Published online Nov 21, 2014. doi: 10.3748/wjg.v20.i43.16029
Revised: July 4, 2014
Accepted: August 13, 2014
Published online: November 21, 2014
The known factors that have contributed to the decline of Helicobacter pylori (H. pylori) eradication rate include antibiotic resistance, poor compliance, high gastric acidity, high bacterial load, and cytochrome P450 2C19 (CYP2C19) polymorphism. Proton pump inhibitor (PPI) is important in the eradication regimen. The principal enzyme implicated in the metabolism of PPIs is CYP2C19. The effects of PPI depend on metabolic enzyme, cytochrome P450 enzymes, and CYP2C19 with genetic differences in the activity of this enzyme (the homozygous EM, heterozygous EM (HetEM), and poor metabolizer). The frequency of the CYP2C19 polymorphism is highly varied among different ethnic populations. The CYP2C19 genotype is a cardinal factor of H. pylori eradication in patients taking omeprazole- based or lansoprazole-based triple therapies. In contrast, the CYP2C19 polymorphism has no significant effect on the rabeprazole-based or esomeprazole-based triple therapies. The efficacy of levofloxacin-based rescue triple therapy might be also affected by the CYP2C19 polymorphism, but CYP2C19 genotypes did not show obvious impact on other levofloxacin-based rescue therapies. Choice of different PPIs and/or increasing doses of PPIs should be individualized based on the pharmacogenetics background of each patient and pharmacological profile of each drug. Other possible factors influencing gastric acid secretion (e.g., IL-1β- 511 polymorphism) would be also under consideration.
Core tip: This manuscript outlines the impact of cytochrome P450 2C19 (CYP2C19) polymorphism on eradication of Helicobacter pylori (H. pylori), including the influences on first line triple therapies, rescue therapies and levofloxacin- based therapies. We suggest that the strategy of eradicating H. pylori should include the examination of CYP2C19 polymorphism, especially for patients receiving rescue therapies or with drug resistances.
-
Citation: Kuo CH, Lu CY, Shih HY, Liu CJ, Wu MC, Hu HM, Hsu WH, Yu FJ, Wu DC, Kuo FC. CYP2C19 polymorphism influences
Helicobacter pylori eradication. World J Gastroenterol 2014; 20(43): 16029-16036 - URL: https://www.wjgnet.com/1007-9327/full/v20/i43/16029.htm
- DOI: https://dx.doi.org/10.3748/wjg.v20.i43.16029
According to Maastricht IV Consensus Report, indications for anti-Helicobacter pylori (H. pylori) therapy include gastroduodenal ulcer disease, gastric low-grade mucosa-associated lymphoid tissue lymphoma, atrophic gastritis, and early gastric cancer following endoscopic resection[1]. The first-line regimen for H. pylori eradication includes proton pump inhibitor (PPI), clarithromycin (CAM), and amoxicillin (AMX), or metronidazole. However, the failure rate of triple anti-H. pylori therapies has increased up to 30%[2,3]. The known factors include antibiotic resistance, poor compliance, high gastric acidity, high bacterial load, and cytochrome P450 2C19 (CYP2C19) polymorphism that contribute to the decline of H. pylori eradication rate[4] So the triple regimen should be abandoned when the CAM-resistance rate in the region is more than 15%-20%, because many studies published recently have demonstrated that the intention to treatment eradication rate is falling short of 80%[5-7]. The same consideration should be also suitable for high levofloxacin resistance area.
According to the recommendation of the Asian Pacific Helicobacter pylori meeting 2012 in Singapore: (1) in areas with low clarithromycin resistance rates, standard triple therapy should be the primary choice, while bismuth-containing quadruple, sequential therapy and concomitant therapy could be alternative first-line therapies; and (2) in areas with high clarithromycin resistance, regimens including bismuth-containing quadruple, sequential therapy and concomitance should be the better choice for first-line regimens. So the antibiotics resistance should be analyzed in the high clarithromycin and/or levofloxacin resistant rate area.
PPI is important in the eradication regimen. Except for the anti-secretory effect, PPI can also increase the efficacy of the antibiotics by decreasing antibiotic decay in the gastric juices, and also possesses direct anti-H. pylori activity[8-11]. The mechanisms whereby PPIs influence the efficacy of eradicating H. pylori include (1) PPIs make acid-labile antibiotics more stable by increasing gastric PH value, especially clarithromycin, thereby increasing concentration and H. pylori sensitivity to antibiotics; and (2) PPIs may alter transport of antibiotics from plasma to gastric juices, increasing luminal concentrations and elevating the success rate of eradication[12]. The importance of potent acid inhibition during eradication therapy has recently been demonstrated[13-17].
