Published online Jun 28, 2010. doi: 10.3748/wjg.v16.i24.3049
Revised: March 25, 2010
Accepted: April 2, 2010
Published online: June 28, 2010
AIM: To identify the clinical outcomes of hepatocellular carcinoma (HCC) patients with inconsistent α-fetoprotein (AFP) levels which were initially high and then low at recurrence.
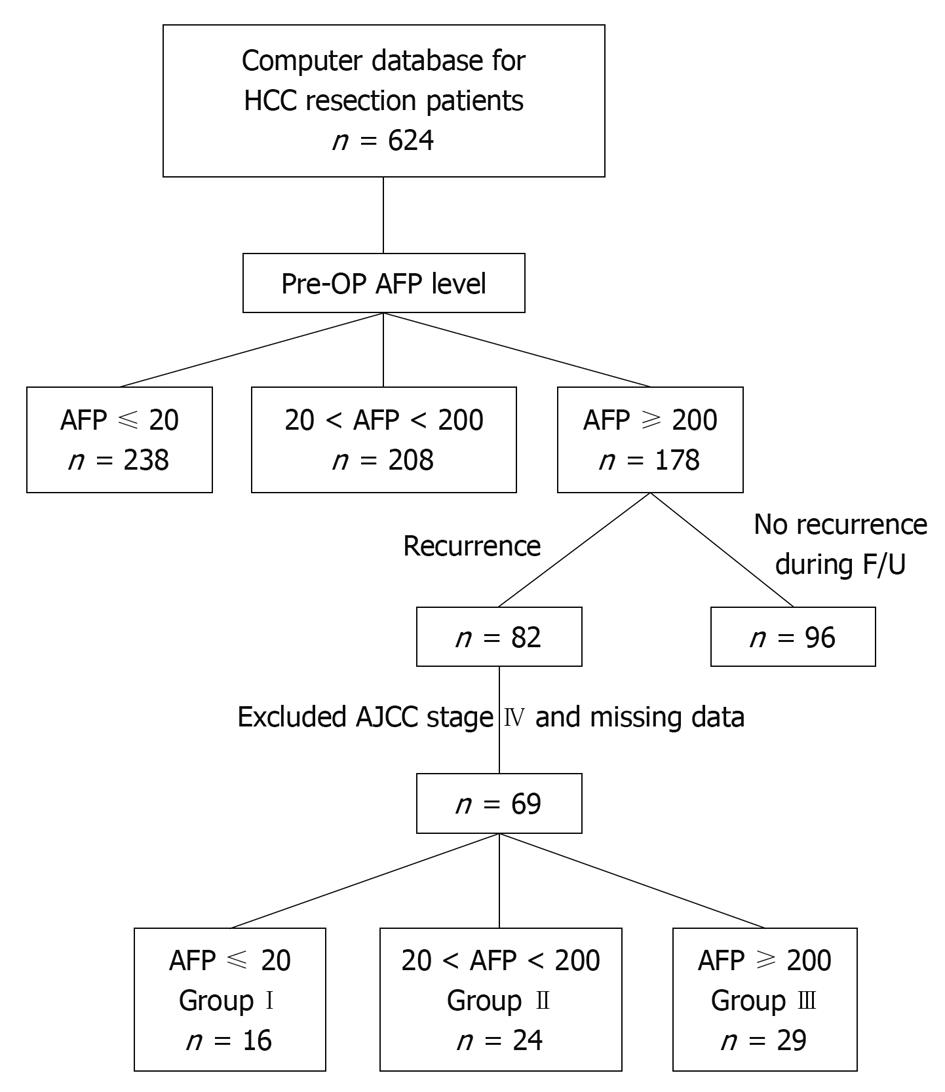
METHODS: We retrospectively included 178 patients who underwent liver resection with high preoperative AFP levels (≥ 200 ng/dL). Sixty-nine HCC patients had recurrence during follow-up and were grouped by their AFP levels at recurrence: group I, AFP ≤ 20 ng/dL (n = 16); group II, AFP 20-200 ng/dL (n = 24); and group III, AFP ≥ 200 ng/dL (n = 29). Their preoperative clinical characteristics, accumulated recurrence rate, and recurrence-to-death survival rate were compared. Three patients, one in each group, underwent liver resection twice for primary and recurrent HCC. AFP immunohistochemistry of primary and recurrent HCC specimens were examined.
RESULTS: In this study, 23% of patients demonstrated normal AFP levels at HCC recurrence. The AFP levels in these patients were initially high. There were no significant differences in clinical characteristics between the three groups except for the mean recurrence interval (21.8 ± 14.6, 12.3 ± 7.7, 8.3 ± 6.6 mo, respectively, P < 0.001) and survival time (40.2 ± 19.9, 36.1 ± 22.4, 21.9 ± 22.0 mo, respectively, P = 0.013). Tumor size > 5 cm, total bilirubin > 1.2 mg/dL, vessel invasion, Child classification B, group III, and recurrence interval < 12 mo, were risk factors for survival rate. Cox regression analysis was performed and vessel invasion, group III, and recurrence interval were independent risk factors. The recurrence interval was significant longer in group I (P < 0.001). The recurrence-to-death survival rate was significantly better in group II (P = 0.016). AFP staining was strong in the primary HCC specimens and was reduced at recurrence in group I specimens.
