Published online Mar 14, 2008. doi: 10.3748/wjg.14.1504
Revised: November 13, 2007
Published online: March 14, 2008
AIM: To investigate apoptosis in human pancreatic cancer cells induced by Triptolide (TL), and the relationship between this apoptosis and expression of caspase-3’ bcl-2 and bax.
METHODS: Human pancreatic cancer cell line SW1990 was cultured in DMEM media for this study. MTT assay was used to determine the cell growth inhibitory rate in vitro. Flow cytometry and TUNEL assay were used to detect the apoptosis of human pancreatic cancer cells before and after TL treatment. RT-PCR was used to detect the expression of apoptosis-associated gene caspase-3’ bcl-2 and bax.
RESULTS: TL inhibited the growth of human pancreatic cancer cells in a dose-and time-dependent manner. TL induced human pancreatic cancer cells to undergo apoptosis with typically apoptotic characteristics. TUNEL assay showed that after the treatment of human pancreatic cancer cells with 40 ng/mL TL for 12 h and 24 h, the apoptotic rates of human pancreatic cancer cells increased significantly. RT-PCR demonstrated that caspase-3 and bax were significantly up-regulated in SW1990 cells treated with TL while bcl-2 mRNA was not.
CONCLUSION: TL is able to induce the apoptosis in human pancreatic cancer cells. This apoptosis may be mediated by up-regulating the expression of apoptosis-associated caspase-3 and bax gene.
- Citation: Zhou GX, Ding XL, Huang JF, Zhang H, Wu SB, Cheng JP, Wei Q. Apoptosis of human pancreatic cancer cells induced by Triptolide. World J Gastroenterol 2008; 14(10): 1504-1509
- URL: https://www.wjgnet.com/1007-9327/full/v14/i10/1504.htm
- DOI: https://dx.doi.org/10.3748/wjg.14.1504
Pancreatic adenocarcinoma is characterized by a poor prognosis and lack of response to conventional therapy. The incidence has shown that no significant sign of decline throughout the past 20 years and almost equals its mortality[1–3]. The 5-year survival rate for this disease is less than 4% and the median survival time after diagnosis is less than 6 mo[23]. Surgical resection of the tumor is still the only effective treatment option, although only 20% of carcinomas of the head of the pancreas are resectable[4].Furthermore, the median survival even after apparent curative resection is only 20 mo, because of early tumor recurrence or rapid metastatic spread[24]. Other treatment options, such as chemotherapy or radiation therapy, provide limited palliation without significant improvement of survival in patients with unresectable pancreatic cancer[2]. Therefore; new targets for chemo-preventive and therapeutic agents need to be identified.
Triptolide (TL), extracts of the Chinese herb Tripterygium Wilfordii hook have potent anti-inflammatory and immunosuppressive properties and have been used successfully in traditional Chinese medicine for the treatment of rheumatoid arthritis and lupus erythematosus[56]. It has been recently reported that TL possesses anti-tumor and proapoptotic activities in many different tumor cell lines, including breast, prostate, lung, and leukemia cells lines[7–13]. TL was also shown to sensitize cells to death induced by a variety of agents, such as Apo2/Trail, TNF-α, and different chemotherapeutic agents[14–16]. In this study, we provide evidence that TL potently inhibits human pancreatic cancer cell lines growth in vitro, suggesting that TL could be used to prevent or treat pancreatic cancer in the future. Considerable studies indicated that TL functioned through a p53-dependent or independent way[11141718]. Recently, Bing et al suggested that TL induced caspase-dependent cell death via the mitochondrial pathway in leukemia cells[19]. However, the cellular and molecular mechanisms underlying TL-induced apoptosis in tumor cells are not fully understood.
The purpose of this study is to investigate the inhibitory effects of TL on apoptosis and angiogenesis of pancreatic cancer in vitro and further to explore whether TL exerts clinical therapeutic value for patients with pancreatic cancer.
The human pancreatic cancer cell line SW1990 was obtained from Professor Wang Xing Peng, Shanghai JiaoTong University (Shanghai, China). SW1990 was cultured in DMEM media. Media was supplemented with 10% FBS and cells grown as monolayers in a humidified atmosphere at 37°C. Crystalline TL (PG490, purity 99%) was obtained from the Institute of Dermatology, Chinese Academy of Medical Sciences (Nanjing, PR China), and prepared as previously described[18].