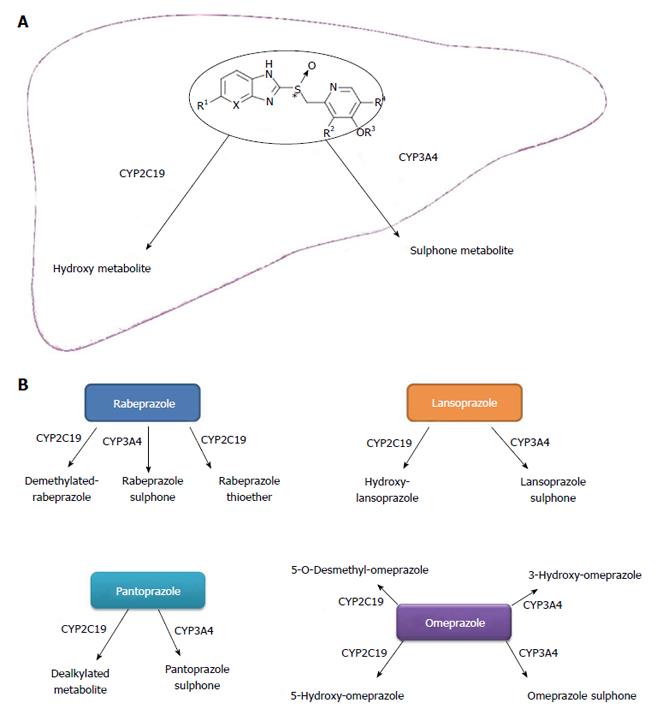
The principal enzyme implicated in the metabolism of PPIs (except rabeprazole) is CYP2C19[18-20] (Figure 1A and B). The effects of PPI depend on the genetic differences of CYP2C19[21]. It is well known that CYP2C19 has polymorphisms in exon 4 (*3) and in exon 5 (*2). Furthermore, the homozygous extensive metabolizer (HomEM) harbors two wild-type alleles (or *1/*1), heterozygous EM (HetEM) carries 1 loss-of-function (LOF) variant allele (frequently *2 or *3), and poor metabolizer (PM) has two LOF variant alleles (*2/*2, or *2/*3)[22,23].
HomEM produces abundance of the enzyme, and metabolizes PPI at high rates. HetEM, with one wild-type and one mutation-type, compromises the rates of PPI metabolism (Table 1).
| Genotype | Ratio (Asian) | Ratio (Caucasian) | Metabolism of PPIs | |
| HomEM | wt/wt | 30%-40% | 70% | At highest rates |
| HetEM | wt/m1 or wt/m2 | 45%-55% | 25%-27% | At moderate rates |
| PM | m1/m2 | 13%-23% | 3%-5% | At lowest rates |
Several trials have been published concerning the effect of the CYP2C19 genotype on eradication of H. pylori by various PPIs-based therapies[24-29]. Cure rates of standard triple therapy depend on the availability of PPI, which itself depends on the CYP2C19 and MDR polymorphisms[1]. Besides these, we would point out the impact of CYP2C19 genotypes on levofloxacin-based first-line and rescue therapies. This was seldom emphasized in previous articles.
For analysis of CYP2C19 genotypes, all enrolled patients’ peripheral blood leukocytes were obtained before the eradication therapy was begun. DNA was extracted from the leukocytes with a commercially available kit (QIAGEN K.K., Tokyo, Japan) and stored until use. Genotyping procedures for identifying the CYP2C19 wild type (wt) gene and two mutated alleles, CYP2C19 m1 and CYP2C19 m2, were performed by a polymerase chain reaction-restriction fragment length polymorphism method with allele-specific primers[30]. However, this technique is expensive and not all medical unit can carry out it. This is one of the limitations for managing H. pylori infection.
The frequency of the CYP2C19 polymorphism is highly varied among different ethnic populations. According to previous reports, Asian people have a higher proportion of poor metabolizers compared to whites[14]. Approximately 2%-6% of Caucasians and 1% of African-Americans have been identified as PM, but this reaches more than 14.0% in Asian populations[31-33]. For example, the frequency of PM in Japanese (19%-23%) is much highe[34-36].
On the other hand, the prevalence rate of HomEM is about 70% for Caucasians, but only 30%-40% for Asians[37]. Since the CYP2C19 genotype is related to different abilities of PPIs to inhibit gastric acid secretion, genotyping of CYP2C19 gene would be a useful tool in the optimization of H. pylori eradication therapy. Therefore, differences of geographic distribution should be taken into consideration in selecting kinds or doses of PPIs for eradication.
PPIs were introduced in 1989; they targeted the gastric H+/ K+-ATPase. PPIs result in a major medical therapeutic advance in the treatment of peptic ulcers and GERD due to more rapid healing of the mucosal lesions and symptom relief.
PPIs are inactive in their native form and are rapidly metabolized by the liver. They are acid-activated prodrugs, and the plasma level of PPIs at the time point of the gastric acid secretion would influent the efficacy of PPIs. The rate of the metabolism is dependent on the character of the cytochrome P450 system, especially S-mephenytoin 4’-hydroxylase (CYP2C19)[38]. CYP3A4 is also an important factor for this[39]. The genetic polymorphisms of CYP2C19 may bring about differences in pharmacokinetics (PK), pharmacodynamics and clinical efficacy of PPIs.
The PK properties of PPIs (area under the plasmic concentration curve (AUC), Cmax and clearance) have significant difference between CYP2C19 PM and HomEM. The ratios of the mean AUC values in PM vs HomEM for omeprazole, pantoprazole, lansoprazole and rabeprazole are 6.3, 6, 4.3 and 1.8 respectively[40]. PM exhibits a 3- to 10-fold higher AUC than HomEM, while HetEM exhibits a 2- to 3-fold higher AUC[32,41].
Omeprazole is converted to hydroxyl and 5-O-demethyl metabolites by CYP2C19, and to the sulfone by CYP3A4[38]. The other PPIs also are extensively metabolized by CYP2C19 and CYP3A4. It has been shown that the mean intragastric pH values were higher in PM than in EMs for both omeprazole and lansoprazole[23]. Rabeprazole showed the least difference, and this is probably due to the metabolism of rabeprazole, which involves a non-enzymatic reduction[40]. Rabeprazole is metabolized to thioether-rabeprazole mainly via a non-enzymatic pathway, with minor involvement of CYP2C19[42]. Esomeprazole is a pure S-isomer of omeprazole, which is different from omeprazole composed of the equal amount of R- and S-isomer. R-omeprazole is more sensitive to CYP2C19 while S-omeprazole is less sensitive[43,44]; therefore, S-omeprazole provides better plasma level of the drug. Besides this, Esomeprazole has minimal first pass metabolism, undergoes less hydroxylation via CYP2C19, and has been shown to have greater gastric acid suppression effect than omeprazole[45,46]. So rabeprazole and esomeprazole appear less influenced by polymorphism of CYP2C19[12,14].