CONCLUSION: Patients in group I with inconsistent AFP levels had a longer recurrence interval and worse recurrence-to-death survival rate than those in group II. This clinical presentation may be caused by a delay in the detection of HCC recurrence.
- Citation: Hsieh CB, Chen TW, Chu CM, Chu HC, Yu CP, Chung KP. Is inconsistency of α-fetoprotein level a good prognosticator for hepatocellular carcinoma recurrence? World J Gastroenterol 2010; 16(24): 3049-3055
- URL: https://www.wjgnet.com/1007-9327/full/v16/i24/3049.htm
- DOI: https://dx.doi.org/10.3748/wjg.v16.i24.3049
Serum α-fetoprotein (AFP) level is widely used as a tumor marker for the diagnosis and detection of both occurrence and recurrence of hepatocellular carcinoma (HCC)[1,2]. For a patient with a history of liver disease, consensus for the diagnosis of HCC can be achieved with an elevated AFP level (≥ 200 ng/dL), along with a dynamic imaging study showing a hepatic tumor, with enhancement in the arterial phase and wash-out in the venous phase[3,4].
A high serum AFP level in HCC also indicates poor tumor differentiation, larger tumor burden, recurrence after tumor resection, and an unfavorable outcome[5-10]. In clinical practice, a dramatic decline in serum AFP level indicates that the tumor has been surgically removed[11]. The AFP level is broadly applicable both pre- and postoperatively.
AFP is secreted in only about 70% of HCC cases, so both false-negative and false-positive rates are high when using AFP as the serological marker for the detection of HCC[12]. In general, patients have an initially high serum AFP level when HCC is diagnosed. Serum AFP level is also considered an effective tumor marker in the surveillance of HCC recurrence[13,14]. Recurrent HCC is expected to have the same bioactivity as the primary tumor and to secrete high levels of AFP, making AFP a seemingly reliable choice. Regular checks of serum AFP levels in patients who initially had high AFP levels to identify HCC recurrence is suggested as a practice guideline by the National Comprehensive Cancer Network (NCCN) version 1, 2009[15].
However, inconsistent AFP levels have been found in patients diagnosed with HCC where initial AFP levels were high, but were normal when HCC recurred. A true relapse of HCC and a second primary tumor due to chromosomal aberration are possible mechanisms[16], and inconsistent AFP levels may differentiate them via AFP secretion bioactivity. If we depend on the AFP level to signal an HCC recurrence, we may miss or delay the diagnosis of recurrence. We may expect the clinical outcome of patients with inconsistent AFP levels to be similar to those with normal AFP levels. However, this clinical presentation has not been investigated before. Therefore, it is important to study the prognosis of this subgroup of HCC patients whose AFP levels are high initially and normal at recurrence. It is possible that the findings of this study could modify the surveillance principle for this subgroup of HCC patients and improve the quality of medical care.
From 1996 to 2008, 642 patients newly diagnosed with HCC who underwent liver resection were entered into a computer database at the Tri-Service General Hospital, a tertiary referral medical center in Taipei, Taiwan. The diagnosis of HCC in all patients was established histologically from surgical specimens. HCC patients with an initial serum AFP level ≥ 200 ng/dL (n = 178) were selected and 82 had HCC recurrence during follow-up. Thirteen patients were excluded because of an American Joint Committee on Cancer (AJCC) stage IV diagnosis, lost to follow-up or missing data. Therefore, 69 patients were included in this study and grouped according to their serum AFP levels when HCC recurred (Figure 1).
Sixteen patients with normal AFP levels (≤ 20 ng/dL) during HCC recurrence were assigned to group I; 24 patients with AFP levels between 20 ng/dL and 200 ng/dL were assigned to group II; and, 29 patients with AFP levels of 200 ng/dL or higher were assigned to group III. In our hospital, the normal AFP level is ≤ 20 ng/dL measured by radioimmunoassay using an anti-AFP antibody (ELSA 2-AFP kit, CIS Bio International, Cedex, France).
