Published online Apr 7, 2012. doi: 10.3748/wjg.v18.i13.1425
Revised: September 17, 2011
Accepted: October 14, 2011
Published online: April 7, 2012
The worldwide incidence and prevalence of cystic echinococcosis have fallen dramatically over the past several decades. Nonetheless, infection with Echinococcus granulosus (E. granulosus) remains a major public health issue in several countries and regions, even in places where it was previously at low levels, as a result of a reduction of control programmes due to economic problems and lack of resources. Geographic distribution differs by country and region depending on the presence in that country of large numbers of nomadic or semi-nomadic sheep and goat flocks that represent the intermediate host of the parasite, and their close contact with the final host, the dog, which mostly provides the transmission of infection to humans. The greatest prevalence of cystic echinococcosis in human and animal hosts is found in countries of the temperate zones, including several parts of Eurasia (the Mediterranean regions, southern and central parts of Russia, central Asia, China), Australia, some parts of America (especially South America) and north and east Africa. Echinococcosis is currently considered an endemic zoonotic disease in the Mediterranean region. The most frequent strain associated with human cystic echinococcosis appears to be the common sheep strain (G1). This strain appears to be widely distributed in all continents. The purpose of this review is to examine the distribution of E. granulosus and the epidemiology of a re-emerging disease such as cystic echinococcosis.
- Citation: Grosso G, Gruttadauria S, Biondi A, Marventano S, Mistretta A. Worldwide epidemiology of liver hydatidosis including the Mediterranean area. World J Gastroenterol 2012; 18(13): 1425-1437
- URL: https://www.wjgnet.com/1007-9327/full/v18/i13/1425.htm
- DOI: https://dx.doi.org/10.3748/wjg.v18.i13.1425
Cystic echinococcosis (CE) is a near-cosmopolitan zoonosis caused by adult or larval stages of tapeworms (cestodes) belonging to the genus Echinococcus (family Taeniidae). Actually, six species of Echinococcus have been recognized, but the most important members of the genus in respect of their public health importance and their geographical distribution are Echinococcus granulosus (E. granulosus) (which causes cystic echinococcosis) and Echinococcus multilocularis (which causes alveolar echinococcosis). Infection with E. granulosus results in the development of one or several unilocular hydatid cysts that in humans develop mainly in the liver (70%), but also lungs (20%) and 10% of cysts can occur almost anywhere in the body (e.g., brain, body musculature, wall of the heart, kidneys, orbit of the eye, marrow cavity of bones). E. multilocularis metacestodes develop as a series of small, interconnected cysts, growing as a metastasising lesion almost exclusively in the liver (98%-100%), but in the later phase of infection distant metastases in other organs may occur.
E. multilocularis is a cestode whose life cycle involves a tapeworm stage during which it lives in the small intestine of carnivores (definitive hosts, usually wild or domestic canids), and a tissue-invading metacestode (larval) stage during which echinococcal cysts develop in internal organs (mainly liver and lungs) of humans and other intermediate hosts as unilocular fluid-filled bladders surrounded by a host-produced layer of granulomatous adventitial reaction. Small vesicles called brood capsules bud internally from the germinal layer and produce multiple protoscolices by asexual division. In humans, the slowly growing echinococcal cysts may reach a volume of several litres and contain many thousands of protoscolices. Moreover, internal septations and daughter cysts may appear over time, disrupting the unilocular pattern typical of the young echinococcal cysts.
Infection of an intermediate host is due to accidental ingestion of tapeworm eggs passed into the environment with faeces from definitive hosts. Transmission of E. granulosus could be due to domestic and wildlife reservoirs, and is influenced by human activities, behaviour, and politics.
CE represents an increasing public health and socio-economic concern in many areas of the world[1-3] and is currently considered an endemic zoonose in the Mediterranean region (MR), in addition to brucellosis, rabies, leishmaniasis and food-borne zoonotic infections[4]. Given a geographic distribution and extent greater than previously believed, several studies have shown that hydatidosis is currently considered an emerging or re-emerging disease[5,6]. The distribution and prevalence of CE depends on the presence in that country of large numbers of nomadic or semi-nomadic sheep and goat flocks that represent the intermediate host of the parasite, and their close contact with the final host, the dog, which mostly provides the transmission of infection to humans.
Molecular studies conducted on mitochondrial DNA (mtDNA) sequences, have shown that E. granulosus complex consists of three species and comprise ten defined strains (genotype G1-10), based on morphology, host specificity and molecular characteristics[7,8]. The intraspecific variants have substantial variation at the genetic level and DNA sequence[9], conferring several characteristics such as life-cycle patterns, host specificity, development rate, antigenicity, transmission dynamics, sensitivity to chemotherapeutic agents, and pathology[10,11]. These characteristics may have important implications for the design and development of vaccines, diagnostic reagents and drugs impacting on the epidemiology and control of echinococcosis[12,13]. Indeed, each Echinococcus species maintains a specific host-adapted genetic identity that only rarely overlaps in some geographical areas[5,11,14].
In this review we discuss aspects of the current epidemiology of E. granulosus complex and highlight worldwide and specific distribution in recognised endemic areas.
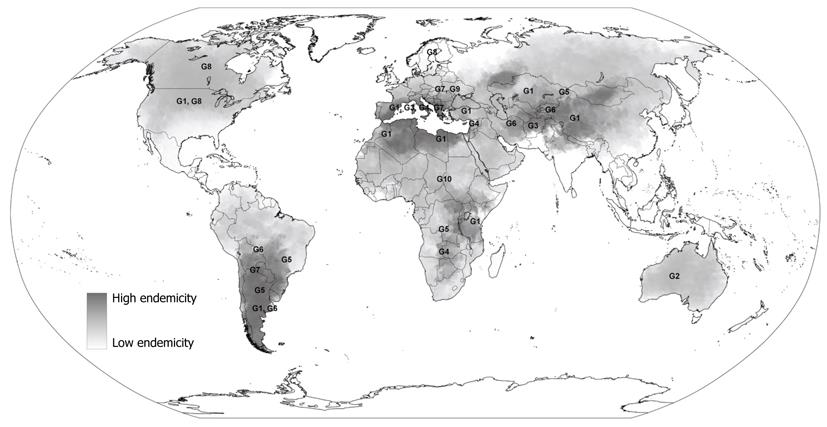
E. granulosus has a worldwide geographical distribution with endemic foci present on every inhabited continent (Figure 1). The greatest prevalence of CE in human and animal hosts is found in countries of the temperate zones, including several parts of Eurasia (the Mediterranean regions, southern and central parts of Russia, central Asia, China), Australia, some parts of America (especially South America) and north and east Africa[2,15].
The distinct genetic types of E. granulosus include two sheep strains (G1 and G2), two bovid strains (G3 and G5), a horse strain (G4), a camelid strain (G6), a pig strain (G7), and a cervid strain (G8). A ninth genotype (G9) has been described in swine in Poland[8,16] and a tenth strain (G10) in reindeer in Eurasia. Among these strains, we have available data for preliminary epidemiological analyses only for some strains. In fact, some of them are still poorly characterised and further research is needed to determine with higher detail their host and geographic ranges and whether their genetic characteristics are conserved between different endemic regions.
The most frequent strain associated with human CE appears to be the common sheep strain (G1). This strain appears to be widely distributed in all continents. Highest rates of infection are recorded in communities involved in extensive sheep farming and epidemiological studies suggest that this genetic variant is the principal strain infecting humans[2,5,9,17]. Consequently, its presence coincides with areas which have high prevalence of human CE such as in Morocco, Tunisia, Kenya, Kazakhstan, western China and Argentina.
The G2 strain is known to be transmitted among sheep and infect humans also, but genetic differences biologically distinguish it from the G1 strain, conferring a different life cycle[18]. It has been found in Australia and previously also documented in Tasmania.
The G3 strain which is diffused among buffalos and transmitted by water, has been recorded in South Asia[19], but no susceptibility among humans has been found.
The G4 strain, formerly known as Echinococcus equinus, appears to infect exclusively equines as intermediate hosts and no human cases have been documented[9,20]. It is known to be diffused in the Mediterranean regions of Spain, Italy, Lebanon, and Syria, as well as in South Africa.
The former cattle strain (G5), known as Echinococcus ortleppi, is transmitted by cattle in Europe, Asia, parts of Africa and South America and only one case in humans has been isolated in past years[21], suggesting a less pathogenic risk for humans than the sheep strain of E. granulosus.
G6-10 strains are poorly distinguished from each other but they are clearly distinct from the common sheep strain[5]. The G6 strain is known to principally affect camels and goats. Animal infection is diffused in the Middle East, Africa, southern Asia and South America[9] and cases of human infection have been found in Nepal, Iran, Mauritania, Kenya and Argentina[5,17].
The G7 strain is transmitted by domestic pigs in Europe (Spain and Italy), Asia and South America, as well as the closely related genetic variant G9 that has been documented to affect Polish patients[16] although the animal reservoir is unknown.
The G8 strains are known to be transmitted between wolves and wild cervids in the northern regions of Europe, Asia and North America. Few cases of human infection have been documented with a lower severity of the disease than CE caused by other forms of E. granulosus[22]. However, transmission between humans of this genetic variant seems to be low and further data is needed to better assess its pathogenicity.
Finally, some other genetic variants which are poorly characterized have been found in several countries. For example, the wildlife “lion” strain transmitted among lions and wild ungulates has been documented in Africa but no human infection has been found.
The most ubiquitous taxa of E. granulosus that occur in North America are the cervid strain (G8) and the sheep strain (G1). The former is diffused in wildlife mainly in Canada, Alaska and Minnesota[23]. The wildlife reservoir was found to be largely diffused among cervids and wolves, coyotes and domestic dogs[24]. EC started to be diagnosed in Canada after the 1950s following the introduction of routine chest X-rays for tuberculosis in some tribes of native Americans (such as Indians and Eskimo) who were identified with pulmonary hydatidosis[24]. In the same period, a review of 101 autochthonous cases of E. granulosus infection in Alaska were documented[22]. It has been estimated that 50% of moose in Ontario and British Columbia are infected with the parasite[25] and that 28%-50% of dogs in the Canadian Northwest Territories are infected with E. granulosus[26]. In humans, pulmonary localization is quite diffuse. Indeed, a recent chart review performed in Alberta documented 22 definite and probable cases, of which 77% were female and 41% aboriginal; 40% had pulmonary involvement and 50% hepatic involvement[27].
Sporadic autochthonous transmission among humans of the sheep strain in the western States of North America such as Arizona, California, New Mexico and Utah has been documented in reports from the 1960s[28]. The source of these E. granulosus infections was Australian sheep dogs imported into Utah in 1938 when the parasite diffused among sheep of this area as well as adjoining states through trading of live sheep[29]. Moreover, another source of infection were immigrants from countries in which echinococcosis disease is highly endemic, historically Icelanders, Italians and Greeks, but in more recent years, mostly persons of Middle Eastern and Asian origin. After the Second World War, foci of transmission involving swine and dogs were reported in several areas such the Mississippi valley due to the close relationship between humans and dogs[30] but transmission of infection appeared to have ended by mid-century[31]. Then, an epidemic focus of sheep and human infection in western states including California, Utah, New Mexico and Arizona in the mid-1960s was traced[28]. Most infections occurred in high-risk groups such as sheep farmers and those involved in home slaughter including Basque-Americans in California[32], Mormons in central Utah[33], and Navajo and Zuni Indians in New Mexico and Arizona[33-35].
