Published online Mar 7, 2014. doi: 10.3748/wjg.v20.i9.2335
Revised: December 11, 2013
Accepted: January 8, 2014
Published online: March 7, 2014
Pancreatic cancer is one of the most aggressive and difficult cancers to treat. Despite numerous research efforts, limited success has been achieved in the therapeutic management of patients with this disease. In the current review, we focus on one component of morphogenesis signaling, Hedgehog (Hh), with the aim of developing novel, effective therapies for the treatment of pancreatic cancer. Hh signaling contributes to the induction of a malignant phenotype in pancreatic cancer and is responsible for maintaining pancreatic cancer stem cells. In addition, we propose a novel concept linking Hh signaling and tumor hypoxic conditions, and discuss the effects of Hh inhibitors in clinical trials. The Hh signaling pathway may represent a potential therapeutic target for patients with refractory pancreatic cancer.
Core tip: Hedgehog (Hh) signaling is involved in the induction of malignant potential in pancreatic cancer, controlling processes of proliferation, invasiveness and tumorigenesis. This phenotypic change is closely associated with the nuclear factor kappa-light-chain-enhancer of activated B cells transcription factor, both in an autocrine and paracrine manner. Hh signaling is also capable of maintaining pancreatic cancer stem cells, and may be activated under conditions of tumor hypoxia. Thus, the Hh signaling pathway may represent a potential therapeutic target for patients with refractory pancreatic cancer and the use of Hh inhibitors will likely play an important role in future therapeutic strategies.
- Citation: Onishi H, Katano M. Hedgehog signaling pathway as a new therapeutic target in pancreatic cancer. World J Gastroenterol 2014; 20(9): 2335-2342
- URL: https://www.wjgnet.com/1007-9327/full/v20/i9/2335.htm
- DOI: https://dx.doi.org/10.3748/wjg.v20.i9.2335
Pancreatic cancer remains one of the deadliest cancers, with an overall survival rate of < 5%[1]. An underlying reason for this may be that few patients undergo curative, surgical operations because of the advanced stage of the cancer at the time of diagnosis. Furthermore, apart from chemotherapy and radiation therapy, there are no effective, alternative therapies for the treatment of refractory pancreatic cancer, and as such, the development of novel therapeutic strategies is urgently required. Recently, it was shown that the Hedgehog (Hh) signaling pathway, which plays a key role in morphogenesis signaling, is re-activated in pancreatic cancer[2]. Hh signaling contributes to tumor aggressiveness, affecting key tumorigenic processes such as proliferation, invasion and progression of cancer cells. Therefore, inhibitors targeting Hh signaling have drawn significant attention as novel, molecularly targeted drugs. Hh signaling components including Patched and Smoothened (Smo) have been detected in almost 70% of human pancreatic cancer specimens and consequently, Hh signaling may play a critical role in the genesis of pancreatic cancer cells[2]. In this review, we summarize recent efforts in the development of new, therapeutic strategies to treat pancreatic cancer, targeting the Hh signaling pathway.
The Hh signaling pathway plays a pivotal role in embryonic patterning and growth control, acting as a morphogen, mitogen and inducing factor of developing organs[3-7]. Hh signaling normally ceases after embryogenesis, however in various cancers, including pancreatic cancer, Hh signaling is re-activated[8]. Therefore, the regulation of Hh signaling in pancreatic cancer likely plays important role in tumorigenesis. The Hh signaling pathway is composed of Hh proteins (sonic Hh; Shh, Indian Hh and Desert Hh), the 12-transmembrane Patched proteins (Patched 1 and Patched 2), the 7-transmembrane protein, Smo and the 5-zinc-finger transcription factors, Gli1, Gli2 and Gli3[9-11]. In the absence of Hh ligand, Patched suppresses Smo, which is the driving protein for Hh signaling, and Gli2 and Gli3 are cleaved by ubiquitin ligases to generate transcriptional repressor isoforms[12-14]. In contrast, in the presence of Hh ligand, inhibition of Smo by Patched is released, Smo is activated, and Gli2 and Gli3 are transmitted to the nucleus as full-length activators leading to the transcription of target genes such as Patched and Gli1[12-14]. Recent studies demonstrated the existence of primary cilia on the cell surface and showed that Smo moves from the cytoplasm to primary cilia in the process of activation[15]. One of the target genes of Hh signaling; Ptch and Gli1 regulate the transcription of the Hh responsive genes by themselves[16]. Other target genes of Hh signaling are the cell cycle regulator Cyclin D1, p21 and N-Myc which plays important role for carcinogenesis and is also typically dysregulated in the cancer cells[7,17,18]. The Hh signaling pathway is unique because several components of this pathway consist of both oncogenes and cancer suppressor genes.