Cells were inoculated onto 96-well plates at the density of 4 × 103 cells/well for 24 h, then treated with various concentrations of TL (100 &mgr;L/well), and incubated for 6, 12, 24 and 48 h, respectively. Then, 5 g/L MTT solution (20 &mgr;L/well) was added to each well, and cells were incubated for an additional 4 h at 37°C. The supernatant was aspirated, and 100 &mgr;L of DMSO was added to the wells to dissolve any precipitate present. The suspension was placed on micro-vibrator for 5 min and the absorbance (A) was then measured at 570 nm by an enzyme immunoassay instrument. Cell inhibitory ratio was calculated by the following formula: Inhibitory ratio (%) = (1-A570 of treated group)/(A570 of control group) × 100%. IC50 was calculated by SAS statistical software.
An early indicator of apoptosis is the rapid translocation and accumulation of the membrane phospholipid phosphatidylserine from the cytoplasmic interface to the extracellular surface. This loss of membrane asymmetry can be detected using the binding properties of Annexin V. To detect apoptosis, Annexin V binding assay was used. After treatment TL 40 ng/mL for 24 h, cells were collected. Cell suspension was added with 10 &mgr;L of fluorescein-conjugated Annexin V (10 &mgr;g/mL) and 10 &mgr;L of PI reagent (50 &mgr;g/mL) and detected by flow cytometry.
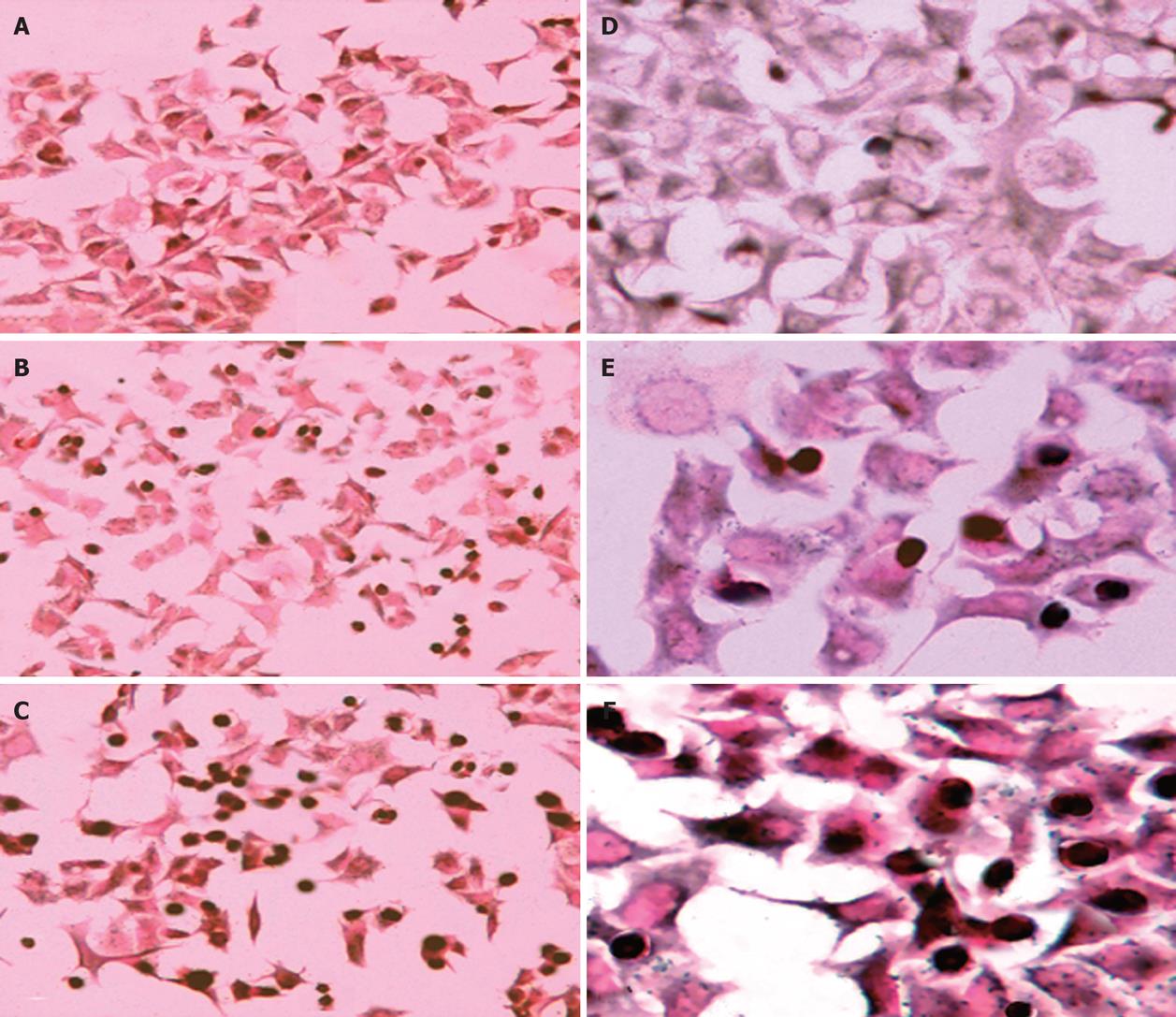