Furuta et al[47] first reported that mutations in exon 4[*3] and exon 5[*2] of CYP2C19 may affect the eradication rates in H. pylori-positive patients using a 1-week triple-therapy regimen with omeprazole, amoxicillin, and clarithromycin in 1998. A previous meta-analysis of 17 studies in Asian patients about the effects of CYP2C19 polymorphisms on the efficacy of PPI-based dual and triple therapy for H. pylori eradication was reported by Padol et al[48]. They found that omeprazole-based regimens were affected by CYP2C19 genotype status, whereas rabeprazole- and lansoprazole-based therapies were not. It also demonstrated significantly lower H. pylori eradication rates in HomEM compared to PM exposed to standard triple or dual therapies.
A meta-analysis showed a significant difference in H. pylori eradication rates between wild-type individuals and carriers of at least one LOF allele (OR = 2.26; 95%CI: 1.58-2.96; P < 0.0001). The difference was also obvious when HomEM and HetEM were compared (OR = 2.79; 95%CI: 1.77-4.41; P < 0.0001). However, when individual agents were analyzed separately, a significant difference was observed for omeprazole and lansoprazole only, whereas no difference between all genotypes was observed for rabeprazole[49].
Although rabeprazole has been suggested to be the PPI least affected by CYP2C19 genotype[50], there are some controversial results. Some studies found the eradication rate of a rabeprazole regimen still correlated with the CYP2C19 genotype[51-53]. Besides this, there were 3 clinical studies[51,54,55] that directly compared H. pylori eradication rates of lansoprazole and rabeprazole according to CYP2C19 polymorphism. They all reported no significant differences of eradication rate between lansoprazole- and rabeprazole- based therapies among different CYP2C19 genotypes. morphism. However, these studies had relatively small sample size and insufficient randomization.
It is reasonable that increasing dosage might overcome the effect of CYP2C19 polymorphism. One study in China demonstrated that increasing the dosage of omeprazole (20-40 mg) would improve the efficacy of eradication[56]. However, other studies did not find a similar dose-dependent effect by use of omeprazole, rabeprazole, and lansoprazole[57,58]; therefore, this strategy needs further survey.
There might be less consistency across the above meta-analysis studies derived from the randomized control trials or cohort studies. Results of one recent large scale meta-analysis analyzed all PPIs-based triple therapies. It showed that a significant difference existed in the H. pylori eradication rates with omeprazole-based triple therapy between the HomEM and PM genotypes, between HomEM and HetEM genotypes, but not between the HetEM and PM genotypes. Similarly, lansoprazole-based triple therapy exhibited a significant difference in the H. pylori eradication rates between HomEM and PM genotypes, between HomEM and HetEM genotypes, but not between HetEM and PM genotypes. Contrary to the above, rabeprazole- or esomeprazole-based triple therapies did not show any significant difference in H. pylori eradication rates among all three genotypes[59].
A previous study reported that fluoroquinolone antibiotics decreased CYP3A- and CYP1A-mediated biotransformation by competitive inhibition, indicating a potential to interact with agents such as PPIs that are metabolized by these enzymes[60]. Thus, it can be reasonable that levofloxacin may inhibit the activity of CYP3A4 and/or CYP2C19, decreasing the metabolism of PPIs, and resulting in higher concentrations of PPIs. However, this hypothesis requires further investigation.
The efficacy of levofloxacin-based rescue triple therapy has been shown to be affected by the CYP2C19 polymorphism[61]. It reveals that the incidence of HomEM and HetEM genotypes was slightly higher in the group with failure of eradication than in the group with success of eradication, but the difference was not significant, while the incidence of the PM genotype was significantly lower in the group with eradication failure than in the group with successful eradication. However, another study disclosed that in patients treated by triple therapies with esomeprazole plus levofloxacin, CYP2C19 genotype had no significant effect on rates of H. pylori eradication[57].
Empirically modified sequential therapy containing levofloxacin and high-dose esomeprazole showed different findings. The eradication rates were not affected by the CYP2C19 polymorphism. The use of a higher dose of esomeprazole could probably overcome the influence of CYP2C19 polymorphisms on the eradication rate of the modified sequential therapy[62]. Besides these, one study showed that the polymorphism of CYP2C19 did not influence quadruple therapy[63].
There are some new types of PPIs, e.g., tenatoprazole, ilaprazole reported in recent years. Tenatoprazole is a novel proton pump inhibitor with a seven-fold longer plasma half-life H(+)/K(+)-ATPase inhibitors[64]. There are no much data about the impact of CYP2C19 polymorphism on tenatoprazole. Ilaprazole is a new proton pump inhibitor and its major metabolite is ilaprazole sulfone. It is predominantly metabolized by CYP3A4/5[65].
There might be some factors other than CYP2C19 also influencing the eradication results. Some studies suggest that the interleukin (IL)-1β-511 polymorphism affects eradication therapy through gastric acid inhibition[25,66,67]. This also disclosed that the cure rate of 1-week standard triple therapy with omeprazole in different IL-1β-511 genotypes ( especially in the IL-1β-511 C/C genotype) was influenced by CYP2C19 genotype status. Other studies have shown that IL-1β-511 genotype-dependent different cure rates of H. pylori infection are observed only in patients with HomEM of CYP2C19, but not in those with HetEM or PM genotype status[26,68]. On the other hand, CYP2C19 genotype-dependent differences in the cure rates of H. pylori infection are observed in patients with the IL-1β- 511 C/C genotype[25,26,68]. Nevertheless, previous studies did not find statistically significant difference of the cure rate among the CYP2C19 genotype subgroups in the IL-1β- 511 C/T and T/T groups. This evidence reveals that IL-1β-511 polymorphism has an impact on CYP2C19 polymorphism influencing eradication of H. pylori.
