Published online Sep 14, 2005. doi: 10.3748/wjg.v11.i34.5327
Revised: February 13, 2005
Accepted: February 18, 2005
Published online: September 14, 2005
AIM: To investigate whether American ginseng (AG, Panax quinquefolium) supplementation was able to improve endurance exercise performance.
METHODS: Thirteen physically active male college students were divided into two groups (AG or placebo) and received supplementation for 4 wk, before the exhaustive running exercise. Treadmill speed was increased to a pace equivalent to 80% VO2max of the subject. A 4-wk washout period followed before the subjects crossed over and received the alternate supplement for the next 4 wk. They then completed a second exhaustive running exercise. The physiological variables that were examined included time to exhaustion and oxygen pulse. Moreover, the plasma creatine kinase (CK) and lactate were measured prior to the exercise, at 15 and 30 min during exercise, immediately after exercise, and 20, 40, 60, and 120 min after exercise.
RESULTS: The major finding of this investigation was that the production plasma CK during the exercise significantly decreased for group AG than for group P. Secondary physiological finding was that 80% VO2max running was not improved over a 4-wk AG supplementation regimen.
CONCLUSION: Supplementation with AG for 4 wk prior to an exhaustive aerobic treadmill running reduced the leakage of CK during exercise, but did not enhance aerobic work capacity. The reduction of plasma CK may be due to the fact that AG is effective for the decrease of skeletal muscle cell membrane damage, induced by exercise during the high-intensity treadmill run.
- Citation: Hsu CC, Ho MC, Lin LC, Su B, Hsu MC. American ginseng supplementation attenuates creatine kinase level induced by submaximal exercise in human beings. World J Gastroenterol 2005; 11(34): 5327-5331
- URL: https://www.wjgnet.com/1007-9327/full/v11/i34/5327.htm
- DOI: https://dx.doi.org/10.3748/wjg.v11.i34.5327
Traditional Chinese medicine plays a key role in the formation of integrative medicine[1]. Ginseng (Genus Panax) root has been a popular Chinese medicine with the belief of restoring Qi or life energy. It is also thought to be a tonic to stimulate appetite, counteract fatigue, boost the immune system, relieve pain and headaches, and improve mental function and physical stamina[2]. The mechanism of action of ginseng is not known, but it is thought to have effects on production of corticotropin and cortisol, immunomodulation, antioxidants and neuroendocrine activity, modulate carbo-hydrate and lipid metabolism, and stimulation of nitric oxidation production in cardiovascular system[3-6].
Adequate dietary supplements or nutritional ergogenic aids are an important means to optimize exercise performance and to ward off fatigue. Ergogenic aids are believed to increase performance by some of the following mechanisms: renewing or increasing energy stores in the body, facilitating the biochemical reactions that yield energy, reducing or neutralizing performance-inhibiting metabolic byproducts, and facilitating recovery[7,8]. Performance in aerobic-type events depends on the ability to maintain a high output per unit of time. There is a growing inclination among athletes to use herbs to improve endurance performance or increase recovery after exercise.
The most studied herb for human aerobic physical performance is ginseng. Although the mechanism underlying the alleged ergogenicity of ginseng on physical performance has not been defined, theories include stimulation of the hypothalamic-pituitary-adrenal cortex axis and increased resistance to the stress of exercise, enhanced myocardial metabolism, increased hemoglobin levels, vasodilatation, increased oxygen extraction by muscles, and improved mito-chondrial metabolism in the muscle, all of which theoretically could enhance aerobic exercise performance[9-13].
In the review studies of Bahrke and Morgan, administration of ginseng or its components enhanced exercise endurance by altering fuel homeostasis during exercise, increased free fatty acid utilization in preference over glucose for cellular energy demands in rats and mice[14,15]. However, the evidence for ginseng as an endurance aerobic exercise ergogenic supplementation in men is variable. As ginseng is touted as a dietary ergogenic aid, incomplete study on performance has yielded little proof to reinforce performance affirmations.
A number of chemically similar steroid glycosides or saponin chemicals, known as ginsenosides, have been identified as active ingredients in ginseng. The original medicinal species of ginseng is Chinese or Korean ginseng (Panax ginseng C.A. Meyer). American ginseng (Panax quinquefolius) contains many of the same compounds, although in slightly different proportions. It is the North American variety of ginseng, which grows in eastern and central USA and Canada.