Cells were seeded and set up in chamber slides (Becton Dickinson, Franklin Lakes, NJ), then analyzed for in site apoptosis using the terminal deoxynucleotidyl transferase (TdT)-mediated d-UTP nick end labeling (TUNEL) method. The DNA fragments in apoptotic cells were labeled at free 3’-OH DNA ends and DNA strand breaks. Incorporated fluorescein was detected by anti-fluorescein antibody conjugated with alkaline phosphatase. After substrate reaction stained cells were analyzed with light microscopy.
The human pancreatic cancer cells were treated in the presence or absence of 40 ng/mL, 80 ng/mL, 160 ng/mL TL for 48 h and total RNA was extracted with TRIzol reagent (Invitrogen Life Technologies). Real-time quantitative PCR was performed by using a cycler (Rocha) and SYBR Green Dye. The primer sequences for genes bcl-2: sense 5’-TCCATGTCTTTGGACAACCA-3’, antisense 5’-CTCCACCAGTGTTCCCATCT-3’; bax: sense 5’-TCCATGTCTTTGGACAACCA-3’, antisense 5’-CTCCACCAGTGTTCCCATCT-3’, caspase-3: sense 5’-AACTGGACTGTGGCATTG-3’, antisense 5’-ACCAGGTGCTGTGGAGTA-3’; β-actin: sense 5’-AAGTACTCCGTGTGGATCGG-3’, antisense 5’-ATGCATTCACCTCCCCTGTG-3’. The experimental procedure was carried out according to the QIAGEN one step RT-PCR Kit. The reaction parameters of bcl-2, bax and caspase-3 were as follows: 94°C 5 min, 94°C 40 s, 56°C 55 s, 72°C 1 min for 36 cycles, 72°C extension 7 min; 94°C 5 min, 94°C 40 s, 58°C 55 s, 72°C 1 min for 36 cycles, 72°C extension 7 min; 94°C 5 min, 94°C 40 s, 50°C 55 s, 72°C 1 min for 36 cycles, 72°C extension 7 min. Ten &mgr;L PCR product was placed onto 1.5% agarose gel and observed by EB staining using Gel-Pro analyzer.
All data were expressed as mean ± SD. Intergroup comparisons were made using one-way analysis of variance (ANOVA). A P value less than 0.05 was considered statistically significant.