The possible best method might be to perform examination of CYP2C19 genotypes before starting eradication. Practically, routine examination of CYP2C19 genotype is not applicable and expensive. A meta-analysis showed that high-dose PPIs increase cure rates by around 6%-10% in comparison with standard doses. Increasing the dose of PPI from twice daily from 20 mg to 40 mg of esomeprazole or rabeprazole twice daily may increase cure rates by 8%-12%[1]. We suggest the following strategies to avoid the influence of CYP2C19 genotype after failure of eradication: (1) select PPI metabolized by the non-enzymatic pathway; and (2) consider increasing dose of CYP2C19 sensitive PPI.
Choice of different PPIs and/or increasing doses of PPIs should be individualized based on the pharmacogenetics background of each patient and pharmacological profile of each drug.
The CYP2C19 variant carriage is an important factor of H. pylori eradication rate in patients taking omeprazole- or lansoprazole-based triple therapies. The efficacy of levofloxacin-based rescue triple therapy might be also affected by the CYP2C19 polymorphism, but CYP2C19 genotypes did not show obvious impact on levofloxacin-based quadruple rescue therapies.
Other possible factors influencing gastric acid secretion (e.g., IL-1β- 511 polymorphisms) should also come under consideration.
P- Reviewer: Chiu CT, Dore MP, Du YQ, Gallelli L, Ierardi E, Shimatani T, Zhang JZ, Zullo A S- Editor: Ma YJ L- Editor: A E- Editor: Liu XM
| 1. | Malfertheiner P, Megraud F, O’Morain CA, Atherton J, Axon AT, Bazzoli F, Gensini GF, Gisbert JP, Graham DY, Rokkas T. Management of Helicobacter pylori infection--the Maastricht IV/Florence Consensus Report. Gut. 2012;61:646-664. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 1541] [Cited by in F6Publishing: 1494] [Article Influence: 124.5] [Reference Citation Analysis (3)] |
| 2. | Fennerty MB, Lieberman DA, Vakil N, Magaret N, Faigel DO, Helfand M. Effectiveness of Helicobacter pylori therapies in a clinical practice setting. Arch Intern Med. 1999;159:1562-1566. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 32] [Cited by in F6Publishing: 36] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 3. | Della Monica P, Lavagna A, Masoero G, Lombardo L, Crocellá L, Pera A. Effectiveness of Helicobacter pylori eradication treatments in a primary care setting in Italy. Aliment Pharmacol Ther. 2002;16:1269-1275. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 51] [Cited by in F6Publishing: 57] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 4. | Kuo CH, Kuo FC, Hu HM, Liu CJ, Wang SS, Chen YH, Hsieh MC, Hou MF, Wu DC. The Optimal First-Line Therapy of Helicobacter pylori Infection in Year 2012. Gastroenterol Res Pract. 2012;2012:168361. [PubMed] [Cited in This Article: ] |
| 5. | Park HG, Jung MK, Jung JT, Kwon JG, Kim EY, Seo HE, Lee JH, Yang CH, Kim ES, Cho KB. Randomised clinical trial: a comparative study of 10-day sequential therapy with 7-day standard triple therapy for Helicobacter pylori infection in naïve patients. Aliment Pharmacol Ther. 2012;35:56-65. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 63] [Cited by in F6Publishing: 63] [Article Influence: 5.3] [Reference Citation Analysis (0)] |
| 6. | Qua CS, Manikam J, Goh KL. Efficacy of 1-week proton pump inhibitor triple therapy as first-line Helicobacter pylori eradication regime in Asian patients: is it still effective 10 years on? J Dig Dis. 2010;11:244-248. [PubMed] [Cited in This Article: ] |
| 7. | Sasaki M, Ogasawara N, Utsumi K, Kawamura N, Kamiya T, Kataoka H, Tanida S, Mizoshita T, Kasugai K, Joh T. Changes in 12-Year First-Line Eradication Rate of Helicobacter pylori Based on Triple Therapy with Proton Pump Inhibitor, Amoxicillin and Clarithromycin. J Clin Biochem Nutr. 2010;47:53-58. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 65] [Cited by in F6Publishing: 73] [Article Influence: 5.2] [Reference Citation Analysis (0)] |
| 8. | Goddard AF, Jessa MJ, Barrett DA, Shaw PN, Idström JP, Cederberg C, Spiller RC. Effect of omeprazole on the distribution of metronidazole, amoxicillin, and clarithromycin in human gastric juice. Gastroenterology. 1996;111:358-367. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 157] [Cited by in F6Publishing: 166] [Article Influence: 5.9] [Reference Citation Analysis (0)] |
| 9. | Erah PO, Goddard AF, Barrett DA, Shaw PN, Spiller RC. The stability of amoxycillin, clarithromycin and metronidazole in gastric juice: relevance to the treatment of Helicobacter pylori infection. J Antimicrob Chemother. 1997;39:5-12. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 167] [Cited by in F6Publishing: 173] [Article Influence: 6.4] [Reference Citation Analysis (1)] |