There is relatively little research that shows a performance benefit of American ginseng (AG) in human beings. The purpose of the present study was to determine, whether 4 wk of oral supplementation with AG had any benefit on endurance exercise and/or recovery after exercise.
Thirteen male volunteers completed this randomized, double blind, crossover experimental research study with a washout period of 4 wk. Both body height and body weight were measured by an auto-anthropometer, Nakamura KN-3000 (Nakamura, Tokyo, Japan). Body weight was measured to the nearest 0.1 kg with subjects not wearing shoes or outerwear. Body weight was recorded to the nearest 0.1 cm. Age, weight, and heights (mean±SD) were 23.0 ± 1.6 years, 70.2 ± 6.3 kg, and 172.5 ± 5.2 cm, respectively. A medical examination was performed on each subject before entering the study. Written voluntary consent to participate was obtained from all subjects after informing them of the purpose of the experiment, the procedure, and the possible risks. This investigation received the approval of the National College of Physical Education and Sports (Taoyuan, Taiwan). All subjects were healthy, physically active, college students with normal dietary habits.
Each subject was instructed to ingest either 4 AG capsules (400 mg AG per capsule) daily or a hydroxymethylcellulose placebo in the same capsule form (both provided by Taiwan Biotech Co., Taiwan), and received his allotment of CS or placebo in 4 weekly portions.
The Panax-bearing ginsenosides content was determined from its degree of concentration in a hot water extract. The compositions of the AG that we used were, ginsenosides Rb1 (8.67%, w/w), Rc (0.99%, w/w), Rd (1.05%, w/w), and Re (5.08%, w/w). At an initial meeting, 4 wk prior to commencing any supplementation, subjects were asked to cease taking any dietary supplementations that contained ginsenosides and were instructed to maintain a consistent diet before and during the experimental period.
VO2max was determined in the pre-experimental period. Each subject came to the laboratory 7 d before the start of the actual study, and performed an incremental running test on a motor-driven treadmill (Quinton Instruments, Model 1 860, Washington, USA) according to the Bruce protocol until exhaustion. In order to determine the baseline endurance performance time, maximum oxygen consumption (VO2max) was determined by the automated system (Model 29C, SensorMedics, Yorba Linda, CA,USA). The VO2max was defined as the attainment of at least two of the three following criteria: (1) an increase of ≤ 140 mL VO2max with an increasing workload; (2) heart rate within 10 beats of age-predicted maximum; and (3) rating of perceived exertion (RPE) greater than 17 using the Borg scale. Heart rate (HR) measured by the Sport Tester (PE 3 000, Polar Electro, Kempele, Finland) were monitored during the treadmill exercise. The RPE was recorded by using the modified Borg scale[16]. Endurance performance time was also recorded at the end of the test for each subject. On the day of the experiment, subjects reported to the laboratory at 7-9 a.m. following a 10-12 h overnight fast, and abstained from rigorous exercise for 48 h prior to the test. An antecubital vein connected to a three-way stopcock with a 10-cm extension tube for blood sampling was inserted in the subjects. The cannula was kept patent by periodic flushing with a sodium chloride solution (9 g/L) and remained in place throughout the experimental period. No heparin was used in the catheter.
Baseline physiological data was collected, while subjects stood quietly on the treadmill (Quinton Instruments, Model 1 860, Washington, USA) for 3 min prior to commencing the running test. Subjects then commenced a 5 min warm-up at a running speed equivalent to 60% VO2max, the treadmill speed was increased to a pace equivalent to 80% VO2max of the subjects. The subjects ran until volitional fatigue. Volitional fatigue was defined as the point at which subjects could no longer maintain the required running speed. VO2max and HR were monitored throughout the exercise and were recorded, and oxygen pulse was calculated from oxygen consumption and heart rate. In addition, oxygen pulse (mLO2/beat) was calculated by dividing oxygen consumption (mLO2/min) by heart rate (beat/min).