Preoperative clinical characteristics were collected from the database and compared. These characteristics included age, gender, tumor size, serum alanine aminotransferase (ALT) level, total serum bilirubin, serum albumin level, platelet count, vessel invasion, tumor number, clinical stage (AJCC, Child-Pugh classification), hepatitis B virus, hepatitis C virus, presence of cirrhosis, mean recurrence interval, mean recurrence-to-death time, mean survival time and location of recurrence. Salvage treatments for recurrent HCC included repeat liver resection, liver transplantation, transarterial chemo-embolization, radio-frequency ablation, and thalidomide therapy. The mean AFP levels at operation (AFP op) and at recurrence (AFP recur) were calculated for these three groups. Intra-hepatic metastasis was defined as recurrent HCC located at the adjacent segment, section and same lobe. In contrast to intra-hepatic metastasis, multicentric recurrence was defined as recurrent HCC located at the contralateral liver lobe. The pathological grade was defined as: grade I - well differentiated, grade II - moderate, and grade III - poorly differentiated, according to the report by the pathologist (Dr. Yu CP).
The mean recurrence interval was defined as the interval between the operation and HCC recurrence. The starting point of overall survival was the operation date, and recurrence-to-death was the date of recurrence diagnosis. The endpoint of accumulated recurrence rate was defined as the date of recurrence and the endpoint of recurrence-to-death survival was the date of death or the last follow-up date if the patients survived. The choice of salvage therapy was according to the willingness of the patient, the degree of tumor progression, and the criteria for treatment guidelines of the Cancer Committee at the Tri-Service General Hospital. The recurrence interval was defined as the day of the initial liver resection to the day of HCC recurrence diagnosis.
Three patients, one from each group, underwent a second liver resection for tumor recurrence. Surgical specimens from both operations in each of these three patients were reviewed by a pathologist (Dr. Yu CP). The specimens were examined by hematoxylin and eosin staining and immunohistochemistry for AFP levels and the differences were compared.
All patients were followed up regularly every month in the first year and every 3 mo thereafter. AFP level was monitored at each follow-up. Abdominal ultrasound imaging or computed tomography (CT) was performed every 3-6 mo in first two years, then annually, according to the NCCN practice guideline[15]. In some patients, magnetic resonance imaging, hepatic angiography, and percutaneous needle biopsy were considered if the CT images were not conclusive for a new hepatic lesion. All clinicopathological data were collected in the computer databases of the Cancer Board at the Tri-Service General Hospital, and the follow-up data were regularly updated. This study was approved by the Institutional Review Board of Tri-Service General Hospital. The follow-up period of this study ended on June 30, 2009.