| 10. | Spengler G, Molnar A, Klausz G, Mandi Y, Kawase M, Motohashi N, Molnar J. Inhibitory action of a new proton pump inhibitor, trifluoromethyl ketone derivative, against the motility of clarithromycin-susceptible and-resistant Helicobacter pylori. Int J Antimicrob Agents. 2004;23:631-633. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 21] [Cited by in F6Publishing: 22] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 11. | Figura N, Crabtree JE, Dattilo M. In-vitro activity of lansoprazole against Helicobacter pylori. J Antimicrob Chemother. 1997;39:585-590. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 25] [Cited by in F6Publishing: 27] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 12. | Kita T, Tanigawara Y, Aoyama N, Hohda T, Saijoh Y, Komada F, Sakaeda T, Okumura K, Sakai T, Kasuga M. CYP2C19 genotype related effect of omeprazole on intragastric pH and antimicrobial stability. Pharm Res. 2001;18:615-621. [PubMed] [Cited in This Article: ] |
| 13. | Kirsch C, Morgner A, Miehlke S. Relevance of cytochrome P450 polymorphisms in the treatment of Helicobacter pylori infection and gastroesophageal reflux disease. Curr Pharmacogenomics. 2006;4:47-56. [DOI] [Cited in This Article: ] [Cited by in Crossref: 9] [Cited by in F6Publishing: 9] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 14. | Kuo CH, Wang SS, Hsu WH, Kuo FC, Weng BC, Li CJ, Hsu PI, Chen A, Hung WC, Yang YC. Rabeprazole can overcome the impact of CYP2C19 polymorphism on quadruple therapy. Helicobacter. 2010;15:265-272. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 30] [Cited by in F6Publishing: 29] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 15. | Furuta T, Shirai N, Kodaira M, Sugimoto M, Nogaki A, Kuriyama S, Iwaizumi M, Yamade M, Terakawa I, Ohashi K. Pharmacogenomics-based tailored versus standard therapeutic regimen for eradication of H. pylori. Clin Pharmacol Ther. 2007;81:521-528. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 132] [Cited by in F6Publishing: 143] [Article Influence: 8.4] [Reference Citation Analysis (0)] |
| 16. | Sugimoto M, Furuta T, Shirai N, Kodaira C, Nishino M, Yamade M, Ikuma M, Watanabe H, Ohashi K, Hishida A. Treatment strategy to eradicate Helicobacter pylori infection: impact of pharmacogenomics-based acid inhibition regimen and alternative antibiotics. Expert Opin Pharmacother. 2007;8:2701-2717. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 25] [Cited by in F6Publishing: 27] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 17. | Sugimoto M, Furuta T, Shirai N, Kodaira C, Nishino M, Ikuma M, Ishizaki T, Hishida A. Evidence that the degree and duration of acid suppression are related to Helicobacter pylori eradication by triple therapy. Helicobacter. 2007;12:317-323. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 85] [Cited by in F6Publishing: 84] [Article Influence: 4.9] [Reference Citation Analysis (0)] |
| 18. | Andersson T, Regårdh CG, Dahl-Puustinen ML, Bertilsson L. Slow omeprazole metabolizers are also poor S-mephenytoin hydroxylators. Ther Drug Monit. 1990;12:415-416. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 120] [Cited by in F6Publishing: 129] [Article Influence: 3.8] [Reference Citation Analysis (0)] |
| 19. | Andersson T, Regårdh CG, Lou YC, Zhang Y, Dahl ML, Bertilsson L. Polymorphic hydroxylation of S-mephenytoin and omeprazole metabolism in Caucasian and Chinese subjects. Pharmacogenetics. 1992;2:25-31. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 129] [Cited by in F6Publishing: 135] [Article Influence: 4.2] [Reference Citation Analysis (0)] |
| 20. | Sohn DR, Kobayashi K, Chiba K, Lee KH, Shin SG, Ishizaki T. Disposition kinetics and metabolism of omeprazole in extensive and poor metabolizers of S-mephenytoin 4’-hydroxylation recruited from an Oriental population. J Pharmacol Exp Ther. 1992;262:1195-1202. [PubMed] [Cited in This Article: ] |
| 21. | Ishizaki T, Horai Y. Review article: cytochrome P450 and the metabolism of proton pump inhibitors--emphasis on rabeprazole. Aliment Pharmacol Ther. 1999;13 Suppl 3:27-36. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 287] [Cited by in F6Publishing: 293] [Article Influence: 11.7] [Reference Citation Analysis (0)] |
| 22. | Tomalik-Scharte D, Lazar A, Fuhr U, Kirchheiner J. The clinical role of genetic polymorphisms in drug-metabolizing enzymes. Pharmacogenomics J. 2008;8:4-15. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 98] [Cited by in F6Publishing: 101] [Article Influence: 5.9] [Reference Citation Analysis (0)] |
| 23. | Hagymási K, Müllner K, Herszényi L, Tulassay Z. Update on the pharmacogenomics of proton pump inhibitors. Pharmacogenomics. 2011;12:873-888. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 57] [Cited by in F6Publishing: 67] [Article Influence: 5.2] [Reference Citation Analysis (0)] |
| 24. | Furuta T, Shirai N, Takashima M, Xiao F, Hanai H, Sugimura H, Ohashi K, Ishizaki T, Kaneko E. Effect of genotypic differences in CYP2C19 on cure rates for Helicobacter pylori infection by triple therapy with a proton pump inhibitor, amoxicillin, and clarithromycin. Clin Pharmacol Ther. 2001;69:158-168. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 191] [Cited by in F6Publishing: 209] [Article Influence: 9.1] [Reference Citation Analysis (0)] |
| 25. | Take S, Mizuno M, Ishiki K, Nagahara Y, Yoshida T, Inaba T, Yamamoto K, Okada H, Yokota K, Oguma K. Interleukin-1beta genetic polymorphism influences the effect of cytochrome P 2C19 genotype on the cure rate of 1-week triple therapy for Helicobacter pylori infection. Am J Gastroenterol. 2003;98:2403-2408. [PubMed] [Cited in This Article: ] |