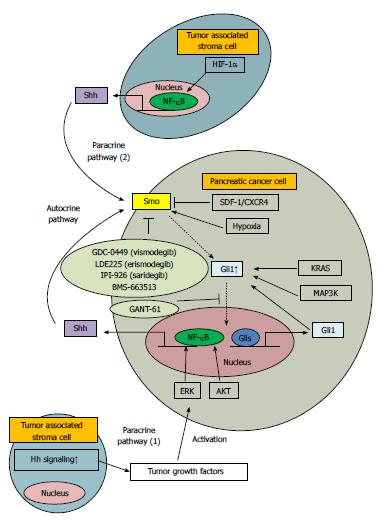
Originally, the relationship between Hh signaling and tumorigenesis was reported following the association of mutations in genes such as Gli1, Patch and Smo in glioblastoma, basal cell carcinoma and rhabdomyosarcoma[19-21]. In pancreatic cancer, ligand-dependent activation of Hh signaling, but not genomic mutation, was first reported[2]. Previous studies have also shown that Shh overexpression is sufficient to initiate pancreatic intraepithelial neoplasia (PanIN)-like precursor lesions[2,22]. At present, this ligand-dependent pathway is thought to be the major mechanism underlying Hh signaling activation. Two distinct ligand-dependent activation pathways exist; autocrine and paracrine. In addition, association between chronic inflammation and the development of cancer has been recognized for several years[23-27]. In both autocrine and paracrine pathways, NF-κB plays a pivotal role. NF-κB is a transcription factor that controls expression of numerous genes involved in inflammation and immune response processes, including proliferation, invasion, adhesion, angiogenesis and apoptosis[28]. In the autocrine pathway, Shh is a direct transcriptional target of NF-κB, and proliferation of pancreatic cancer cells is accelerated via overexpression of Shh[29,30]. In the paracrine paradigm, tumor-associated stroma is important as a microenvironmental factor[31,32]. In one paracrine pathway, stroma cells surrounding pancreatic ductal adenocarcinoma cells, secrete tumor-growth factors through stromal Hh signaling activation[31]. This may explain why low concentrations of Hh signaling antagonist are sufficient to inhibit tumor growth [paracrine pathway (1), Figure 1][31]. In an alternative paracrine pathway, NF-κB-activated monocytes located in the tumor stromal area produce Shh, which stimulates the Hh signaling pathway in pancreatic cancer [paracrine pathway (2), Figure 1][33]. Inhibition of Hh signaling targets pancreatic stellate cells in the tumor-associated stroma, specifically reducing pancreatic tumor growth and metastasis[34,35]. In addition, Singh et al[36] showed that CXCL12/CXCR4 protein signaling induces Shh expression in pancreatic cancer via extracellular regulated kinase (ERK) and Akt kinase-mediated activation of NF-κB. Some other molecules affected by the activation of Hh signaling may also contribute to the induction of malignant potential in pancreatic cancer. Decrease in Cyclin D1by the inhibition of Hh signaling induces the G0/G1 arrest and inhibits cell proliferation[37]. Matrix metalloproteinase (MMP)-9 and MMP-2 locate the downstream of Gli1 and are involved with the invasiveness in pancreatic cancer[38,39].
Solid tumor cancer stem cells were first identified in breast cancer as CD24-/lowCD44+ cells[40]. CD44+CD24+epithelial-specific antigen (ESA)+ pancreatic cancer cells are reported to exhibit the stem cell characteristics of self-renewal and the ability to produce differentiated progeny[41]. Most importantly, cancer stem cells (CSCs) are characterized by features of resistance towards conventional chemotherapy and radiotherapy[42-45]. Pancreatic CSCs exhibit upregulation of Shh[46]. Recently, inhibition of Hh signaling was reported to inhibit the self-renewal of pancreatic CSCs and reverse chemoresistance[47]. Subsequent studies demonstrated that various agents were capable of inhibiting pancreatic CSCs via suppression of Hh signaling. For example, Tang et al[48] revealed that epigallocatechin-3-gallate, an active compound in green tea, inhibits the self-renewal capacity of pancreatic CSCs via inhibition of Hh signaling components including Smo, Ptch, Gli1 and Gli2. Other groups demonstrated that sulforaphane, a component of dietary cruciferous vegetables, decreases pancreatic CSC self-renewal via inhibition of Hh signaling components, Smo, Gli1 and Gli2[49,50]. Han et al[51] has revealed that suppression of Hh signaling by arsenic trioxide leads to the inhibition of the viability of pancreatic CSCs using animal models. A better understanding of the molecular pathways driving CSCs will lead to the development of effective, new therapeutic approaches for the treatment of pancreatic cancer.
As previously discussed, there are numerous reports describing CD44+CD24+ double positive cells in pancreatic CSCs. However to date, there have been relatively few studies investigating CD24 or CD44 molecules alone as therapeutic targets in pancreatic CSCs. CD24 is a unique molecule because it is described as a marker of pancreatic CSCs, whereas it is expressed at low levels or is absent in breast CSCs. CD24 is thought to act as an adhesion molecule[52,53]. Recently, truncated Gli1 was shown to induce clinically more aggressive cancer via the increased expression of CD24[54]. Ringel et al[55] showed that constitutive expression of CD44 variants may also be associated with the malignant state of invasive pancreatic carcinoma. However the precise roles CD24 and CD44 in pancreatic CSCs remain unclear.
Pancreatic cancer is thought to occur under high levels of hypoxia[56]. Therefore, a detailed understanding of the hypoxic microenvironment is crucial for developing effective therapeutic approaches to treat this malignancy. Previous studies have shown that the oxygen concentration in venous blood and deep tumor environments is 5.3% and 1.3%, respectively[57,58]. Thus, to accurately analyze the molecular mechanisms underlying pancreatic cancer, experiments performed under hypoxic conditions are required. The relationship between hypoxia and Hh signaling activation was first reported in 2011, with a study showing that hypoxia activates Hh signaling pathway by upregulating Smo transcription[38]. Thereafter, it was reported that hypoxia induces epithelial to mesenchymal transition (EMT) via activation of Hh signaling[59]. Interestingly, under hypoxic conditions, activation of Hh signaling is independent of hypoxia inducible factor (HIF)-1α and is also ligand-independent, with no observable increase in Shh[38,59]. Conversely, Spivak-Kroizman et al[60] showed that hypoxia and desmoplasia led to more aggressive and therapy-resistant tumors via activation of Hh signaling by Shh, due to HIF-1α activation in the stroma. The mechanisms underlying activation of Hh signaling under hypoxic conditions remains unclear. However, given that Hh signaling is activated under tumor hypoxic conditions, this pathway may represent an important therapeutic target. Indeed, protein-bound polysaccharide decreases invasiveness and proliferation in pancreatic cancer by inhibition of Hh signaling, especially under hypoxia[39].