Blood samples were taken prior to the exercise, at 15 and 30 min during exercise, immediately after exercise, and 20, 40, 60, and 120 min after exercise. At each sampling time, about 5 mL of venous blood was taken. Whole blood for determination of hematocrit and hemoglobin was collected in EDTA tubes cooled at -4°C and examined within 4 h after venepuncture to correct relative changes in plasma volume by using hematocrit and hemoglobin values from each test, according to the methods described by Dill and Costill[17]. The other venous blood was to obtain EDTA-plasma and stored at -20°C for later analysis. The plasma creatine kinase (CK) and plasma lactate were measured by a spectrophotometer technique (Johnson & Johnson DT-60II, Orthoclinical Diagnostics, Rochester, NY,USA) by means of ultraviolet test kits (Orthoclinical Diagnostics, Rochester, NY,USA).
SPSS 11.0 for Windows statistical program was used to perform all analyses. The independent variables between the two supplements (AG vs placebo), including an endurance run time to exhaustion and VO2max after supplements were compared using Student’s t-test for paired data. Differences in oxygen pulse and plasma CK, and plasma lactate values between the two treatment levels were analyzed by factorial (two-way, time×treatment) ANOVA with repeated measures. A Tukey’s post hoc test was used to locate any significant difference. Significance was accepted at the P < 0.05 level. All data are presented as mean±SD.
There was no difference between groups in VO2max and the run time to exhaustion (Table 1).
| Group | VO2max (mL/min/kg) | Time to exhaustion (s) |
| AG | 44.6 ± 3.1 | 2 279.5 ± 252.7 |
| P | 45.1 ± 5.0 | 2 218.3 ± 345.9 |
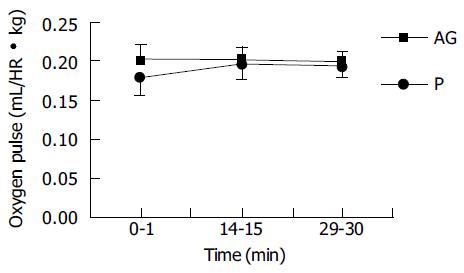
Data of oxygen consumption were collected in the time period of 0-1, 14-15, and 29-30 min. The oxygen pulse had no significant difference between groups during these periods (Figure 1).
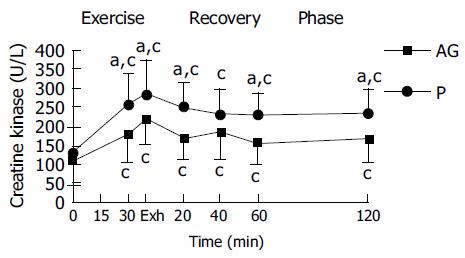
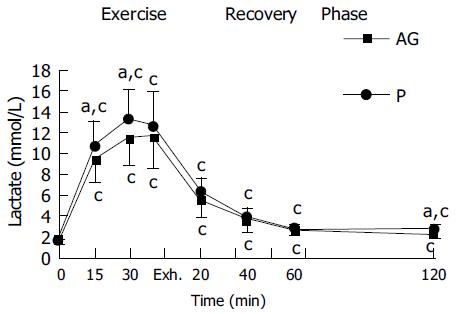
In both placebo and AG groups, plasma CK and plasma lactate were significantly increased with the duration of exercise and reached a peak at exhaustive time (Figures 2 and 3). Blood lactate concentration at 15, 30 min of exercise, and 120 min after exercise in AG group (9.3 ± 2.1, 11.4 ± 2.7, and 2.2 ± 0.4 mmol/L) were significantly lower than in the placebo group (10.6 ± 2.4, 13.3 ± 2.7, and 2.6 ± 0.4 mmol/L), respectively (Figure 3). Moreover, plasma CK activity at 30 min of exercise, immediately, 20, 60, and 120 min after exercise in the AG group (181.2 ± 78.0, 217.5 ± 64.0, 167.5 ± 56.2, 155.3 ± 57.8, and 167.7 ± 61.5 U/L) were significantly lower than in the placebo group (254.3 ± 81.6, 280.2 ± 90.0, 246.5 ± 66.3, 227.8 ± 58.4, and 231.9 ± 67.8 U/L), respectively (Figure 2).
