Published online Nov 14, 2005. doi: 10.3748/wjg.v11.i42.6689
Revised: February 12, 2005
Accepted: February 15, 2005
Published online: November 14, 2005
AIM: To elucidate the immunologic parameters for the outcome of patients with malignant tumors, especially esophageal squamous cell carcinoma (ESCC) associated with high malignant potential.
METHODS: Clinicopathologic features were compared between patients with lower and higher CD4 and CD8 values as well as CD4/CD8 ratio in peripheral blood.
RESULTS: The survival rate of patients with higher CD4 value was significantly better than that in patients with lower CD4 value (P = 0.039). The survival rate of patients with higher CD8 value was significantly worse than that of patients with lower CD8 value (P = 0.026). Similarly, the survival rate of patients with higher CD4/CD8 ratio was significantly better than that of patients with lower CD4/CD8 ratio (P = 0.042). Additionally, multivariate analysis demonstrated that lower CD8 and lower CD4/CD8 ratio were factors independently associated with worse prognosis of patients.
CONCLUSION: All the immunologic parameters can predict the outcome of patients with ESCC.
- Citation: Nozoe T, Maehara Y, Sugimachi K. Preoperative sorting of circulating T lymphocytes in patients with esophageal squamous cell carcinoma: Its prognostic significance. World J Gastroenterol 2005; 11(42): 6689-6693
- URL: https://www.wjgnet.com/1007-9327/full/v11/i42/6689.htm
- DOI: https://dx.doi.org/10.3748/wjg.v11.i42.6689
Impaired immunity is well known to be correlated with the tumorigenesis and/or progressive behavior of human tumors[1-3]. Therefore, it is important to assess the immunologic dynamics of patients with mali-gnant tumors, especially esophageal carcinoma.
We have reported the significance of preoperative assessment of such immunological parameters as serum C-reactive protein concentration[4], prognostic nutritional index[5], and phytohemagglutinin (PHA) response test[6] as a prognostic indicator in esophageal carcinoma.
CD8+, cytotoxic T lymphocytes, plays an immunologic role as the specific tumor terminator and CD4+, helper T lymphocyte, serves the function of controlling CD8+ T-cell-dependent tumor termination[7]. However, only a few investigations are available on the clinicopathologic significance of these lymphocytes in controlling esophageal carcinoma[8-10].
It was reported that lower CD4/CD8 ratio in per-ipheral blood can be used as an indicator for worse prognosis of patients with esophageal carcinoma[11]. In the current study, we investigated the clinical significance of the serum values of CD4 and CD8, and the CD4/CD8 ratio in patients with esophageal squamous cell carcinoma (ESCC).
One hundred and thirty-four patients (118 men and 16 women) with ESCC, who underwent esophageal resection and reconstruction of the digestive tract in our institute between 1990 and 1997, were enrolled in this study. The patients had a median age of 62 years (range, 41-82 years).
Follow-up was continued until their death. The interval of follow-up ranged from 29 d to 8 years and 9 mo averaged 2 years and 11 mo. Serum values of lymphocyte sub-populations, CD4 and CD8, were measured as previously described[11].
Pathological features were presented according to the guidelines for clinical and pathologic studies on carcinoma of the esophagus established by the Japanese Society for Esophageal Diseases[12], and clinical stages were determined by the TNM classification of malignant tumors approved by the International Union Against Cancer[13]. Clinicopathologic features were compared between patients with lower and higher values of CD4 and CD8 as well as CD4/CD8 ratio.
Chi-square test and Student’s t test were used to compare the clinicopathologic data. The cumulative survival rates were calculated by the Kaplan-Meier method and the survival curves were tested by the Mantel-Cox method. P<0.05 was considered statistically significant.