Pancreatic cancer is often refractory to standard treatments, and many patients are unable to undergo surgery because of the advanced stage of disease at the time of diagnosis. Chemotherapy using gemcitabine and 5-FU derivatives, Tegafur-Gimeracil-Oteracil Potassium (S-1), are often used in Japan. However, combined use of Hh inhibitors with gemcitabine or 5-FU may induce chemoresistance[37]. One reason may be that gemcitabine and 5-FU are sensitive to S-phase and that Hh inhibitor often induces G1 arrest in cancer cells[37]. Conversely, several groups have shown that combined treatment with Hh inhibitors and gemcitabine has a synergistic effect on tumor growth in a xenograft model[61]. Combined use of Hh inhibitors and cisplatin, a cell cycle independent drug, may also have a synergistic effect[37]. Molecular targeting drug is now well established and the combined use of Hh inhibitors and other targeted drugs is currently being studied and utilized. For example, there is a possible synergistic relationship between Hh and epidermal growth factor receptor (EGFR) signaling pathways in pancreatic cancer[62-64]. Although combination therapy with Hh inhibitors remains controversial, these findings will be essential for developing new effective therapeutic strategies. Radiation is considered the third therapeutic strategy for the treatment of pancreatic cancer. Recently, focal radiation in combination with Hh inhibitors exhibited synergistic effects on reducing lymph node metastasis in pancreatic cancer[65]. Immunotherapy is anticipated as the fourth line of therapy after surgery, chemotherapy and radiation. In this approach, activated lymphocytes and dendritic cells (DCs) derived from patients with advanced cancer are often used. Recently, it was reported that Hh signaling is revitalized in activated lymphocytes and DCs derived from patients with advanced cancer and used for immunotherapy, and that this plays a pivotal role in the maintenance of their functions[66,67]. Therefore, Hh inhibitors may not have a synergistic effect when combined with immunotherapy.
Within the class of Hh inhibitors, recent drug development has focused on Smo inhibitors. Although exact patients’ outcome has not been reported yet, Sekulic et al[68] has shown that the independently assessed response rate was 30% and 43%, and the median duration of response was 7.6 mo using two-cohort study with GDC-0449 (visnodegib) in metastatic and locally advanced basal-cell carcinoma. GDC-0449 and IPI-926 (saridegib) are currently under phase II clinical trials in metastatic, advanced and recurrent pancreatic cancer[69] and BMS-663513 is under phase I clinical trial[70]. A recent study demonstrated that LDE225 (erismodegib), a Smo antagonist, suppresses tumor growth and prolongs survival in a murine model of islet cell neoplasms[71]. Furthermore, GANT-61, a Gli transcription factor inhibitor, has been shown to inhibit pancreatic cancer stem cell growth[72]. An overview of Hh signaling inhibitors is shown in Figure 1. More recently, inhibition of Hh signaling has received significant attention as an anti-tumor strategy. Based on this, the relationship between Hh signaling and various materials has been reported. For instance, resveratrol, 3,4’,5-trihydroxystilbene inhibits proliferation and induces apoptosis via Hh signaling in pancreatic cancer[73]. Curcumin, a phenolic compound extracted from Zigiberaceae turmeric, reverses EMT of pancreatic cancer by inhibiting Hh signaling[74]. Triparanol, a known cholesterol biosynthesis inhibitor blocking the 24-dehydrocholesterol reductase, suppresses pancreatic cancer tumor growth by deregulation of Hh signaling[75].
Gli1 is both a transcription factor and a target gene, as shown in previous reviews, and crosstalk between Hh signaling and other pathways has been demonstrated[8]. Gli1 is activated via several kinds of signaling pathways. In pancreatic cancer, various signaling pathways including KRAS[76], ERK[36], AKT[36], MAP3K[77] and SDF-1/CXCR4[78] are associated with Hh signaling (Figure 1). Because Gli1 is located downstream in many of these pathways, it may represent a better therapeutic target.
In this review, we have summarized the development of pancreatic cancer treatment, with specific focus on the Hh signaling pathway. The Hh signaling pathway may represent an important therapeutic target in pancreatic cancer because this pathway is activated in the majority of pancreatic cancers and both ligand-dependent and independent inhibitors are effective. Hh inhibitor can successfully inhibit tumor growth and invasiveness in vitro and can be a promising drug, however, in clinical trial, it is not easy to verify the effectiveness of Hh signaling inhibitor. This reason may be that the actual function of Hh signaling molecules are not fully understood[79,80].
Hh signaling inhibitors should be effective in cancers in which Hh components are mutated such as basal cell carcinoma, basal cell nevus syndrome and medulloblastoma because Hh signaling is constitutively activated[81]. And in these cancers, Hh signaling inhibitors may become the first use drug in future clinical life. However, for other tumors, appropriate combination therapy may be required for the effective therapy. In January 2012, the Smo inhibitor, vismodegib, was clinically approved for the first time by the US Food and Drug Administration, for the treatment of unresectable or metastatic basal cell carcinomas of the skin[82]. Hh signaling inhibitors will now be used in pancreatic cancer as a monotherapy and in combination therapy with other chemodrugs, molecularly targeted drugs or radiation therapy.
We thank Ms Kaori Nomiyama for her skillful technical assistance.