Continuous clinical data were analyzed using the Student’s t test and ANOVA, and the categorical data were analyzed using Fisher’s exact test or the χ2 test. Kaplan-Meier and Cox regression analyses were performed to determine the survival curves, and the survival relative ratio between the three groups of HCC patients. P < 0.05 was considered statistically significant. All statistical analyses were performed using statistical software (SPSS version 15.0 for Windows, Chicago, IL, USA).
Of the 69 HCC patients with initially high AFP levels who underwent liver resection, 16 had normal serum AFP levels (≤ 20 ng/dL) at recurrence. There were no significant differences between the three groups in terms of clinical characteristics such as age, gender, tumor stage, virology status, tumor pattern, biochemical examination results, and cirrhotic status (Table 1). Significant differences between the three groups were observed for recurrence intervals, survival times and location of recurrence (P < 0.001, P = 0.013, P = 0.001, respectively).
| Group I (n = 16) | Group II (n = 24) | Group III (n = 29) | P | |
| Age (yr) | 59.7 ± 12.5 | 57.1 ± 16.6 | 48.8 ± 16.3 | 0.055 |
| Gender (M/F) | 10/6 | 12/12 | 19/10 | 0.498 |
| Tumor size (cm) | 5.64 ± 3.07 | 4.80 ± 3.40 | 5.08 ± 3.11 | 0.696 |
| ALT (U/L) | 74.1 ± 50.9 | 77.2 ± 84.4 | 60.6 ± 44.1 | 0.615 |
| T Bil (mg/dL) | 0.79 ± 0.32 | 0.88 ± 0.49 | 0.96 ± 0.54 | 0.496 |
| Albumin (g/dL) | 3.74 ± 0.60 | 3.99 ± 0.50 | 3.74 ± 0.55 | 0.196 |
| Plat (103/μL) | 154.0 ± 65.8 | 134.9 ± 68.3 | 181.1 ± 94.5 | 0.155 |
| Vessel invasion (+/-) | 4/12 | 6/18 | 8/21 | 0.971 |
| Tumor number (S/M) | 9/7 | 16/8 | 15/14 | 0.541 |
| AJCC I/II/III | 6/2/8 | 14/4/6 | 9/6/14 | 0.282 |
| Child A/B | 13/3 | 22/2 | 23/6 | 0.445 |
| HBV (+/-) | 12/4 | 15/9 | 25/4 | 0.241 |
| HCV (+/-) | 5/11 | 7/14 | 6/23 | 0.678 |
| Cirrhosis (+/-) | 10/6 | 14/10 | 20/9 | 0.720 |
| AFP (op) (ng/dL) | 1582 ± 2645 | 3049 ± 7215 | 2776 ± 6696 | 0.751 |
| AFP (recur) (ng/dL) | 7.5 ± 5.2 | 86.8 ± 57.9 | 8162.3 ± 15 250.7 | 0.006 |
| Recur interval (mo) | 21.8 ± 14.6 | 12.3 ± 7.7 | 8.3 ± 6.6 | < 0.001 |
| Recur-to-death (mo) | 18.4 ± 19.5 | 24.1 ± 19.8 | 13.6 ± 19.6 | 0.162 |
| Survival time (mo) | 40.2 ± 19.9 | 36.1 ± 22.4 | 21.9 ± 22.0 | 0.013 |
| Intra-hepatic recur | 2/14 | 8/16 | 17/8 | 0.001 |
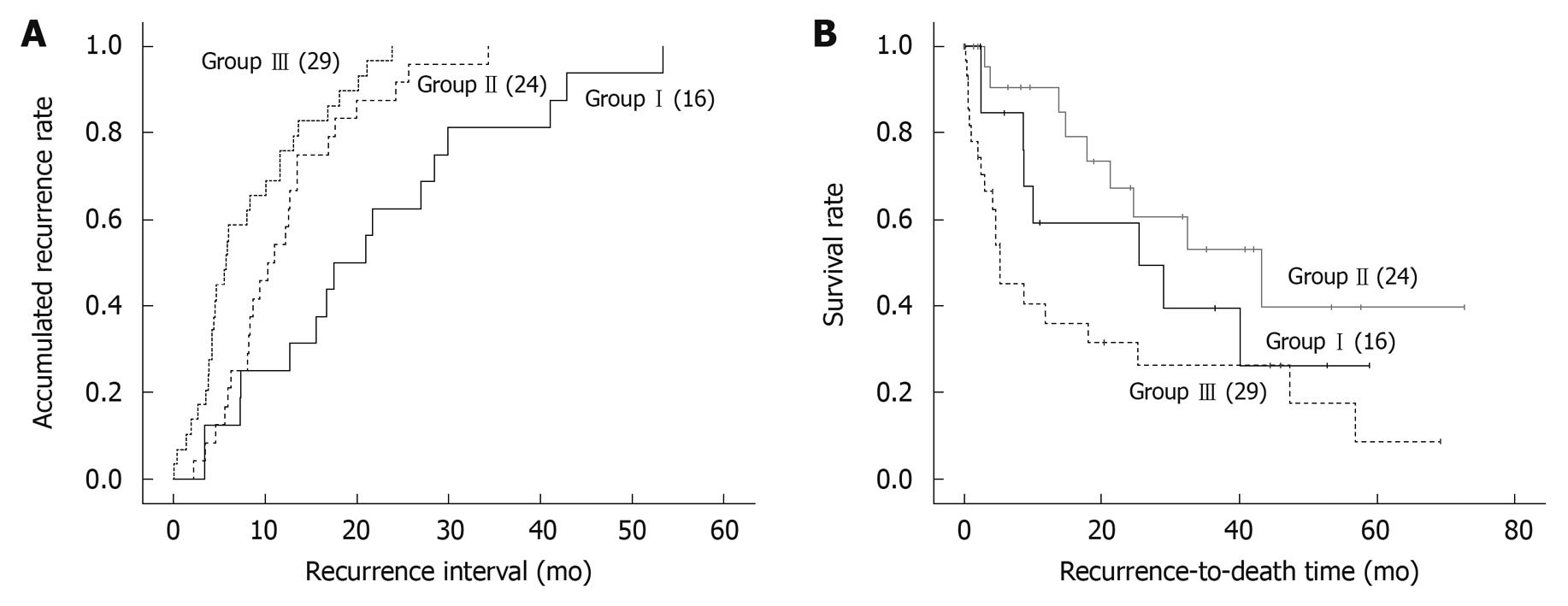
The accumulated recurrence rates in the three groups were calculated using the Kaplan-Meier method (Figure 2A). Accumulated recurrence rates were calculated from the first liver resection date to the recurrence date. Recurrence took place in all patients and was compared among groups I, II, and III (P < 0.001). The relative ratios [95% confidence interval (CI)] of groups II and III compared with group I patients were 0.244 (0.119-0.498) and 0.591 (0.340-1.027), respectively, with group I as the reference.