| 26. | Sugimoto M, Furuta T, Shirai N, Ikuma M, Hishida A, Ishizaki T. Influences of proinflammatory and anti-inflammatory cytokine polymorphisms on eradication rates of clarithromycin-sensitive strains of Helicobacter pylori by triple therapy. Clin Pharmacol Ther. 2006;80:41-50. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 25] [Cited by in F6Publishing: 28] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 27. | Kuwayama H, Asaka M, Sugiyama T, Fukuda Y, Aoyama N, Hirai Y, Fujioka T. Rabeprazole-based eradication therapy for Helicobacter pylori: a large-scale study in Japan. Aliment Pharmacol Ther. 2007;25:1105-1113. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 40] [Cited by in F6Publishing: 31] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 28. | Yang JC, Yang YF, Uang YS, Lin CJ, Wang TH. Pharmacokinetic- pharmacodynamic analysis of the role of CYP2C19 genotypes in short-term rabeprazole-based triple therapy against Helicobacter pylori. Br J Clin Pharmacol. 2009;67:503-510. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 11] [Cited by in F6Publishing: 12] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 29. | Serrano D, Torrado S, Torrado-Santiago S, Gisbert JP. The influence of CYP2C19 genetic polymorphism on the pharmacokinetics/- pharmacodynamics of proton pump inhibitor-containing Helicobacter pylori treatments. Curr Drug Metab. 2012;13:1303-1312. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 15] [Cited by in F6Publishing: 15] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 30. | De Morais SM, Wilkinson GR, Blaisdell J, Meyer UA, Nakamura K, Goldstein JA. Identification of a new genetic defect responsible for the polymorphism of (S)-mephenytoin metabolism in Japanese. Mol Pharmacol. 1994;46:594-598. [PubMed] [Cited in This Article: ] |
| 31. | Ieiri I, Kubota T, Urae A, Kimura M, Wada Y, Mamiya K, Yoshioka S, Irie S, Amamoto T, Nakamura K. Pharmacokinetics of omeprazole (a substrate of CYP2C19) and comparison with two mutant alleles, C gamma P2C19m1 in exon 5 and C gamma P2C19m2 in exon 4, in Japanese subjects. Clin Pharmacol Ther. 1996;59:647-653. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 78] [Cited by in F6Publishing: 81] [Article Influence: 2.9] [Reference Citation Analysis (0)] |
| 32. | Klotz U, Schwab M, Treiber G. CYP2C19 polymorphism and proton pump inhibitors. Basic Clin Pharmacol Toxicol. 2004;95:2-8. [PubMed] [Cited in This Article: ] |
| 33. | Goldstein JA, Ishizaki T, Chiba K, de Morais SM, Bell D, Krahn PM, Evans DA. Frequencies of the defective CYP2C19 alleles responsible for the mephenytoin poor metabolizer phenotype in various Oriental, Caucasian, Saudi Arabian and American black populations. Pharmacogenetics. 1997;7:59-64. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 241] [Cited by in F6Publishing: 249] [Article Influence: 9.2] [Reference Citation Analysis (0)] |
| 34. | Küpfer A, Preisig R. Pharmacogenetics of mephenytoin: a new drug hydroxylation polymorphism in man. Eur J Clin Pharmacol. 1984;26:753-759. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 281] [Cited by in F6Publishing: 291] [Article Influence: 7.3] [Reference Citation Analysis (0)] |
| 35. | Wedlund PJ, Aslanian WS, McAllister CB, Wilkinson GR, Branch RA. Mephenytoin hydroxylation deficiency in Caucasians: frequency of a new oxidative drug metabolism polymorphism. Clin Pharmacol Ther. 1984;36:773-780. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 194] [Cited by in F6Publishing: 198] [Article Influence: 5.0] [Reference Citation Analysis (0)] |
| 36. | Horai Y, Nakano M, Ishizaki T, Ishikawa K, Zhou HH, Zhou BI, Liao CL, Zhang LM. Metoprolol and mephenytoin oxidation polymorphisms in Far Eastern Oriental subjects: Japanese versus mainland Chinese. Clin Pharmacol Ther. 1989;46:198-207. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 153] [Cited by in F6Publishing: 164] [Article Influence: 4.7] [Reference Citation Analysis (0)] |
| 37. | Ishizaki T, Sohn DR, Kobayashi K, Chiba K, Lee KH, Shin SG, Andersson T, Regårdh CG, Lou YC, Zhang Y. Interethnic differences in omeprazole metabolism in the two S-mephenytoin hydroxylation phenotypes studied in Caucasians and Orientals. Ther Drug Monit. 1994;16:214-215. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 77] [Cited by in F6Publishing: 84] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 38. | Furuta T, Shirai N, Sugimoto M, Nakamura A, Hishida A, Ishizaki T. Influence of CYP2C19 pharmacogenetic polymorphism on proton pump inhibitor-based therapies. Drug Metab Pharmacokinet. 2005;20:153-167. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 186] [Cited by in F6Publishing: 191] [Article Influence: 10.1] [Reference Citation Analysis (0)] |
| 39. | Fock KM, Ang TL, Bee LC, Lee EJ. Proton pump inhibitors: do differences in pharmacokinetics translate into differences in clinical outcomes? Clin Pharmacokinet. 2008;47:1-6. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 61] [Cited by in F6Publishing: 66] [Article Influence: 4.1] [Reference Citation Analysis (0)] |
| 40. | Ogawa R, Echizen H. Drug-drug interaction profiles of proton pump inhibitors. Clin Pharmacokinet. 2010;49:509-533. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 115] [Cited by in F6Publishing: 117] [Article Influence: 8.4] [Reference Citation Analysis (0)] |