As well as in the United States, all genetic variants of the E. granulosus complex have been introduced into South America with domestic animals imported from other regions, such as Europe. The principal strain of E. granulosus is the sheep strain (G1), widely diffused in Peru, Chile, Argentina and Brazil[2].
In the central Peruvian Andes, the prevalence of hydatidosis in livestock has been noted to be 89% in sheep and 80% in cattle in a livestock raising community[36]. Among definitive hosts, the prevalence of infection in dogs in endemic areas has been reported to range from 32% to 46%[36-38] and from 46% to 88%[38-40]. The recorded surgical incidence of CE in the central and southern Peruvian Andes has been noted to be 1-2 cases per 100 000 inhabitants[36] and the prevalence of asymptomatic CE between 3% and 9.3% in rural villages in the central Peruvian highlands[38]. However, a study in a coastal city of Peru reported an annual surgical incidence of 32 per 10 000 for 1998[37] leading to the conclusion that incidence of CE is significantly under-reported. Recently, a re-emergence of transmission has been documented after the failure of previous control activities[41].
Chile is an endemic area for E. granulosus infection. During 2000, the prevalence of bovine, sheep and canine hydatidosis for the entire country decreased to 22.3%, 6.3% and 11%, respectively[42], after a control program[2,43,44]. With regard to human infection, although the overall incidence of diagnosed disease has been assessed as 2-2.5 cases per 100 000 inhabitants between 1992 and 2004, taking under-notification into account, the incidence has been estimated at 10 per 100 000. A major endemic area for EC is the southern part of Chile where annual surgical incidence ranged from 6 to 20 cases per 100 000 in August 2005 but reaching 162 per 100 000 in some regions[45].
In Argentina, several strains of E. granulosus and E. ortleppi have been found in different host animals and humans such as the sheep strain (G1) (mostly infecting humans), the Tasmanian sheep strain (G2), the cattle strain (G5) and the camel strain (G6)[14,46], while the pig strain (G7) has been detected in pigs and dogs but not humans[46]. The prevalence of EC affecting livestock has been documented as reaching 7% of cattle, 12.5% of sheep, 9.8% of pigs and 6.0% of goats[2]. In humans, prevalence rates depend on the endemicity of the area, ranging from 1.4 per 100 000 to 404, 260 and 30 cases per 100 000 in Neuquen, Chubut and Rio Negro (regions of Patagonia), respectively[42].
In southern Brazil only sheep strain and E. ortleppi have been recorded[47,48], although the most endemic area is the southern part of Brazil. Indeed, a recent analysis of hydatidosis prevalence in animals in this area reported a prevalence of infection to be 25.5% of cattle, 30.2% of sheep[42] and from 11.4% to 38% of dogs[49]. Data about human hydatidosis documented a seroprevalence of 6% in the rural population and 3.5% in the urban population of Sena Madureira[50]. However, the few data available to allow conclusions on epidemiology of different taxa often depend on control activities that are inconsistent in their consideration of the economic and public health impact of echinococcosis in these areas.
The most common strain currently found in Australia is the G1, while the G2 strain was previously also found in Tasmania[7,8,51]. This G2 strain probably evolved as a genetically modified variant after a Tasmanian hydatid control campaign aimed to strictly control helminthic diffusion among dogs. Thus, this genetic variant became dominant because of the limited gene pool on an island[18]. However, the absence of diffusion of the hydatid infection in wildlife and the intense hydatid control programmes allowed the eradication of E. granulosus from Tasmania in the middle 1990s[52].
In Australia several areas have been documented at high risk of transmission of E. granulosus, especially in wildlife. The definitive hosts most commonly involved in transmission in south eastern Australia are represented by the wild dog[53,54], while the most common intermediate hosts are grey kangaroos and wallabies[54]. Western Australia, south of Perth, is another active area of transmission of E. granulosus[55]. In this region, similar intermediate hosts have been found[10] while in northern Western Australia the source of infection has yet to be confirmed[56].
However, wildlife reservoirs play the main role in maintaining a constant source of transmission for domestic livestock, domestic dogs and humans[53,54,57-60]. Recent analyses assessed infection in wild dogs caught in the outer suburbs of Townsville, Queensland[61], and in those examined from the Maroochy Shire, eastern Queensland[62]. Sheep infection is still common in farms with a high number of poorly managed domestic dogs; additionally livestock are often hunted by wild dogs contaminating the pasture with eggs of E. granulosus[53]. However, dog and sheep infection prevalence seems to be decreasing over the last years[60], although recent surveys reported a re-emergence of domestic transmission of E. granulosus in some rural areas of south eastern regions where it was found that 29% of 344 rural dogs in New South Wales and 18% of 218 Victorian dogs tested positive[63].
Annually, new cases of human hydatidosis appear stable, numbering between 80 and 100 among the entire country[60,64]. Human transmission has traditionally been a public health problem of rural people due to E. granulosus infected domestic animals, but there is increasing potential for accidental exposure of urban residents due to the infiltration in urban centres by infected wildlife definitive hosts such as foxes and wild dogs. In fact, these animals are attracted to public recreation areas commonly frequented by urban residents to scavenge food scraps[61,65]. Thus, urban residents could accidentally have direct contact with E. granulosus eggs through wild dog or fox faeces or via coprophagous flies when visiting parks and forests for recreational purposes. Furthermore, it has been documented that there has been a potential infection of the dogs of recreational pig hunters living in urban centres[66].
The reporting of hydatidosis or echinococcosis does not depend on any monitoring system but only on individual case reports. Thus, assessing accurate prevalence and incidence, as well as trend changes over time, is still difficult to achieve.
The G1 strain, infecting sheep, goats, cattle and camels, is the most common genetic variant documented in Iran[67]. On the other hand, the G6 strain has also been found in camels, sheep and cattle in the same area[67]. Both of these were diagnosed in human hydatid infection confirming the pathogenicity of G6 for humans[67].
In Kazakstan, it has been assessed that the prevalence of infection in sheep ranges between 20%-25% in 1-year-old sheep and 74%-80% in sheep 6 years old and over. Among wild and village dogs, the prevalence of infection is 23% and 6%, respectively[68]. Although the highest worm burdens have been recorded in rural dogs, only those closer to human habitation are responsible for transmitting disease to humans[68]. Human infection has increased since the middle 1990s till present time from 200 surgical cases annually to the current level of nearly 1000 cases per year[69,70]. Similar trends in human cases have been assessed in all other Central Asian countries. However, no detailed data is available about transmission and diffusion of E. granulosus infection in Central Asian countries.
Hydatidosis is a serious public health problem in Turkey where E. granulosus infection in dogs ranges between 0.32% and 40%[71]. The predominant genotype of E. granulosus in Turkey is the G1 strain with a prevalence infection rate in farm animals ranging from 26.6% to 50.9% in sheep, from 13.3% to 35.68% in cattle, and reaching 22.1% in goats, 44.31% in cows and 24.39% in bulls in the most endemic areas such the Budur region[72], the Kirikkal region[73], the Afyonkarahisar district[74], and the Sivas region[75]. Lower rates in sheep (3.5%) and cattle (11.6%) have been found in less endemic areas such as Thrace region[76]. Surgical cases of human hydatidosis have been estimated to range from 0.87 to 6.6 per 100 000 inhabitants between 1987 and 1994[71]. A more recent survey based on hospital, regional and ministerial documents showed that, from 2001 to 2005, a total of 14 789 CE surgical cases were recorded with a higher incidence in the Middle Anatolian region (38.57%) and lower in the Black Sea region[77].
Several regions of the Arab peninsula such as Syria, Israel and Palestine are considered endemic for E. granulosus. In fact, hydatidosis is mostly associated with main risk factors such as livestock production, raising of sheep and nomadic tribal life that characterize northern Syria, northern Israel and western Palestine. Epidemiological evidence in Syria showed a prevalence of E. granulosus infection ranging between 9% and 15% in dogs and between 5% and 17% in livestock[78]; in Israel, ranging between 5.4% to 14.2% in dogs and between 4.56% and 10% in sheep[79,80]; and in Palestine, ranging between 7.9% and 14.3% in dogs[81]. Human infection rates have been assessed in individual studies. Annual surgical prevalence recorded from the Al-Maqased Hospital in Jerusalem was documented to be 1.76 per 100 000 inhabitants in the middle 1990s[81], while in hospitals of the Palestinian West Bank this value was 3.1 per 100 000 inhabitants, with the highest rates of 4.9, 5.0 and 5.1 per 100 000 inhabitants found in Hebron, Jericho and Bethlehem, respectively[82]. In an epidemiological study conducted in northern Israel a cumulative infection rate of 1.5 per 100 000 inhabitants was found[83], while in another study conducted in a Bedouin group from southern Israel this rate was 0.68%[83].
China is one of the most important endemic regions of CE[2]. The sheep strain (G1) and the camel strain (G6) are the only two E. granulosus strains found in China[84], both of them infectious to humans[85]. The most endemic areas for Echinococcus spp. have been recognized as the provinces and autonomous regions stretching from western Xinjiang[86], Ningxia and Inner Mongolia, with the highest prevalence rates occurring in pastoral communities of the eastern Tibetan plateau[87-89] (south western Qinghai and north western Sichuan) and the Tibetan autonomous area of south Gansu[90], located in western and northwestern China[85,91-95]. Infection by cysts of E. granulosus can be found in organs of ungulate intermediate hosts[96-99]. High prevalence of hydatid infection has been reported in sheep and yaks (99%), cattle (88%) and pigs (70%)[90]. In fact, in the western and northwestern pastoral areas of China, livestock pastoralism is a major industry with a total of 350 million sheep and other domesticated large herbivores including horses, camels, and red deer[90]. On the other hand, the definitive host is mainly represented by canids, predominantly the domestic dog. Indeed, they are kept in large populations in northwestern China for pastoralism and cultural reasons[87]. Given the close contact with local people, dogs are considered the most important definitive host transmitting E. granulosus to humans[2,87]. However, in certain rural regions, wild canids such as wolves and foxes are involved in the sylvatic cycle[2].
The first human CE was reported in China in 1905[86]. Over the last century, about 35 000 cases of human cystic echinococcosis have been treated surgically in China. However, given the documented 21 560 cases in Xinjiang alone with a prevalence of 80 cases/100 000 inhabitants[86], it has been assessed that an underestimation occurred in past years. Now, it has been estimated that about one million existing cases of human echinococcosis occur in China[100]. Of these, about 70% present with chronic cystic lesions of the liver as well as in other organs including the brain[101]. The infection rate of females has been assessed to be considerably higher than that of males because of their role in the home activities including feeding dogs, collecting yak dung for fuel, and milking livestock[87,102]. Thus, nomadic or seminomadic pastoral lifestyle is one of the most important risk factors for CE in China, especially in western and northwestern areas where livestock pastoralism is a major industry[90], and women are more frequently exposed to the definitive hosts of CE. Consequently, adults have much higher infection rates than children[87], and the infection rate increases with age[102].