The risk factors for clinical outcome related to survival are shown in Table 2. The six factors affecting clinical outcome by Cox proportion hazard analysis were tumor size (> 5 cm), total bilirubin (> 1.2 mg/dL), vessel invasion (+), recurrence interval (< 12 mo), Child-Pugh classification (B), and group I and III. When Cox regression analysis was performed, vessel invasion, group III, and recurrence interval were identified as independent risk factors.
| Univariate analysis | Multivariate analysis | |||||
| RR | 95% CI | P | RR | 95% CI | P | |
| Age (yr) > 50/ ≤ 50 | 1.023 | 0.532-1.968 | 0.95 | |||
| Gender (M/F) | 0.640 | 0.325-1.262 | 0.20 | |||
| Tumor size (cm) > 5/ ≤ 5 | 0.444 | 0.230-0.856 | 0.02 | 0.836 | 0.404-1.729 | 0.63 |
| ALT (U/L) > 40/ ≤ 40 | 0.929 | 0.471-1.831 | 0.83 | |||
| T Bil (mg/dL) > 1.2/ ≤ 1.2 | 0.357 | 0.175-0.730 | 0.005 | 0.430 | 0.159-1.168 | 0.10 |
| Albumin (g/dL) > 3.5/ ≤ 3.5 | 1.674 | 0.867-3.232 | 0.13 | |||
| Plat (103/μL) > 10/ ≤ 10 | 1.600 | 0.808-3.169 | 0.18 | |||
| Vessel invasion (–/+) | 0.470 | 0.228-0.969 | 0.04 | 0.251 | 0.096-0.658 | 0.005 |
| Tumor number (S/M) | 1.073 | 0.556-2.070 | 0.83 | |||
| Child B/A | 0.372 | 0.182-0.759 | 0.007 | 0.452 | 0.153-1.339 | 0.15 |
| HBV (-/+) | 0.598 | 0.262-1.363 | 0.22 | |||
| HCV (-/+) | 1.514 | 0.688-3.334 | 0.30 | |||
| Cirrhosis (-/+) | 0.885 | 0.452-1.734 | 0.72 | |||
| Group I reference | 0.005 | 0.04 | ||||
| II/I | 0.371 | 0.162-0.846 | 0.02 | 2.142 | 0.569-7.000 | 0.02 |
| III/I | 0.318 | 0.144-0.700 | 0.004 | 0.582 | 0.254-3.033 | 0.12 |
| AFP (op) (ng/dL) > 200/ ≤ 200 | 0.048 | 0.000-14 716.4 | 0.64 | |||
| AFP (recur) (ng/dL) > 200/ ≤ 200 | 0.341 | 0.177-0.653 | 0.001 | 0.851 | 0.301-1.389 | 0.45 |
| Recur interval (mo) > 12/ ≤ 12 | 2.550 | 1.265-5.145 | 0.009 | 2.438 | 1.112-5.348 | 0.03 |
| Path grade I reference | 0.42 | |||||
| II/I | 0.821 | 0.376-1.795 | 0.62 | |||
| III/I | 0.504 | 0.197-1.291 | 0.15 | |||
The recurrence-to-death survival rates were calculated from the recurrence date to the last follow-up date and compared among groups I , II, and III as shown in Figure 2B (P = 0.016). Group II had a significantly better survival rate than the other two groups. The relative ratios (95% CI) of groups II and III compared with group I were 0.550 (0.241-1.253) and 0.337 (0.153-0.743), respectively.
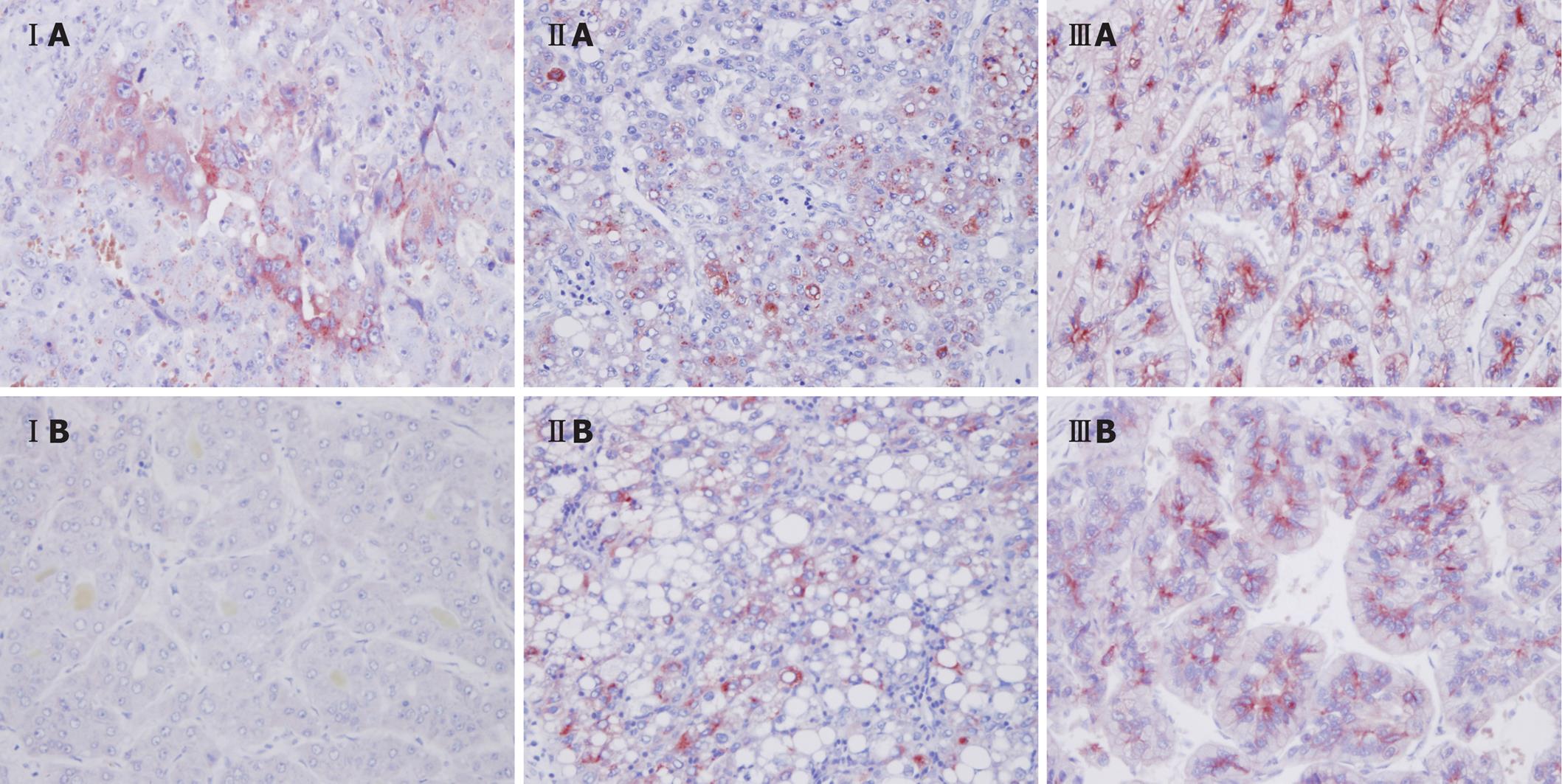
We compared the AFP immunohistochemistry results for a pair of specimens from each group. All groups revealed strong AFP staining in the primary HCC liver resection specimens. In the liver resection specimens from recurrent HCC, groups II and III showed strong AFP staining, but the specimen from group I showed reduced staining (Figure 3).