| 41. | Klotz U. Clinical impact of CYP2C19 polymorphism on the action of proton pump inhibitors: a review of a special problem. Int J Clin Pharmacol Ther. 2006;44:297-302. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 94] [Cited by in F6Publishing: 99] [Article Influence: 5.5] [Reference Citation Analysis (0)] |
| 42. | Adachi K, Katsube T, Kawamura A, Takashima T, Yuki M, Amano K, Ishihara S, Fukuda R, Watanabe M, Kinoshita Y. CYP2C19 genotype status and intragastric pH during dosing with lansoprazole or rabeprazole. Aliment Pharmacol Ther. 2000;14:1259-1266. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 113] [Cited by in F6Publishing: 116] [Article Influence: 4.8] [Reference Citation Analysis (0)] |
| 43. | Abelö A, Andersson TB, Antonsson M, Naudot AK, Skånberg I, Weidolf L. Stereoselective metabolism of omeprazole by human cytochrome P450 enzymes. Drug Metab Dispos. 2000;28:966-972. [PubMed] [Cited in This Article: ] |
| 44. | Andersson T, Hassan-Alin M, Hasselgren G, Röhss K, Weidolf L. Pharmacokinetic studies with esomeprazole, the (S)-isomer of omeprazole. Clin Pharmacokinet. 2001;40:411-426. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 169] [Cited by in F6Publishing: 161] [Article Influence: 7.3] [Reference Citation Analysis (0)] |
| 45. | Hassan-Alin M, Andersson T, Bredberg E, Röhss K. Pharmacokinetics of esomeprazole after oral and intravenous administration of single and repeated doses to healthy subjects. Eur J Clin Pharmacol. 2000;56:665-670. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 97] [Cited by in F6Publishing: 99] [Article Influence: 4.1] [Reference Citation Analysis (0)] |
| 46. | Dent J. Review article: pharmacology of esomeprazole and comparisons with omeprazole. Aliment Pharmacol Ther. 2003;17 Suppl 1:5-9. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 22] [Cited by in F6Publishing: 23] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 47. | Furuta T, Ohashi K, Kamata T, Takashima M, Kosuge K, Kawasaki T, Hanai H, Kubota T, Ishizaki T, Kaneko E. Effect of genetic differences in omeprazole metabolism on cure rates for Helicobacter pylori infection and peptic ulcer. Ann Intern Med. 1998;129:1027-1030. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 253] [Cited by in F6Publishing: 227] [Article Influence: 8.7] [Reference Citation Analysis (0)] |
| 48. | Padol S, Yuan Y, Thabane M, Padol IT, Hunt RH. The effect of CYP2C19 polymorphisms on H. pylori eradication rate in dual and triple first-line PPI therapies: a meta-analysis. Am J Gastroenterol. 2006;101:1467-1475. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 112] [Cited by in F6Publishing: 112] [Article Influence: 6.2] [Reference Citation Analysis (0)] |
| 49. | Shi S, Klotz U. Proton pump inhibitors: an update of their clinical use and pharmacokinetics. Eur J Clin Pharmacol. 2008;64:935-951. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 216] [Cited by in F6Publishing: 228] [Article Influence: 14.3] [Reference Citation Analysis (1)] |
| 50. | Williams MP, Pounder RE. Review article: the pharmacology of rabeprazole. Aliment Pharmacol Ther. 1999;13 Suppl 3:3-10. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 56] [Cited by in F6Publishing: 56] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 51. | Inaba T, Mizuno M, Kawai K, Yokota K, Oguma K, Miyoshi M, Take S, Okada H, Tsuji T. Randomized open trial for comparison of proton pump inhibitors in triple therapy for Helicobacter pylori infection in relation to CYP2C19 genotype. J Gastroenterol Hepatol. 2002;17:748-753. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 58] [Cited by in F6Publishing: 58] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 52. | Horai Y, Kimura M, Furuie H, Matsuguma K, Irie S, Koga Y, Nagahama T, Murakami M, Matsui T, Yao T. Pharmacodynamic effects and kinetic disposition of rabeprazole in relation to CYP2C19 genotypes. Aliment Pharmacol Ther. 2001;15:793-803. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 94] [Cited by in F6Publishing: 96] [Article Influence: 4.2] [Reference Citation Analysis (0)] |
| 53. | Lin CJ, Yang JC, Uang YS, Chern HD, Wang TH. Time-dependent amplified pharmacokinetic and pharmacodynamic responses of rabeprazole in cytochrome P450 2C19 poor metabolizers. Pharmacotherapy. 2003;23:711-719. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 16] [Cited by in F6Publishing: 16] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 54. | Kawabata H, Habu Y, Tomioka H, Kutsumi H, Kobayashi M, Oyasu K, Hayakumo T, Mizuno S, Kiyota K, Nakajima M. Effect of different proton pump inhibitors, differences in CYP2C19 genotype and antibiotic resistance on the eradication rate of Helicobacter pylori infection by a 1-week regimen of proton pump inhibitor, amoxicillin and clarithromycin. Aliment Pharmacol Ther. 2003;17:259-264. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 57] [Cited by in F6Publishing: 59] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 55. | Miki I, Aoyama N, Sakai T, Shirasaka D, Wambura CM, Maekawa S, Kuroda K, Tamura T, Kita T, Sakaeda T. Impact of clarithromycin resistance and CYP2C19 genetic polymorphism on treatment efficacy of Helicobacter pylori infection with lansoprazole- or rabeprazole-based triple therapy in Japan. Eur J Gastroenterol Hepatol. 