 6-8 weeks | Stent can be adjusted | Difficult to adjust once inserted. Second stent or laser debulking for tumour overgrowth |
| Enhancement of | Yes | No | No |
| dysphagia relief with radiotherapy | |||
A future direction may be the combination of laser palliation of dysphagia with radical chemoradiotherapy in inoperable patients. This approach is attractive as many patients with advanced disease present with severe malnutrition caused by their dysphagia. Radical treatment is not possible in a cachectic patient, but if dysphagia is overcome, patients regain weight and are able to tolerate intensive therapy. Long term data are lacking but early results suggest that this approach can lead to prolonged survival in at least some patients who have previously been thought to be terminally ill.
The situation with the small percentage of rectal and recto-sigmoid cancers that are not suitable for surgery is very similar[5]. Endoscopic laser therapy can simply and effectively relieve symptoms due to exophytic tumour, usually as a day case procedure with minimal sedation. Bleeding, obstruction, mucus discharge and tenesmus can be helped, and the effect can be prolonged by the addition of palliative, external beam radiotherapy, although laser treatment cannot help pelvic pain or obstruction due to extraluminal tumour. Expanding metal stents are now available to relieve some distal colonic strictures, but they are unlikely to find an important role in palliating low rectal cancers.
Lasers were first used with flexible endoscopes in the mid 1970s for the control of haemorrhage. Endoscopic treatment with the NdYAG laser significantly reduced the rebleeding rate after haemorrhage from peptic ulcers in a number of studies. Later trials showed that injection sclerotherapy worked just as well, and was simpler and cheaper. The only benign lesions for which endoscopic laser therapy retains an important role for control of blood loss are vascular lesions like hereditary telangiectasia, angiodysplasia and watermelon stomach. These can be managed by sclerotherapy and various thermocoagulatory modalities such as argon plasma coagulation, but laser treatment is effective, simpler to apply and needs less treatment sessions, especially when multiple lesions are present.
High power NdYAG laser is used during endoscopy, with short shots of a second or two, typically at 50-80 W. The laser fibre is held away from the surface of the target to vaporize and coagulate tumour tissue under direct vision. An alternative approach is to insert the tip of the laser fibre directly into the target area and to use a much lower power (3-5 W) to gently "cook" diseased tissue over a period of several minutes. This is known as interstitial laser photocoagulation (ILP). There is no selectivity of effect. Both normal and malignant tissue will be necrosed if heated to a high enough temperature for long enough. ILP can be used for the percutaneous treatment of small hepatic metastases (particularly from previously resected, primary colorectal cancers) in patients who are unsuitable or unfit for partial hepatectomy[6]. The key to using this technique succesfully is the imaging. Fibers can be inserted through needles positioned percutaneously under ultrasound, CT (computerised tomography) or MR (magnetic resonance) imaging. The best way to assess the effect is on contrast enhanced CT scans taken 24 h after treatment, which show laser necrosed areas as new zones of non-enhancement. The size of the zone of necrosis depends on the number of laser fibres used, but it is possible to necrose lesions up to about 4-5 cm in diameter. Treated areas heal mainly with regeneration of normal liver. ILP is only appropriate for hepatic metastases in patients with a small number of clearly identifiable lesions and no evidence of extrahepatic disease, but for these individuals, it may slow down the progression of their disease, and is likely to be complimentary to chemotherapy. Radiofrequency heating can achieve a similar thermal effect without the need for lasers.
Photodynamic therapy (PDT) is a technique for producing localised necrosis of tissue with light after prior administration of a photosensitising agent[7]. Drugs that are activated by red light are usually chosen as red light penetrates deeply into tissue, but mucosal disease (e.g. dysplasia in Barrett's oesophagus or squamous oesophagus) might be conveniently treated with green light, which penetrates only 1-2 mm. On their own, neither the light nor the photosensitising drug produces any effect. The biological effect is photochemical with no increase in tissue temperature, so is quite different from the thermal techniques discussed above. ILP and PDT are compared in Table 3. In particular, PDT produces remarkably little effect on connective tissue like collagen, so it is possible to get full thickness necrosis in the wall of the gastrointestinal tract with minimal risk of perforation[8]. The effects produced vary with the photosensitiser used and may prove of value in a surprisingly wide range of conditions. Potential applications are summarised in Table 4.