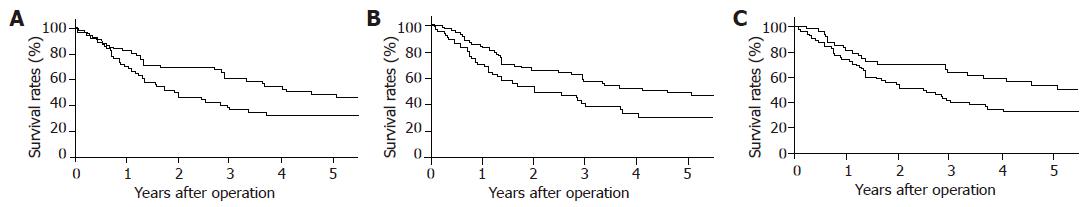
Among the clinicopathologic factors, the mean age of patients with higher CD4 value (group H-CD4) was significantly lower than that of patients with lower CD4 value (group L-CD4, P = 0.046). However, no significant difference was observed in other factors including tumor-related factors (Table 1). The 1-, 3-, and 5-year survival rates were 82.2%, 60.4% and 48.4%, respectively, in group H-CD4 and 70.8%, 36.9% and 32.7%, respectively, in group L-CD4 (P = 0.039, Figure 1A).
| Group H-CD4 | Group L-CD4 | P | |
| Variables | (n = 68) | (n = 66) | |
| Gender | |||
| Male | 57 (83.8) | 61 (92.4) | 0.205 |
| Female | 11 (16.2) | 5 (7.6) | |
| Age | 60.9±8.8 | 64.1+9.6 | 0.046 |
| Location of tumor | |||
| Upper | 12 (17.6) | 11 (16.7) | 0.845 |
| Middle | 38 (55.9) | 40 (60.6) | |
| Lower | 18 (26.5) | 15 (22.7) | |
| Degree of differentiation | |||
| Well | 4 (5.9) | 6 (9.1) | 0.620 |
| Moderately | 57 (83.8) | 51 (77.3) | |
| Poorly | 7 (10.3) | 9 (13.6) | |
| Tumor size (cm) | 5.3+2.7 | 5.4+2.5 | 0.970 |
| Depth of tumor | |||
| Tis | 2 (2.9) | 2 (3.0) | 0.051 |
| T1a | 6 (8.8) | 5 (7.6) | |
| T1b | 15 (22.1) | 9 (13.6) | |
| T2 | 14 (20.6) | 9 (13.6) | |
| T3 | 15 (22.1) | 33 (50.0) | |
| T4 | 16 (23.5) | 8 (12.2) | |
| Lymph nodes metastasis | |||
| Positive | 24 (35.3) | 31 (53.0) | 0.231 |
| Negative | 44 (64.7) | 35 (47.0) | |
| Lymphatic invasion | |||
| Positive | 12 (17.6) | 18 (27.3) | 0.259 |
| Negative | 56 (82.4) | 48 (72.7) | |
| Venous invasion | |||
| Positive | 5 (7.4) | 11 (16.7) | 0.163 |
| Negative | 63 (92.6) | 55 (83.3) | |
| TNM stage | |||
| 0 | 2 (2.9) | 2 (3.0) | 0.749 |
| I | 17 (25.0) | 11 (16.7) | |
| IIA | 16 (23.5) | 18 (27.3) | |
| IIB | 10 (14.7) | 8 (12.2) | |
| III | 23 (33.8) | 27 (40.8) | |
| Curability | |||
| Curative resection | 52 (76.5) | 56 (84.8) | 0.314 |
| Non curative resection | 16 (23.5) | 10 (15.2) |
No significant difference was found in the clini-copathologic factors between patients with higher (group H-CD8) and lower CD8 value (group L-CD8, Table 2). The 1-, 3-, and 5-year survival rates were 69.7%, 38.5% and 30.0%, respectively, in group H-CD8 and 82.7%, 57.7%, and 49.0%, respectively, in group L-CD8 (P = 0.026, Figure 1B).