2003;15:27-33. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 47] [Cited by in F6Publishing: 49] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 56. | Yang JC, Wang HL, Chern HD, Shun CT, Lin BR, Lin CJ, Wang TH. Role of omeprazole dosage and cytochrome P450 2C19 genotype in patients receiving omeprazole-amoxicillin dual therapy for Helicobacter pylori eradication. Pharmacotherapy. 2011;31:227-238. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 34] [Cited by in F6Publishing: 37] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 57. | Pan X, Li Y, Qiu Y, Tang Q, Qian B, Yao L, Shi R, Zhang G. Efficacy and tolerability of first-line triple therapy with levofloxacin and amoxicillin plus esomeprazole or rabeprazole for the eradication of Helicobacter pylori infection and the effect of CYP2C19 genotype: a 1-week, randomized, open-label study in Chinese adults. Clin Ther. 2010;32:2003-2011. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 23] [Cited by in F6Publishing: 29] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 58. | Lee JH, Jung HY, Choi KD, Song HJ, Lee GH, Kim JH. The Influence of CYP2C19 Polymorphism on Eradication of Helicobacter pylori: A Prospective Randomized Study of Lansoprazole and Rabeprazole. Gut Liver. 2010;4:201-206. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 32] [Cited by in F6Publishing: 33] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 59. | Tang HL, Li Y, Hu YF, Xie HG, Zhai SD. Effects of CYP2C19 loss-of-function variants on the eradication of H. pylori infection in patients treated with proton pump inhibitor-based triple therapy regimens: a meta-analysis of randomized clinical trials. PLoS One. 2013;8:e62162. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 63] [Cited by in F6Publishing: 70] [Article Influence: 6.4] [Reference Citation Analysis (0)] |
| 60. | Kuo CH, Hu HM, Kuo FC, Hsu PI, Chen A, Yu FJ, Tsai PY, Wu IC, Wang SW, Li CJ. Efficacy of levofloxacin-based rescue therapy for Helicobacter pylori infection after standard triple therapy: a randomized controlled trial. J Antimicrob Chemother. 2009;63:1017-1024. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 65] [Cited by in F6Publishing: 69] [Article Influence: 4.6] [Reference Citation Analysis (0)] |
| 61. | Liou JM, Chen CC, Chen MJ, Chang CY, Fang YJ, Lee JY, Sheng WH, Wang HP, Wu MS, Lin JT. Empirical modified sequential therapy containing levofloxacin and high-dose esomeprazole in second-line therapy for Helicobacter pylori infection: a multicentre clinical trial. J Antimicrob Chemother. 2011;66:1847-1852. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 40] [Cited by in F6Publishing: 41] [Article Influence: 3.2] [Reference Citation Analysis (0)] |
| 62. | Wu DC, Hsu PI, Tseng HH, Tsay FW, Lai KH, Kuo CH, Wang SW, Chen A. Helicobacter pylori infection: a randomized, controlled study comparing 2 rescue therapies after failure of standard triple therapies. Medicine (Baltimore). 2011;90:180-185. [PubMed] [Cited in This Article: ] |
| 63. | Kuo CH, Hsu PI, Kuo FC, Wang SS, Hu HM, Liu CJ, Chuah SK, Chen YH, Hsieh MC, Wu DC. Comparison of 10 day bismuth quadruple therapy with high-dose metronidazole or levofloxacin for second-line Helicobacter pylori therapy: a randomized controlled trial. J Antimicrob Chemother. 2013;68:222-228. [PubMed] [Cited in This Article: ] |
| 64. | Li H, Meng L, Liu F, Wei JF, Wang YQ. H+/K+-ATPase inhibitors: a patent review. Expert Opin Ther Pat. 2013;23:99-111. [PubMed] [Cited in This Article: ] |
| 65. | Seo KA, Lee SJ, Kim KB, Bae SK, Liu KH, Kim DH, Shin JG. Ilaprazole, a new proton pump inhibitor, is primarily metabolized to ilaprazole sulfone by CYP3A4 and 3A5. Xenobiotica. 2012;42:278-284. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 26] [Cited by in F6Publishing: 26] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 66. | Jung HC, Kim JM, Song IS, Kim CY. Helicobacter pylori induces an array of pro-inflammatory cytokines in human gastric epithelial cells: quantification of mRNA for interleukin-8, -1 alpha/beta, granulocyte-macrophage colony-stimulating factor, monocyte chemoattractant protein-1 and tumour necrosis factor-alpha. J Gastroenterol Hepatol. 1997;12:473-480. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 99] [Cited by in F6Publishing: 98] [Article Influence: 3.6] [Reference Citation Analysis (0)] |
| 67. | El-Omar EM, Carrington M, Chow WH, McColl KE, Bream JH, Young HA, Herrera J, Lissowska J, Yuan CC, Rothman N. Interleukin-1 polymorphisms associated with increased risk of gastric cancer. Nature. 2000;404:398-402. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 1690] [Cited by in F6Publishing: 1614] [Article Influence: 67.3] [Reference Citation Analysis (0)] |
| 68. | McLellan RA, Drobitch RK, Monshouwer M, Renton KW. Fluoroquinolone antibiotics inhibit cytochrome P450-mediated microsomal drug metabolism in rat and human. Drug Metab Dispos. 1996;24:1134-1138. [PubMed] [Cited in This Article: ] |