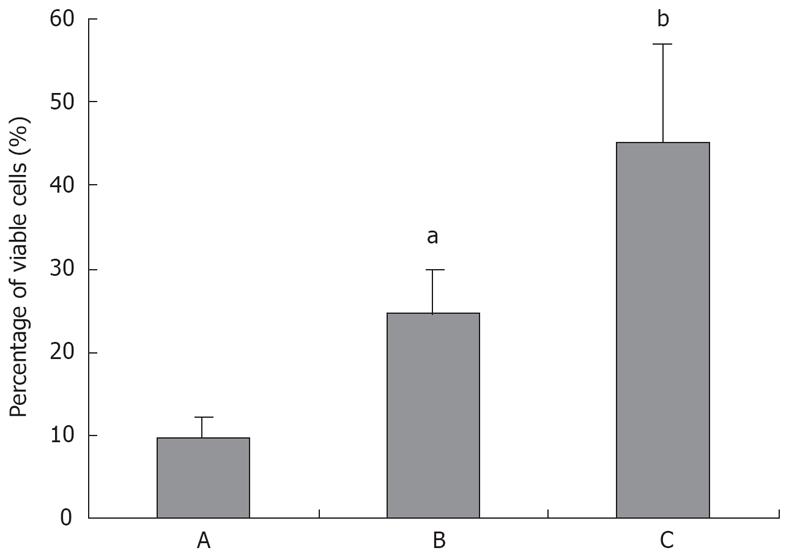
The human pancreatic cancer cells were exposed to increasing concentrations (20 ng/mL to 160 ng/mL) of TL for 6 h to 48 h, respectively. The human pancreatic cancer cells showed death in a dose- and time-dependent manner. The data are summarized in Figure 1.
Although previous studies reported that TL treatment showed anti-proliferation and induction of apoptosis in some cancer cells, there is heretofore no data on the usage of TL in pancreatic cancer. In the present study, we tested the effects of TL on the viability of the human pancreatic cancer cell line SW1990. Our results showed that treatment with TL resulted in a dose-dependent decrease in viable cells in SW1990 cells (Figure 2). After treatment with 160 ng/mL TL for 24 h, the percentage of dead cells was 45.1% in TL cells, while that was only 9.6% in the control group.
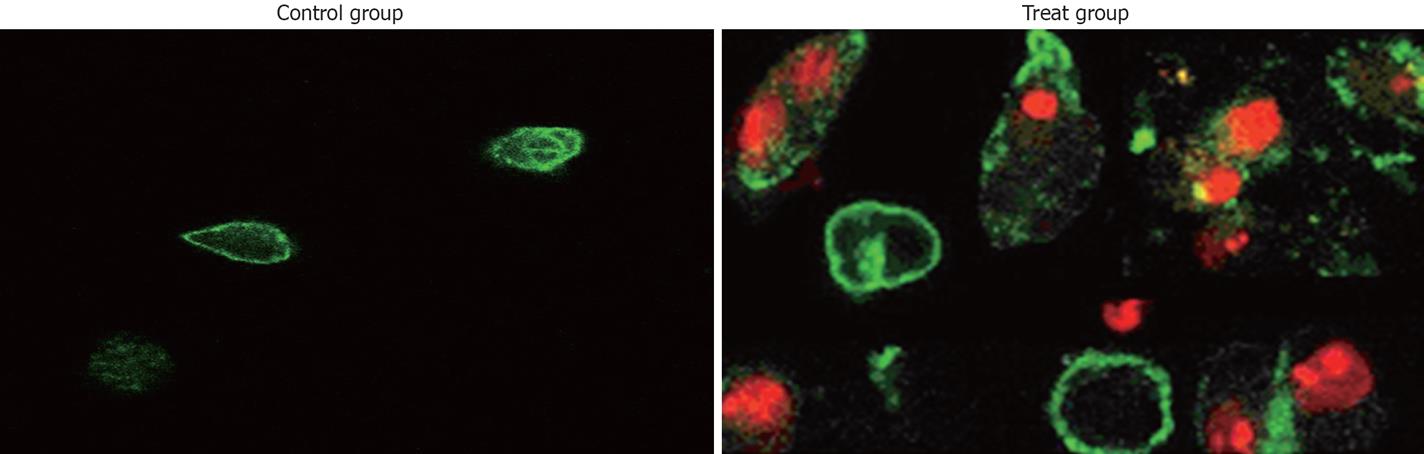
We examined whether the reduced cell viability by TL in pancreatic cancer cell lines was due to induction of apoptosis, because SW1990 cell line seemed to be more sensitive to TL treatment, we chose that cell line and analyzed apoptosis using Annexin V/propidium iodine staining. FCS analysis revealed typical apoptotic phenotype in cells treated with 40 ng/mL TL for 24 h (Figure 3), in contrast, control cells without TL treatment displayed a small quantity of apoptosis. Moreover, we performed TUNEL assay and demonstrated that 40 ng/mL TL treatment could significantly induce apoptosis (Figure 4). These data provided first evidence that TL can be used to inhibit human pancreatic cell line growth in vitro and its effect is associated with induction apoptosis.