| ILP | PDT | |
| Nature of biological effect | Thermal | Photochemical |
| Wavelength of light used | Infrared (805-1064 nm) | Green (510-530 nm) Red (630-675 nm) |
| Typical laser power per fibre | 3-5 W | 0.1-0.3 W (higher for illuminating hollow organs) |
| Effect on connective tissue | Destroyed | Largely unaffected |
| Healing | Resorption and scarring, some regeneration | Regeneration, sometimes with scarring |
| Selectivity of necrosis between tissue of origin of tumour and other adjacent tissues | None | Possible between mucosa and underlying muscle in hollow organs |
| Selectivity of necrosis between tumour and tissue of origin of tumour | None | Minimal |
| Cumulative toxicity | None | None |
| Small, inoperable tumours in endoscopically accessible sites |
| Areas of dysplasia, especially in Barrett's oesophagus |
| Localised pancreatic cancer |
| Bile duct cancer |
| As an adjunct to surgery |
| Palliation of advanced cancers-controversial |
| Eradication of Helicobacter pylori-speculative |
PDT is an attractive option for treating small tumours of the gastrointestinal tract in patients who are unsuitable for surgery. In a series of 123 patients with early oesophageal cancers treated with PDT using the photosensitiser porfimer sodium (Photofrin), a complete response (no evidence of tumour on endoscopy or biopsy) was seen in 87% at 6 months[9]. The disease specific survival was 75% although the overall 5 year survival was only 25%, This means that in the majority of the patients, the cancer was not the main cause of death. These patients were inoperable due to comorbidity and this minimally invasive therapy was well tolerated. Care must be taken not to treat too extensive a lesion as circumferential scarring in the muscle layer can cause a stricture. Strictures occured in 35% of the patients in this series, although they did all respond to dilatation. PDT can be applied at any endoscopically accessible site in the upper or lower gastrointestinal tract, but it cannot treat any lesion that has spread beyond the site of origin as, for example, to local lymph nodes.
Although the light for PDT is applied locally, the drug is given systemically, which means that the whole body is photosensitised, including the skin. This can be a problem, as there is a risk of skin damage due to drug activation by ambient light. With the photosensitiser porfimer sodium, patients must avoid bright sunlight for up to 3 months, although with the sensitiser mTHPC (meso-tetrahydroxyphenyl chlorin) it is 2-3 weeks and with ALA (5-amino laevulinic acid), it is only 1-2 days.
PDT has been proposed for the palliation of advanced malignant dysphagia. This was the first application for which PDT was licensed in the USA (using porfimer sodium). PDT does provide some relief in this situation, but there are very few cases that can be helped by PDT if NdYAG laser therapy or stent insertion fail, and it is certainly not desirable to make patients photosensitive for much of their remaining life[10]. PDT may be of value to treat tumour that has grown over or through a stent that cannot be adjusted and which cannot tolerate the heat from a NdYAG laser. In general terms, it seems more logical to licence PDT for treating early oesophageal cancers, as has been done by the Japanese authorities, although the UK seems more likely to follow the American pattern, at least initially.