The research was designed to determine whether or not supplementation with AG for 4 wk prior to 80% VO2max run would increase the time to exhaustion and/or have any ergogenic benefit in healthy male college students. The variables that were examined included time to exhaustion, oxygen pulse, plasma CK, and plasma lactate. To our knowledge, this is the first study of AG in human clinical trials to determine endurance aerobic physical performance. The major finding of this investigation was that the production of plasma CK during exercise significantly decreased for group AG over group P (P < 0.05). Secondary physiological findings suggested that 80% VO2max running was not improved over a 4-wk AG supplementation regimen.
The CK marker is used to determine muscle damage. The amount of plasma CK of healthy adults at rest is approximately 40-200 U/L for men[18]. In this study, the plasma CK conce-ntrations before exercise are within the normal range in both groups and have no significant difference between groups (AG group, 108.1 ± 62.4; P group, 138.6 ± 94.6, respectively).
Although most studies indicate that muscle injury is assessed through prolonged endurance exercise, it is obvious that it can be caused during high intensity short-term exercise as well[19,20]. During running exercise, the extensor muscles of the lower limb performed eccentric actions as the foot touches the ground and the dorsiflexors of the ankle contract eccentrically. In eccentric exercise, the contracting muscle is forcibly lengthened as it develops tension, potentially causing damage. Therefore, endurance exercise, such as an exhaustive running exercise can induce damage and pathological alteration in skeletal muscle.
With high-intensity exercise, the high-force contractions cause muscle cell injury early in the exercise period[20]. Mechanical rupture of muscle fiber is one of the major mechanisms to explain how the muscle injury was induced by exercise. This stress on the cross bridges of the myofiber causes disruption of the muscle fibrils leading to Z-line in disintegration or Z-line streaming[21]. Moreover, strenuous exercise can also disrupt the sarcolemma and sarcoplasmic reticulum[22]. Mair et al[23] demonstrated a transient rise in the serum concentrations of muscle proteins such as CK, an indicator of muscle damage due to sarcolemma disruption, which cause a leakage of CK into the blood.
In this study, the total CK activity significantly increased in both groups during exhaustive running exercise. We assign this to plasma CK increasing in both groups of the subjects due to exercise-induced skeletal muscle damage. The increases in plasma CK in this study indicate that muscle damage had occurred during the exhaustive running in both groups.
Intense exercise may increase the production of free radicals or reactive oxygen species. A free radical prefers to steal electrons from the lipid membrane of a cell, initiating a free radical attack on the cell known as lipid peroxidation. Kanter et al[24] have demonstrated that post-exercise plasma CK elevations may be related to an exercise-induced lipid peroxidation. The American ginseng Panax quinquefolium exhibits effective antioxidant, free radical scavenging activity, and inhibiting lipid peroxidation[25-29].
Lactate is an important indicator of muscle performance under stress. Lactate levels rise as intensity increases during exercise. The lower blood lactate concentration during running exercise presumably reflects a lower intramuscular lactate concentration and an increased relative contribution of anaerobic metabolism to ATP production. In this study, the AG group exhibited lower plasma levels of lactate at 15, 30 min of exercise than that in the placebo group. It seems unlikely to be due to amelioration of oxygen extraction from the blood by the working muscles as a consequence of AG supplementation, since VO2max and oxygen pulse was unaffected by prior supplementation. Decreased blood lactate accumulation is not necessarily a result of muscle tissue anti-hypoxia. Factors other than an increased cellular PO2, a decrease in intracellular calcium concentration, decreased activation of glycogen phosphorylase, or decreased intrac-ellular pH can also cause a decrease in intramuscular lactate production[30]. Otherwise, the rate of efflux of lactate from the contractile muscle could be decreased due to the decreased muscle cell membrane permeability after exercise-induced damage[31].
In conclusion, a 4-wk AG supplementation reduced the leakage of CK from skeletal sarcoplasm into blood streaming during an exhaustive treadmill run, but did not enhance aerobic work capacity. The reduction of plasma CK level may be due to AG that is effective for the decrease of skeletal muscle cell membrane damage induced by exercise during the high intensity treadmill run. In the future, we will investigate if the reduction in CK efflux is simply an indication of increased sarcolemma stability or whether the muscles are in fact receiving less damage.
We wish to extend our deep appreciation to all participating subjects for their dedication to this study.