P- Reviewers: Ceyhan C, Fan Y S- Editor: Wen LL L- Editor: A E- Editor: Wang CH
| 1. | Jemal A, Siegel R, Ward E, Hao Y, Xu J, Thun MJ. Cancer statistics, 2009. CA Cancer J Clin. 2009;59:225-249. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 7953] [Cited by in F6Publishing: 8022] [Article Influence: 534.8] [Reference Citation Analysis (2)] |
| 2. | Thayer SP, di Magliano MP, Heiser PW, Nielsen CM, Roberts DJ, Lauwers GY, Qi YP, Gysin S, Fernández-del Castillo C, Yajnik V. Hedgehog is an early and late mediator of pancreatic cancer tumorigenesis. Nature. 2003;425:851-856. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 1151] [Cited by in F6Publishing: 1138] [Article Influence: 54.2] [Reference Citation Analysis (0)] |
| 3. | Ingham PW, McMahon AP. Hedgehog signaling in animal development: paradigms and principles. Genes Dev. 2001;15:3059-3087. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 2300] [Cited by in F6Publishing: 2268] [Article Influence: 98.6] [Reference Citation Analysis (0)] |
| 4. | McMahon AP, Ingham PW, Tabin CJ. Developmental roles and clinical significance of hedgehog signaling. Curr Top Dev Biol. 2003;53:1-114. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 681] [Cited by in F6Publishing: 677] [Article Influence: 32.2] [Reference Citation Analysis (0)] |
| 5. | Hooper JE, Scott MP. Communicating with Hedgehogs. Nat Rev Mol Cell Biol. 2005;6:306-317. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 578] [Cited by in F6Publishing: 620] [Article Influence: 32.6] [Reference Citation Analysis (0)] |
| 6. | Mukherjee S, Frolova N, Sadlonova A, Novak Z, Steg A, Page GP, Welch DR, Lobo-Ruppert SM, Ruppert JM, Johnson MR. Hedgehog signaling and response to cyclopamine differ in epithelial and stromal cells in benign breast and breast cancer. Cancer Biol Ther. 2006;5:674-683. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 113] [Cited by in F6Publishing: 127] [Article Influence: 7.1] [Reference Citation Analysis (0)] |
| 7. | Cohen MM. The hedgehog signaling network. Am J Med Genet A. 2003;123A:5-28. [PubMed] [Cited in This Article: ] |
| 8. | Onishi H, Katano M. Hedgehog signaling pathway as a therapeutic target in various types of cancer. Cancer Sci. 2011;102:1756-1760. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 69] [Cited by in F6Publishing: 73] [Article Influence: 5.6] [Reference Citation Analysis (0)] |
| 9. | Jiang J, Hui CC. Hedgehog signaling in development and cancer. Dev Cell. 2008;15:801-812. [PubMed] [DOI] [Cited in This Article: ] [Cited by in F6Publishing: 5] [Reference Citation Analysis (0)] |
| 10. | Bai CB, Stephen D, Joyner AL. All mouse ventral spinal cord patterning by hedgehog is Gli dependent and involves an activator function of Gli3. Dev Cell. 2004;6:103-115. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 340] [Cited by in F6Publishing: 349] [Article Influence: 17.5] [Reference Citation Analysis (0)] |
| 11. | Kasper M, Regl G, Frischauf AM, Aberger F. GLI transcription factors: mediators of oncogenic Hedgehog signalling. Eur J Cancer. 2006;42:437-445. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 278] [Cited by in F6Publishing: 308] [Article Influence: 17.1] [Reference Citation Analysis (0)] |
| 12. | Stecca B, Ruiz I Altaba A. Context-dependent regulation of the GLI code in cancer by HEDGEHOG and non-HEDGEHOG signals. J Mol Cell Biol. 2010;2:84-95. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 188] [Cited by in F6Publishing: 200] [Article Influence: 14.3] [Reference Citation Analysis (0)] |
| 13. | Wang B, Fallon JF, Beachy PA. Hedgehog-regulated processing of Gli3 produces an anterior/posterior repressor gradient in the developing vertebrate limb. Cell. 2000;100:423-434. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 816] [Cited by in F6Publishing: 780] [Article Influence: 32.5] [Reference Citation Analysis (0)] |
| 14. | Pan Y, Bai CB, Joyner AL, Wang B. Sonic hedgehog signaling regulates Gli2 transcriptional activity by suppressing its processing and degradation. Mol Cell Biol. 2006;26:3365-3377. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 392] [Cited by in F6Publishing: 656] [Article Influence: 36.4] [Reference Citation Analysis (0)] |
| 15. | Corbit KC, Aanstad P, Singla V, Norman AR, Stainier DY, Reiter JF. Vertebrate Smoothened functions at the primary cilium. Nature. 2005;437:1018-1021. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 1114] [Cited by in F6Publishing: 1097] [Article Influence: 57.7] [Reference Citation Analysis (0)] |
| 16. | Freeman M. Feedback control of intercellular signalling in development. Nature. 2000;408:313-319. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 401] [Cited by in F6Publishing: 446] [Article Influence: 18.6] [Reference Citation Analysis (0)] |
| 17. | Pasca di Magliano M, Hebrok M. Hedgehog signalling in cancer formation and maintenance. Nat Rev Cancer. 2003;3:903-911. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 650] [Cited by in F6Publishing: 678] [Article Influence: 32.3] [Reference Citation Analysis (0)] |
| 18. | Gill PS, Rosenblum ND. Control of murine kidney development by sonic hedgehog and its GLI effectors. Cell Cycle. 2006;5:1426-1430. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 42] [Cited by in F6Publishing: 46] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 19. | Kinzler KW, Bigner SH, Bigner DD, Trent JM, Law ML, O’Brien SJ, Wong AJ, Vogelstein B. Identification of an amplified, highly expressed gene in a human glioma. Science. 1987;236:70-73. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 475] [Cited by in F6Publishing: 757] [Article Influence: 20.5] [Reference Citation Analysis (0)] |