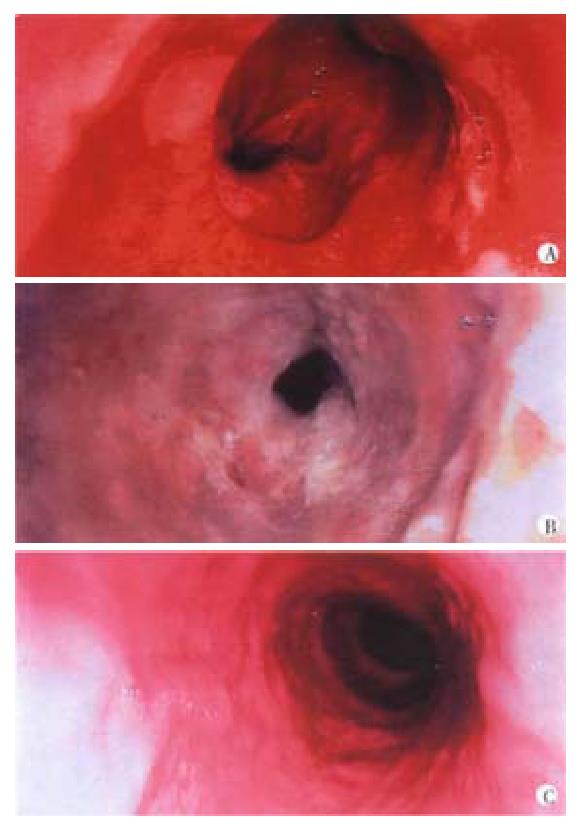
ALA is a naturally occurring substance which is converted in vivo through a series of derivatives to haem via protoporphyrin IX (PPIX). PPIX is a photosensitiser, and after exogenous administration of ALA, enough PPIX accumulates to produce a PDT effect on activation with red or green light[7] (Figure 2). However, in contrast to porfimer sodium and mTHPC, PPIX accumulates primarily in the mucosa of hollow organs. This makes it possible to destroy mucosa (normal or abnormal) without damaging the underlying muscle layer. This is exactly what is required in the treatment of Barrett's oesophagus with dysplasia. In a recent report, using ALA PDT, high grade dysplasia was cleared in 10 of 10 and carcinoma in situ was cleared in 17 out of 22 patients with Barrett's oesophagus[11] and in a randomised placebo controlled trial, this modality had a significant impact on patient outcome[12]. There were no oesophageal strictures, in contrast to 35% of 100 patients with high grade dysplasia or intramucosal carcinoma arising in Barrett's oesophagus who were treated with PDT using porfimer sodium[13]. However, ALA-PDT brings its own problems, as the depth of necrosis does not exceed 2 mm and this is not always sufficient. It is reassuring that the necrosed, neoplastic Barrett's mucosa healed with regeneration of normal squamous mucosa, but follow up biopsies showed that in some cases, there was still untreated columnar mucosa under the new squamous mucosa.
Endoscopic management of Barrett's oesophagus is still a difficult problem. Thermal ablation with an argon plasma coagulation or KTP or NdYAG laser involves moving a small therapeutic beam across the area to be treated under direct endoscopic vision. It is easy to undertreat, and leave abnormal mucosa, or overtreat, with the risk of muscle scarring or even perforation. With PDT, balloon light delivery systems are available which illuminate all the relevant mucosa evenly, and there is very little risk of perforation, but using porfimer sodium, there is a high risk of a stricture as there is no selectivity of effect between the mucosa and underlying layers. PDT with ALA is the only technique at present that seems able to produce a selective effect, necrosing mucosa but not the underlying muscle, but to date, the results are no better than using other ablative techniques. More research is needed to find reliable ways of ensuring that the full thickness of the abnormal mucosa is ablated. In the present situation, surgery is still the treatment of choice for patients with high grade dysplasia or early carcinoma in Barrett's oesophagus. PDT should be reserved for those who are unfit for surgery. Endoscopic treatment of Barrett's oesophagus without evidence of dysplasia should be limited to the context of clinical trials. Since dysplasia and even cancer has been reported to occur in areas treated by PDT which have reverted to 'normal' squamous epithelium after treatment, long term surveillance endoscopy is still needed in patients treated this way.