Science Editor Guo SY Language Editor Elsevier HK
Co-first-authors: Cheng-Chen Hsu and Min-Chen Ho
Co-correspondents: Cheng-Chen Hsu
| 1. | Lu AP, Jia HW, Xiao C, Lu QP. Theory of traditional Chinese medicine and therapeutic method of diseases. World J Gastroenterol. 2004;10:1854-1856. [PubMed] [Cited in This Article: ] |
| 2. | Coleman CI, Hebert JH, Reddy P. The effects of Panax ginseng on quality of life. J Clin Pharm Ther. 2003;28:5-15. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 97] [Cited by in F6Publishing: 75] [Article Influence: 3.6] [Reference Citation Analysis (0)] |
| 3. | Tode T, Kikuchi Y, Hirata J, Kita T, Nakata H, Nagata I. Effect of Korean red ginseng on psychological functions in patients with severe climacteric syndromes. Int J Gynaecol Obstet. 1999;67:169-174. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 76] [Cited by in F6Publishing: 76] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 4. | Yuan CS, Attele AS, Wu JA, Lowell TK, Gu Z, Lin Y. Panax quinquefolium L. inhibits thrombin-induced endothelin release in vitro. Am J Chin Med. 1999;27:331-338. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 13] [Cited by in F6Publishing: 14] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 5. | Ji LL, Peterson DM. Aging, exercise, and phytochemicals: promises and pitfalls. Ann N Y Acad Sci. 2004;1019:453-461. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 15] [Cited by in F6Publishing: 15] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 6. | Tan BK, Vanitha J. Immunomodulatory and antimicrobial effects of some traditional chinese medicinal herbs: a review. Curr Med Chem. 2004;11:1423-1430. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 222] [Cited by in F6Publishing: 230] [Article Influence: 12.1] [Reference Citation Analysis (0)] |
| 7. | Lawrence ME, Kirby DF. Nutrition and sports supplements: fact or fiction. J Clin Gastroenterol. 2002;35:299-306. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 25] [Cited by in F6Publishing: 27] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 8. | Maughan RJ, King DS, Lea T. Dietary supplements. J Sports Sci. 2004;22:95-113. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 126] [Cited by in F6Publishing: 94] [Article Influence: 4.7] [Reference Citation Analysis (0)] |
| 9. | Kim DH, Moon YS, Jung JS, Min SK, Son BK, Suh HW, Song DK. Effects of ginseng saponin administered intraperitoneally on the hypothalamo-pituitary-adrenal axis in mice. Neurosci Lett. 2003;343:62-66. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 48] [Cited by in F6Publishing: 50] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 10. | Zhang HG, Li XH, Yang ZC. Effects of Panax notoginseng saponins on myocardial Gsalpha mRNA expression and ATPase activity after severe scald in rats. Burns. 2003;29:541-546. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 14] [Cited by in F6Publishing: 15] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 11. | Bae JW, Lee MH. Effect and putative mechanism of action of ginseng on the formation of glycated hemoglobin in vitro. J Ethnopharmacol. 2004;91:137-140. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 19] [Cited by in F6Publishing: 17] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 12. | Li Z, Chen X, Niwa Y, Sakamoto S, Nakaya Y. Involvement of Ca2+ -activated K+ channels in ginsenosides-induced aortic relaxation in rats. J Cardiovasc Pharmacol. 2001;37:41-47. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 39] [Cited by in F6Publishing: 41] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 13. | Wang BX, Zhou QL, Yang M, Wang Y, Cui ZY, Liu YQ, Ikejima T. Hypoglycemic mechanism of ginseng glycopeptide. Acta Pharmacol Sin. 2003;24:61-66. [PubMed] [Cited in This Article: ] |
| 14. | Bahrke MS, Morgan WP. Evaluation of the ergogenic properties of ginseng. Sports Med. 1994;18:229-248. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 76] [Cited by in F6Publishing: 77] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 15. | Bahrke MS, Morgan WR. Evaluation of the ergogenic properties of ginseng: an update. Sports Med. 2000;29:113-133. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 97] [Cited by in F6Publishing: 96] [Article Influence: 4.0] [Reference Citation Analysis (0)] |