| 20. | Gailani MR, Bale AE. Developmental genes and cancer: role of patched in basal cell carcinoma of the skin. J Natl Cancer Inst. 1997;89:1103-1109. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 85] [Cited by in F6Publishing: 90] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 21. | Xie J, Murone M, Luoh SM, Ryan A, Gu Q, Zhang C, Bonifas JM, Lam CW, Hynes M, Goddard A. Activating Smoothened mutations in sporadic basal-cell carcinoma. Nature. 1998;391:90-92. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 1005] [Cited by in F6Publishing: 1300] [Article Influence: 50.0] [Reference Citation Analysis (0)] |
| 22. | Berman DM, Karhadkar SS, Maitra A, Montes De Oca R, Gerstenblith MR, Briggs K, Parker AR, Shimada Y, Eshleman JR, Watkins DN. Widespread requirement for Hedgehog ligand stimulation in growth of digestive tract tumours. Nature. 2003;425:846-851. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 969] [Cited by in F6Publishing: 951] [Article Influence: 45.3] [Reference Citation Analysis (0)] |
| 23. | Garcea G, Dennison AR, Steward WP, Berry DP. Role of inflammation in pancreatic carcinogenesis and the implications for future therapy. Pancreatology. 2005;5:514-529. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 77] [Cited by in F6Publishing: 88] [Article Influence: 4.6] [Reference Citation Analysis (0)] |
| 24. | Farrow B, Evers BM. Inflammation and the development of pancreatic cancer. Surg Oncol. 2002;10:153-169. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 235] [Cited by in F6Publishing: 263] [Article Influence: 12.0] [Reference Citation Analysis (0)] |
| 25. | Jura N, Archer H, Bar-Sagi D. Chronic pancreatitis, pancreatic adenocarcinoma and the black box in-between. Cell Res. 2005;15:72-77. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 56] [Cited by in F6Publishing: 64] [Article Influence: 3.4] [Reference Citation Analysis (0)] |
| 26. | Luo JL, Maeda S, Hsu LC, Yagita H, Karin M. Inhibition of NF-kappaB in cancer cells converts inflammation- induced tumor growth mediated by TNFalpha to TRAIL-mediated tumor regression. Cancer Cell. 2004;6:297-305. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 468] [Cited by in F6Publishing: 455] [Article Influence: 22.8] [Reference Citation Analysis (0)] |
| 27. | Greten FR, Eckmann L, Greten TF, Park JM, Li ZW, Egan LJ, Kagnoff MF, Karin M. IKKbeta links inflammation and tumorigenesis in a mouse model of colitis-associated cancer. Cell. 2004;118:285-296. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 1859] [Cited by in F6Publishing: 1905] [Article Influence: 95.3] [Reference Citation Analysis (0)] |
| 28. | Karin M, Greten FR. NF-kappaB: linking inflammation and immunity to cancer development and progression. Nat Rev Immunol. 2005;5:749-759. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 2227] [Cited by in F6Publishing: 2298] [Article Influence: 120.9] [Reference Citation Analysis (0)] |
| 29. | Nakashima H, Nakamura M, Yamaguchi H, Yamanaka N, Akiyoshi T, Koga K, Yamaguchi K, Tsuneyoshi M, Tanaka M, Katano M. Nuclear factor-kappaB contributes to hedgehog signaling pathway activation through sonic hedgehog induction in pancreatic cancer. Cancer Res. 2006;66:7041-7049. [PubMed] [Cited in This Article: ] |
| 30. | Kasperczyk H, Baumann B, Debatin KM, Fulda S. Characterization of sonic hedgehog as a novel NF-kappaB target gene that promotes NF-kappaB-mediated apoptosis resistance and tumor growth in vivo. FASEB J. 2009;23:21-33. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 91] [Cited by in F6Publishing: 101] [Article Influence: 6.3] [Reference Citation Analysis (0)] |
| 31. | Yauch RL, Gould SE, Scales SJ, Tang T, Tian H, Ahn CP, Marshall D, Fu L, Januario T, Kallop D. A paracrine requirement for hedgehog signalling in cancer. Nature. 2008;455:406-410. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 748] [Cited by in F6Publishing: 780] [Article Influence: 48.8] [Reference Citation Analysis (0)] |
| 32. | Tian H, Callahan CA, DuPree KJ, Darbonne WC, Ahn CP, Scales SJ, de Sauvage FJ. Hedgehog signaling is restricted to the stromal compartment during pancreatic carcinogenesis. Proc Natl Acad Sci USA. 2009;106:4254-4259. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 307] [Cited by in F6Publishing: 332] [Article Influence: 22.1] [Reference Citation Analysis (0)] |
| 33. | Yamasaki A, Kameda C, Xu R, Tanaka H, Tasaka T, Chikazawa N, Suzuki H, Morisaki T, Kubo M, Onishi H. Nuclear factor kappaB-activated monocytes contribute to pancreatic cancer progression through the production of Shh. Cancer Immunol Immunother. 2010;59:675-686. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 34] [Cited by in F6Publishing: 40] [Article Influence: 2.7] [Reference Citation Analysis (0)] |
| 34. | Hwang RF, Moore TT, Hattersley MM, Scarpitti M, Yang B, Devereaux E, Ramachandran V, Arumugam T, Ji B, Logsdon CD. Inhibition of the hedgehog pathway targets the tumor-associated stroma in pancreatic cancer. Mol Cancer Res. 2012;10:1147-1157. [PubMed] [Cited in This Article: ] |