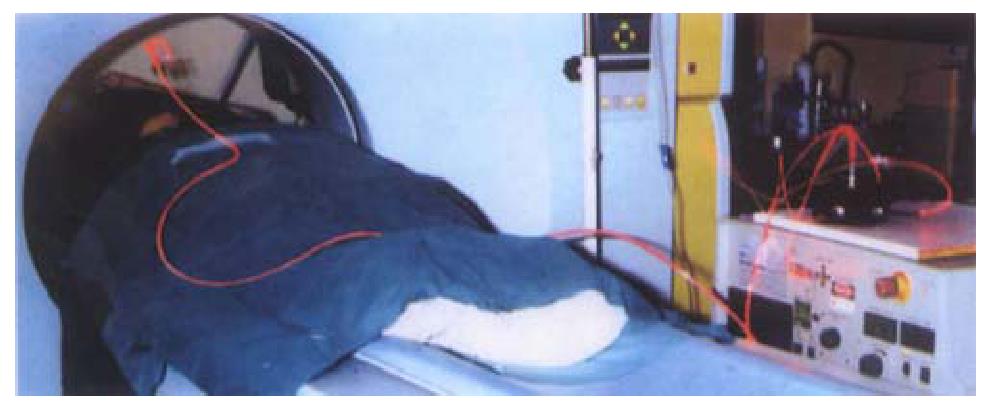
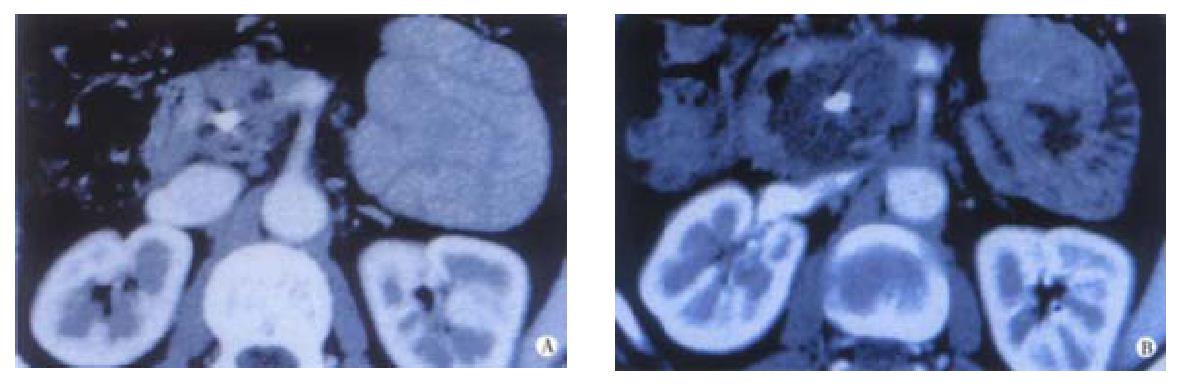
Most of the applications of PDT in gastroenterology have been for lesions of the luminal gut. Interest is now growing in using this modality to treat localised tumours of the pancreas and bile ducts. Animal studies have shown that the pancreas and adjacent normal tissues can tolerate PDT and that necrosis can be produced in cancers transplanted into the hamster pancreas. These results justified a pilot clinical study[14]. The technique is shown in Figure 3. Sixteen patients with small, localised, pancreatic cancers, not involving major blood vessels, were sensitised intravenously with mTHPC. Three days later, up to 4 needles were inserted into the tumour percutaneously under computerised tomography (CT) guidance and laser fibres passed through the needles to activate the drug in the tumour. Contrast enhanced CT scans a few days later showed new areas of devascularisation in the pancreas consistent with PDT induced necrosis (Figure 4). There were no serious complications, and most patients were out of hospital within a week. The median survival from diagnosis was 12.5 months with the longest surviving subject living for 34 months. These preliminary data are encouraging but randomised trials are needed to establish just how successful this aproach will be. At present, it is far too early to judge what role PDT may develop in the management of this unpleasant cancer.
The median survival in patients with unresectable hilar cholangiocarcinoma is 4-6 months due to refractory cholestasis or sepsis. PDT may have a role in the treatment of bile duct cancers, particularly for relief of obstructive jaundice when biliary stents are ineffective or become occluded. Two small uncontrolled studies have shown prolongation of survival in this group of patients to more than one year and interstingly, areas of tumour not directly illuminated have also undergone regression, presumably due to conduction of light through the biliary tree[15,16]. Larger, controlled studies are needed in this area.
A logical application of PDT is as an adjunct to surgery, to destroy small tumour deposits that are not visible to the naked eye or which involve areas that can not be resected. One randomised trial has been reported looking at PDT as an adjunct to resection of rectal cancers, but there was no difference between the two groups in the incidence of local recurrence[17].
A more speculative application of PDT is for the treatment of Helicobacter pylori. With the increasing incidence of antibiotic resistance, it would be attractive to have an alternative therapy and all sites colonised by H. pylori are easily accessible endoscopically for light delivery. The organism is certainly sensitive to PDT in culture, using methylene blue as the photosensitiser, and preliminary ex vivo experiments have also given encouraging results[18], but it would take considerable technical ingenuity to get adequate drug and light to all relevant sites to make this worth trying clinically.
Tissue spectroscopy is a way of interrogating tissue to obtain a characteristic 'tissue signature' that reveals its underlying histology. A number of spectroscopic techniques exist but all exploit the interaction between specific properties of light and the tissues under interrogation.