| Group H-CD8 | Group L-CD8 | P | |
| Variables | (n = 64) | (n = 70) | |
| Gender | |||
| Male | 58 (90.6) | 60 (85.7) | 0.543 |
| Female | 6 (9.4) | 10 (14.3) | |
| Age | 63.4+9.6 | 61.6+9.1 | 0.265 |
| Location of tumor | |||
| Upper | 9 (14.1) | 14 (20.0) | 0.635 |
| Middle | 38 (59.4) | 40 (57.1) | |
| Lower | 17 (26.5) | 16 (22.9) | |
| Degree of differentiation | |||
| Well | 4 (6.3) | 6 (8.6) | 0.810 |
| Moderately | 53 (82.8) | 55 (78.6) | |
| Poorly | 7 (10.9) | 9 (12.8) | |
| Tumor size (cm) | 5.4+2.4 | 5.3+2.8 | 0.930 |
| Depth of tumor | |||
| Tis | 2 (3.1) | 2 (2.8) | 0.899 |
| T1a | 4 (6.3) | 7 (10.0) | |
| T1b | 11 (17.2) | 13 (18.6) | |
| T2 | 10 (15.6) | 13 (18.6) | |
| T3 | 26 (40.6) | 22 (31.4) | |
| T4 | 11 (17.2) | 13 (18.6) | |
| Lymph nodes metastasis | |||
| Positive | 29 (45.3) | 26 (37.1) | 0.382 |
| Negative | 35 (54.7) | 44 (62.9) | |
| Lymphatic invasion | |||
| Positive | 11 (17.2) | 19 (27.1) | 0.241 |
| Negative | 53 (82.8) | 51 (72.9) | |
| Venous invasion | |||
| Positive | 9 (14.1) | 7 (10.0) | 0.647 |
| Negative | 55 (85.9) | 63 (90.0) | |
| TNM stage | |||
| 0 | 2 (3.1) | 2 (2.8) | 0.858 |
| I | 12 (18.8) | 16 (22.9) | |
| IIA | 15 (23.4) | 19 (27.1) | |
| IIB | 8 (12.5) | 10 (14.3) | |
| III | 27 (42.2) | 23 (32.9) | |
| Curability | |||
| Curative resection | 51 (79.7) | 57 (81.4) | 0.830 |
| Non curative resection | 13 (20.3) | 13 (18.6) |
Significant difference between patients with higher (group H-CD4/8) and lower CD4/CD8 ratio (group L-CD4/8) was observed only in gender proportion (P = 0.036, Table 3). The 1-, 3-, and 5-year survival rates were 80.9%, 62.9%, and 52.9%, respectively, in group H-CD4/8 and 74.0%, 40.3% and 33.0%, respectively, in group L-CD4/8 (P = 0.042, Figure 1C).
| Group H-CD4/8 | Group L-CD4/8 | P | |
| Variables | (n = 48) | (n = 86) | |
| Gender | |||
| Male | 38 (79.2) | 80 (93.0) | 0.036 |
| Female | 10 (20.8) | 6 (7.0) | |
| Age | 61.4+8.9 | 63.0+9.6 | 0.322 |
| Location of tumor | |||
| Upper | 10 (20.8) | 13 (15.1) | 0.675 |
| Middle | 26 (54.2) | 52 (60.5) | |
| Lower | 12 (25.0) | 21 (24.4) | |
| Degree of differentiation | |||
| Well | 5 (10.4) | 5 (5.8) | 0.459 |
| Moderately | 36 (75.0) | 72 (83.7) | |
| Poorly | 7 (14.6) | 9 (10.5) | |
| Tumor size (cm) | 4.9+2.5 | 5.6+2.7 | 0.125 |
| Depth of tumor | |||
| Tis | 2 (4.2) | 2 (2.3) | 0.213 |
| T1a | 4 (8.3) | 7 (8.1) | |
| T1b | 11 (22.9) | 13 (15.1) | |
| T2 | 8 (16.7) | 15 (17.5) | |
| T3 | 11 (22.9) | 37 (43.0) | |
| T4 | 12 (25.0) | 12 (14.0) | |
| Lymph nodes metastasis | |||
| Positive | 16 (33.3) | 39 (34.9) | 0.241 |
| Negative | 32 (66.7) | 47 (65.1) | |
| Lymphatic invasion | |||
| Positive | 12 (25.0) | 18 (20.9) | 0.745 |
| Negative | 36 (75.0) | 68 (79.1) | |
| Venous invasion | |||
| Positive | 5 (10.4) | 11 (12.8) | 0.647 |
| Negative | 43 (89.6) | 75 (87.2) | |
| TNM stage | |||
| 0 | 2 (4.2) | 2 (2.3) | 0.858 |
| I | 13 (27.1) | 15 (17.5) | |
| IIA | 11 (22.9) | 23 (26.7) | |
| IIB | 6 (12.5) | 12 (14.0) | |
| III | 16 (33.3) | 34 (39.5) | |
| Curability | |||