| 35. | Lonardo E, Frias-Aldeguer J, Hermann PC, Heeschen C. Pancreatic stellate cells form a niche for cancer stem cells and promote their self-renewal and invasiveness. Cell Cycle. 2012;11:1282-1290. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 134] [Cited by in F6Publishing: 138] [Article Influence: 11.5] [Reference Citation Analysis (0)] |
| 36. | Singh AP, Arora S, Bhardwaj A, Srivastava SK, Kadakia MP, Wang B, Grizzle WE, Owen LB, Singh S. CXCL12/CXCR4 protein signaling axis induces sonic hedgehog expression in pancreatic cancer cells via extracellular regulated kinase- and Akt kinase-mediated activation of nuclear factor κB: implications for bidirectional tumor-stromal interactions. J Biol Chem. 2012;287:39115-39124. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 89] [Cited by in F6Publishing: 96] [Article Influence: 8.0] [Reference Citation Analysis (0)] |
| 37. | Onishi H, Morifuji Y, Kai M, Suyama K, Iwasaki H, Katano M. Hedgehog inhibitor decreases chemosensitivity to 5-fluorouracil and gemcitabine under hypoxic conditions in pancreatic cancer. Cancer Sci. 2012;103:1272-1279. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 29] [Cited by in F6Publishing: 35] [Article Influence: 2.9] [Reference Citation Analysis (0)] |
| 38. | Onishi H, Kai M, Odate S, Iwasaki H, Morifuji Y, Ogino T, Morisaki T, Nakashima Y, Katano M. Hypoxia activates the hedgehog signaling pathway in a ligand-independent manner by upregulation of Smo transcription in pancreatic cancer. Cancer Sci. 2011;102:1144-1150. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 61] [Cited by in F6Publishing: 71] [Article Influence: 5.5] [Reference Citation Analysis (0)] |
| 39. | Onishi H, Morisaki T, Nakao F, Odate S, Morisaki T, Katano M. Protein-bound polysaccharide decreases invasiveness and proliferation in pancreatic cancer by inhibition of hedgehog signaling and HIF-1α pathways under hypoxia. Cancer Lett. 2013;335:289-298. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 18] [Cited by in F6Publishing: 23] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 40. | Al-Hajj M, Wicha MS, Benito-Hernandez A, Morrison SJ, Clarke MF. Prospective identification of tumorigenic breast cancer cells. Proc Natl Acad Sci USA. 2003;100:3983-3988. [PubMed] [Cited in This Article: ] |
| 41. | Li C, Heidt DG, Dalerba P, Burant CF, Zhang L, Adsay V, Wicha M, Clarke MF, Simeone DM. Identification of pancreatic cancer stem cells. Cancer Res. 2007;67:1030-1037. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 2377] [Cited by in F6Publishing: 2352] [Article Influence: 138.4] [Reference Citation Analysis (0)] |
| 42. | Hermann PC, Huber SL, Herrler T, Aicher A, Ellwart JW, Guba M, Bruns CJ, Heeschen C. Distinct populations of cancer stem cells determine tumor growth and metastatic activity in human pancreatic cancer. Cell Stem Cell. 2007;1:313-323. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 1987] [Cited by in F6Publishing: 1789] [Article Influence: 105.2] [Reference Citation Analysis (0)] |
| 43. | Bar EE, Chaudhry A, Lin A, Fan X, Schreck K, Matsui W, Piccirillo S, Vescovi AL, DiMeco F, Olivi A. Cyclopamine-mediated hedgehog pathway inhibition depletes stem-like cancer cells in glioblastoma. Stem Cells. 2007;25:2524-2533. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 448] [Cited by in F6Publishing: 479] [Article Influence: 28.2] [Reference Citation Analysis (0)] |
| 44. | Mueller MT, Hermann PC, Witthauer J, Rubio-Viqueira B, Leicht SF, Huber S, Ellwart JW, Mustafa M, Bartenstein P, D’Haese JG. Combined targeted treatment to eliminate tumorigenic cancer stem cells in human pancreatic cancer. Gastroenterology. 2009;137:1102-1113. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 250] [Cited by in F6Publishing: 272] [Article Influence: 18.1] [Reference Citation Analysis (0)] |
| 45. | Di J, Duiveman-de Boer T, Figdor CG, Torensma R. Eradicating cancer cells: struggle with a chameleon. Oncotarget. 2011;2:99-101. [PubMed] [Cited in This Article: ] |
| 46. | Lee CJ, Dosch J, Simeone DM. Pancreatic cancer stem cells. J Clin Oncol. 2008;26:2806-2812. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 255] [Cited by in F6Publishing: 283] [Article Influence: 17.7] [Reference Citation Analysis (0)] |
| 47. | Huang FT, Zhuan-Sun YX, Zhuang YY, Wei SL, Tang J, Chen WB, Zhang SN. Inhibition of hedgehog signaling depresses self-renewal of pancreatic cancer stem cells and reverses chemoresistance. Int J Oncol. 2012;41:1707-1714. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 63] [Cited by in F6Publishing: 73] [Article Influence: 6.1] [Reference Citation Analysis (0)] |
| 48. | Tang SN, Fu J, Nall D, Rodova M, Shankar S, Srivastava RK. Inhibition of sonic hedgehog pathway and pluripotency maintaining factors regulate human pancreatic cancer stem cell characteristics. Int J Cancer. 2012;131:30-40. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 139] [Cited by in F6Publishing: 158] [Article Influence: 12.2] [Reference Citation Analysis (0)] |
| 49. | Li SH, Fu J, Watkins DN, Srivastava RK, Shankar S. Sulforaphane regulates self-renewal of pancreatic cancer stem cells through the modulation of Sonic hedgehog-GLI pathway. Mol Cell Biochem. 2013;373:217-227. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 104] [Cited by in F6Publishing: 97] [Article Influence: 8.1] [Reference Citation Analysis (0)] |