All tissues exhibit a natural fluorescence. In luminal organs, dysplastic mucosa has a different fluorescence spectrum to normal tissue and this can be detected using specially modified endoscopes to emit blue light (either laser or a broader band source) and very sensitive cameras that detect green or red light. The technique is already proving useful for detecting early lesions in the bronchial tree. The accuracy of this technique is also under investigation for dysplasia in Barrett's oesophagus or in chronic ulcerative colitis but in both cases, the findings are confounded by the weak signals generated and the small differences between the fluorescence signal of normal, inflamed and dysplastic tissues. Studies are also underway to enhance the differences between normal and dysplasia by giving a drug exogenously that is selectively concentrated in the areas of dysplasia but this does not as yet appear to be beneficial. Although the idea is promising, real clinical application still seems a long way away.
This technique exploits the difference in scattering of light of various wavelengths by different sized particles-the same principle that explains why the sky is blue and sunsets are red. It uses white light passed down a probe placed through the biopsy channel of an endoscope. This is a point measurement, like a biopsy but has the advantage of almost instantaneous diagnosis. Again, this is in early stages of development, but it is cheap, does not require complex equipment and initial reports suggest a high degree of accuracy[19,20].
Thermal laser is an established tool for endoscopic palliation of advanced gastrointestinal tract cancers. Interstitial laser photocoagulation may help in the management of patients with a small number of isolated hepatic metastases. However, the most important new applications of lasers being developed in gastroenterology are in photodynamic therapy, particularly for the endoscopic treatment of dysplasia in Barrett's oesophagus; for the treatment of small inoperable tumours in the gastrointestinal tract and in the pancreatobiliary system. Finally, light based 'optical' methods are under investigation for instantaneous diagnosis in the gut and these may enable more accurate and faster diagnosis than have been available hitherto. The latter techniques are only at an early stage of clinical trials, but if these studies are successful, PDT and optical diagnosis could find an important role in gastroenterology practice.
Edited by Ma JY
| 1. | Bown SG. Palliation of malignant dysphagia: surgery, radiotherapy, laser, intubation alone or in combination. Gut. 1991;32:841-844. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 76] [Cited by in F6Publishing: 79] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 2. | Cwikiel W, Tranberg KG, Cwikiel M, Lillo-Gil R. Malignant dysphagia: palliation with esophageal stents--long-term results in 100 patients. Radiology. 1998;207:513-518. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 96] [Cited by in F6Publishing: 98] [Article Influence: 3.8] [Reference Citation Analysis (0)] |
| 3. | Lovat LB, Mathou N, Thorpe SM, Gertner D, Winslet MC, Bown SG. Relief of dysphagia with self expanding metal stents is far from perfect. Gut. 2000;46:W22. [Cited in This Article: ] |
| 4. | Sculpher MJ, Sargeant IR, Loizou LA, Thorpe SM, Spencer GM, Bown SG. A cost analysis of Nd: YAG laser ablation versus endoscopic intubation for the palliation of malignant dysphagia. Eur J Cancer. 1995;31A:1640-1646. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 7] [Cited by in F6Publishing: 9] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 5. | Brunetaud JM, Maunoury V, Ducrotte P, Cochelard D, Cortot A, Paris JC. Palliative treatment of rectosigmoid carcinoma by laser endoscopic photoablation. Gastroenterology. 1987;92:663-668. [PubMed] [Cited in This Article: ] |
| 6. | Amin Z, Donald JJ, Masters A, Kant R, Steger AC, Bown SG, Lees WR. Hepatic metastases: interstitial laser photocoagulation with real-time US monitoring and dynamic CT evaluation of treatment. Radiology. 1993;187:339-347. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 216] [Cited by in F6Publishing: 224] [Article Influence: 7.2] [Reference Citation Analysis (0)] |