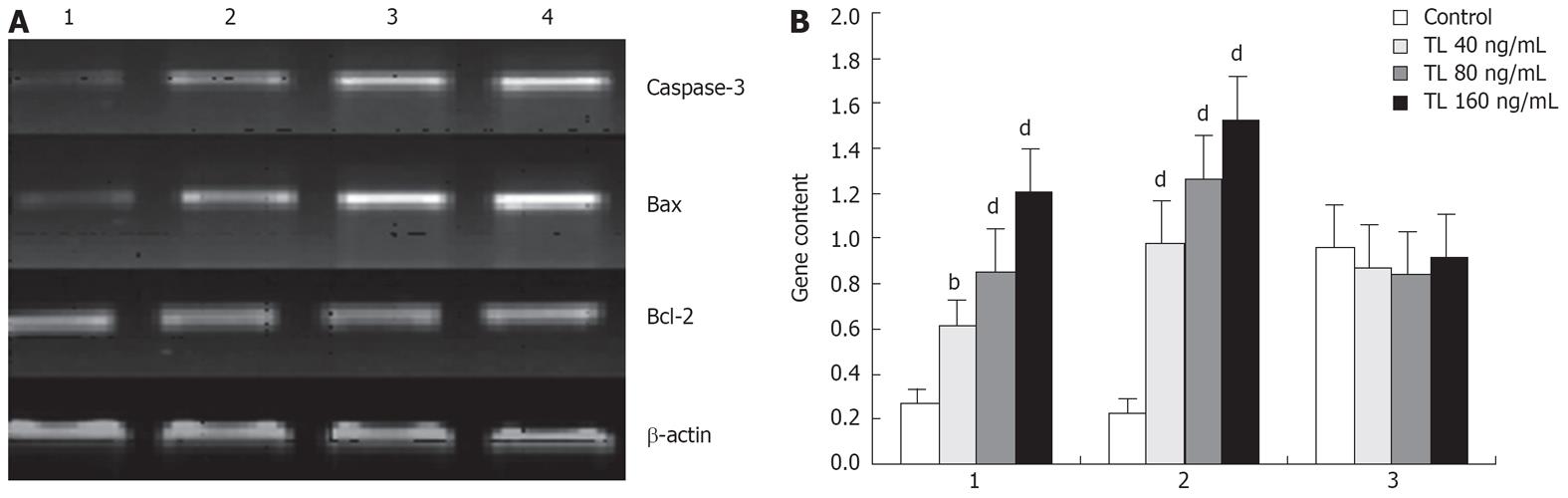
After exposure to 40 ng/mL, 80 ng/mL, 160 ng/mL TL for 48 h, caspase-3 and bax mRNA expression were up-regulated remarkably in TL-treated cells. In contrast, the expression of bcl-2 mRNA was not affected by TL (Figure 5).
Recent studies revealed many properties of TL relevant to anticancer activity, and anti-inflammatory activity besides. Theproliferative and proapoptotic activity of TL has been shown with many different types of cancer cells in vitro and in vivo [821–27]. We therefore decided to test the effects of Triptolide on primary cultures of pancreatic cancer cells to see if this compound would show antitumor activity. In this study, we found that human pancreatic cancer cells treated with TL showed death in a dose- and time-dependent manner in the MTT assay. This suggested TL was able to restrain the growth of human pancreatic cancer cells. It was found that TL has the potential to induce the apoptosis of human pancreatic cancer cells.
TL has potent anticancer activity. However, the mechanism by which TL exerts its anticancer activities remains unclear. To explore the molecular mechanisms involved in the anticancer activity of TL, we have examined the effect of TL on the growth of pancreatic carcinoma SW1990 cells. After exposure to 40 ng/mL, 80 ng/mL, 160 ng/mL TL for 48 h, treatment of SW1990 cells with TL caused a marked up-regulation of caspase-3 and bax mRNA expression. In contrast, the expression of bcl-2 mRNA was not affected by TL. Thus it is likely that TL-induced apoptosis of pancreatic carcinoma SW1990 cells has no relation with bcl-2. The study by Wang et al[28] has shown that treatment of pancreatic cancer cells with gypenosides extracted from TL does not affect the Bcl-2 expression. This is the reason for our focusing on Bax proteins in exploring the effects of TL on mitochondria-dependent cell death pathway. It is shown that the expression of the Bax in SW1990 cells can be up-regulated by TL in our study. Meanwhile the expression of Bcl-2 was not affected by TL treatment.