The increasing number of diagnosed cases may reflect improved diagnostic methods and improved outreach programs. In fact, China is now recognized as a new focus for echinococcosis research.
Although most regions of Africa are poorly researched and limited information is available, several taxa have been found in the African countries[19,103,104]. The most common strain is the G1, highly diffused in the North and East African sheep raising areas. Moreover, the exclusive presence of the camel strain (G6) has been documented. In addition, wild strains such as the E. equinus (the “horse strain”)[105] and the “lion strain”[106] have been found in South Africa. However, the nature of Echinococcus in African wildlife is poorly documented.
In a recent study carried out in Libya, 25.8% of stray dogs and 21% of owned dogs have been assessed to be positive for EC[107] while another study found a prevalence of 58% of hydatidosis in the same area[108]. Nevertheless, several surveys assessed that other animals also, especially camels, are frequently infected by E. granulosus[109,110], while infection rates in livestock varied from 1.7% to 33.4% in sheep, 1.0% to 13.9% in cattle, 1.4% to 40.0% in camels and 0% to 18% in goats[111-113], often associated with human cases[114]. The sheep strain has been considered the most common genetic variant diffused among humans in another survey, reporting a prevalence rate of 1.7% of 20 200 patients screened by ultrasound for hydatid cysts in 36 villages along the northern coast of Libya[115] and an incidence rate of 4.2 cases per 100 000 inhabitants in Eastern Libya[112]. Indeed, in a genetic survey conducted on 179 isolates from humans collected in the border area of northwestern Kenya and south-eastern Sudan, only one was associated with the camel strain (G6) while the remaining were the common sheep strain (G1)[17]. On the other hand, other surveys conducted in central Sudan[110,116] and Egypt[117] documented the presence of human echinococcosis cases diagnosed as G6 and at least two other distinct strains (camel and equine)[118].
CE is currently of low endemicity in Egypt with a mean prevalence in dogs ranging between 3.2% in urban areas and 6% in rural areas[119]. Higher prevalence has been documented in Cairo with about 15% of dogs infected[120]. Among ruminants, confirming earlier results[121], recent data demonstrated an overall prevalence infection rate of 0.3% in sheep and goats, 0.68% in pigs, 6.4% in cows and buffaloes, 2.53% in camels[121] and 10.62% in donkeys[122]. In humans, a retrospective hospital study showed an annual surgical incidence ranging between 1.34 and 2.60 per 100 000 inhabitants[123].
In Tunisia, echinococcosis is a major public health problem due to its high prevalence and morbidity. Molecular analysis has demonstrated that the most common genetic variants of E. granulosus circulating in Tunisia are the G1 sheep strain and the G6 camel strain[124,125]. Sheep breeding is a significant risk factor, being practised by 94.7% of patients vs 58.3% of the farming population[126]. A series of studies carried out between 1999 and 2007 assessed that the prevalence of E. granulosus infection reached 10.41% in lambs (6-12 mo), 75.42% in sheep aged 1-2 years and 83.83 to 100% in sheep over 2 years old[127]; and 10.1% of camels[124] and 40% of sheep in a further analysis conducted in North-East Tunisia[128]. Despite the lack of recent published data, the last report of EC in humans reported an annual surgical incidence of hydatidosis of about 15 per 100 000 inhabitants[129].
In Algeria similar strain distribution has been found, identifying the sheep strain G1 infecting sheep, cattle and humans and the camel strain G6 infecting camels[130]. Dogs likely represent the main source of infection for farm animals and humans[19] with a prevalence rate of 24.8% in camels, 13.9% in cattle and 6.0% in horses[131]. Despite poor data regarding recently reported human infection, it is documented that more than 700 surgical cases are notified each year to the Ministry of Health. Last published work assessed that the annual incidence of human EC reached 3.6-4.6 per 100 000 inhabitants[132].
Morocco is considered an endemic area for echinococcosis. A genotype almost similar to the common G1 sheep strain with some nucleotide variations was found in camels and horses. Infection rate in dogs ranges from 22.0% to 62.8%, depending on the region[133]. In a more recent analysis, CE infection prevalence rates have been documented to be 10.58% in sheep, 1.88% in goats, 22.98% in cattle, 12.03% in camels and 17.80% in equines, mostly in Middle Atlas (48.72% in cattle) and in North West (37.61% in cattle and 31.65% in sheep)[134]. In humans, an annual rate of 4.55 surgical cases per 100 000 inhabitants has been documented in 2006, with a higher prevalence in the middle Atlas mountainous region[135].
With the exception of Malta and the area controlled by the Government in southern Cyprus, where the disease has been practically eliminated, all the Mediterranean regions including the Arab peninsula countries are facing problems due to CE. Indeed, in Cyprus CE had an annual surgical incidence rate of 12.9 per 100 000 inhabitants before the first eradication program implemented in the 1970s and, subsequently, a second program in the 1990s[136]. In the northern part of Cyprus, disease rates decreased from 1.95% in dogs examined in 1998-1999 to 0.012% in 2000-2003, from 23.58% to 6.61% in cattle, from 5.31% to 1.53% in sheep, while in goats rates were consistently below 0.5% and remained at 0.13%. On the other hand, the south part of Cyprus that maintained its control programme was able to keep positive testing levels at virtually 0%[137].
In Europe, E. granulosus is present in most countries with the exception of Ireland, Iceland and Denmark. EC of animals is rare in northern and central Europe with the exception of cervid-transmitted echinococcosis in Finland and pig-transmitted echinococcosis in regions further east. The cervid strain in Finland was found to differ genetically from the previously described North American cervid strain G8, and was identified as a new strain, G10[138]. Transmission has been documented to occur mostly between wolves, reindeer and elks[139].
The most endemic areas have been documented to be the Mediterranean regions where annual incidence rates for human CE of 4-8 per 100 000 have been reported, and parts of Eastern Mediterranean countries such as Bulgaria[2]. In some other eastern regions such as Poland, Slovakia and Ukraine, the pig strains (G6-G10) often occur as animal and sometimes human CE[140,141]. In Serbia and Montenegro the most frequent intermediate hosts for E. granulosus are pigs, with a percentage of infected animals ranging between 4.6% and 57.6%[142] but no information is available about human infection. Although several other countries such as Albania[78], Bosnia and Herzegovina[143,144] are recognized as endemic for CE, none of them have available published data on the exact incidence of CE in livestock, carnivores or humans.
In Greece, investigation of the prevalence and the genotype of E. granulosus in sheep and goats in Peloponnesus (southern Greece) revealed that sheep were infected by the G1 (sheep) strain and the G3 (buffalo) strain, while the 20 goats examined harboured the G7 (pig) strain[145]. The prevalence of CE in farm animals ranged from the mid 1980s to the mid 1990s between 82% and 56.6% in cattle, 80% and 100% in sheep, 24% and 15.4% in goats and 5% and 9.3% in pigs, while surgical human cases reached 12.9 per 100 000 inhabitants in 1984 and up to 29% in 1999[146]. Furthermore, surveillance in livestock species since 1998 has documented a prevalence of 31.3% in sheep, 10.3% in goats, 0.6% in pigs and 0% in cattle[146]. Finally, a more recent survey conducted on sheep in central Greece from 2002 to 2006, revealed an incidence rate of 39.3%[147]. In humans, the overall incidence rate was estimated to have increased from 9.77 per 100 000 in 1967[148] to 10.59 per 100 000 inhabitants in 1983[149]; results which were confirmed in another survey where an incidence of 12.7 per 100 000 inhabitants (varying from 11.6 to 13.35) has been reported[150]. Incidence rates steadily declined in the most recent survey carried out in 2007 where they have been documented to be 0.122 per 100 000 inhabitants[151]. Published data for the entire country are not available but according to personal communications with surgeons it is estimated that approximately 800 cases of cystic echinococcosis are diagnosed each year, of which between 300 and 400 of them were undergoing surgical treatment.
In Western Europe, the sheep strain (G1) is the principal cause of human CE. In the past, the cattle-based transmission cycle of E. ortlepp in Germany and Switzerland has been documented[2,152], but now cases are reduced to sporadic occurrence and only a single case from a human patient in the Netherlands has been reported[21].
In the United Kingdom, the parasite has a restricted distribution, being found mainly in mid and southern Wales[2,152]. Recently, a re-emergence of E. granulosus in Wales has been reported, noting a rise in prevalence in rural dogs between 1989 and 2002 of 3.4% to 8.1%[153].
In Spain, CE is an endemic disease in north-eastern, central and western parts of the country, with prevalence rates rising in the last few years. The most common strains found in these areas were the sheep strain (G1) infecting sheep, cattle, goats, pigs, wild boars and humans, the pig strain (G7) infecting pigs, goats and wild boars, and E. equinus (old G4 strain) infecting horses[154]. In the province of Alava, two recent surveys documented prevalence of E. granulosus infection of 8% in the dog definitive hosts[155] and 15% in Iberian wolves[156]. In the municipality of Madrid, it has been assessed that hydatidosis affected 2.88% of sheep[157]. In Laroja region, the overall prevalence has been calculated to reach 20.3% in adult sheep and up to 23% in sheep and cows in the northeastern, central and western parts of the country[158].
With regard to human hydatidosis, a higher incidence of surgical cases occurs in Salamanca, with 10.8/100 000 inhabitants affected between the end of the 1980s and 2000[159]. On the other hand, in the Laroja region, prevalence of CE decreased from 19 to 4 cases per 100 000 inhabitants until 2000[158] and in the rest of the country it ranges between 1.1 and 3.4 cases per 100 000 inhabitants[159].
In France, a surveillance system in the mid 1990s revealed a prevalence of hydatidosis of 2.5% in livestock and less than 0.28 per 100 000 in humans[160]. A higher annual incidence has been documented in Corsica (10/100 000) and eastern regions (4.5/100 000 inhabitants)[78]. In recent years, the European Centre for Disease Prevention and Control reported 17 human cases in 2005.
Italy is considered a medium to high risk country for echinococcosis. The G1 (sheep), G2 (Tasmanian sheep), G3 (buffalo), G4 (horse), and G7 (pig) genotypes of E. granulosus are commonly found in livestock of several regions of Italy, especially in the southern part (such as in the Campania region), in Sardinia and in Sicily[3]. Indeed, the prevalence rate of E. granulosus in sheep has been reported to be 5%-28% in Basilicata, 22% in Abruzzo and 47% in Tuscany[3]. In Sicily, CE was found in 67.1% of cattle, 11.13%-57.6% of sheep and 5.6%-19% of shepherd dogs[161,162]. CE prevalence of infection in Sardinia has been assessed to be 70%-92.8% of sheep, 9.4% of cattle, 9.4%-11.1% of pigs, 1% of horses and 3%-19% of dogs[3,163-166]. In Campania, the prevalence rate in cattle has been reported to range from 10.4%[167] to 14.8%[163] while in buffalos this ranges from 10%[168] to 18.6%[169].