Twenty-three percent of the patients in our study had inconsistent serum AFP levels between the time of initial HCC diagnosis and its recurrence. These patients had a longer recurrence interval. However, they did not have a better recurrence-to-death survival than patients with higher AFP levels at recurrence. We propose the following possible explanations: First, there was de novo HCC after the first resection, and the behavior or biological activity of the recurrent tumor was more malignant than the first. Second, a delay in the detection of the recurrent tumor as a result of normal AFP levels decreased the recurrence-to-death survival. This meant that these patients seemed to have a longer recurrence interval due to the delayed diagnosis caused by normal AFP levels. Once HCC recurrence was diagnosed via clinical symptoms, the recurrent tumor was advanced, which then shortened the time from recurrence to death.
In general, the principle we follow in HCC patients after resection is according to the NCCN guideline. For patients with initially high AFP levels, such as the patients of our study, the AFP level is followed every 3 mo, and imaging studies are carried out every 3-6 mo in the first two years. After 2 years, the AFP level is followed every 3 mo and imaging studies are carried out annually. If the AFP level is elevated, suggesting HCC recurrence then further intervention is arranged. However, in the group with inconsistent AFP levels, the imaging studies were carried out annually due to normal AFP levels. Once HCC recurrence based on the NCCN guideline is diagnosed, the recurrence will be detected late resulting in poor quality medical care and legal problems.
In our patients, some underwent resection twice for removal of HCC. The first and second specimens from liver resection did not show consistent histopathological findings in a patient from group I, but did show similar histopathological findings in patients from groups II and III. Although these paired pathological findings are not conclusive, they do hint at an inconsistent pathological presentation. In a previous study, the authors proposed that the chromosomal changes and clonality relationship between primary and recurrent HCC could be proved using a comparative genomic hybridization technique[16]. In that study, 35% of HCC patients were considered to have a second primary HCC. In our study, the mechanism of HCC recurrence in group I patients may be due to de novo HCC with low secretion of AFP. This mechanism in groups II and III may be due to intra-hepatic metastasis from the primary HCC. Many possible reasons for this phenomenon have been reported, including de novo HCC[16,17], a biologic tumor changed by dedifferentiation[18], or a multicentric origin[19].
In our study, we used Cox regression analysis of the risk factors for accumulated recurrence rate and no confounders were identified. Patients with inconsistent AFP levels had a longer accumulated recurrence rate than those in groups II and III. The second primary HCC might have needed a longer time to develop, resulting in a longer recurrence period. In groups II and III, the recurrent tumor was probably from an intra-hepatic metastasis or residual HCC resulting in a shorter recurrence interval than that suggested by other articles[20,21]. The recurrence-to-death survival rate of patients with inconsistent AFP levels was worse than that in group II where the AFP level was consistent.