| 16. | Borg GA. Perceived exertion: a note on "history" and methods. Med Sci Sports. 1973;5:90-93. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 274] [Cited by in F6Publishing: 249] [Article Influence: 4.9] [Reference Citation Analysis (0)] |
| 17. | Dill DB, Costill DL. Calculation of percentage changes in volumes of blood, plasma, and red cells in dehydration. J Appl Physiol. 1974;37:247-248. [PubMed] [Cited in This Article: ] |
| 18. | Tietz NW. Clinical guide to laboratory tests. 3rd ed. Philadelphia: Saunders 1995; 180. [Cited in This Article: ] |
| 19. | Byrd SK. Alterations in the sarcoplasmic reticulum: a possible link to exercise-induced muscle damage. Med Sci Sports Exerc. 1992;24:531-536. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 60] [Cited by in F6Publishing: 60] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 20. | Fridén J, Lieber RL. Structural and mechanical basis of exercise-induced muscle injury. Med Sci Sports Exerc. 1992;24:521-530. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 254] [Cited by in F6Publishing: 243] [Article Influence: 7.6] [Reference Citation Analysis (0)] |
| 21. | MacIntyre DL, Reid WD, McKenzie DC. Delayed muscle soreness. The inflammatory response to muscle injury and its clinical implications. Sports Med. 1995;20:24-40. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 212] [Cited by in F6Publishing: 233] [Article Influence: 8.0] [Reference Citation Analysis (0)] |
| 22. | Clarkson PM, Hubal MJ. Exercise-induced muscle damage in humans. Am J Phys Med Rehabil. 2002;81:S52-S69. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 792] [Cited by in F6Publishing: 767] [Article Influence: 34.9] [Reference Citation Analysis (0)] |
| 23. | Mair J, Mayr M, Müller E, Koller A, Haid C, Artner-Dworzak E, Calzolari C, Larue C, Puschendorf B. Rapid adaptation to eccentric exercise-induced muscle damage. Int J Sports Med. 1995;16:352-356. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 59] [Cited by in F6Publishing: 60] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 24. | Kanter MM, Lesmes GR, Kaminsky LA, La Ham-Saeger J, Nequin ND. Serum creatine kinase and lactate dehydrogenase changes following an eighty kilometer race. Relationship to lipid peroxidation. Eur J Appl Physiol Occup Physiol. 1988;57:60-63. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 86] [Cited by in F6Publishing: 83] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 25. | Li J, Huang M, Teoh H, Man RY. Panax quinquefolium saponins protects low density lipoproteins from oxidation. Life Sci. 1999;64:53-62. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 31] [Cited by in F6Publishing: 34] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 26. | Li JP, Huang M, Teoh H, Man RY. Interactions between Panax quinquefolium saponins and vitamin C are observed in vitro. Mol Cell Biochem. 2000;204:77-82. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 14] [Cited by in F6Publishing: 14] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 27. | Kitts DD, Wijewickreme AN, Hu C. Antioxidant properties of a North American ginseng extract. Mol Cell Biochem. 2000;203:1-10. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 271] [Cited by in F6Publishing: 280] [Article Influence: 11.7] [Reference Citation Analysis (0)] |
| 28. | Fu Y, Ji LL. Chronic ginseng consumption attenuates age-associated oxidative stress in rats. J Nutr. 2003;133:3603-3609. [PubMed] [Cited in This Article: ] |
| 29. | Ng TB, Liu F, Wang HX. The antioxidant effects of aqueous and organic extracts of Panax quinquefolium, Panax notoginseng, Codonopsis pilosula, Pseudostellaria heterophylla and Glehnia littoralis. J Ethnopharmacol. 2004;93:285-288. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 84] [Cited by in F6Publishing: 72] [Article Influence: 3.6] [Reference Citation Analysis (0)] |
| 30. | Connett RJ, Honig CR, Gayeski TE, Brooks GA. Defining hypoxia: a systems view of VO2, glycolysis, energetics, and intracellular PO2. J Appl Physiol (1985). 1990;68:833-842. [PubMed] [Cited in This Article: ] |
| 31. | Newham DJ, Jones DA, Edwards RH. Large delayed plasma creatine kinase changes after stepping exercise. Muscle Nerve. 1983;6:380-385. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 177] [Cited by in F6Publishing: 185] [Article Influence: 4.5] [Reference Citation Analysis (0)] |