Infection of E. granulosus in animals seems to occur also in several regions of the centre of Italy while north regions could be considered of low endemicity. Indeed, in Central Italy medium prevalence values usually range from 20.2% to 47%-81.18% in sheep, from 7.34% to 15.3% in cattle, and reach 71.97% in goats, and 0.82% in pigs[170-172]. In Abruzzo, prevalence infection rates in sheep and cattle are 20.2% and 15.3%, respectively[163]. On the other hand, in Emilia Romagna the prevalences were low for several animals: 0.39%-0.54% in cattle, 0.30% in sheep, 0.39% in goats, 0.34% in horses and 0.95 per million in pigs[173]. In dogs and wolves retrieved along the whole Apennines the prevalence of E. granulosus infection has been noted to be 31% and 15%, respectively[172,174].
Despite these findings, the overall national occurrence of CE in farm animals can be considered low with prevalence rates of 0.52% of cattle, 1.30% of sheep, 0.6% of goats, 3.86% of sheep and goats, 0.0013% of pigs and 0.019% of horses[175]. On the other hand, human hydatidosis represents a serious public health problem, with an incidence of 1.3 cases per 100 000 inhabitants, a maximum of 4-8 cases per 100 000 inhabitants in Sardinia[176], and the occurrence of over 1000 cases requiring surgery each year[177]. Endemic zones reflect animal infection, with higher incidence rates in Sardinia and Sicily, medium in the Central-South regions, and a sporadic diffusion in the northern part of the country where this disease plays a minor role (prevalence < 1%). Annual mean incidence rates of surgical cases have been reported to be 6.6-10.6 per 100 000 inhabitants in Sardinia[178,179], 1.57-5.6 in Emilia Romagna[180,181], 1.22 in Lombardia, 2.30 in Sicily[182], 1.76 in Basilicata, 0.46 in Campania and 2.33 in Apulia[178].
Risk factors for infection are now considered to be widespread use of extensive or semi-extensive sheep farming (echinococcosis being a work-related disease), illegal slaughtering, and high numbers of sheepdogs and other types of dogs[183].
Given the wide geographic distribution, CE caused by E. granulosus is a re-emerging disease in several countries and regions, even in places where it was previously at low levels. Evidence suggests this is a result of a reduction of control programmes due to economic problems and lack of resources, leading to severe disease, considerable economic loss and, definitely, a public health problem of increasing concern.
Peer reviewer: Vezali Elena, MD, Department of Hepatology, “Hygeia” Diagnostic and Therapeutic Center of Athens, Eruthrou Staurou 4, Marousi 15123, Greece
S- Editor Tian L L- Editor Logan S E- Editor Zhang DN
| 1. | Eckert J, Conraths FJ, Tackmann K. Echinococcosis: an emerging or re-emerging zoonosis? Int J Parasitol. 2000;30:1283-1294. [DOI] [Cited in This Article: ] [Cited by in Crossref: 242] [Cited by in F6Publishing: 236] [Article Influence: 9.8] [Reference Citation Analysis (0)] |
| 2. | Eckert J, Schantz PM, Gasser RB, Torgerson PR, Bessonov AS, Movsessian SO, Thakur A, Grimm F, Nikogossian MA. Geographic distribution and prevalence. WHO/OIE Manual on Echinococcosis in Humans and Animals: a Public Health Problem of Global Concern. Paris: Office International des Epizooties Paris 2001; 100-142. [Cited in This Article: ] |
| 3. | Garippa G, Varcasia A, Scala A. Cystic echinococcosis in Italy from the 1950s to present. Parassitologia. 2004;46:387-391. [PubMed] [Cited in This Article: ] |
| 4. | Sadjjadi SM. Present situation of echinococcosis in the Middle East and Arabic North Africa. Parasitol Int. 2006;55 Suppl:S197-S202. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 202] [Cited by in F6Publishing: 193] [Article Influence: 10.2] [Reference Citation Analysis (0)] |
| 5. | Thompson RC, McManus DP. Towards a taxonomic revision of the genus Echinococcus. Trends Parasitol. 2002;18:452-457. [DOI] [Cited in This Article: ] [Cited by in Crossref: 321] [Cited by in F6Publishing: 331] [Article Influence: 15.0] [Reference Citation Analysis (0)] |
| 6. | Torgerson PR, Karaeva RR, Corkeri N, Abdyjaparov TA, Kuttubaev OT, Shaikenov BS. Human cystic echinococcosis in Kyrgystan: an epidemiological study. Acta Trop. 2003;85:51-61. [DOI] [Cited in This Article: ] [Cited by in Crossref: 62] [Cited by in F6Publishing: 65] [Article Influence: 3.1] [Reference Citation Analysis (0)] |
| 7. | Pearson M, Le TH, Zhang LH, Blair D, Dai THN, McManus DP. Molecular taxonomy and strain analysis in Echinococcus. Cestode Zoonoses: echinococcosis and cysticercosis NATO Science Series. Series 1: life and Behavioural Sciences, vol. 341. Amsterdam: IOS Press 2002; 205-219. [Cited in This Article: ] |
| 8. | McManus DP, Thompson RC. Molecular epidemiology of cystic echinococcosis. Parasitology. 2003;127 Suppl:S37-S51. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 152] [Cited by in F6Publishing: 156] [Article Influence: 7.8] [Reference Citation Analysis (0)] |
| 9. | Thompson RCA, McManus DP. Aetiology: parasites and life-cycles. WHOI/OIE, Paris. Manual on echinococcosis in humans and animals: a public health problem of global concern. Geneva: World Organisation for Animal Health 2001; 1-19. [Cited in This Article: ] |
| 10. | Thompson RC, Lymbery AJ. The nature, extent and significance of variation within the genus Echinococcus. Adv Parasitol. 1988;27:209-258. [DOI] [Cited in This Article: ] [Cited by in Crossref: 104] [Cited by in F6Publishing: 109] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 11. | Thompson RCA. Biology and systematics of Echinococcus. Echinococcus and hydatid disease. Wallingford: CAB International 1995; 1-50. [Cited in This Article: ] |
| 12. | Thompson RC, Lymbery AJ. Echinococcus: biology and strain variation. Int J Parasitol. 1990;20:457-470. [DOI] [Cited in This Article: ] [Cited by in Crossref: 42] [Cited by in F6Publishing: 43] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 13. | Bowles J, Blair D, McManus DP. A molecular phylogeny of the genus Echinococcus. Parasitol. 1995;110:317-328. [DOI] [Cited in This Article: ] [Cited by in Crossref: 147] [Cited by in F6Publishing: 148] [Article Influence: 5.1] [Reference Citation Analysis (0)] |
| 14. | Haag KL, Ayala FJ, Kamenetzky L, Gutierrez AM, Rosenzvit M. Livestock trade history, geography, and parasite strains: the mitochondrial genetic structure of Echinococcus granulosus in Argentina. J Parasitol. 2004;90:234-239. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 36] [Cited by in F6Publishing: 34] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 15. | Yang YR, Sun T, Li Z, Zhang J, Teng J, Liu X, Liu R, Zhao R, Jones MK, Wang Y. Community surveys and risk factor analysis of human alveolar and cystic echinococcosis in Ningxia Hui Autonomous Region, China. Bull World Health Organ. 2006;84:714-721. [PubMed] [Cited in This Article: ] |
| 16. | Scott JC, Stefaniak J, Pawlowski ZS, McManus DP. Molecular genetic analysis of human cystic hydatid cases from Poland: identification of a new genotypic group (G9) of Echinococcus granulosus. Parasitology. 1997;114:37-43. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 108] [Cited by in F6Publishing: 109] [Article Influence: 4.0] [Reference Citation Analysis (0)] |
| 17. | Dinkel A, Njoroge EM, Zimmermann A, Wälz M, Zeyhle E, Elmahdi IE, Mackenstedt U, Romig T. A PCR system for detection of species and genotypes of the Echinococcus granulosus-complex, with reference to the epidemiological situation in eastern Africa. Int J Parasitol. 2004;34:645-653. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 139] [Cited by in F6Publishing: 129] [Article Influence: 6.5] [Reference Citation Analysis (0)] |
| 18. | McManus DP, Bryant C. Biochemistry, physiology and molecular biology of Echinococcus. Echinococcus and hydatid disease. Wallingford: CAB International 1995; 135-181. [Cited in This Article: ] |
| 19. | Macpherson CNL, Wachira TWM. Cystic echinococcosis in Africa south of the Sahara. Compendium of Cystic Echinococcosis in Africa and in Middle Eastern Countries with special Reference to Morocco. Provo: Brigham Young University 1997; 245-277. [Cited in This Article: ] |
| 20. | Thompson RC, Lymbery AJ, Constantine CC. Variation in Echinococcus: towards a taxonomic revision of the genus. Adv Parasitol. 1995;35:145-176. [DOI] [Cited in This Article: ] [Cited by in Crossref: 100] [Cited by in F6Publishing: 106] [Article Influence: 3.7] [Reference Citation Analysis (0)] |
| 21. | Bowles J, van KF, McManus D. Cattle strain of Echinococcus granulosus and human infection. Lancet. 1992;339:1358. [DOI] [Cited in This Article: ] [Cited by in Crossref: 40] [Cited by in F6Publishing: 45] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 22. | Wilson JF, Diddams AC, Rausch RL. Cystic hydatid disease in Alaska. A review of 101 autochthonous cases of Echinococcus granulosus infection. Am Rev Respir Dis. 1968;98:1-15. [PubMed] [Cited in This Article: ] |
| 23. | Peterson RO. Wolf Ecology and Prey Relationships on Isle Royale. Washington DC: United States Printing Office 1997; . [Cited in This Article: ] |
| 24. | Webster GA, Cameron TW. Epidemiology and diagnosis of echinococcosis in Canada. Can Med Assoc J. 1967;96:600-607. [PubMed] [Cited in This Article: ] |
| 25. | Lamy AL, Cameron BH, LeBlanc JG, Culham JA, Blair GK, Taylor GP. Giant hydatid lung cysts in the Canadian northwest: outcome of conservative treatment in three children. J Pediatr Surg. 1993;28:1140-1143. [DOI] [Cited in This Article: ] [Cited by in Crossref: 31] [Cited by in F6Publishing: 33] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 26. | Moore RD, Urschel JD, Fraser RE, Nakai SS, Geeraert AJ. Cystic hydatid lung disease in northwest Canada. Can J Surg. 1994;37:20-22. [PubMed] [Cited in This Article: ] |
| 27. | Somily A, Robinson JL, Miedzinski LJ, Bhargava R, Marrie TJ. Echinococcal disease in Alberta, Canada: more than a calcified opacity. BMC Infect Dis. 2005;5:34. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 22] [Cited by in F6Publishing: 27] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 28. | Schantz PM, Chai J, Craig PS, Eckert J, Jenkins DJ, Macpherson CNL, Thakur A. Epidemiology and Control of hydatid disease. Echinococcus and Hydatid Disease. Wallingford: CAB International 1995; 231-233. [Cited in This Article: ] |