Our previous study clearly indicated that TL was able to inhibit 5-LOX gene expression in pancreatic cell lines, which contributes to TL proapoptotic activity[29]. Wang et al[28] examined the effect of TL on the growth of pancreatic carcinoma PANC-1 and cervical adenocarcinoma HeLa cells. They found that TL potently suppressed cell growth and induced apoptosis in HeLa and PANC-1 cells, which was indicated by nuclear fragmentation and blebbing. In both HeLa and PANC-1 cells, apoptosis induced by TL was associated with activation of caspase-3 and caspase-8, and with cleavage of poly (ADP-ribose) polymerase and Bid. Moreover, in HeLa cells, caspase-9 is also significantly activated in response to TL. Interestingly, substitution of the 14-OH of TL with an acetyl group abrogated both its anticancer and its antiinflammatory activities. Wang et al[26] studies suggest that TL may exert its anticancer effects by initiating apoptosis through both death-receptor- and mitochondria-mediated pathways. Their results indicate that both the apoptosis-promoting and the antiinflammatory activities of TL depend on the 14-OH group. Liu et al have shown that caspase 3 is responsible for TL-induced Dendritic cell apoptosis, supported by observations that TL can activate caspase 3 and that specific inhibition of caspase 3 can abrogate the apoptotic effects of TL on Dendritic cell[28].
The present study demonstrated that TL was able to induce the apoptosis in Human pancreatic cancer. This apoptosis may be mediated by up-regulating the expression of apoptosis-associated gene caspase-3 and bax. TL may be used as a potential anti-pancreatic carcinoma chemotherapeutic drug.
Pancreatic adenocarcinoma is of a poor prognosis and lack of response to conventional therapy. Among the treatment options, surgical resection of the tumor is still the most effective. The 5-year survival rate for this disease is very low even after curative resection. Therefore; new targets for chemopreventive and therapeutic agents need to be identified. Triptolide (TL) is an extract of the Chinese herb Tripterygium Wilfordii hook. It has been reported that TL possesses anti-tumor and proapoptotic activities in many different tumor cell lines, which functions through p53-dependent or independently[10121516]. Recently, Bing suggested that TL induced caspase-dependent apoptosis via the mitochondrial pathway in leukemia cells. However, the cellular and molecular mechanisms underlying TL-induced apoptosis in tumor cells have not been fully understood.
Pancreatic adenocarcinoma remains one of the most lethal of malignancies. The incidence of pancreatic cancer has steadily increased over the past four decades. Satisfactory treatment is available only for the minority of patients who present with very early-stage disease. There are several articles that TL possesses anti-tumor and proapoptotic activities in many different tumor cell lines, including breast, prostate, lung, and leukemia cells line. TL was also shown to sensitize cells to death induced by a variety of agents, such as Apo2/Trail, TNF-α, and different chemotherapeutic agents
This study gives us new knowledge of TL and proof that TL was able to induce apoptosis in human pancreatic cancer. This apoptosis may be mediated by up-regulating the expression of apoptosis-associated gene caspase-3 and bax. TL may be used as a potential anti-pancreatic carcinoma chemotherapeutic drug and as a clinical application in the future.
The results from this study confirm that TL potently induces the apoptosis of human pancreatic cancer cells, which may be mediated by up-regulating the expression of apoptosis-associated gene caspase-3 and bax. It can be seen from this article that TL may be used to prevent or treat pancreatic cancer in the future.
TL extracts of the Chinese herb Tripterygium Wilfordii hook have potent anti-inflammatory and immunosuppressive properties and have been used successfully in traditional Chinese medicine for the treatment of rheumatoid arthritis and lupus erythematosus.