| 50. | Rodova M, Fu J, Watkins DN, Srivastava RK, Shankar S. Sonic hedgehog signaling inhibition provides opportunities for targeted therapy by sulforaphane in regulating pancreatic cancer stem cell self-renewal. PLoS One. 2012;7:e46083. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 84] [Cited by in F6Publishing: 92] [Article Influence: 7.7] [Reference Citation Analysis (0)] |
| 51. | Han JB, Sang F, Chang JJ, Hua YQ, Shi WD, Tang LH, Liu LM. Arsenic trioxide inhibits viability of pancreatic cancer stem cells in culture and in a xenograft model via binding to SHH-Gli. Onco Targets Ther. 2013;6:1129-1138. [PubMed] [DOI] [Cited in This Article: ] [Cited by in F6Publishing: 1] [Reference Citation Analysis (0)] |
| 52. | Kristiansen G, Sammar M, Altevogt P. Tumour biological aspects of CD24, a mucin-like adhesion molecule. J Mol Histol. 2004;35:255-262. [PubMed] [Cited in This Article: ] |
| 53. | Aigner S, Sthoeger ZM, Fogel M, Weber E, Zarn J, Ruppert M, Zeller Y, Vestweber D, Stahel R, Sammar M. CD24, a mucin-type glycoprotein, is a ligand for P-selectin on human tumor cells. Blood. 1997;89:3385-3395. [PubMed] [Cited in This Article: ] |
| 54. | Cao X, Geradts J, Dewhirst MW, Lo HW. Upregulation of VEGF-A and CD24 gene expression by the tGLI1 transcription factor contributes to the aggressive behavior of breast cancer cells. Oncogene. 2012;31:104-115. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 86] [Cited by in F6Publishing: 92] [Article Influence: 7.1] [Reference Citation Analysis (0)] |
| 55. | Ringel J, Jesnowski R, Schmidt C, Ringel J, Köhler HJ, Rychly J, Batra SK, Löhr M. CD44 in normal human pancreas and pancreatic carcinoma cell lines. Teratog Carcinog Mutagen. 2001;21:97-106. [PubMed] [Cited in This Article: ] |
| 56. | Koong AC, Mehta VK, Le QT, Fisher GA, Terris DJ, Brown JM, Bastidas AJ, Vierra M. Pancreatic tumors show high levels of hypoxia. Int J Radiat Oncol Biol Phys. 2000;48:919-922. [PubMed] [Cited in This Article: ] |
| 57. | Caldwell CC, Kojima H, Lukashev D, Armstrong J, Farber M, Apasov SG, Sitkovsky MV. Differential effects of physiologically relevant hypoxic conditions on T lymphocyte development and effector functions. J Immunol. 2001;167:6140-6149. [PubMed] [Cited in This Article: ] |
| 58. | Höckel S, Schlenger K, Vaupel P, Höckel M. Association between host tissue vascularity and the prognostically relevant tumor vascularity in human cervical cancer. Int J Oncol. 2001;19:827-832. [PubMed] [Cited in This Article: ] |
| 59. | Lei J, Ma J, Ma Q, Li X, Liu H, Xu Q, Duan W, Sun Q, Xu J, Wu Z. Hedgehog signaling regulates hypoxia induced epithelial to mesenchymal transition and invasion in pancreatic cancer cells via a ligand-independent manner. Mol Cancer. 2013;12:66. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 95] [Cited by in F6Publishing: 110] [Article Influence: 10.0] [Reference Citation Analysis (0)] |
| 60. | Spivak-Kroizman TR, Hostetter G, Posner R, Aziz M, Hu C, Demeure MJ, Von Hoff D, Hingorani SR, Palculict TB, Izzo J. Hypoxia triggers hedgehog-mediated tumor-stromal interactions in pancreatic cancer. Cancer Res. 2013;73:3235-3247. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 137] [Cited by in F6Publishing: 144] [Article Influence: 13.1] [Reference Citation Analysis (0)] |
| 61. | Bahra M, Kamphues C, Boas-Knoop S, Lippert S, Esendik U, Schüller U, Hartmann W, Waha A, Neuhaus P, Heppner F. Combination of hedgehog signaling blockage and chemotherapy leads to tumor reduction in pancreatic adenocarcinomas. Pancreas. 2012;41:222-229. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 20] [Cited by in F6Publishing: 22] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 62. | Qin CF, Hao K, Tian XD, Xie XH, Yang YM. Combined effects of EGFR and Hedgehog signaling pathway inhibition on the proliferation and apoptosis of pancreatic cancer cells. Oncol Rep. 2012;28:519-526. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 7] [Cited by in F6Publishing: 12] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 63. | Eberl M, Klingler S, Mangelberger D, Loipetzberger A, Damhofer H, Zoidl K, Schnidar H, Hache H, Bauer HC, Solca F. Hedgehog-EGFR cooperation response genes determine the oncogenic phenotype of basal cell carcinoma and tumour-initiating pancreatic cancer cells. EMBO Mol Med. 2012;4:218-233. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 126] [Cited by in F6Publishing: 132] [Article Influence: 11.0] [Reference Citation Analysis (0)] |
| 64. | Chitkara D, Singh S, Kumar V, Danquah M, Behrman SW, Kumar N, Mahato RI. Micellar delivery of cyclopamine and gefitinib for treating pancreatic cancer. Mol Pharm. 2012;9:2350-2357. [PubMed] [Cited in This Article: ] |
| 65. | Gu D, Liu H, Su GH, Zhang X, Chin-Sinex H, Hanenberg H, Mendonca MS, Shannon HE, Chiorean EG, Xie J. Combining hedgehog signaling inhibition with focal irradiation on reduction of pancreatic cancer metastasis. Mol Cancer Ther. 2013;12:1038-1048. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 35] [Cited by in F6Publishing: 46] [Article Influence: 4.2] [Reference Citation Analysis (0)] |