| 29. | Crellin JR, Andersen FL, Schantz PM, Condie SJ. Possible factors influencing distribution and prevalence of Echinococcus granulosus in Utah. Am J Epidemiol. 1982;116:463-474. [PubMed] [Cited in This Article: ] |
| 30. | Pappaioanou M, Schwabe CW, Sard DM. An evolving pattern of human hydatid disease transmission in the United States. Am J Trop Med Hyg. 1977;26:732-742. [PubMed] [Cited in This Article: ] |
| 31. | Hutchison WF. Studies on the hydatid worm, Echinococcus granulosus. II. Prevalence in Missippi. Am J Trop Med Hyg. 1960;9:612-615. [PubMed] [Cited in This Article: ] |
| 32. | Schantz PM, Clérou RP, Liu IK, Schwabe CW. Hydatid disease in the central valley of California. Transmission of infection among dogs, sheep, and man in Kern county. Am J Trop Med Hyg. 1970;19:823-830. [PubMed] [Cited in This Article: ] |
| 33. | Andersen FL. Introduction to cystic echinococcosis and description of cooperative research project in Morocco. Compendium on Cystic Echinococcosis. Brigham: Young Univ Press; 1997; 1-17. [Cited in This Article: ] |
| 34. | Schantz PM, von Reyn CF, Welty T, Schultz MG. Echinococcosis in Arizona and New Mexico. Survey of hospital records, 1969-1974. Am J Trop Med Hyg. 1976;25:312-317. [PubMed] [Cited in This Article: ] |
| 35. | Schantz PM, Alstine CV, Blacksheep A, Sinclair S. Prevalence of Echinococcus granulosus and other cestodes in dogs on the Navajo reservation in Arizona and New Mexico. Am J Vet Res. 1977;38:669-670. [PubMed] [Cited in This Article: ] |
| 36. | Moro PL, McDonald J, Gilman RH, Silva B, Verastegui M, Malqui V, Lescano G, Falcon N, Montes G, Bazalar H. Epidemiology of Echinococcus granulosus infection in the central Peruvian Andes. Bull World Health Organ. 1997;75:553-561. [PubMed] [Cited in This Article: ] |
| 37. | Moro PL, Lopera L, Cabrera M, Cabrera G, Silva B, Gilman RH, Moro MH. Short report: endemic focus of cystic echinococcosis in a coastal city of Peru. Am J Trop Med Hyg. 2004;71:327-329. [PubMed] [Cited in This Article: ] |
| 38. | Moro PL, Gilman RH, Verastegui M, Bern C, Silva B, Bonilla JJ. Human hydatidosis in the central Andes of Peru: evolution of the disease over 3 years. Clin Infect Dis. 1999;29:807-812. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 30] [Cited by in F6Publishing: 27] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 39. | Moro PL, Bonifacio N, Gilman RH, Lopera L, Silva B, Takumoto R, Verastegui M, Cabrera L. Field diagnosis of Echinococcus granulosus infection among intermediate and definitive hosts in an endemic focus of human cystic echinococcosis. Trans R Soc Trop Med Hyg. 1999;93:611-615. [DOI] [Cited in This Article: ] [Cited by in Crossref: 46] [Cited by in F6Publishing: 46] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 40. | Lopera L, Moro PL, Chavez A, Montes G, Gonzales A, Gilman RH. Field evaluation of a coproantigen enzyme-linked immunosorbent assay for diagnosis of canine echinococcosis in a rural Andean village in Peru. Vet Parasitol. 2003;117:37-42. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 19] [Cited by in F6Publishing: 13] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 41. | Dueger EL, Gilman RH. Prevalence, intensity, and fertility of ovine cystic echinococcosis in the central Peruvian Andes. Trans R Soc Trop Med Hyg. 2001;95:379-383. [DOI] [Cited in This Article: ] [Cited by in Crossref: 31] [Cited by in F6Publishing: 34] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 42. | Panamerican Health Organization. Report of the Southern Cone subregional project on echinococcosis control and surveillance Argentina, Brasil, Chile and Uruguay: first meeting; 2004 July 7-9; Montevideo, Uruguay. Montevideo: OPS 2004; . [Cited in This Article: ] |
| 43. | Alvarez F, Tamayo R, Ernst S. Estimación de la prevalencia de echinococcosis canina en la XII Región, Chile, 2002. Parasitol Latinoam. 2005;60:74-77. [Cited in This Article: ] |
| 44. | Serra I, Araneda J, Araya C, Serra V. [Regional analysis of human and animal hydatidosis in Chile, 1989-1993]. Bol Chil Parasitol. 1996;51:3-12. [PubMed] [Cited in This Article: ] |
| 45. | Apt W, Perez C, Galdamez E, Campano S, Vega F, Vargas D, Rodriguez J, Retamal C, Cortes P, Zulantay I. Echinococcosis/hydatidosis in the VII Region of Chile: diagnosis and educational intervention. Rev Panam Salud Publica. 2000;7:8-16. [DOI] [Cited in This Article: ] [Cited by in Crossref: 18] [Cited by in F6Publishing: 19] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 46. | Kamenetzky L, Gutierrez AM, Canova SG, Haag KL, Guarnera EA, Parra A, Garcia GE, Rosenzvit MC. Several strains of Echinococcus granulosus infect livestock and humans in Argentina. Infect Genet Evol. 2002;2:129-136. [DOI] [Cited in This Article: ] [Cited by in Crossref: 104] [Cited by in F6Publishing: 106] [Article Influence: 4.8] [Reference Citation Analysis (0)] |
| 47. | Haag KL, Araújo AM, Gottstein B, Siles-Lucas M, Thompson RC, Zaha A. Breeding systems in Echinococcus granulosus (Cestoda; Taeniidae): selfing or outcrossing? Parasitology. 1999;118:63-71. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 62] [Cited by in F6Publishing: 64] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 48. | De la Rue ML, Silva KLMV, Dinkel A, Mackenstedt U, Romig T. New data on Echinococcus pp.in southern Brazil. Int Arch Hydatidosis. 2004;35:61. [Cited in This Article: ] |
| 49. | Farias LN, Malgor R, Cassaravilla C, Braganca C, de la Rue ML. Echinococcosis in southern Brazil: efforts toward implementation of a control program in Santana do Livramento, Rio Grande do Sul. Rev Inst Med Trop Sao Paulo. 2004;46:153-156. [DOI] [Cited in This Article: ] [Cited by in Crossref: 11] [Cited by in F6Publishing: 12] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 50. | Pastore R, Vitali LH, Macedo Vde O, Prata A. [A serological survey of the infection by Echinococcus sp. in the municipality of Sena Madureira, AC]. Rev Soc Bras Med Trop. 2003;36:473-477. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 9] [Cited by in F6Publishing: 10] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 51. | Jenkins DJ. Hydatid control in Australia: where it began, what we have achieved and where to from here. Int J Parasitol. 2005;35:733-740. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 36] [Cited by in F6Publishing: 37] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 52. | Beard TC, Bramble AJ, Middleton MJ. Eradication in Our Lifetime: A Log Book of the Tasmanian Hydatid Control Programs, 1962-1996. Hobart: Department of Primary Industries, Water & Environment 2001; . [Cited in This Article: ] |
| 53. | Grainger HJ, Jenkins DJ. Transmission of hydatid disease to sheep from wild dogs in Victoria, Australia. Int J Parasitol. 1996;26:1263-1270. [DOI] [Cited in This Article: ] [Cited by in Crossref: 29] [Cited by in F6Publishing: 30] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 54. | Jenkins DJ, Morris B. Echinococcus granulosus in wildlife in and around the Kosciuszko National Park, south-eastern Australia. Aust Vet J. 2003;81:81-85. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 46] [Cited by in F6Publishing: 45] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 55. | Jenkins DJ, Macpherson CN. Transmission ecology of Echinococcus in wild-life in Australia and Africa. Parasitology. 2003;127 Suppl:S63-S72. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 50] [Cited by in F6Publishing: 52] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 56. | Lymbery AJ, Thompson RC, Kruger JG. The geographic distribution of hydatid infection in cattle in Western Australia. Aust Vet J. 1995;72:430-432. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 11] [Cited by in F6Publishing: 12] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 57. | Hope M, Bowles J, Prociv P, McManus DP. A genetic comparison of human and wildlife isolates of Echinococcus granulosus in Queensland: public health implications. Med J Aust. 1992;156:27-30. [PubMed] [Cited in This Article: ] |
| 58. | Jenkins DJ, Morris B. Unusually heavy infections of Echinococcus granulosus in wild dogs in south-eastern Australia. Aust Vet J. 1991;68:36-37. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 21] [Cited by in F6Publishing: 22] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 59. | Thompson RC, Nott DB, Squire J, Rennell D. Evidence that the Australian sylvatic strain of Echinococcus granulosus is infective to humans. Med J Aust. 1987;146:396-397. [PubMed] [Cited in This Article: ] |
| 60. | Jenkins DJ, Power K. Human hydatidosis in New South Wales and the Australian Capital Territory, 1987-1992. Med J Aust. 1996;164:18-21. [PubMed] [Cited in This Article: ] |
| 61. | Brown B, Copeman DB. Zoonotic importance of parasites in wild dogs caught in the vicinity of Townsville. Aust Vet J. 2003;81:700-702. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 30] [Cited by in F6Publishing: 31] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 62. | Jenkins DJ, Allen L, Goullet M. Encroachment of Echinococcus granulosus into urban areas in eastern Queensland, Australia. Aust Vet J. 2008;86:294-300. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 29] [Cited by in F6Publishing: 30] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 63. | Jenkins DJ, McKinlay A, Duolong HE, Bradshaw H, Craig PS. Detection of Echinococcus granulosus coproantigens in faeces from naturally infected rural domestic dogs in south eastern Australia. Aust Vet J. 2006;84:12-16. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 13] [Cited by in F6Publishing: 14] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 64. | Jenkins DJ. Echinococcus in Australia. Echinococcosis in Central Asia: problems and solutions. Almaty, Duir: INTAS Network Project 01-0505 2004; 125-130. [Cited in This Article: ] |
| 65. | Jenkins DJ, Craig NA. The role of foxes Vulpes vulpes in the epidemiology of Echinococcus granulosus in urban environments. Med J Aust. 1992;157:754-756. [PubMed] [Cited in This Article: ] |