Elevated serum AFP is useful for the diagnosis of HCC, and also implies a large tumor burden at presentation[6]. A low serum AFP level at recurrence in patients with an initially high AFP level at HCC diagnosis may be a good prognostic factor of survival[6]. However, this was not supported by our results. This poor outcome may be caused by a biological change in the malignancy or delayed diagnosis. Physicians need to diagnose recurrence as early as possible by regular surveillance to improve patient outcome. In the NCCN practice guidelines, version 1, 2009[15], surveillance for HCC recurrence in patients with initially elevated AFP levels includes checking AFP levels every 3 mo and performing imaging studies annually for 2 years after resection[15]. When physicians follow the NCCN guidelines, they should bear in mind that AFP level is not as sensitive as generally believed for tumor recurrence in this subgroup of HCC patients, similar to group I patients in our study. The results of our study showed that regular checks of AFP levels should be conducted every 1-3 mo, followed by imaging studies every 3 mo for patients with an initially elevated AFP level. Regular imaging studies should not be omitted even when the serum AFP level is normal. The results of our study are important for reminding practicing physicians that regular imaging is still the benchmark in the surveillance of HCC patients.
There were some limitations in this study. First, these preliminary data were from one medical center and the conclusion needs to be validated by a meta-analysis of multicentric data. Second, the diagnosis of HCC recurrence was not proven by pathologic findings. We diagnosed HCC recurrence by dynamic imaging studies, hepatic angiography, and clinical disease progression. In a further study, tumor biopsy should be performed before salvage therapy if possible. The results of histochemical staining in the recurrent tumor could explain the exact mechanism of this clinical presentation in patients with inconsistent AFP levels.
In conclusion, 23% of our selected patients demonstrated an inconsistency in serum AFP level between initial HCC diagnosis before resection and HCC recurrence diagnosis. To improve medical care and avoid missed or delayed diagnoses, regular checks of AFP level and imaging studies every three months are strongly recommended during follow-up, even for patients with normal serum AFP levels.
Serum α-fetoprotein (AFP) level is widely used as a tumor marker for the diagnosis and detection of both occurrence and recurrence of hepatocellular carcinoma (HCC). A high serum AFP level in HCC also indicates a poor outcome. However, inconsistent AFP levels have been found, where patients’ AFP levels were initially high when HCC was diagnosed, but were normal when HCC recurred. There is no research on the prognosis of this subgroup of HCC patients.
When clinical physicians follow the National Comprehensive Cancer Network guideline, they should bear in mind that AFP level is not as sensitive as generally believed for tumor recurrence in this subgroup of HCC patients. Regular checks of AFP level and imaging studies every three months are strongly recommended during follow-up, even for patients with normal serum AFP levels.
This is the first article to investigate the clinical outcomes of patients with inconsistent AFP levels. The clinical outcomes in this subgroup of HCC patients were not as good as those with normal AFP levels. To improve medical care and avoid missed or delayed diagnoses, clinical physicians should bear these findings in mind.
The authors modified the surveillance principle for this subgroup of HCC patients following the results of this study to improve the quality of medical care.
This is a retrospective analysis of a cohort of patients with HCC, it maybe interesting for the readers.
Peer reviewers: James Neuberger, Professor, Liver Unit, Queen Elizabeth Hospital, Birmingham B15 2TH, United Kingdom; Wei Tang, MD, EngD, Assistant Professor, H-B-P Surgery Division, Artificial Organ and Transplantation Division, Department of Surgery, Graduate School of Medicine, The University of Tokyo, Tokyo 113-8655, Japan
S- Editor Wang JL L- Editor Webster JR E- Editor Lin YP
| 1. | Chen DS, Sung JL. Serum alphafetoprotein in hepatocellular carcinoma. Cancer. 1977;40:779-783. [Cited in This Article: ] |
| 2. | Peng SY, Lai PL, Chu JS, Lee PH, Tsung PT, Chen DS, Hsu HC. Expression and hypomethylation of alpha-fetoprotein gene in unicentric and multicentric human hepatocellular carcinomas. Hepatology. 1993;17:35-41. [Cited in This Article: ] |