This article is of considerable interest and potential importance in demonstrating in vitro activity of the herbal extract TL against a human pancreatic cell line by inducing apoptosis. This work should stimulate additional in vitro studies in human cell lines, experiments involving animal models of pancreatic cancer, and, possibly, clinical testing of TL.
| 1. | Greenlee RT, Murray T, Bolden S, Wingo PA. Cancer statistics, 2000. CA Cancer J Clin. 2000;50:7-33. [Cited in This Article: ] |
| 2. | Howard TJ. Pancreatic adenocarcinoma. Curr Probl Cancer. 1996;20:281-328. [Cited in This Article: ] |
| 3. | Parker SL, Tong T, Bolden S, Wingo PA. Cancer statistics, 1996. CA Cancer J Clin. 1996;46:5-27. [Cited in This Article: ] |
| 4. | Yeo CJ, Cameron JL. Improving results of pancreati-coduodenectomy for pancreatic cancer. World J Surg. 1999;23:907-912. [Cited in This Article: ] |
| 5. | Tao XL, Sun Y, Dong Y, Xiao YL, Hu DW, Shi YP, Zhu QL, Dai H, Zhang NZ. A prospective, controlled, double-blind, cross-over study of tripterygium wilfodii hook F in treatment of rheumatoid arthritis. Chin Med J (Engl). 1989;102:327-332. [Cited in This Article: ] |
| 6. | Ma J, Dey M, Yang H, Poulev A, Pouleva R, Dorn R, Lipsky PE, Kennelly EJ, Raskin I. Anti-inflammatory and immunosuppressive compounds from Tripterygium wilfordii. Phytochemistry. 2007;68:1172-1178. [Cited in This Article: ] |
| 7. | Chen BJ. Triptolide, a novel immunosuppressive and anti-inflammatory agent purified from a Chinese herb Tripterygium wilfordii Hook F. Leuk Lymphoma. 2001;42:253-265. [Cited in This Article: ] |
| 8. | Shamon LA, Pezzuto JM, Graves JM, Mehta RR, Wangcharoentrakul S, Sangsuwan R, Chaichana S, Tuchinda P, Cleason P, Reutrakul V. Evaluation of the mutagenic, cytotoxic, and antitumor potential of triptolide, a highly oxygenated diterpene isolated from Tripterygium wilfordii. Cancer Lett. 1997;112:113-117. [Cited in This Article: ] |
| 9. | Wei YS, Adachi I. Inhibitory effect of triptolide on colony formation of breast and stomach cancer cell lines. Zhongguo Yaoli Xuebao. 1991;12:406-410. [Cited in This Article: ] |
| 10. | Yang S, Chen J, Guo Z, Xu XM, Wang L, Pei XF, Yang J, Underhill CB, Zhang L. Triptolide inhibits the growth and metastasis of solid tumors. Mol Cancer Ther. 2003;2:65-72. [Cited in This Article: ] |
| 11. | Kiviharju TM, Lecane PS, Sellers RG, Peehl DM. Antiproliferative and proapoptotic activities of triptolide (PG490), a natural product entering clinical trials, on primary cultures of human prostatic epithelial cells. Clin Cancer Res. 2002;8:2666-2674. [Cited in This Article: ] |
| 12. | Yang Y, Liu Z, Tolosa E, Yang J, Li L. Triptolide induces apoptotic death of T lymphocyte. Immunopharmacology. 1998;40:139-149. [Cited in This Article: ] |
| 13. | Phillips PA, Dudeja V, McCarroll JA, Borja-Cacho D, Dawra RK, Grizzle WE, Vickers SM, Saluja AK. Triptolide induces pancreatic cancer cell death via inhibition of heat shock protein 70. Cancer Res. 2007;67:9407-9416. [Cited in This Article: ] |
| 14. | Lee KY, Chang W, Qiu D, Kao PN, Rosen GD. PG490 (triptolide) cooperates with tumor necrosis factor-alpha to induce apoptosis in tumor cells. J Biol Chem. 1999;274:13451-13455. [Cited in This Article: ] |
| 15. | Lee KY, Park JS, Jee YK, Rosen GD. Triptolide sensitizes lung cancer cells to TNF-related apoptosis-inducing ligand (TRAIL)-induced apoptosis by inhibition of NF-kappaB activation. Exp Mol Med. 2002;34:462-468. [Cited in This Article: ] |