| 66. | Thompson RC, Lymbery AJ, Hobbs RP, Elliot AD. Hydatid disease in urban areas of Western Australia: an unusual cycle involving western grey kangaroos (Macropus fuliginosus), feral pigs and domestic dogs. Aust Vet J. 1988;65:188-190. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 23] [Cited by in F6Publishing: 24] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 67. | Harandi MF, Hobbs RP, Adams PJ, Mobedi I, Morgan-Ryan UM, Thompson RC. Molecular and morphological characterization of Echinococcus granulosus of human and animal origin in Iran. Parasitology. 2002;125:367-373. [PubMed] [Cited in This Article: ] |
| 68. | Shaikenov BSh, Torgerson P. Changes in the epidemiology of echinococcosis in Kazakhstan. Echinococcosis in central Asia: problems and solutions. Almaty, Duir: INTAS Network Project 01-0505 2004; 3-12. [Cited in This Article: ] |
| 69. | Torgerson PR, Shaikenov BS, Kuttubaev O. Cystic echinococcosis in Central Asia: New epidemic in Kazakhstan and Kyrgystan. Cestode Zoonoses: Echinococcosis and cysticercosis. Oxford: IOS Press 2000; 99-105. [Cited in This Article: ] |
| 70. | Torgerson PR, Shaikenov BS, Baitursinov KK, Abdybekova AM. The emerging epidemic of echinococcosis in Kazakhstan. Trans R Soc Trop Med Hyg. 2002;96:124-128. [DOI] [Cited in This Article: ] [Cited by in Crossref: 40] [Cited by in F6Publishing: 42] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 71. | Altintas N. Past to present: echinococcosis in Turkey. Acta Trop. 2003;85:105-112. [DOI] [Cited in This Article: ] [Cited by in Crossref: 84] [Cited by in F6Publishing: 90] [Article Influence: 4.3] [Reference Citation Analysis (0)] |
| 72. | Umur S. Prevalence and economic importance of cystic echinococcosis in slaughtered ruminants in Burdur, Turkey. J Vet Med B Infect Dis Vet Public Health. 2003;50:247-252. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 38] [Cited by in F6Publishing: 40] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 73. | Yildiz K, Tunçer C. [Prevalence of hydatid cysts in cattle in the province of Kirikkale.]. Turkiye Parazitol Derg. 2005;29:247-250. [PubMed] [Cited in This Article: ] |
| 74. | Köse M, Sevimli FK. Prevalence of cystic echinococcosis in slaughtered cattle in Afyonkarahisar. Turkiye Parazitol Derg. 2008;32:27-30. [PubMed] [Cited in This Article: ] |
| 75. | Aciöz M, Celiksöz A, Ozçelik S, Değerli S. [Prevalence of cyst hydatic in slaughtered cattle between April and May 2005 in Sivas]. Turkiye Parazitol Derg. 2008;32:205-207. [PubMed] [Cited in This Article: ] |
| 76. | Esatgil MU, Tüzer E. Prevalence of hydatidosis in slaughtered animals in Thrace, Turkey. Turkiye Parazitol Derg. 2007;31:41-45. [PubMed] [Cited in This Article: ] |
| 77. | Utuk AE, Simsek S, Koroglu E, McManus DP. Molecular genetic characterization of different isolates of Echinococcus granulosus in east and southeast regions of Turkey. Acta Trop. 2008;107:192-194. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 59] [Cited by in F6Publishing: 63] [Article Influence: 3.9] [Reference Citation Analysis (0)] |
| 78. | Seimenis A, Battelli G. Epidemiological situation and surveillance. Information Circular-WHO Mediterrean Zoonoses Control Centre, Issue dedicated on cystic echinococcosis in the Mediterranean countries. 2003;6-8. [Cited in This Article: ] |
| 79. | Shimshony A. Epidemiology of emerging zoonoses in Israel. Emerg Infect Dis. 1997;3:229-238. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 16] [Cited by in F6Publishing: 17] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 80. | Furth M. Echinococcosis in Northern Israel: prevalence in domestic and wild animals. International Symposium on Environmental Adaptation of Echinococcus. 1998;18-20. [Cited in This Article: ] |
| 81. | Abdel-Hafez SK, Kamhawi S. Cystic echinococcosis in the Levant countries (Jordan, Palestinian Authority, Israel, Syria, and Lebanon). Compendium on Cystic Echinococcosis in Africa and Middle Eastern Countries with Special Reference to Morocco. Pravo: Brigham Young University 1997; 292-316. [Cited in This Article: ] |
| 82. | Abu-Hasan N, Daragmeh M, Adwan K, Al-Qaoud K, Abdel-Hafez SK. Human cystic echinococcosis in the West Bank of Palestine: surgical incidence and seroepidemiological study. Parasitol Res. 2002;88:107-112. [DOI] [Cited in This Article: ] [Cited by in Crossref: 20] [Cited by in F6Publishing: 21] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 83. | Youngster I, Hoida G, Craig PS, Sneir R, El-On J. Prevalence of cystic echinococcosis among Muslim and Jewish populations in southern Israel. Acta Trop. 2002;82:369-375. [DOI] [Cited in This Article: ] [Cited by in Crossref: 12] [Cited by in F6Publishing: 14] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 84. | Zhang YL, Bart JM, Wen H, Ma XD, Miao YQ, Lin RY. Molecular evidence of sheep (G1) and camel (G6) strains of Echinococcus granulosus in Xinjiang, China. Zhongguo Ji Sheng Chong Bing Fang Zhi Zazhi. 2005;18:333-335. [Cited in This Article: ] |
| 85. | Bart JM, Abdukader M, Zhang YL, Lin RY, Wang YH, Nakao M, Ito A, Craig PS, Piarroux R, Vuitton DA. Genotyping of human cystic echinococcosis in Xinjiang, PR China. Parasitology. 2006;133:571-579. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 64] [Cited by in F6Publishing: 65] [Article Influence: 3.6] [Reference Citation Analysis (0)] |
| 86. | Chi P, Zhang W, Zhang Z, Hasyet M, Liu F, Ding Z, Andersen FL, Tolley HD, Schantz PM. Cystic echinococcosis in the Xinjiang/Uygur Autonomous Region, People's Republic of China. I. Demographic and epidemiologic data. Trop Med Parasitol. 1990;41:157-162. [PubMed] [Cited in This Article: ] |
| 87. | Tiaoying L, Jiamin Q, Wen Y, Craig PS, Xingwang C, Ning X, Ito A, Giraudoux P, Wulamu M, Wen Y. Echinococcosis in Tibetan populations, western Sichuan Province, China. Emerg Infect Dis. 2005;11:1866-1873. [PubMed] [Cited in This Article: ] |
| 88. | Qiu JM, Liu F, Schantz PM, Ito A, Delker C, He JG. Epidemiological survey of hydatidosis in Tibetan areas of western Sichuan province. Archivos Internacionales de la Hidatidosis. 1999;33:84. [Cited in This Article: ] |
| 89. | Chai JJ. Epidemiological studies on cystic echinococcosis in China--a review. Biomed Environ Sci. 1995;8:122-136. [PubMed] [Cited in This Article: ] |
| 90. | Craig PS. Epidemiology of echinococcosis in Western China. Echinococcosis in central Asia: problems and solutions. Almaty, Dauir: INTAS Network Project 01-0505 2004; 43-58. [Cited in This Article: ] |
| 91. | Giraudoux P, Craig PS, Delattre P, Bao G, Bartholomot B, Harraga S, Quere JP, Raoul F, Wang Y, Shi D. Interactions between landscape changes and host communities can regulate Echinococcus multilocularis transmission. Parasitol. 2003;127 Suppl:S121-S131. [DOI] [Cited in This Article: ] [Cited by in Crossref: 97] [Cited by in F6Publishing: 95] [Article Influence: 4.8] [Reference Citation Analysis (0)] |
| 92. | Giraudoux P, Pleydell D, Raoul F, Quéré JP, Wang Q, Yang Y, Vuitton DA, Qiu J, Yang W, Craig PS. Transmission ecology of Echinococcus multilocularis: what are the ranges of parasite stability among various host communities in China? Parasitol Int. 2006;55 Suppl:S237-S246. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 58] [Cited by in F6Publishing: 59] [Article Influence: 3.1] [Reference Citation Analysis (0)] |
| 93. | Xiao N, Qiu J, Nakao M, Li T, Yang W, Chen X, Schantz PM, Craig PS, Ito A. Echinococcus shiquicus n. sp., a taeniid cestode from Tibetan fox and plateau pika in China. Int J Parasitol. 2005;35:693-701. [PubMed] [Cited in This Article: ] |
| 94. | Yang YR, Rosenzvit MC, Zhang LH, Zhang JZ, McManus DP. Molecular study of Echinococcus in west-central China. Parasitology. 2005;131:547-555. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 59] [Cited by in F6Publishing: 61] [Article Influence: 3.4] [Reference Citation Analysis (0)] |
| 95. | Tang CT, Wang YH, Peng WF, Tang L, Chen D. Alveolar Echinococcus species from Vulpes corsac in Hulunbeier, Inner Mongolia, China, and differential development of the metacestodes in experimental rodents. J Parasitol. 2006;92:719-724. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 15] [Cited by in F6Publishing: 18] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 96. | Musinov M. The epizootiology of echinococcosis in animals in Uzbekistan. Med Parazitol (. Mosk). 1998;40-41. [Cited in This Article: ] |
| 97. | Oksanen A, Laaksonen S. Faecal survey of dogs for echinococcosis and other internal parasites in north-eastern Finland. Suomen Elainlaakarilehti. 1995;101:171-172. [Cited in This Article: ] |
| 98. | Kilani M, Lancia A, Jemli MH, Darghouth MA, Jomaa I, Diouani F. Prevalence of Echinococcus granulosus in captured dogs in Tunisia (parasitic necropsy). Archivos Internacionales de la Hidatidosis. 1997;32:276. [Cited in This Article: ] |
| 99. | Kimura H, Furuya K, Kawase S, Sato C, Takahashi K, Uraguchi K. Epidemiology of alveolar echinococcosis in Hokkaido, Japan. Archivos Internacionales de la Hidatidosis. 1999;33:85-89. [Cited in This Article: ] |
| 100. | Ito A, Romig T, Takahashi K. Perspective on control options for Echinococcus multilocularis with particular reference to Japan. Parasitol. 2003;127 Suppl:S159-S172. [DOI] [Cited in This Article: ] [Cited by in Crossref: 57] [Cited by in F6Publishing: 56] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 101. | Wen H, Yang WG. Public health importance of cystic echinococcosis in China. Acta Trop. 1997;67:133-145. [DOI] [Cited in This Article: ] [Cited by in Crossref: 37] [Cited by in F6Publishing: 41] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 102. | Wang Q, Qiu J, Yang W, Schantz PM, Raoul F, Craig PS, Giraudoux P, Vuitton DA. Socioeconomic and behavior risk factors of human alveolar echinococcosis in Tibetan communities in Sichuan, People's Republic of China. Am J Trop Med Hyg. 2006;74:856-862. [PubMed] [Cited in This Article: ] |
| 103. | Ibrahem MM, Gusbi AM. Cystic echinococcosis in North Africa (excluding Morocco): veterinary aspects. Compendium of Cystic Echinococcosis in Africa and in Middle Eastern Countries with special Reference to Morocco. Provo: Brigham Young University 1997; 207-222. [Cited in This Article: ] |
| 104. | Kachani M, Ouhelli H, Kadiri A, El Hasnaoui M. Prevalence of hydatid cysts in livestock in Morocco and potential role of these intermediate hosts in transmission of cystic echinococcosis. Compendium of Cystic Echinococcosis in Africa and in Middle Eastern Countries with special Reference to Morocco. Provo: Brigham Young University 1997; 156-168. [Cited in This Article: ] |