| 66. | Onishi H, Morisaki T, Kiyota A, Koya N, Tanaka H, Umebayashi M, Katano M. The Hedgehog inhibitor cyclopamine impairs the benefits of immunotherapy with activated T and NK lymphocytes derived from patients with advanced cancer. Cancer Immunol Immunother. 2013;62:1029-1039. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 7] [Cited by in F6Publishing: 7] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 67. | Onishi H, Morisaki T, Kiyota A, Koya N, Tanaka H, Umebayashi M, Katano M. The Hedgehog inhibitor suppresses the function of monocyte-derived dendritic cells from patients with advanced cancer under hypoxia. Biochem Biophys Res Commun. 2013;436:53-59. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 3] [Cited by in F6Publishing: 4] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 68. | Sekulic A, Migden MR, Oro AE, Dirix L, Lewis KD, Hainsworth JD, Solomon JA, Yoo S, Arron ST, Friedlander PA. Efficacy and safety of vismodegib in advanced basal-cell carcinoma. N Engl J Med. 2012;366:2171-2179. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 947] [Cited by in F6Publishing: 950] [Article Influence: 79.2] [Reference Citation Analysis (0)] |
| 69. | Kelleher FC. Hedgehog signaling and therapeutics in pancreatic cancer. Carcinogenesis. 2011;32:445-451. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 80] [Cited by in F6Publishing: 97] [Article Influence: 6.9] [Reference Citation Analysis (0)] |
| 70. | Sandhiya S, Melvin G, Kumar SS, Dkhar SA. The dawn of hedgehog inhibitors: Vismodegib. J Pharmacol Pharmacother. 2013;4:4-7. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 56] [Cited by in F6Publishing: 60] [Article Influence: 5.5] [Reference Citation Analysis (0)] |
| 71. | Fendrich V, Wiese D, Waldmann J, Lauth M, Heverhagen AE, Rehm J, Bartsch DK. Hedgehog inhibition with the orally bioavailable Smo antagonist LDE225 represses tumor growth and prolongs survival in a transgenic mouse model of islet cell neoplasms. Ann Surg. 2011;254:818-823; discussion 823. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 29] [Cited by in F6Publishing: 31] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 72. | Fu J, Rodova M, Roy SK, Sharma J, Singh KP, Srivastava RK, Shankar S. GANT-61 inhibits pancreatic cancer stem cell growth in vitro and in NOD/SCID/IL2R gamma null mice xenograft. Cancer Lett. 2013;330:22-32. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 110] [Cited by in F6Publishing: 127] [Article Influence: 10.6] [Reference Citation Analysis (0)] |
| 73. | Mo W, Xu X, Xu L, Wang F, Ke A, Wang X, Guo C. Resveratrol inhibits proliferation and induces apoptosis through the hedgehog signaling pathway in pancreatic cancer cell. Pancreatology. 2011;11:601-609. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 58] [Cited by in F6Publishing: 59] [Article Influence: 4.9] [Reference Citation Analysis (0)] |
| 74. | Sun XD, Liu XE, Huang DS. Curcumin reverses the epithelial-mesenchymal transition of pancreatic cancer cells by inhibiting the Hedgehog signaling pathway. Oncol Rep. 2013;29:2401-2407. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 73] [Cited by in F6Publishing: 78] [Article Influence: 7.1] [Reference Citation Analysis (0)] |
| 75. | Bi X, Han X, Zhang F, He M, Zhang Y, Zhi XY, Zhao H. Triparanol suppresses human tumor growth in vitro and in vivo. Biochem Biophys Res Commun. 2012;425:613-618. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 9] [Cited by in F6Publishing: 10] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 76. | Nolan-Stevaux O, Lau J, Truitt ML, Chu GC, Hebrok M, Fernández-Zapico ME, Hanahan D. GLI1 is regulated through Smoothened-independent mechanisms in neoplastic pancreatic ducts and mediates PDAC cell survival and transformation. Genes Dev. 2009;23:24-36. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 298] [Cited by in F6Publishing: 313] [Article Influence: 20.9] [Reference Citation Analysis (0)] |
| 77. | An Y, Cai B, Chen J, Lv N, Yao J, Xue X, Tu M, Tang D, Wei J, Jiang K. MAP3K10 promotes the proliferation and decreases the sensitivity of pancreatic cancer cells to gemcitabine by upregulating Gli-1 and Gli-2. Cancer Lett. 2013;329:228-235. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 14] [Cited by in F6Publishing: 17] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 78. | Li X, Ma Q, Xu Q, Liu H, Lei J, Duan W, Bhat K, Wang F, Wu E, Wang Z. SDF-1/CXCR4 signaling induces pancreatic cancer cell invasion and epithelial-mesenchymal transition in vitro through non-canonical activation of Hedgehog pathway. Cancer Lett. 2012;322:169-176. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 132] [Cited by in F6Publishing: 175] [Article Influence: 14.6] [Reference Citation Analysis (0)] |
| 79. | Low JA, de Sauvage FJ. Clinical experience with Hedgehog pathway inhibitors. J Clin Oncol. 2010;28:5321-5326. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 148] [Cited by in F6Publishing: 158] [Article Influence: 11.3] [Reference Citation Analysis (0)] |
| 80. | Merchant AA, Matsui W. Targeting Hedgehog--a cancer stem cell pathway. Clin Cancer Res. 2010;16:3130-3140. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 348] [Cited by in F6Publishing: 379] [Article Influence: 27.1] [Reference Citation Analysis (0)] |
| 81. | Sahebjam S, Siu LL, Razak AA. The utility of hedgehog signaling pathway inhibition for cancer. Oncologist. 2012;17:1090-1099. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 39] [Cited by in F6Publishing: 42] [Article Influence: 3.5] [Reference Citation Analysis (0)] |
| 82. | Atwood SX, Chang AL, Oro AE. Hedgehog pathway inhibition and the race against tumor evolution. J Cell Biol. 2012;199:193-197. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 84] [Cited by in F6Publishing: 93] [Article Influence: 8.5] [Reference Citation Analysis (0)] |