| Curative resection | 36 (75.0) | 72 (83.7) | 0.830 |
| Non curative resection | 12 (25.0) | 14 (16.3) |
Multivariate analysis demonstrated that lower CD8 (95%CI, 2.07, 1.26–3.38; P = 0.004) and lower CD4/CD8 ratio (95%CI, 1.73, 1.02-2.93; P = 0.043) were factors independently associated with worse prognosis of patients.
With the development of monoclonal antibodies in detecting lymphocytes subpopulation[14], lymphocyte subtypes in peripheral blood were examined to investigate their functions in immune-surveillance. Among the subpopulations of lymphocytes, investigations of cancer immunology have been focused on CD8, suppressor/cytotoxic T lymphocyte responses. Attention has also been paid to CD4, helper/inducer T lymphocytes, as a critical component of the anti-tumor immune response[15].
Tumor-specific immune response depends on the function of activated CD4 cells[16], and therefore the deficiency in the function of activated CD4 cells might be directly correlated with the immune-deficiency of the host. CD4 helper/inducer T lymphocytes produce lymphokines, thus promoting the cytotoxic activity of CD8 T lymphocytes[17,18]. Therefore, activation of both CD4 and CD8 can exert a synergistic immune response to the termination of tumor cells.
Though some investigations have demonstrated an immunologic anti-tumor effect of CD4 and CD8[8], the clinical significance of CD4/CD8 ratio in tumor infiltrating lymphocytes and/or in peripheral blood as an indicator of progressive gastrointestinal tumor and/or worse prognosis of patients has been occasionally reported[19-21]. Diederichsen et al[19] reported that low CD4/CD8 ratio in tumor infiltrating lymphocytes is an independent prognostic indicator in patients with colorectal carcinoma. Decrease of the CD4/CD8 ratio is correlated with progressive behavior of the tumor indicated by such tumor-related factors as stage of the tumor, tumor invasion, lymph node metastasis, and size of the tumor in gastric cancer[20]. Moreover, severe pre-operative cellular immune-suppression, where CD4/CD8 ratio was less than 1.0, is a predictive parameter for mor-tality in patients with gastric cancer[21].
CD8 expression in TIL in tumor tissue can serve the function of suppressing the proliferation of ESCC[9], and similarly CD8 infiltration into the tumor is an independent prognostic indicator for ESCC[10]. Recently, increase of the number of CD4 and CD8 T lymphocytes in tumor nests and stroma has been found to be an independent indicator of favorable prognosis of patients with ESCC[8].
These results suggest that CD8 T-lymphocyte in-filtration, as have been investigated in some other tumors[22,23], plays a pivotal role in immune-potential against ESCCs.
However, it was reported that the prognosis of patients with lung carcinoma associated with more CD8 expressing T cells within cancer nests is significantly worse than that of patients with tumors of fewer CD8 expressing T cells[24]. High percentage of activated CD8-positive cells in postoperative peripheral blood is an indicator of worse prognosis for renal cell carcinoma[25].