| 105. | Kumaratilake LM, Thompson RC, Eckert J. Echinococcus granulosus of equine origin from different countries possess uniform morphological characteristics. Int J Parasitol. 1986;16:529-540. [DOI] [Cited in This Article: ] [Cited by in Crossref: 53] [Cited by in F6Publishing: 55] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 106. | Verstr AJ. Review of Echinococcus species in South Africa. Onderstepoort J Vet Res. 1965;32:7-118. [PubMed] [Cited in This Article: ] |
| 107. | Buishi IE, Njoroge EM, Bouamra O, Craig PS. Canine echinococcosis in northwest Libya: assessment of coproantigen ELISA, and a survey of infection with analysis of risk-factors. Vet Parasitol. 2005;130:223-232. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 56] [Cited by in F6Publishing: 59] [Article Influence: 3.1] [Reference Citation Analysis (0)] |
| 108. | Ben Musa NA, Sadek GS. Prevalence of echinococcosis in street dogs in Tripole District, Libya. J Egypt Soc Parasitol. 2007;37:793-800. [PubMed] [Cited in This Article: ] |
| 109. | Kassem HH, Gdoura NK. Hydatidosis in camels (Camelus dromedarius) slaughtered at Sirt Abattoir, Libya. J Egypt Soc Parasitol. 2006;36:1-10. [Cited in This Article: ] |
| 110. | Elmahdi IE, Ali QM, Magzoub MM, Ibrahim AM, Saad MB, Romig T. Cystic echinococcosis of livestock and humans in central Sudan. Ann Trop Med Parasitol. 2004;98:473-479. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 38] [Cited by in F6Publishing: 40] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 111. | Al-Khalidi NW. Cystic echinococcosis (hydatidosis) in sheep, goats, cattle and camels in Shahat Abattoir, Al-Jabal, Libya. In: Proceedings of the Third Annual Meeting for Animal Production Under Arid Conditions, United Arab Emirates University, vol. 1 1998; 143-149. [Cited in This Article: ] |
| 112. | Tashani OA, Zhang LH, Boufana B, Jegi A, McManus DP. Epidemiology and strain characteristics of Echinococcus granulosus in the Benghazi area of eastern Libya. Ann Trop Med Parasitol. 2002;96:369-381. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 47] [Cited by in F6Publishing: 46] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 113. | Ibrahem MM, Crellin JR. Prevalence of cystic echinococcosis in camels (Camelus dromedarius) in Libya. J Helminthol. 1998;72:27-31. [DOI] [Cited in This Article: ] [Cited by in Crossref: 36] [Cited by in F6Publishing: 37] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 114. | Bardonnet K, Piarroux R, Schneegans F, Dia L, Beurdeley A, Vuitton DA. Hydatidosis in Mauritania: a camel strain infectious to humans. Int Arch Hydatidosis. 2001;34:20. [Cited in This Article: ] |
| 115. | Shambesh MA, Craig PS, Macpherson CN, Rogan MT, Gusbi AM, Echtuish EF. An extensive ultrasound and serologic study to investigate the prevalence of human cystic echinococcosis in northern Libya. Am J Trop Med Hyg. 1999;60:462-468. [PubMed] [Cited in This Article: ] |
| 116. | Omer RA, Dinkel A, Romig T, Mackenstedt U, Elamin M, Elnahas A, Aradaib I. Strains characterization of human hydatidosis in Sudan. Int Arch Hydatidosis. 2004;35:41. [Cited in This Article: ] |
| 117. | Azab ME, Bishara SA, Helmy H, Oteifa NM, El-Hoseiny LM, Ramzy RM, Ahmed MA. Molecular characterization of Egyptian human and anima Echinococcus granulosus isolates by RAPD-PCR technique. J Egypt Soc Parasitol. 2004;34:83-96. [PubMed] [Cited in This Article: ] |
| 118. | Derbala AA. Current status of hydatidosis/echinococcosis: guidelines for surveillance, siology, diagnosis and recommendations for prevention and control in Egypt. 29th World Congress of the World Small Animal Vet. Ass.. 2004;. [Cited in This Article: ] |
| 119. | El Shazly AM, Awad SE, Nagaty IM, Morsy TA. Echinococcosis in dogs in urban and rural areas in Dakahlia Governorate, Egypt. J Egypt Soc Parasitol. 2007;37:483-492. [PubMed] [Cited in This Article: ] |
| 120. | Mazyad SA, Mahmoud LH, Hegazy MM. Echinococcosis granulosus in stray dogs and Echino-IHAT in the hunters in Cairo, Egypt. J Egypt Soc Parasitol. 2007;37:523-532. [PubMed] [Cited in This Article: ] |
| 121. | Haridy FM, Ibrahim BB, Elshazly AM, Awad SE, Sultan DM, El-Sherbini GT, Morsy TA. Hydatidosis granulosus in Egyptian slaughtered animals in the years 2000-2005. J Egypt Soc Parasitol. 2006;36:1087-1100. [PubMed] [Cited in This Article: ] |
| 122. | Haridy FM, Abdel Gawad AG, Ibrahim BB, Hassan AA, El-Sherbi GT, El Shazly AM, Morsy TA. Zoonotic hydatidosis in donkeys: post mortum examination in the Zoo, Giza, Egypt. J Egypt Soc Parasitol. 2008;38:305-312. [PubMed] [Cited in This Article: ] |
| 123. | Kandeel A, Ahmed ES, Helmy H, El Setouhy M, Craig PS, Ramzy RM. A retrospective hospital study of human cystic echinococcosis in Egypt. East Mediterr Health J. 2004;10:349-357. [PubMed] [Cited in This Article: ] |
| 124. | Lahmar S, Debbek H, Zhang LH, McManus DP, Souissi A, Chelly S, Torgerson PR. Transmission dynamics of the Echinococcus granulosus sheep-dog strain (G1 genotype) in camels in Tunisia. Vet Parasitol. 2004;121:151-156. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 49] [Cited by in F6Publishing: 52] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 125. | M'rad S, Filisetti D, Oudni M, Mekki M, Belguith M, Nouri A, Sayadi T, Lahmar S, Candolfi E, Azaiez R. Molecular evidence of ovine (G1) and camel (G6) strains of Echinococcus granulosus in Tunisia and putative role of cattle in human contamination. Vet Parasitol. 2005;129:267-272. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 54] [Cited by in F6Publishing: 51] [Article Influence: 2.7] [Reference Citation Analysis (0)] |
| 126. | Benabid M, Chahed MK, Nouira R, Galai Y, Bourabtine A, Aoun K. Knowledge, behaviour and implications on hydatidosis in Tunisia. Rev Tun Infectiol. 2007;4:22-428. [Cited in This Article: ] |
| 127. | Lahmar S, Kilani M, Torgerson PR, Gemmell MA. Echinococcus granulosus larvae in the livers of sheep in Tunisia: the effects of host age. Ann Trop Med Parasitol. 1999;93:75-81. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 26] [Cited by in F6Publishing: 39] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 128. | Lahmar S, Chéhida FB, Pétavy AF, Hammou A, Lahmar J, Ghannay A, Gharbi HA, Sarciron ME. Ultrasonographic screening for cystic echinococcosis in sheep in Tunisia. Vet Parasitol. 2007;143:42-49. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 42] [Cited by in F6Publishing: 41] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 129. | Anon T. The surgical incidence rate of hydatidosis in Tunisia (1988-1992). Report of the D.S.S.B. (Direction de Santé et des Soins de base). Tunis: Ministry Public Health 1993; . [Cited in This Article: ] |
| 130. | Bart JM, Bardonnet K, Elfegoun MC, Dumon H, Dia L, Vuitton DA, Piarroux R. Echinococcus granulosus strain typing in North Africa: comparison of eight nuclear and mitochondrial DNA fragments. Parasitology. 2004;128:229-234. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 35] [Cited by in F6Publishing: 35] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 131. | Bardonnet K, Benchikh-Elfegoun MC, Bart JM, Harraga S, Hannache N, Haddad S, Dumon H, Vuitton DA, Piarroux R. Cystic echinococcosis in Algeria: cattle act as reservoirs of a sheep strain and may contribute to human contamination. Vet Parasitol. 2003;116:35-44. [DOI] [Cited in This Article: ] [Cited by in Crossref: 43] [Cited by in F6Publishing: 49] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 132. | Shambesh MK. Human cystic echinococcosis in North Africa (excluding Morocco). Compendium on Cystic Echinococcosis in Africa and in Middle Eastern Countries with Special Reference to Morocco. Provo: Brigham Young University 1997; 223-244. [Cited in This Article: ] |
| 133. | Ouhelli H, Kadiri A, El Hasnaoui M, Kachani M. Prevalence of Echinococcus granulosus in dogs in Morocco and potential role of dogs in transmission of cystic echinococcosis. Compendium on Cystic Echinococcosis in Africa and in Middle Eastern Countries with Special Reference to Morocco. Provo: Brigham Young University 1997; 145-155. [Cited in This Article: ] |
| 134. | Azlaf R, Dakkak A. Epidemiological study of the cystic echinococcosis in Morocco. Vet Parasitol. 2006;137:83-93. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 111] [Cited by in F6Publishing: 111] [Article Influence: 6.2] [Reference Citation Analysis (0)] |
| 135. | Kachani M, Macpherson CN, Lyagoubi M, Berrada M, Bouslikhane M, Kachani F, El HM. Public health education/importance and experience from the field. Educational impact of community-based ultrasound screening surveys. Acta Trop. 2003;85:263-269. [DOI] [Cited in This Article: ] [Cited by in Crossref: 23] [Cited by in F6Publishing: 25] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 136. | Economides P, Christofi G. Evaluation of control programmes for echinococcosis/hydatidosis in Cyprus. Rev Sci Tech. 2000;19:784-792. [PubMed] [Cited in This Article: ] |
| 137. | Christofi G, Deplazes P, Christofi N, Tanner I, Economides P, Eckert J. Screening of dogs for Echinococcus granulosus coproantigen in a low endemic situation in Cyprus. Vet Parasitol. 2002;104:299-306. [DOI] [Cited in This Article: ] [Cited by in Crossref: 43] [Cited by in F6Publishing: 45] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 138. | Lavikainen A, Lehtinen MJ, Meri T, Hirvelä-Koski V, Meri S. Molecular genetic characterization of the Fennoscandian cervid strain, a new genotypic group (G10) of Echinococcus granulosus. Parasitology. 2003;127:207-215. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 166] [Cited by in F6Publishing: 169] [Article Influence: 8.0] [Reference Citation Analysis (0)] |
| 139. | Hirvela-Koski V, Haukisalmi V, Kilpela SS, Nylund M, Koski P. Echinococcus granulosus in Finland. Vet Parasitol. 2003;111:175-192. [DOI] [Cited in This Article: ] [Cited by in Crossref: 32] [Cited by in F6Publishing: 33] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 140. | Kedra AH, TKach VV, Swiderski Z, Pawlowski Z, Emets A, Pawlowski J. Molecular characterisation of Echinococcus granulosus from a wild boar. Acta Parasitol. 2000;45:121-122. [Cited in This Article: ] |