| 3. | Kudo M, Okanoue T. Management of hepatocellular carcinoma in Japan: consensus-based clinical practice manual proposed by the Japan Society of Hepatology. Oncology. 2007;72 Suppl 1:2-15. [Cited in This Article: ] |
| 4. | Bruix J, Sherman M. Management of hepatocellular carcinoma. Hepatology. 2005;42:1208-1236. [Cited in This Article: ] |
| 5. | Peng SY, Chen WJ, Lai PL, Jeng YM, Sheu JC, Hsu HC. High alpha-fetoprotein level correlates with high stage, early recurrence and poor prognosis of hepatocellular carcinoma: significance of hepatitis virus infection, age, p53 and beta-catenin mutations. Int J Cancer. 2004;112:44-50. [Cited in This Article: ] |
| 6. | Tangkijvanich P, Anukulkarnkusol N, Suwangool P, Lertmaharit S, Hanvivatvong O, Kullavanijaya P, Poovorawan Y. Clinical characteristics and prognosis of hepatocellular carcinoma: analysis based on serum alpha-fetoprotein levels. J Clin Gastroenterol. 2000;31:302-308. [Cited in This Article: ] |
| 7. | Soresi M, Magliarisi C, Campagna P, Leto G, Bonfissuto G, Riili A, Carroccio A, Sesti R, Tripi S, Montalto G. Usefulness of alpha-fetoprotein in the diagnosis of hepatocellular carcinoma. Anticancer Res. 2003;23:1747-1753. [Cited in This Article: ] |
| 8. | Farinati F, Marino D, De Giorgio M, Baldan A, Cantarini M, Cursaro C, Rapaccini G, Del Poggio P, Di Nolfo MA, Benvegnù L. Diagnostic and prognostic role of alpha-fetoprotein in hepatocellular carcinoma: both or neither? Am J Gastroenterol. 2006;101:524-532. [Cited in This Article: ] |
| 9. | Poon RT, Fan ST, Lo CM, Liu CL, Wong J. Intrahepatic recurrence after curative resection of hepatocellular carcinoma: long-term results of treatment and prognostic factors. Ann Surg. 1999;229:216-222. [Cited in This Article: ] |
| 10. | Choi GH, Kim DH, Kang CM, Kim KS, Choi JS, Lee WJ, Kim BR. Prognostic factors and optimal treatment strategy for intrahepatic nodular recurrence after curative resection of hepatocellular carcinoma. Ann Surg Oncol. 2008;15:618-629. [Cited in This Article: ] |
| 11. | Choi D, Lim HK, Rhim H, Kim YS, Yoo BC, Paik SW, Joh JW, Park CK. Percutaneous radiofrequency ablation for recurrent hepatocellular carcinoma after hepatectomy: long-term results and prognostic factors. Ann Surg Oncol. 2007;14:2319-2329. [Cited in This Article: ] |
| 12. | Zhou L, Liu J, Luo F. Serum tumor markers for detection of hepatocellular carcinoma. World J Gastroenterol. 2006;12:1175-1181. [Cited in This Article: ] |
| 13. | Chen JG, Parkin DM, Chen QG, Lu JH, Shen QJ, Zhang BC, Zhu YR. Screening for liver cancer: results of a randomised controlled trial in Qidong, China. J Med Screen. 2003;10:204-209. [Cited in This Article: ] |
| 14. | Wong LL, Limm WM, Severino R, Wong LM. Improved survival with screening for hepatocellular carcinoma. Liver Transpl. 2000;6:320-325. [Cited in This Article: ] |
| 15. | NCCN Clinical Practice Guidelines in Oncology. Hepatobiliary cancers (Version 1, 2009). Available from: http://www.nccn.org/professionals/physician_gls/PDF/hepatobiliary.pdf. [Cited in This Article: ] |
| 16. | Chen YJ, Yeh SH, Chen JT, Wu CC, Hsu MT, Tsai SF, Chen PJ, Lin CH. Chromosomal changes and clonality relationship between primary and recurrent hepatocellular carcinoma. Gastroenterology. 2000;119:431-440. [Cited in This Article: ] |
| 17. | Chen PJ, Chen DS, Lai MY, Chang MH, Huang GT, Yang PM, Sheu JC, Lee SC, Hsu HC, Sung JL. Clonal origin of recurrent hepatocellular carcinomas. Gastroenterology. 1989;96:527-529. [Cited in This Article: ] |
| 18. | Ding SF, Jalleh RP, Wood CB, Bowles L, Delhanty JD, Dooley J, Habib NA. Different DNA changes in primary and recurrent hepatocellular carcinoma. Gut. 1992;33:1433-1435. [Cited in This Article: ] |
| 19. | Okusaka T, Okada S, Nose H, Ishii H, Nakasuka H, Nakayama H, Nagahama H, Yoshimori M, Shimada K, Yamamoto J. The prognosis of patients with hepatocellular carcinoma of multicentric origin. Hepatogastroenterology. 1996;43:919-925. [Cited in This Article: ] |
| 20. | Furihata T, Sawada T, Kita J, Iso Y, Kato M, Rokkaku K, Shimoda M, Kubota K. Serum alpha-fetoprotein level per tumor volume reflects prognosis in patients with hepatocellular carcinoma after curative hepatectomy. Hepatogastroenterology. 2008;55:1705-1709. [Cited in This Article: ] |
| 21. | Shirabe K, Takenaka K, Gion T, Shimada M, Fujiwara Y, Sugimachi K. Significance of alpha-fetoprotein levels for detection of early recurrence of hepatocellular carcinoma after hepatic resection. J Surg Oncol. 1997;64:143-146. [Cited in This Article: ] |