The different methods used to evaluate the value or expression of CD8, histological type of the tumor, or balance between immunologic dynamics of the tumor and the host might explain this possible discrepancy in the significance of CD8 T lymphocytes in anti-tumor immune.
In the current study, the decreased CD4/CD8 ratio as well as the increased CD8 and the decreased CD4 in peripheral blood could predict the worse prognosis in patients with ESCCs. Preoperative coexistence of impaired immunity could influence the postoperative complications[5]. The incidence of postoperative complications is an independent indicator of worse prognosis in patients with esophageal carcinoma[26]. Therefore, preoperative impaired immunity seems not to be negligible as the cause of death, other than esophageal carcinoma.
Assessment of preoperative immunity in patients seems to be of great importance in predicting the subsequent outcome of patients with ESCCs.
Science Editor Wang XL and Guo SY Language Editor Elsevier HK
| 1. | Neuner A, Schindel M, Wildenberg U, Muley T, Lahm H, Fischer JR. Prognostic significance of cytokine modulation in non-small cell lung cancer. Int J Cancer. 2002;101:287-292. [PubMed] [DOI] [Cited in This Article: ] |
| 2. | Takagi K, Yamamori H, Morishima Y, Toyoda Y, Nakajima N, Tashiro T. Preoperative immunosuppression: its relationship with high morbidity and mortality in patients receiving thoracic esophagectomy. Nutrition. 2001;17:13-17. [PubMed] [DOI] [Cited in This Article: ] |
| 3. | Eilber FR, Morton DL. Impaired immunologic reactivity and recurrence following cancer surgery. Cancer. 1970;25:362-367. [PubMed] [DOI] [Cited in This Article: ] |
| 4. | Nozoe T, Saeki H, Sugimachi K. Significance of preoperative elevation of serum C-reactive protein as an indicator of prognosis in esophageal carcinoma. Am J Surg. 2001;182:197-201. [PubMed] [DOI] [Cited in This Article: ] |
| 5. | Nozoe T, Kimura Y, Ishida M, Saeki H, Korenaga D, Sugimachi K. Correlation of pre-operative nutritional condition with post-operative complications in surgical treatment for oesophageal carcinoma. Eur J Surg Oncol. 2002;28:396-400. [DOI] [Cited in This Article: ] |
| 6. | Nozoe T, Korenaga D, Ohga T, Futatsugi M, Maehara Y. Suppression of the phytohemagglutinin response to lymphocytes is an independent prognosticator in patients with squamous cell carcinoma of the esophagus. Ann Thorac Surg. 2003;76:260-265. [PubMed] [DOI] [Cited in This Article: ] |
| 7. | Shiku H. Importance of CD4+ helper T-cells in antitumor immunity. Int J Hematol. 2003;77:435-438. [PubMed] [DOI] [Cited in This Article: ] |
| 8. | Cho Y, Miyamoto M, Kato K, Fukunaga A, Shichinohe T, Kawarada Y, Hida Y, Oshikiri T, Kurokawa T, Suzuoki M. CD4+ and CD8+ T cells cooperate to improve prognosis of patients with esophageal squamous cell carcinoma. Cancer Res. 2003;63:1555-1559. [PubMed] [Cited in This Article: ] |
| 9. | Takeno S, Noguchi T, Kikuchi R, Wada S, Sato T, Uchida Y. Immunohistochemical study of leukocyte infiltration and expression of hsp70 in esophageal squamous cell carcinoma. Oncol Rep. 2001;8:585-590. [PubMed] [Cited in This Article: ] |
| 10. | Schumacher K, Haensch W, Röefzaad C, Schlag PM. Prognostic significance of activated CD8(+) T cell infiltrations within esophageal carcinomas. Cancer Res. 2001;61:3932-3936. [PubMed] [Cited in This Article: ] |