| 16. | Chang WT, Kang JJ, Lee KY, Wei K, Anderson E, Gotmare S, Ross JA, Rosen GD. Triptolide and chemotherapy cooperate in tumor cell apoptosis. A role for the p53 pathway. J Biol Chem. 2001;276:2221-2227. [Cited in This Article: ] |
| 17. | Jiang XH, Wong BC, Lin MC, Zhu GH, Kung HF, Jiang SH, Yang D, Lam SK. Functional p53 is required for triptolide-induced apoptosis and AP-1 and nuclear factor-kappaB activation in gastric cancer cells. Oncogene. 2001;20:8009-8018. [Cited in This Article: ] |
| 18. | Chan EW, Cheng SC, Sin FW, Xie Y. Triptolide induced cytotoxic effects on human promyelocytic leukemia, T cell lymphoma and human hepatocellular carcinoma cell lines. Toxicol Lett. 2001;122:81-87. [Cited in This Article: ] |
| 19. | Carter BZ, Mak DH, Schober WD, McQueen T, Harris D, Estrov Z, Evans RL, Andreeff M. Triptolide induces caspase-dependent cell death mediated via the mitochondrial pathway in leukemic cells. Blood. 2006;108:630-637. [Cited in This Article: ] |
| 20. | Liu Q, Chen T, Chen H, Zhang M, Li N, Lu Z, Ma P, Cao X. Triptolide (PG-490) induces apoptosis of dendritic cells through sequential p38 MAP kinase phosphorylation and caspase 3 activation. Biochem Biophys Res Commun. 2004;319:980-986. [Cited in This Article: ] |
| 21. | Wei YS, Adachi I. Inhibitory effect of triptolide on colony formation of breast and stomach cancer cell lines. Zhongguo Yaoli Xuebao. 1991;12:406-410. [Cited in This Article: ] |
| 22. | Chan EW, Cheng SC, Sin FW, Xie Y. Triptolide induced cytotoxic effects on human promyelocytic leukemia, T cell lymphoma and human hepatocellular carcinoma cell lines. Toxicol Lett. 2001;122:81-87. [Cited in This Article: ] |
| 23. | Kupchan SM, Court WA, Dailey RG Jr, Gilmore CJ, Bryan RF. Triptolide and tripdiolide, novel antileukemic diterpenoid triepoxides from Tripterygium wilfordii. J Am Chem Soc. 1972;94:7194-7195. [Cited in This Article: ] |
| 24. | Qiu D, Zhao G, Aoki Y, Shi L, Uyei A, Nazarian S, Ng JC, Kao PN. Immunosuppressant PG490 (triptolide) inhibits T-cell interleukin-2 expression at the level of purine-box/nuclear factor of activated T-cells and NF-kappaB transcriptional activation. J Biol Chem. 1999;274:13443-13450. [Cited in This Article: ] |
| 25. | Yinjun L, Jie J, Yungui W. Triptolide inhibits transcription factor NF-kappaB and induces apoptosis of multiple myeloma cells. Leuk Res. 2005;29:99-105. [Cited in This Article: ] |
| 26. | Yang S, Chen J, Guo Z, Xu XM, Wang L, Pei XF, Yang J, Underhill CB, Zhang L. Triptolide inhibits the growth and metastasis of solid tumors. Mol Cancer Ther. 2003;2:65-72. [Cited in This Article: ] |
| 27. | Lin J, Chen LY, Lin ZX, Zhao ML. The effect of triptolide on apoptosis of glioblastoma multiforme (GBM) cells. J Int Med Res. 2007;35:637-643. [Cited in This Article: ] |
| 28. | Wang X, Matta R, Shen G, Nelin LD, Pei D, Liu Y. Mechanism of triptolide-induced apoptosis: Effect on caspase activation and Bid cleavage and essentiality of the hydroxyl group of triptolide. J Mol Med. 2006;84:405-415. [Cited in This Article: ] |
| 29. | Zhou GX, Ding XL, Huang JF, Zhang H, Wu SB. Suppression of 5-lipoxygenase gene is involved in triptolide-induced apoptosis in pancreatic tumor cell lines. Biochim Biophys Acta. 2007;1770:1021-1027. [Cited in This Article: ] |
| 30. | Liu Q, Chen T, Chen H, Zhang M, Li N, Lu Z, Ma P, Cao X. Triptolide (PG-490) induces apoptosis of dendritic cells through sequential p38 MAP kinase phosphorylation and caspase 3 activation. Biochem Biophys Res Commun. 2004;319:980-986. [Cited in This Article: ] |