| 141. | Snábel V, D'Amelio S, Mathiopoulos K, Turceková L, Dubinský P. Molecular evidence for the presence of a G7 genotype of Echinococcus granulosus in Slovakia. J Helminthol. 2000;74:177-181. [PubMed] [Cited in This Article: ] |
| 142. | Ivanovic S, Pavlovic I. Conference Technologija mesa (Yugoslavia). Meat Technol. 1999;40:302-303. [Cited in This Article: ] |
| 143. | Obradović Z, Zerem E, Beslagić Z, Susić A. [Echinococcosis in Bosnia and Herzegovina]. Med Arh. 2006;60:259-262. [PubMed] [Cited in This Article: ] |
| 144. | Guska S, Cerimagic Z, Pilav I. Conservative surgical treatment of pulmonary hydatid disease in children. Med Arh. 2007;61:11-15. [Cited in This Article: ] |
| 145. | Varcasia A, Tosciri G, Pedes T, Pipia AP, Marrosu R, Scala A, Garippa G. Cystic echinococcosis in pigs and wald boars of Sardinia (Italy). Proc 6th International Symposium on the Mediterranean Pig. 2007;. [Cited in This Article: ] |
| 146. | Sotiraki S, Himonas C, Korkoliakou P. Hydatidosis-echinococcosis in Greece. Acta Trop. 2003;85:197-201. [DOI] [Cited in This Article: ] [Cited by in Crossref: 41] [Cited by in F6Publishing: 44] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 147. | Christodoulopoulos G, Theodoropoulos G, Petrakos G. Epidemiological survey of cestode-larva disease in Greek sheep flocks. Vet Parasitol. 2008;153:368-373. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 52] [Cited by in F6Publishing: 56] [Article Influence: 3.5] [Reference Citation Analysis (0)] |
| 148. | Karpathios T, Fretzayas A, Nicolaidou P, Papadellis F, Vassalos M, Tselentis J, Thomaidis T, Matsaniotis N. Statistical aspects of hydatid disease in Greek adults. Am J Trop Med Hyg. 1985;34:124-128. [PubMed] [Cited in This Article: ] |
| 149. | Matsaniotis N, Karpathios T, Koutoyzis J, Nicolaidou P, Fretzayas A, Papadellis F, Thomaidis T. Hydatid disease in Greek children. Am J Trop Med Hyg. 1983;32:1075-1078. [PubMed] [Cited in This Article: ] |
| 150. | Papadopoulos G. Echinococcosis/hydatidosis in the world. Epizootiological and epidemiological analysis: problems in the Mediterranean area. Abstr. XIII Congreso International de Hidatologia. Madrid 1985; 21-24. [Cited in This Article: ] |
| 151. | Office International des Epizooties. World Animal Health Information Data Base. Office International des Epizooties. 2007; Available from: http://www.oie.int//wahis/public.php. [Cited in This Article: ] |
| 152. | Eckert J, Thompson RC. Echinococcus strains in Europe: a review. Trop Med Parasitol. 1988;39:1-8. [PubMed] [Cited in This Article: ] |
| 153. | Buishi I, Walters T, Guildea Z, Craig P, Palmer S. Reemergence of canine Echinococcus granulosus infection, Wales. Emerg Infect Dis serial online, 2005-04; 11(4). Available from: http//www.cdc.gov/ncidod/EID/vol11no4-0178.htm. [Cited in This Article: ] |
| 154. | Daniel Mwambete K, Ponce-Gordo F, Cuesta-Bandera C. Genetic identification and host range of the Spanish strains of Echinococcus granulosus. Acta Trop. 2004;91:87-93. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 60] [Cited by in F6Publishing: 61] [Article Influence: 3.1] [Reference Citation Analysis (0)] |
| 155. | Benito A, Carmena D, Joseph L, Martínez J, Guisantes JA. Dog echinococcosis in northern Spain: comparison of coproantigen and serum antibody assays with coprological exam. Vet Parasitol. 2006;142:102-111. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 36] [Cited by in F6Publishing: 33] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 156. | Sobrino R, Gonzalez LM, Vicente J, Fernández de Luco D, Garate T, Gortázar C. Echinococcus granulosus (Cestoda, Taeniidae) in the Iberian wolf. Parasitol Res. 2006;99:753-756. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 19] [Cited by in F6Publishing: 21] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 157. | Rodrigo MR, Canizares FJ, Nieto GA, Lopez FCR, Alcalde MS. Ovine Hydatidosis in the Community of Madrid (Spain). Rev Panam Salud Publica. 1997;5:376-379. [Cited in This Article: ] |
| 158. | Jimenez S, Perez A, Gil H, Schantz P, Ramalle E, Juste R. Progress in control of cystic echinococcosis in La Rioja, Spain: decline in infection prevalences in human and animal hosts and economic costs and benefits. Acta Trop. 2002;83:213-221. [DOI] [Cited in This Article: ] [Cited by in Crossref: 51] [Cited by in F6Publishing: 55] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 159. | Pardo J, Muro A, Galindo I, Cordero M, Carpio A, Siles-Lucas M. [Hydatidosis in the province of Salamanca (Spain): should we let down our guard?]. Enferm Infecc Microbiol Clin. 2005;23:266-269. [DOI] [Cited in This Article: ] [Cited by in Crossref: 23] [Cited by in F6Publishing: 30] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 160. | Bichet H, Dorchies P. [Estimation of the prevalence of bovine hydatid cyst in the south Pyrenees]. Parasite. 1998;5:61-68. [PubMed] [Cited in This Article: ] |
| 161. | Poglayen G, Brianti E, Russo A, Gaglio G, Sorgi C, Giannetto S. Old dreams, new vision: Cystic echinococosis in Sicily. WAAVP. 2003;19:164. [Cited in This Article: ] |
| 162. | Giannetto S, Poglayen G, Brianti E, Sorgi C, Gaglio G, Canu S, Virga A. An epidemiological updating on cystic echinococcosis in cattle and sheep in Sicily, Italy. Parassitologia. 2004;46:423-424. [PubMed] [Cited in This Article: ] |
| 163. | Garippa G. Updates on cystic echinococcosis (CE) in Italy. Parassitologia. 2006;48:57-59. [PubMed] [Cited in This Article: ] |
| 164. | Varcasia A, Canu S, Lightowlers MW, Scala A, Garippa G. Molecular characterization of Echinococcus granulosus strains in Sardinia. Parasitol Res. 2006;98:273-277. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 57] [Cited by in F6Publishing: 57] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 165. | Scala A, Garippa G, Varcasia A, Tranquillo VM, Genchi C. Cystic echinococcosis in slaughtered sheep in Sardinia (Italy). Vet Parasitol. 2006;135:33-38. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 52] [Cited by in F6Publishing: 53] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 166. | Varcasia A, Garippa G, Scala A. The diagnosis of Echinococcus granulosus in dogs. Parassitologia. 2004;46:409-412. [PubMed] [Cited in This Article: ] |
| 167. | Rinaldi L, Maurelli MP, Capuano F, Perugini AG, Veneziano V, Cringoli S. Molecular update on cystic echinococcosis in cattle and water buffaloes of southern Italy. Zoonoses Public Health. 2008;55:119-123. [Cited in This Article: ] |
| 168. | Capuano F, Rinaldi L, Maurelli MP, Perugini AG, Veneziano V, Garippa G, Genchi C, Musella V, Cringoli G. Cystic echinococcosis in water buffaloes: epidemiological survey and molecular evidence of ovine (G1) and buffalo (G3) strains. Vet Parasitol. 2006;137:262-268. [Cited in This Article: ] |
| 169. | Cringoli G, Rinaldi L, Musella V, Veneziano V, Maurelli MP, Di Pietro F, Frisiello M, Di Pietro S. Geo-referencing livestock farms as tool for studying cystic echinococcosis epidemiology in cattle and water buffaloes from southern Italy. Geospat Health. 2007;2:105-111. [PubMed] [Cited in This Article: ] |
| 170. | Bio C, Fagiolo A. Incidence of Hydatidosis in sheep slaughterhouse in the province of Mazzo, Italy. Parassitologia. 2004;46:28. [Cited in This Article: ] |
| 171. | Piergili Fioretti D, Diaferia M, Veronesi F, Sammarone E. Distribution of hydatidosis in slaughtered animals in Umbria region from 1995 to 2004: a retrospective analysis. Parassitologia. 2004;46:437-438. [PubMed] [Cited in This Article: ] |
| 172. | Giangaspero A, Paoletti B, Gatti A, Iorio R, Traversa D, Capelli G, Manfredi MT, Varcasia A, Garippa G. The epidemiological scenario on echinococcosis in the Abruzzo region. Parassitologia. 2006;48:338. [Cited in This Article: ] |
| 173. | Faggioli P, Baldelli R, Battelli G. Cystic echinococcosis in Italy: prevalence in food-producing animals slaughtered in the Emilia-Romagna Region. 6th National Conference of Parasitology of the Bulgarian Society for Parasitology. 2001;121. [Cited in This Article: ] |
| 174. | Guberti V, Bolognini M, Lanfranchi P, Battelli G. Echinococcus granulosus in the wolf in Italy. Parassitologia. 2004;46:425-427. [PubMed] [Cited in This Article: ] |
| 175. | Guberti V; EFSA. The Community Summary Report on Trends and Sources of Zoonoses, Zoonotic Agents, Antimicrobial Resistance and Foodborne Outbreaks in the European Union in 2006. EFSA J. 2006;130:207-216. [Cited in This Article: ] |
| 176. | Pozio E. Epidemiology and control prospects of foodborne parasitic zoonoses in the European Union. Parassitologia. 2008;50:17-24. [PubMed] [Cited in This Article: ] |
| 177. | Dionigi G, Carrafiello G, Recaldini C, Sessa F, Boni L, Rovera F, Dionigi R. Laparoscopic resection of a primary hydatid cyst of the adrenal gland: a case report. J Med Case Reports. 2007;1:61. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 14] [Cited by in F6Publishing: 19] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 178. | Gabriele F, Bortoletti G, Conchedda M, Palmas C, Ecca AR. [Human cystic hydatidosis in Italy: a public health emergency? Past to present]. Parassitologia. 2004;46:39-43. [PubMed] [Cited in This Article: ] |
| 179. | Conchedda M, Antonelli A, Caddori A, Seu V, Gabriele F. Human Cystic Echinococcosis in Sardinia from 2001 to 2005 based on Hospital discharge Reports. Parassitologia. 2008;50:40. [Cited in This Article: ] |
| 180. | Ostanello F, Piccolomini LL, Martinello F, Vizioli M, Battelli G. [Echinococcosis/hydatidosis in Emilia-Romagna: a study of hospital admissions in the period of 1989-1993]. Epidemiol Prev. 1997;21:41-47. [PubMed] [Cited in This Article: ] |
| 181. | Battelli G, Ostanello F, Baldelli R, Di Francesco A, Grilli R, Vizioli M. Human echinococcosis in the Emilia-Romagna region (northern Italy) in the years 1997 to 2002: an updating. Parassitologia. 2004;46:415-416. [PubMed] [Cited in This Article: ] |
| 182. | Silva KT. Malaria eradication as a legacy of colonial discourse: the case of Sri Lanka. Parassitologia. 1994;36:149-163. [PubMed] [Cited in This Article: ] |
| 183. | Kern P, Bardonnet K, Renner E, Auer H, Pawlowski Z, Ammann RW, Vuitton DA, Kern P. European echinococcosis registry: human alveolar echinococcosis, Europe, 1982-2000. Emerg Infect Dis. 2003;9:343-349. [PubMed] [Cited in This Article: ] |