| 11. | Tsutsui S, Sonoda K, Sumiyoshi K, Kitamura K, Toh Y, Kitamura M, Kuwano H, Sugimachi K, Okamura S. Prognostic significance of immunological parameters in patients with esophageal cancer. Hepatogastroenterology. 1996;43:501-509. [PubMed] [Cited in This Article: ] |
| 12. | Japanese Society for Esophageal Diseases. Guide lines for the clinical and pathological studies on carcinoma of the esophagus, 9th ed. Tokyo: Kanehara Company 1999; . [Cited in This Article: ] |
| 13. | LH Sobin, Wittekind C, editors . International Union Against Cancer. TNM classification of malignant tumours, 5th ed. New York: Wiley-Liss 1997; p 54-58. [Cited in This Article: ] |
| 14. | Reinherz EL, Kung PC, Goldstein G, Schlossman SF. Separation of functional subsets of human T cells by a monoclonal antibody. Proc Natl Acad Sci USA. 1979;76:4061-4065. [PubMed] [DOI] [Cited in This Article: ] |
| 15. | Pardoll DM, Topalian SL. The role of CD4+ T cell responses in antitumor immunity. Curr Opin Immunol. 1998;10:588-594. [PubMed] [DOI] [Cited in This Article: ] |
| 16. | Chen L, Linsley PS, Hellström KE. Costimulation of T cells for tumor immunity. Immunol Today. 1993;14:483-486. [PubMed] [DOI] [Cited in This Article: ] |
| 17. | Toes RE, Ossendorp F, Offringa R, Melief CJ. CD4 T cells and their role in antitumor immune responses. J Exp Med. 1999;189:753-756. [PubMed] [DOI] [Cited in This Article: ] |
| 18. | Qin Z, Richter G, Schüler T, Ibe S, Cao X, Blankenstein T. B cells inhibit induction of T cell-dependent tumor immunity. Nat Med. 1998;4:627-630. [PubMed] [DOI] [Cited in This Article: ] |
| 19. | Diederichsen AC, Hjelmborg Jv, Christensen PB, Zeuthen J, Fenger C. Prognostic value of the CD4+/CD8+ ratio of tumour infiltrating lymphocytes in colorectal cancer and HLA-DR expression on tumour cells. Cancer Immunol Immunother. 2003;52:423-428. [PubMed] [DOI] [Cited in This Article: ] |
| 20. | Lee WJ, Chang KJ, Lee CS, Chen KM. Selective depression of T-lymphocyte subsets in gastric cancer patients: an implication of immunotherapy. J Surg Oncol. 1994;55:165-169. [PubMed] [DOI] [Cited in This Article: ] |
| 21. | Rey-Ferro M, Castaño R, Orozco O, Serna A, Moreno A. Nutritional and immunologic evaluation of patients with gastric cancer before and after surgery. Nutrition. 1997;13:878-881. [PubMed] [DOI] [Cited in This Article: ] |
| 22. | Oshikiri T, Miyamoto M, Shichinohe T, Suzuoki M, Hiraoka K, Nakakubo Y, Shinohara T, Itoh T, Kondo S, Katoh H. Prognostic value of intratumoral CD8+ T lymphocyte in extrahepatic bile duct carcinoma as essential immune response. J Surg Oncol. 2003;84:224-228. [PubMed] [DOI] [Cited in This Article: ] |
| 23. | Naito Y, Saito K, Shiiba K, Ohuchi A, Saigenji K, Nagura H, Ohtani H. CD8+ T cells infiltrated within cancer cell nests as a prognostic factor in human colorectal cancer. Cancer Res. 1998;58:3491-3494. [PubMed] [Cited in This Article: ] |
| 24. | Wakabayashi O, Yamazaki K, Oizumi S, Hommura F, Kinoshita I, Ogura S, Dosaka-Akita H, Nishimura M. CD4( ) T cells in cancer stroma, not CD8( ) T cells in cancer cell nests, are associated with favorable prognosis in human non-small cell lung cancers. Cancer Sci. 2003;94:1003-1009. [DOI] [Cited in This Article: ] |