| 7. | Dougherty TJ, Gomer CJ, Henderson BW, Jori G, Kessel D, Korbelik M, Moan J, Peng Q. Photodynamic therapy. J Natl Cancer Inst. 1998;90:889-905. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 4038] [Cited by in F6Publishing: 3662] [Article Influence: 140.8] [Reference Citation Analysis (0)] |
| 8. | Barr H, Tralau CJ, Boulos PB, MacRobert AJ, Tilly R, Bown SG. The contrasting mechanisms of colonic collagen damage between photodynamic therapy and thermal injury. Photochem Photobiol. 1987;46:795-800. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 103] [Cited by in F6Publishing: 110] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 9. | Sibille A, Lambert R, Souquet JC, Sabben G, Descos F. Long-term survival after photodynamic therapy for esophageal cancer. Gastroenterology. 1995;108:337-344. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 168] [Cited by in F6Publishing: 147] [Article Influence: 5.1] [Reference Citation Analysis (0)] |
| 10. | Bown SG, Millson CE. Photodynamic therapy in gastroenterology. Gut. 1997;41:5-7. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 25] [Cited by in F6Publishing: 20] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 11. | Gossner L, Stolte M, Sroka R, Rick K, May A, Hahn EG, Ell C. Photodynamic ablation of high-grade dysplasia and early cancer in Barrett's esophagus by means of 5-aminolevulinic acid. Gastroenterology. 1998;114:448-455. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 300] [Cited by in F6Publishing: 295] [Article Influence: 11.3] [Reference Citation Analysis (0)] |
| 12. | Ackroyd R, Brown NJ, Davis MF, Stephenson TJ, Marcus SL, Stoddard CJ, Johnson AG, Reed MW. Photodynamic therapy for dysplastic Barrett's oesophagus: a prospective, double blind, randomised, placebo controlled trial. Gut. 2000;47:612-617. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 147] [Cited by in F6Publishing: 152] [Article Influence: 6.3] [Reference Citation Analysis (0)] |
| 13. | Overholt BF, Panjehpour M, Haydek JM. Photodynamic therapy for Barrett's esophagus: follow-up in 100 patients. Gastrointest Endosc. 1999;49:1-7. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 364] [Cited by in F6Publishing: 321] [Article Influence: 12.8] [Reference Citation Analysis (0)] |
| 14. | Rogowska AZ, Whitelaw DE, Lees WR, Gillams AR, Ripley PM, Buonaccorsi GA. Photodynamic therapy for palliation of unresectable pancreatic cancer. Gut. 1999;44:W191. [Cited in This Article: ] |
| 15. | Berr F, Wiedmann M, Tannapfel A, Halm U, Kohlhaw KR, Schmidt F, Wittekind C, Hauss J, Mössner J. Photodynamic therapy for advanced bile duct cancer: evidence for improved palliation and extended survival. Hepatology. 2000;31:291-298. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 153] [Cited by in F6Publishing: 158] [Article Influence: 6.6] [Reference Citation Analysis (0)] |
| 16. | Ortner MA, Liebetruth J, Schreiber S, Hanft M, Wruck U, Fusco V, Müller JM, Hörtnagl H, Lochs H. Photodynamic therapy of nonresectable cholangiocarcinoma. Gastroenterology. 1998;114:536-542. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 209] [Cited by in F6Publishing: 174] [Article Influence: 6.7] [Reference Citation Analysis (0)] |
| 17. | Ansell , JK , Abulafi AM, Allardice JT, De Jode ML, Grahn MF, Williams NS. Adjuvant intraoperative photodynamic therapy for colorectal cancer. Br J Surg. 1996;83:694. [Cited in This Article: ] |
| 18. | Bown SG, Lovat LB. The biology of photodynamic therapy in the gastrointestinal tract. Gastrointest Endosc Clin N Am. 2000;10:533-550. [PubMed] [Cited in This Article: ] |
| 19. | Lovat LB, Pickard D, Novelli M, Ripley PM, Francis H, Bigo IJ. A novel 'optical biopsy' technique using elastic scattering spectroscopy for dysplasia and cancer in Barrett's oesophagus. Gut. 2000;46:TH1. [Cited in This Article: ] |
| 20. | Messmann H, Knüchel R, Bäumler W, Holstege A, Schölmerich J. Endoscopic fluorescence detection of dysplasia in patients with Barrett's esophagus, ulcerative colitis, or adenomatous polyps after 5-aminolevulinic acid-induced protoporphyrin IX sensitization. Gastrointest Endosc. 1999;49:97-101. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 90] [Cited by in F6Publishing: 89] [Article Influence: 3.6] [Reference Citation Analysis (0)] |